Abstract
Recent evidence supports ‘the neurotrophin hypothesis of depression' in its prediction that brain-derived neurotrophic factor (BDNF) is involved in depression. However, some key questions remain unanswered, including whether abnormalities in BDNF persist beyond the clinical state of depression, whether BDNF levels are related to the clinical features of depression and whether distinct antidepressants affect BDNF levels equally. We addressed these questions and investigated serum BDNF levels in 962 depressed patients, 700 fully remitted persons (⩾6 months) and 382 healthy controls. We found serum BDNF levels to be low in antidepressant-free depressed patients relative to controls (P=0.007) and to depressed patients who were treated with an antidepressant (P=0.001). BDNF levels of fully remitted persons (whether unmedicated or treated with an antidepressant) were comparable to those of controls. Analyzing the sample of antidepressant-free depressed patients showed that BDNF levels were unrelated to the core clinical features of depression such as its severity or first versus a recurrent episode. The antidepressant associated upregulation of serum BDNF in depressed patients was confined to selective serotonin reuptake inhibitors (SSRIs) (P=0.003) and St John's wort (P=0.03). Our results suggest that low serum levels of BDNF are a state abnormality that is evident during depression and normalizes during remission. Increases in serum levels of BDNF during antidepressant treatment appear to be confined to some antidepressants and do not parallel clinical characteristics, such as the severity of depressive symptoms.
Keywords: depression, brain-derived neurotrophic factor, antidepressants, BDNF
Introduction
Brain-derived neurotrophic factor (BDNF) is a neurotrophin that has been linked to the viability of neurons in brain circuits that regulate emotion, memory, learning, sleep and appetite.1, 2 The neurotrophin hypothesis of depression is based on these functions of BDNF and postulates that depression results from stress-induced decreases in BDNF expression and that antidepressants are efficacious because they increase BDNF expression.3, 4 Consistent with this hypothesis are the findings that depression is accompanied by decreased central and peripheral levels of BDNF,5, 6, 7 and that antidepressants elicit an increase in BDNF levels in animal models for depression8 and in depressed humans.6, 7 Together with the latency of weeks before antidepressants become clinically effective,9 these observations shaped the hypothesis that the efficacy of antidepressants depend on neuroadaptive changes that are brought about by changes in BDNF signaling.3
Taken together, there is reason to believe that BDNF is involved in depression and in antidepressant action. Results inconsistent with the neurotrophin hypothesis, however, also have been reported. There are, for example, studies that did not detect alternations in BDNF in depressed persons or in the course of treatment with an antidepressant.10, 11, 12 In addition, some questions remain unanswered so that the neurotrophin hypothesis is at best incomplete.13 A major question that largely remains to be answered is whether low BDNF levels persist beyond the clinical state of depression.14, 15 A second question is whether BDNF levels are related to the clinical features of depression, such as a first versus a recurrent episode.16, 17 Yet a third outstanding question is whether all classes of antidepressants affect BDNF levels equally.6, 7
We studied, cross-sectionally, serum BDNF levels of depressed patients, remitted depressed persons and never depressed persons. Our efforts had three concerns: (1) to compare serum BDNF levels of antidepressant-free and antidepressant treated current and fully remitted depressed patients and never depressed persons, (2) to explore the associations between some of the core clinical features of depression and serum BDNF levels and (3) to evaluate the association between the use of several distinct classes of antidepressants and serum BDNF levels.
Materials and methods
Patients and sample collection
Patients were from the Netherland Study of Depression and Anxiety (NESDA). Full details on the rationale, objectives and protocol of NESDA are described in a previous paper by Penninx et al.18 In brief, NESDA is a prospective cohort study (N=2981) that recruited patients in mental health care, primary care and in the general population. Included were persons with a depressive and/or an anxiety disorder, persons with a depressive and/or an anxiety disorder in remission and persons without a history or current depressive or anxiety disorders. Persons who were diagnosed with psychotic, bipolar I or II, obsessive compulsive or severe alcohol use disorder were not eligible. Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) diagnoses19 were assigned on the basis of responses to the Composite International Diagnostic Interview 2.1 lifetime version20 that was administered by trained interviewers. At baseline, participants provided blood samples, underwent a medical examination and gave written informed consent for the study that was approved by the Ethical Committees of the participating institutes.
Our study enrolled 2044 persons (68.6% of the NESDA sample). On the basis of the diagnosis, antidepressant use and the availability of BDNF data, we created five groups: antidepressant-free depressed patients (n=541), antidepressant-treated depressed patients (n=421), antidepressant-free remitted depressed persons (n=539), antidepressant-treated remitted depressed persons (n=161) and healthy persons who served as controls (n=382). Depressed patients met the criteria for a depressive episode within the last 6 months (n=541). The majority of these patients had a current diagnosis of depression (n=388), but some (n=153) had a diagnosis of depression 1–6 months prior to baseline and did not fulfill all criteria in the past month. Persons who were in full remission of depression were diagnosed with major depressive disorder (MDD) somewhere in their lives, but had been free of depression and anxiety during at least 6 months. Persons were included in the control group when they had: (1) no lifetime mood or anxiety disorders, (2) no documented family history of depression or anxiety and (3) a low score (⩽14) on the inventory of depressive symptoms.21
Antidepressants
Data on the use of antidepressants were acquired through drug container observation and self-report. Use of an antidepressant was defined as intake of minimally the daily dose as recommended by the World Health Organization22 during the last month on at least 50% of the days. We coded for the use of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, noradrenergic and specific serotonergic antidepressants (NaSSAs) and St John's wort (Hypericum perforatum). The duration of use was expressed in months.
Clinical features of MDD
All patients were characterized on the symptom severity of depression using the Inventory of Depressive Symptom.21 Patient samples were further characterized on having a first or a recurrent depressive episode, the presence of comorbid anxiety, the age at onset of depression, the recency of depression, the chronicity of depression and on the presence of suicide ideation. The Composite International Diagnostic Interview20 served as source of information on the presence of a first or a recurrent depressive episode, the presence of a comorbid anxiety, age at onset of depression (that is the age at which the first episode occurred) and the recency of depression (that is fulfilling criteria in the past month versus fulfilling criteria in the past 6 months but not in the past month). Depression was considered chronic if symptoms had been present ⩾24 months during the last 5 years, which was assessed using the Life Chart method.23 The scale for suicide ideation24 was used to examine the presence (yes versus no) of suicide ideation during the past week.
BDNF protein measurements
A measure of 50 ml of blood was withdrawn into vacuum tubes between 0730 and 0930 hours after an overnight fast. After blood collection, serum was separated and stored at −85°C until it was assayed. BDNF protein levels were measured using the Emax Immuno Assay system from Promega according to the manufacturer's protocol (Madison, WI, USA), in one laboratory (Maastricht University) by one technician who was blind to the diagnoses. Undiluted serum was acid treated as this reliably increased the detectable BDNF in a dilution-dependent way. Greiner Bio-One high affinity 96-well plates were used. Serum samples were diluted 100 times, and the absorbency was read in duplicate using a Bio-Rad (Hercules, CA, USA) Benchmark microplate reader at 450 nm. The intra- and inter-assay coefficients of variation were found to be within 3 and 9%, respectively. Four persons had BDNF values that were below the reliable detection threshold of 1.56 ng ml−1. These values were set at the lower detection limit. Positive outliers (mean+3 s.d., n=6) were trimmed to the mean+3 s.d. value. There were no differences between persons with missing and non-missing BDNF with regard to gender (P=0.71), age (P=0.67) and diagnoses (P=0.33).
Covariates
Potential variance due to gender, age and educational level was controlled for in all analyses. In addition, we controlled for body mass index (BMI), physical activity and smoking as these variables are associated with BDNF25, 26, 27 and mood.28, 29, 30 Data on weight and height were collected, and BMI was calculated (weight/height2). Information on physical activity was gathered using the International Physical Activity Questionnaire31 and expressed as the number of met-minutes (that is the ratio of the amount of energy expenditure during activity to the energy expenditure at rest). Smoking status was dichotomized as current versus non-smoker. Time of the morning blood withdrawal and duration of serum storage were controlled for since BDNF levels vary according to variation on these variables.32, 33
Statistical analyses
All computations were performed in SPSS version 17.0 (SPSS, Chicago, IL, USA). BDNF values were controlled for basic covariates in all analyses. Effect sizes on pairwise comparisons were presented as Cohen's d.34 A two-tailed α level of 0.05 was used to determine statistical significance.
Analysis of variance (ANOVA) was used to compare BDNF levels of antidepressant-free depressed patients and antidepressant-treated depressed patients, antidepressant-free patients and antidepressant-treated persons who were in remission (⩾6 months) and controls. Post hoc tests between the groups were performed following a significant F-statistic using Tukey's test.
A multivariable regression analysis was used to identify whether the clinical features of depression were associated with BDNF levels. Regression was performed in patient groups in which the mean BDNF level deviated significantly from the control group. Pearson correlation coefficients between predictors and BDNF levels were also calculated. Basic covariates were entered in the first step of regression. In the second step, the clinical features of depression were entered. The regression model was fit using method enter. Tolerance of the predictors and normality of error variances was verified.
To establish whether the use of an antidepressant effected BDNF levels equally in current and remitted depression, a 2 (currently depressed versus depression in (full) remission) × 2 (antidepressants; yes or no) ANOVA was performed. Potential antidepressant-specific associations between the use of SSRI, tricyclic antidepressant, serotonin-norepinephrine reuptake inhibitor, NaSSA and St John's wort and BDNF levels were evaluated by contrasting BDNF levels of persons who used one of these agents against the BDNF level of the antidepressant-free persons. Analyses were repeated with the severity of depressive symptoms and the duration of antidepressant use as covariates.
Results
Demographics and clinical features
Demographical and clinical features among the five groups are given in Table 1. ANOVA and χ2 tests showed that, compared with controls, depressed and remitted persons were more likely to be female, to be older, to have received fewer years of education and to smoke. BMI was higher in current and remitted antidepressant-treated depressed persons compared with controls and to antidepressant-free depressed and remitted persons. The amount of physical activity was low in the antidepressant treated currently depressed group relative to the other groups. Post hoc comparisons on demographical and clinical features between the current and remitted depressed groups are given in Table 1.
Table 1. Demographic and clinical characteristics (percentages (%) or mean±s.d.) of participants by depression diagnosis (never, current and remitted) and antidepressant use (yes versus no).
| Characteristic | Controls (n=382) | Current MDD no antidepressants (n=541) | Current MDD antidepressants (n=421) | Remitted MDD no antidepressants (n=539) | Remitted MDD antidepressants (n=161) | P-value |
|---|---|---|---|---|---|---|
| Female (%) | 61.0 | 66.7 | 67.0 | 71.1 | 70.8 | <0.05 |
| Age | 45.7±12.3 | 39.8±12.6 | 42.6±11.0 | 43.1±12.9 | 45.4±10.8 | <0.001a,b |
| Education (years) | 13.4±3.3 | 11.9±3.2 | 11.7±3.3 | 12.6±3.1 | 12.1±3.3 | <0.001 |
| BMI | 25.4±4.6 | 25.5±5.4 | 26.3±5.6 | 25.3±4.6 | 26.6±5.6 | <0.01a,b |
| Mean met-minutes (weeks)c | 3.7±3.0 | 3.5±3.3 | 3.2±3.3 | 3.8±3.1 | 3.1±2.8 | <0.01b |
| Smoker (%) | 16.5 | 38.7 | 46.0 | 35.5 | 34.3 | <0.001a |
| Alcohol dependent (%) | 5.4 | 23.3 | 20.0 | 17.0 | 13.7 | <0.001 |
| Depression severity, IDS | 5.3±3.5 | 29.6±12.7 | 34.5±13.1 | 16.8±10.3 | 20.3±10.6 | <0.001a,b |
| Age of onset of MDD | NA | 26.1±12.3 | 27.4±12.6 | 27.6±12.2 | 28.2±11.7 | 0.35 |
| Chronic MDD (%)d | NA | 27.5 | 38.3 | 11.1 | 18.7 | <0.001a,b |
| >1 episode of MDD (%) | NA | 63.6 | 58.2 | 54.6 | 61.5 | <0.05a |
| Comorbid anxiety (%)e | NA | 42.2 | 47.7 | NA | NA | <0.05 |
| Suicide ideation (%) | NA | 22.4 | 29.3 | 5.2 | 6.2 | <0.001a |
| Antidepressant medication | ||||||
| SSRI (%) | NA | NA | 62.7 | NA | 65.8 | 0.27 |
| SNRI (%) | NA | NA | 16.4 | NA | 13.0 | 0.06 |
| TCA (%) | NA | NA | 8.1 | NA | 13.0 | 0.19 |
| NaSSA (%) | NA | NA | 8.6 | NA | 2.5 | <0.05 |
| St John's wort (%) | NA | NA | 4.3 | NA | 5.6 | 0.32 |
| Duration of use (months) | NA | NA | 7.5±4.9 | NA | 10.9±3.5 | <0.001 |
Abbreviations: BMI, body mass index; IDS, inventory of depressive symptoms; MDD, major depressive disorder; NaSSA, noradrenergic and specific serotonergic antidepressant; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Indicates a statistically significant difference (at P<0.05) between the antidepressant treated and antidepressant free current MDD groups.
Indicates a statistically significant difference (at P<0.05) between the antidepressant treated and antidepressant free remitted MDD groups.
Mean met-minutes (that is ratio of energy expenditure during activity to energy expenditure at rest) divided by 1000.
Symptoms were considered chronic if they were present for at least 24 months during the last 5 years.
Included social phobia, panic disorder with and without agoraphobia, agoraphobia and generalized anxiety disorder.
BDNF levels in persons with current or remitted depression and controls
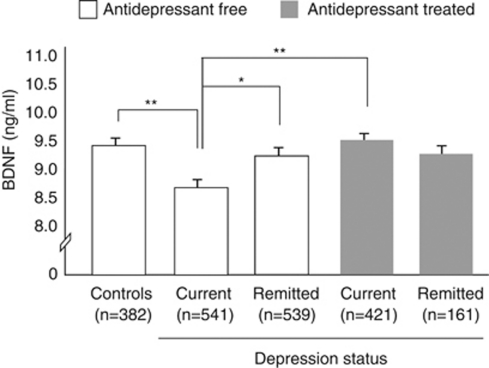
An ANOVA model showed a main effect of diagnostic status on serum levels of BDNF (F1,1578=4.09, P=0.01). Pairwise comparisons (see Figure 1) indicated that serum BDNF levels were low in antidepressant-free depressed patients compared with controls (d=0.19), to antidepressant-free persons who were in full remission (d=0.15) and to antidepressant-treated depressed patients (d=0.23). BDNF levels of antidepressant-free persons who were in full remission and depressed patients who were treated with an antidepressant were comparable to those of controls.
Figure 1.
Plotted are mean serum brain-derived neurotrophic factor (BDNF) levels by diagnoses and antidepressant use. Error bars reflect the standard error of the mean. *Denotes statistical significance at P<0.05. **Denotes statistical significance at P<0.01.
BDNF and the clinical features of MDD
The exploration of the association between the clinical features of MDD and serum BDNF was restricted to the antidepressant free currently depressed group, as BDNF levels in this group were low relative to controls.
Pearson's correlation showed that female gender and being in the early remission phase of depression (1–6 months) versus having a current episode were negatively associated with serum BDNF. Age, BMI, age at onset of MDD and the presence of comorbid anxiety were positively associated to serum BDNF (Table 2).
Table 2. Results of correlation and multivariable regression analyses of demographical and clinical characteristics with serum levels of BDNF in antidepressant free patients with MDD.
| ra | B | 95% CI B | β | t | P-value | |
|---|---|---|---|---|---|---|
| Gender (1=male, 2=female) | −0.13** | −0.65 | −1.24 to −0.06 | −0.10 | −2.15 | 0.03 |
| Age (continuous, years) | 0.17** | 0.03 | 0.01 to 0.06 | 0.11 | 1.98 | 0.04 |
| Education (continuous, years) | −0.04 | −0.01 | −0.09 to 0.08 | −0.005 | −0.11 | 0.91 |
| BMI (continuous) | 0.13** | 0.06 | 0.01 to 0.10 | 0.09 | 1.97 | 0.04 |
| Met-minutes (continuous, IPAQ) | −0.02 | −0.001 | −0.01 to 0.01 | −0.009 | −0.21 | 0.83 |
| Smoker (1=no, 2=yes) | −0.02 | −0.07 | −0.04 to 0.02 | −0.02 | −0.44 | 0.66 |
| Time of Blood withdrawal (continuous)b | −0.04 | −0.004 | −0.12 to 0.02 | −0.04 | −1.11 | 0.23 |
| Duration of serum storage (continuous, days) | 0.02 | 0.14 | −0.40 to 0.68 | 0.02 | 0.49 | 0.62 |
| MDD status (1=current, 2=early remitted)c | −0.11* | −0.15 | −0.50 to 0.25 | −0.04 | −0.64 | 0.52 |
| MDD severity (continuous, IDS) | 0.03 | −0.007 | −0.04 to 0.02 | −0.06 | −1.12 | 0.24 |
| MDD type (1=single episode, 2=recurrent) | 0.01 | 0.05 | −0.56 to 0.66 | 0.007 | 0.15 | 0.88 |
| Comorbid anxiety disorder (1=no, 2=yes) | 0.08* | 0.31 | −0.36 to 0.97 | 0.05 | 0.91 | 0.36 |
| Age at onset MDD (continuous) | 0.14** | 0.08 | −0.04 to 0.18 | 0.07 | 1.25 | 0.21 |
| Chronic MDD (1=no, 2=yes) | 0.07 | 0.19 | −0.49 to 0.87 | 0.03 | 0.55 | 0.58 |
| Suicide ideation (1=no, 2=yes) | 0.06 | 0.59 | −0.13 to 1.33 | 0.07 | 1.51 | 0.12 |
Abbreviations: BDNF, brain-derived neurotrophic factor; BMI, body mass index; CI, confidence interval; IDS, inventory of depressive symptoms; IPAQ, international physical activity questionnaire; MDD, major depressive disorder.
*Denotes statistical significance of the univariate correlation at P<0.05.
**Denotes statistical significance of the univariate correlation at P<0.01.
Univariate correlation with serum levels of BDNF; Pearson's r for continuous variables and Spearman's ρ for variables.
In minutes from 0600 hours.
The presence of a current (1 month) versus an early remission (1–6 months of remission) diagnosis.
Basic covariates were entered in the first step of the multivariable regression analysis, followed by the clinical features that were entered in step two. Tolerance of the predictors was high (all>0.70), indicating that our individual predictors were not redundant with one another. Error variances were normally distributed. Results of the first step showed that gender and age were significant predictors of BDNF levels. Women had lower levels of BDNF compared with men (β=−0.10, P=0.02) and older patients had higher levels of BDNF (β=0.11, P=0.002) compared with younger patients. Results of the second step showed that none of the clinical features (listed in Table 2) was significantly associated with serum BDNF. Gender and age preserved its significance. BMI emerged as a significant (positive) predictor of serum BDNF. Table 2 presents the results of the second step of the regression analysis.
BDNF and the use of antidepressants
A 2 (currently depressed versus depression in (full) remission) × 2 (antidepressant use; yes versus no) ANOVA showed that diagnostic status interacted with antidepressant use (F1,1578=4.19, P=0.03), indicating that the use of an antidepressant during a depressive episode was associated with higher BDNF levels, whereas in the remission phase, the use of an antidepressant did not show such an association (see Figure 2). Main effects of diagnostic status and antidepressant use were not observed.
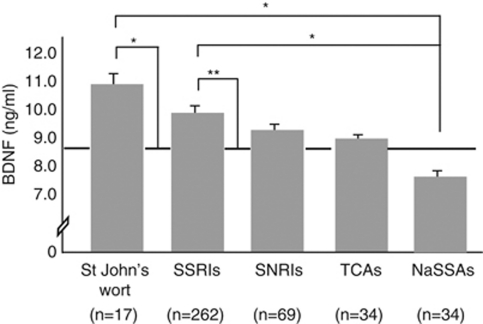
Figure 2.
Plotted are mean serum brain-derived neurotrophic factor (BDNF) levels by specific class of antidepressant (St John's wort, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs) and noradrenergic and specific serotonergic antidepressants (NaSSAs)). The dashed line indicates the mean BDNF level of the antidepressant-free depressed group. Error bars reflect the standard error of the mean. *Denotes statistical significance at P<0.05. **Denotes statistical significance at P<0.01.
To uncover potential differences between various classes of antidepressants, we compared BDNF levels of depressed patients who used SSRIs, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, NaSSAs or St John's wort among each other and with BDNF level of antidepressant-free depressed patients. This analysis was restricted to the currently depressed group as the effect of the use of an antidepressant on serum BDNF levels was confined to this group. In this group, 67% (n=282) used antidepressant for longer than 12 weeks. We observed a main effect of group (F5,941=4.29, P<0.001). Post hoc comparisons (see Figure 2) showed that, relative to not using an antidepressant, the use of SSRIs (d=0.39) and St John's wort (d=0.63) was associated with high levels of BDNF. The use of a NaSSA was associated with low levels of BDNF relative to SSRI (d=0.54) and St John's wort (d=0.85) use. Analyses were run with and without co-varying for the severity of depressive symptoms and for the duration of antidepressant use. These analyses revealed a similar pattern of results. Furthermore, serum BDNF levels were unrelated to treatment duration (r=−0.02, P=0.65), which might suggest that our findings were not driven by the duration of antidepressant use.
Discussion
Largely in accord with previous findings6, 7 and with the neurotrophin hypothesis of depression,3, 4 our data showed that serum BDNF levels were low in antidepressant-free depressed patients compared with healthy controls. Our data further showed that BDNF levels were low in depressed patients who were not on antidepressant medication compared with antidepressant-free persons who were in full remission and that BDNF levels of this latter group were comparable to those of controls. Herewith, we establish as one of the first14 that low levels of BDNF in serum are a state characteristic for depression. In line with one study that reported low levels of BDNF in euthymic patients,15 we found that patients who were in early remission (1–6 months) had serum BDNF levels that were comparable to those of currently depressed patients. Thus, serum BDNF levels remain low after clinical improvement has set in. This could indicate that low levels of BDNF are a consequence of depressive symptoms that persist into early remission. Alternatively, the low levels of BDNF during early remission might also represent a scar of a depressive episode. These explanations could not be fully elucidated in the current study and longitudinal designs clearly are essential to understand this issue.
We were unable to replicate the earlier findings that a higher depression severity,17, 35 having a recurrent compared with a first episode of MDD16 and the occurrence of suicide ideation36, 37 are accompanied by lower levels of BDNF. In fact, we even found that the early remission phase, which was accompanied by a lower symptom severity of depression (mean inventory of depressive symptoms scores were 22.4±11.4 versus 32.4±12.1 in early remitted and currently depressed patients respectively), was associated with somewhat lower BDNF levels compared with the current depressive state. The other clinical features (that is age at onset of depression, the presence of comorbid anxiety and the chronicity of depression) also were unrelated to serum BDNF in multivariable analyses. These findings, given the size of the current cohort, give us confidence in excluding the clinical features of depression as potential correlates of serum BDNF levels. This might be an important conclusion, as it hints that other (than specifically depression related) factors may be at play in the relative fall of BDNF levels during a depressive episode. Interestingly, being male and having a higher BMI were found to be positively associated with BDNF among antidepressant-free depressed patients. Although these findings were unsought, they parallel the results of some previous studies,17, 38, 39 and they give ground to interesting hypotheses. For example, as weight loss is a prime behavioral abnormality of depression19 and often a residual symptom in early remission40, 41 it could be that, alternations in BDNF levels are mediated by (transient) changes in eating behavior during, or in the aftermath of, a depressive episode. Likewise, weight gain is a documented side effect of antidepressant treatment,42, 43 and thus the absence of weight loss could potentially explain the absence of a relative fall of BDNF in depressed patients during treatment with an antidepressant.
Alternative factors that have been proposed to underlie the low levels of BDNF during depression are exposure to stressful life events. Two studies, for example, found that adverse life events are associated with lower peripheral BDNF levels within a depressed and bipolar patient samples.44, 45 Therefore, it seems worthwhile to integrate a wider range of variables, notably (early) adverse life events, but also genetic variants and their interactions with environmental variables46 in models that study the link between BDNF and depression.
In addition, we found that serum BDNF levels were higher in antidepressant-treated patients compared with patients who were antidepressant free. This finding largely is in accord with previous findings.6, 7 We were able to expand previous findings by showing that the use of an antidepressant is associated with increased serum BDNF during a depressive episode but not during remission. This suggests that antidepressant-induced increases in BDNF occur in a disease state when BDNF functioning might be defective and not in remission when BDNF functioning is normalized. In addition, we found the increase in serum BDNF levels to be a specific associate of the use of SSRIs and St John's wort and not of the use of serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants or NaSSAs. Although not directly confirmed, this finding might be explained by increased availability of extra-synaptic levels of serotonin. It is known that serotonin stimulates the expression of BDNF.47, 48 In line with this, we found the highest BDNF levels in patients who were treated with an agent that generally leads to an increase in the availability of serotonin (that is SSRIs and St John's wort).49, 50 Furthermore, we found the lowest levels of BDNF in patients who were treated with agents that have little or no impact on the availability of serotonin (that is NaSSAs).43, 51 Nevertheless, this antidepressant-specific finding seems at odds with the specific prediction of the neurotrophin hypothesis, stating that increases in BDNF levels are a key mediator for an antidepressant response to occur.3 According to this prediction, one might expect that antidepressants that are known to be about equally efficacious in the treatment of the symptoms of depression50, 51, 52 would have similar effects on serum BDNF levels. Yet another finding that seems hard to reconcile with the neurotrophin hypothesis is that the group of depressed persons who used antidepressants (prolonged and frequently) had the highest BDNF levels, but also the highest symptom severity of depression. This suggests, to our belief that increases in peripheral BDNF levels do not parallel clinical effectiveness, or at least have no direct effects on the depression characteristics such as its severity. Such a conclusion on the absence of direct effects could also be drawn on the findings that the severity of a depressive episode was unrelated to serum BDNF levels and that persons who were in early remission had similar levels of BDNF yet marked lower levels of depression severity compared with depressed patients.
Caution, however, is warranted when interpreting our findings on the associations between the use of an antidepressant and serum levels of BDNF because our patients were not randomly assigned to the various drugs (or no drug) conditions. Thus, our findings might be confounded by indication. An additional limitation of our study is that we relied on data that were collected in a single wave, precluding any form of causality. Furthermore, we measured serum levels of BDNF and assume that these measurements mirror the amount of BDNF in the brain. This assumption is validated on preclinical work that showed that cortical and peripheral levels of BDNF are correlated53, 54, 55 but remains complicated, because in addition to neurons, several other tissues serve as sources of BDNF in serum.54 However, various strengths of our study seem evident and these include the use of multivariable techniques and the large sample size (that relates positive to all previous studies and to two previous meta-analyses6, 7 as well).
In conclusion, we believe that our data indicate that low levels of BDNF in blood serum are a state characteristic of depression and thus an abnormality that is evident during the clinical state and the early remission phase of depression but not when the symptoms of depression are in full remission. Our findings further suggest that some of the core clinical features of depression are unrelated to serum levels of BDNF. Finally, increases in serum levels of BDNF appear to be a specific pharmacological effect of a subset of antidepressants that does not parallel depression characteristics such as the severity of depression.
Acknowledgments
We thank Robin Struijk (Maastricht University) for determining serum levels of BDNF in our sample. The NESDA study infrastructure is financed by the Geestkracht program of ZonMW, the Dutch Scientific Organisation-Medical Sciences (Grant no. 10.000.1002) and by complementary funding from participating mental healthcare institutions (GGZ Buitenamstel, GGZ Drenthe, GGZ Friesland, GGZ Geestgronden, GGZ Rivierduinen and Lentis) and Universities (Leiden University Medical Center, University Medical Center Groningen and VU University Medical Center). BDNF measurements were financed with NWO (Dutch Scientific Organisation) VIDI-grant (Grant no. 016.085.353) awarded to Dr Elzinga. Contribution of Dr Oude Voshaar was made possible by a NWO Clinical Fellowship (Grant no. 907.0023.1).
The authors declare no conflict of interest.
References
- Duman RS, Malberg J, Nakagawa S, D'Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2000;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brains of suicide victims. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analyses of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ. Treatment of mood disorders. Nat Neurosci. 2002;6:1068–1070. doi: 10.1038/nn943. [DOI] [PubMed] [Google Scholar]
- Basterzi AD, Yazici K, Aslan E, Delialioglu N, Tasdelen B, Acar ST, et al. Effects of fluoxetine and venlafaxine on serum brain-derived neurotrophic factor levels in depressed patients. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:281–285. doi: 10.1016/j.pnpbp.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Bonaccorso S, Ricciardi A, Scaccianoce S, Panaccione I, Wang L, et al. Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, ecitalopram, or venlafaxine. J Psych Res. 2009;43:247–254. doi: 10.1016/j.jpsychires.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenhorn AA, Schulte-Hebruggen O, Danker-Hopfe H, Malbranc M, Hartung H-D, Anders D. Serun neurotrophins—a study on the time course and influencing factors in a large old sample. Neurobiol Aging. 2007;28:1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Groves JO. Is it time to reassess the BDNF hypothesis of depression. Mol Psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- Trajkovska V, Vinberg M, Aznar S, Knudsen GM, Kessing LV. Whole blood BDNF levels in healthy twins discordant for affective disorder: association to life events and neuroticism. J Affect Disord. 2008;108:165–169. doi: 10.1016/j.jad.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Serritella C, Martiadis V, Maj M. Decreased levels of brainderived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders. Bipolar Disord. 2008;10:95–100. doi: 10.1111/j.1399-5618.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- Lee B-H, Kim Y, Park S-H, Kim Y-K. Decreased plasma BDNF level in depressive patients. J Affect Dis. 2007;101:239–244. doi: 10.1016/j.jad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Beekman AT, Smit JH, Nolen WA, Spinhoven P, Cuijpers P, et al. NESDA Research Consortium The Netherlands Study of Depression and Anxiety (NESDA): rational, objectives and methods. Int J Methods Psychiatr Res. 2008;17:121–140. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders4th edn.American Psychiatric Association: Washington, DC; 1994 [Google Scholar]
- Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The multicentere WHO/ADAMHA field trials. Br J Psychiatry. 1991;159:645–653. doi: 10.1192/bjp.159.5.645. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology 2008 ; http://www.whocc.no/atcddd/. Accessed March 2010.
- Lyketsos CG, Nestadt G, Cwi J, Heithoff K, Eaton WW. The life chart interview: a standardized method to describe the course of psychopathology. Int J Methods Psychiatr Res. 1994;4:143–155. [Google Scholar]
- Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- Suwa M, Kishimoto H, Nofuji Y, Sasaki H, Radak Z, Kumagai S. Serum brain-derived neurotrophic factor level is increased and associated with obesity in newly diagnosed female patients with type 2 diabetes mellitus. Metabolism. 2006;55:852–857. doi: 10.1016/j.metabol.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Rojas-Vega S, Strüder HK, Wahrmann BV, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121:59–65. doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- Kim TS, Kim DJ, Lee H, Kim YK. Increased plasma brain-derived neurotrophic factor levels in chronic smokers following unaided smoking cessation. Neurosci Lett. 2007;423:53–57. doi: 10.1016/j.neulet.2007.05.064. [DOI] [PubMed] [Google Scholar]
- Simon GE, von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AHS, Cronkite R, Moos R. Physical activity, exercise coping, and depression in a 10-year cohort study of depressed patients. J Affect Disord. 2006;93:79–85. doi: 10.1016/j.jad.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. Arch Gen Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Begliuomini S, Lenzi E, Ninni F, Casarosa E, Merlini S, Pluchino N, et al. Plasma brain-derived neurorophic factor daily variations in men: correlation with cortisol circadian rhythm. J Endocrinol. 2008;197:429–435. doi: 10.1677/JOE-07-0376. [DOI] [PubMed] [Google Scholar]
- Trajkovska V, Marcussen AB, Vinberg M, Hartvig P, Aznar S, Knudsen GM. Measurements of brain-derived neurotrophic factor: methodological aspects and demographical data. Brain Res Bull. 2007;73:143–149. doi: 10.1016/j.brainresbull.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates: Hillsdale, NJ; 1988. [Google Scholar]
- Shimuzu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alternations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Deveci A, Aydemir O, Taskin O, Taneli F, Esen-Danaci A. Serum BDNF levels in suicide attempters related to psychosocial stressors: a comparative study with depression. Neuropsychobiol. 2007;56:93–97. doi: 10.1159/000111539. [DOI] [PubMed] [Google Scholar]
- Kim Y-K, Lee H-P, Won S-D, Park E-U, Lee H-Y, Lee B-H, et al. Low plasma BDNF is associated with suicidal behavior in major depression. Progress Neuro-Psychopharmacol Biol Psychiatry. 2007;31:78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Fabrazzo M, Martiadis V, Serritella C, Pannuto M, Maj M. Circulating brain-derived neurotrophic factor is decreased in women with anorexia and bulimia nervosa but not in women with binge eating disorder: relationships to co-morbid depression, psychopathology and hormonal variables. Psychol Med. 2005;35:897–905. doi: 10.1017/s0033291704003368. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Hashimoto K, Shimuzu E, Kumakiri C, Koizumi H, Okamura N, et al. Decreased levels of serum brain-derived neurotrophic factor in female patients with eating disorders. Biol Psychiatry. 2003;54:485–490. doi: 10.1016/s0006-3223(02)01746-8. [DOI] [PubMed] [Google Scholar]
- Paykel ES. The clinical interview for depression development, reliability and validity. J Affect Disord. 1985;8:85–96. doi: 10.1016/0165-0327(85)90014-x. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- Kachur SG, Hannan CL, Ward KE. Antidepressant-induced weight gain. Med Health Res. 2005;88:359–361. [PubMed] [Google Scholar]
- Antilla SAK, Leinonen EVJ. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249–264. doi: 10.1111/j.1527-3458.2001.tb00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer-Sant'Anna M, Tramontina J, Andreazza AC, Cereser K, da Costa S, Santin A, et al. Traumatic life events in bipolar disorder: impact on BDNF levels and psychopathology. Bipolar Disord. 2007;9 (S1:128–135. doi: 10.1111/j.1399-5618.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Stein LM, Lopes RP, Teixeira AL, Bauer ME. Low plasma Brainderived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression—acpreliminary report. Biol Psychiatry. 2008;64:281–285. doi: 10.1016/j.biopsych.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RH, Schofield R, et al. Interactions between BDNF val66met polymorphism predicts brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacol. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- Mann JJ. The medical management of depression. N Engl J Med. 2005;353:1819–1834. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- Gaster B, Holroyd J. St John's wort for depression. Arch Intern Med. 2000;160:152–156. doi: 10.1001/archinte.160.2.152. [DOI] [PubMed] [Google Scholar]
- Kent JM. SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet. 2000;355:911–918. doi: 10.1016/S0140-6736(99)11381-3. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, et al. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry. 2009;42:270–276. doi: 10.1055/s-0029-1224162. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species Int J Neuropsychopharmacol 2010. e-pub ahead of print 7 July 2010; doi: 10.1017/S1461145710000738 [DOI] [PubMed]