Abstract
The lens is an ideal model system for the study of macromolecular aging and its consequences for cellular function, since there is no turnover of lens fibre cells. To examine biochemical processes that take place in the lens and that may also occur in other long-lived cells, membranes were isolated from defined regions of human lenses that are synthesised at different times during life, and assayed for the presence of tightly bound cytosolic proteins using quantitative iTRAQ proteomics technology. A majority of lens beta crystallins and all gamma crystallins became increasingly membrane bound with age, however, the chaperone proteins alpha A and alpha B crystallin, as well as the thermally-stable protein, βB2 crystallin, did not. Other proteins such as brain-associated signal protein 1 and paralemmin 1 became less tightly bound in the older regions of the lens. It is evident that protein–membrane interactions change significantly with age. Selected proteins that were formerly cytosolic become increasingly tightly bound to cell membranes with age and are not removed even by treatment with 7 M urea. It is likely that such processes reflect polypeptide denaturation over time and the untoward binding of proteins to membranes may alter membrane properties and contribute to impairment of communication between older cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-010-9198-9) contains supplementary material, which is available to authorized users.
Keywords: Human lens, Aging, Protein denaturation, Membrane binding, Lens barrier
Introduction
The activities of various channels and other membrane components can become impaired (Michaelis et al. 1996; Gao et al. 1998; Michels et al. 2003) with age. This may have significant consequences for the functioning of the cell and for the tissue as a whole; however, the reasons for impairment are poorly understood. Clearly, age-related post-translational modifications can alter protein function (Forster et al. 1996; Derham and Harding 1997; Bulteau et al. 2000). Another contributing factor may be the binding of protein aggregates to the membranes of older cells. Several age-related diseases are associated with protein misfolding and aggregation (Dobson 2001) and in the case of Alzheimer’s disease, a considerable body of research has focussed on the interaction of the amyloid β peptides with membranes (McLaurin and Chakrabartty 1996; Aisenbrey et al. 2008; Wong et al. 2009) and it is now thought that membrane binding of oligomers of these peptides may underlie their cytotoxicity (Ji et al. 2001; Wong et al. 2009).
The lens represents a convenient tissue to examine the interaction of denatured proteins with membranes, because of its unique growth pattern. Cells are added continuously throughout life to the lens that was present at birth and there is no significant turnover of macromolecules thereafter (Lynnerup et al. 2008). Thus, the lens contains a complete diary record of proteins, and possibly membranes, from in utero to the present. Over a period of decades, some of the cytosolic proteins within the lens cells are modified and denature; a process that may be responsible for well-known age-related conditions of the lens such as presbyopia and nuclear cataract (Heys et al. 2007). It has been proposed that a major step in cataract formation is the formation of a barrier at middle age that impedes the diffusion of antioxidants from the metabolically active, outer cortex, into the lens centre (Sweeney and Truscott 1998; Moffat et al. 1999). This barrier leaves long-lived proteins in the lens centre more prone to oxidative modification and denaturation.
The present study takes advantage of the unique growth pattern of the ocular lens, and the resultant ages of the tissues themselves, to examine the interactions of cytosolic proteins with plasma membranes. In this study, membrane binding of proteins was characterised in defined regions of the lens that are formed at different stages of life using quantitative proteomics iTRAQ technology (Zieske 2006). The iTRAQ procedure allows for both identification and relative quantitation of proteins present in multiple samples. In the first 4-plex iTRAQ experiments, proteins from a specific region of the lens that is formed post-natally, i.e. the barrier region, were compared between two young lenses and two older lenses. In a second complementary 8-plex iTRAQ experiment, proteins from four regions dissected from each of two individual lenses, one aged 20 years and one 52 years, were compared. This 8-plex experiment provided a comparison of age-related changes within a single lens, and also of the effect of age on identical regions from two separate lenses. In two of the lens regions that are formed in utero, the profiles therefore represent the cumulative effect of five decades of exposure of these macromolecules to physiological pH and temperature. Overall the data revealed a pronounced and progressive increase in the binding to the membranes of some, but not all, cytosolic proteins with age.
Materials and methods
Human lenses
Normal human lenses were obtained from the Lions Eye Bank (Sydney, Australia). Human lenses were handled in accordance with the tenets of the declaration of Helsinki, with ethics approval from Sydney University (Ethics #7292). All lenses were collected with 12 h of death and stored at −80°C until use.
Lens dissection
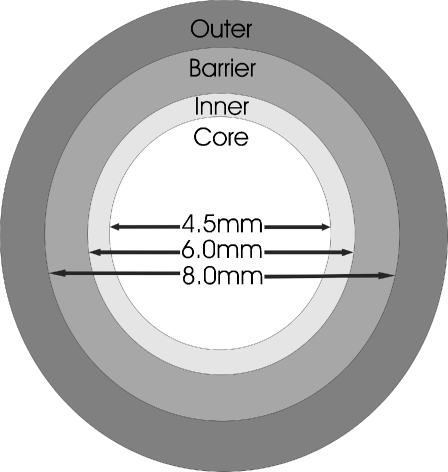
Human lenses aged 20, 21, 22, 23, 24, 72 75, 76, and 77 (4-plex) and 20 and 52 (8-plex) were decapsulated and then dissected into four regions: outer cortex (>8.0 mm), barrier (6.0–8.0 mm), inner (4.5–6.0 mm) and core (4.5 mm), by the sequential use of three trephines. Firstly, an 8-mm trephine was used to remove the outer cortical tissue, then a 6-mm trephine was used to isolate the barrier region. One millimetre from each end of the inner/core billet was removed using a scalpel and the inner and core tissues were separated by use of a 4.5-mm trephine. A diagram of the dissected regions is displayed in Fig. 1.
Fig. 1.
Cross-section through the visual axis of the lens highlighting the four regions isolated by dissection
Isolation of lens membranes
Each lens region was homogenised in 600 μL of 10 mM phosphate buffer pH 7, containing 0.1 mM EGTA and protease inhibitor cocktail (Roche) and then centrifuged. The supernatant was removed and the pellet re-homogenised in the same phosphate buffer an additional two times. The pellet was then homogenised in 200 μL of 10 mM Tris-buffer, pH 8.0 containing 4 M urea. The supernatant was removed and the pellet re-homogenised in the 4 M urea buffer. The resultant pellet was homogenised in 200 μl of 10 mM Tris-buffer, pH 8.0 containing 7 M urea. The supernatant was removed and the pellet washed with milliQ™ water (100 μL). The membrane-containing pellet was lyophilized. All centrifugation steps were at 25,000×g (30 min, 4°C)
iTRAQ labelling
In this study, several independent iTRAQ experiments were conducted, three using 4-plex and the most recent with 8-plex technology.
Each lens membrane preparation was solubilized in 25 μL of 1% (w/v) Triton X-100 (Sigma) in 0.5 M tetraethylammonium bicarbonate (Fluka), and the protein concentration was determined by the BCA protein assay (Thermo Scientific). In each iTRAQ experiment, an equal amount of protein was labelled from each sample; the amount determined by the lowest available protein in the set of four or eight samples. The final protein concentration in each of the samples ranged between 1.5 and 8 μg/μL depending on the sample set and region. Each sample was reduced, alkylated, and digested according to the 4-plex or 8-plex iTRAQ protocol (Applied Biosystems).
Modification of Lys or Arg residues would affect the tryptic digestion used for iTRAQ analysis and such modifications have been described in older lens proteins, however, since the degree of modification is of the order of micromoles or millimoles/mole of Lys (Ahmed et al. 2003; Fan et al. 2009) this is unlikely to influence significantly the analytical method used here
Each sample (four or eight samples) was then subjected to the corresponding iTRAQ labelling protocol. The labelling was performed on each sample with the assigned iTRAQ tagging reagent 114, 115, 116, and 117, for the 4-plex experiment and 113, 114, 115, 116, 117, 118, 119, and 121 for the 8-plex experiment. After 1 or 2 h of labelling, the reactions were quenched with 10 μL of 1 M Tris pH 8.0. After labelling, all iTRAQ-labelled samples were combined, and the combined sample dried on a Speed Vac.
Two dimensions of chromatographic separation were employed to fractionate the labelled peptides. Firstly, strong cation exchange (SCX) chromatography using a polysulfoethyl A column (200 × 2.1 mm i.d.; PolyLC) at 250 μL/min was accomplished with off-line fraction collection at 5 min intervals. Peptide elution was detected by absorbance at 214 nm. The SCX elution gradient was performed as follows: The labelled peptides were loaded at 100% low salt buffer (10 mM KH2PO4 in 25% acetonitrile (ACN); pH 2.7–3.0). Peptides were eluted with a 20-min gradient from 0% to 10% high salt buffer (10 mM KH2PO4 and 0.5 M KCl in 25% ACN; pH 2.7–3.0) followed by a 40-min gradient from 10% to 50% high salt buffer. This was followed by isocratic elution at 50% high salt buffer. A final ramp to 100% high salt buffer was used to ready the column for subsequent runs. Collected SCX fractions were lyophilized to dryness.
Peptides present in the SCX fractions were further fractionated by reversed-phase HPLC using an Ultimate LC system (Dionex) for either on-line liquid chromatography-mass spectroscopy (LC-MS)/mass spectroscopy (MS) analysis or off-line spotting of fractions for matrix-assisted laser desorption/ionization (MALDI) MS/MS experiments. The peptides were separated over a 75 μm × 15 cm C18 column (Vydac) using a 50 min gradient from 12% solvent B (85% ACN, 10% isopropanol and 0.1% trifluoroacetic acid (TFA)) to 41% solvent B, followed by a rapid increase to 95% solvent B (5 min). Absorbance at 214 nm was used to monitor peptide elution at a flow rate of 600 nL/min. For off-line spotting of MALDI target plates, the eluate was mixed with a MALDI matrix solution at a ratio of 1:2 (v/v, eluate to matrix solution). The MALDI matrix solution was 8 mg/mL α-cyano-4-hydroxy-cinnamic acid (Bruker Daltonics) in 70% ACN/0.1% TFA, with 0.15 mg/mL of ammonium citrate.
Mass spectrometry
On-line LC-MS/MS spectra were acquired using an Applied Biosystems QSTAR (Q-TOF) instrument. Eluted peptides were directed into the ion spray source and mass spectra were acquired over a m/z range of 400–1,700. The top 2 most abundant ions in each mass spectrum were selected for MS/MS analysis. Off-line, spotted MALDI target plates were analyzed using a 4800 MALDI TOF-TOF mass spectrometer (Applied Biosystems). For the MALDI experiment MS spectra were collected over a m/z range of 1,000–4,000 to identify parent ions and the top 15 most intense ions were selected for MS/MS analysis.
Data analysis and presentation
QStar and 4800 data were processed using GPS Explorer v. 3.6 or Protein Pilot v. 2.1 (Applied Biosystems). Peptide data for the 4-plex experiment were searched against the NCBInr (Homo sapiens) protein database downloaded on 28 Nov 2005. Peptide data for the 8-plex experiment were searched against the RefSeq human protein database downloaded on 02 Sept 2008 (ftp://ftp.ncbi.nih.gov/refseq/H_sapiens/mRNA_Prot/) appended with a reversed database. S-methylmethanethiosulfonate-labelling of cysteine, iTRAQ labelling of lysine and peptide N-termini were used as fixed modifications for the search. Methionine oxidation and iTRAQ labelling of tyrosine were set as variable modifications. Search results, including peptide/protein identifications as well as iTRAQ reporter ion areas, were tabulated and utilised directly (4-plex) or exported into the iQuantitator software (8-plex) (Schwacke et al. 2009). The 4-plex data are presented as percent change based on average values from three separate iTRAQ experiments using both QStar and 4800 instrumentation.
iTRAQ 8-plex analysis
Age- and region-dependent changes in protein expression employed the model-base approach described in (Hill et al. 2008) and implemented in the iQuantitator (Schwacke et al. 2009) software package. For this application, the model described in (Hill et al. 2008) was adapted to include a protein–region–age interaction term. The iQuantitator software, a tool that provides estimates of treatment-dependent expression change using the Bayesian statistical framework and Monte Carlo Markov Chain sampling, was used to estimate age-dependent differences in expression for each region and differences between each age–region combination, relative to the young barrier case (the reference use in earlier studies). Tandem MS summary reports for the 8-plex iTRAQ experiment included data from 12,058 spectra. Of those, 7,729 were eliminated because of low confidence spectra (Mascot confidence below 70%) or no protein identification, 22 were eliminated due to missing reporter ion areas or areas below threshold, 225 were eliminated as degenerate, 716 had iTRAQ-modified tyrosines, and 262 were hits from the decoy database. The remaining 3,104 spectra, with high confidence identifications were used in the analysis. The sample set was thinned by a factor of 200 leaving 10,000 samples from which comparisons were performed. For each comparison, credible intervals were estimated from differences in the sampler output. All calculations were performed using the R Statistical Computing environment (R version 2.6.2) (http://www.R-project.org).
Expression curves
Each individual curve (red or green) displays the frequency of peptides from that protein that appear at a specific expression fold change, i.e. a histogram. The expression fold change is determined by the iTRAQ ratio of that peptide compared with the young barrier signal (reference signal) to provide a direct comparison to the 4-plex data. Thus, the young barrier signal is set to 1.0. The circles represent the average expression fold change (old vs. young) for a given region and the horizontal lines represent the “credible interval”. If the credible interval line does not touch the vertical 1.0 expression fold change line, then there is a significant difference with age in that region.
Results
Comparison of lens barrier regions
The most abundant proteins within lens fibre cells are the crystallins. In human lenses, the major crystallins are αA-, αB-, βA1-/A3-, βA4-, βΒ1-, βΒ2-, βΒ3-, γS-, γC- and γD-crystallin (Lampi et al. 1997; Bloemendal et al. 2004). Small amounts of γB-crystallin are also present. The two α-crystallins, which are members of the small heat shock protein family (Horwitz 1992), account for approximately 40% of total lens protein (Horwitz 2003), with the β- and γ-crystallins representing approximately 30% each (Bron et al. 2000).
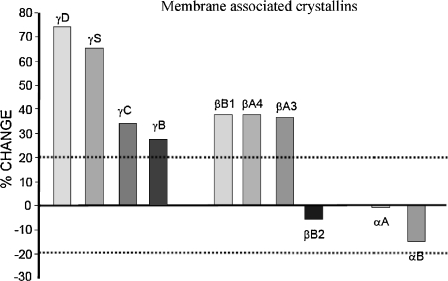
An initial study was conducted on the barrier region (Fig. 1) only using 4-plex iTRAQ technology. The barrier region, which is the part of the lens that is synthesised post-natally and in early childhood (Koretz et al. 1994; Kuszak 1995), was dissected from two young and two old lenses per experiment (a) 22, 24 vs 72, 72; (b) 22, 23 vs 76, 77 and (c) 20, 21 vs 75, 77). The rationale was to compare membrane-associated proteins from the same lens region before and after the formation of the barrier to diffusion that forms at middle age (Sweeney and Truscott 1998; Moffat et al. 1999). If proteins were significantly increased in this region from older lenses, they could be candidates for involvement in barrier formation. The average iTRAQ ratios from these experiments are reported in Fig. 2.
Fig. 2.
Proteins tightly bound to membranes in the barrier region. 4-Plex iTRAQ data shown as averages from three experiments on lenses aged (22, 24 vs 72, 72), (22, 23 vs 76, 77) and (20, 21 vs 75, 77). The 20% lines are shown as a conservative estimate to suggest that changes above or below the lines are likely to be significant. The percent of change refers to the average change in abundance of a particular crystallin in the older lenses relative to the younger lenses
A wide range of proteins was detected including enzymes, cytoskeletal and intrinsic membrane proteins (data not shown). Despite the fact that the fibre cell membranes had been extracted with 4 M urea, and then with 7 M urea, all of the major crystallins were detected and the amounts of most of these structural proteins increased with lens age. There was, however, a marked degree of selectivity in terms of the subunits that were bound (Fig. 2). For example, all of the gamma crystallin polypeptides were found to increase with age, as were βB1-, βA3- and βA4-crystallin. By contrast, neither of the two alpha crystallin subunits, nor βB2-crystallin, were observed to increase. This result suggests that increased membrane binding of selected proteins could be involved in the formation of the barrier that develops in the human lens at middle age, and which acts to restrict the diffusion of small molecules, such as glutathione, from the cortex to the lens interior (Sweeney and Truscott 1998) where it is required to provide a reducing environment.
Comparison of four lens regions
In order to determine if the process of membrane binding is confined to the barrier region, or is part of a more general age-related phenomenon taking place in all regions of the lens, the recently introduced 8-plex iTRAQ technology was employed. This strategy allows eight separate samples to be compared. Four geometric regions were therefore dissected from each of two lenses aged 20 and 52 (see Fig. 1, Experimental procedures). These regions are denoted as the core, inner, barrier and outer, and correspond to distinct developmental stages. The core, also known as the foetal nucleus, is the part of the lens that is formed early in utero. The inner region (infantile nucleus) is synthesised in the later months prior to birth. The barrier region (described above) is laid down in childhood. The corresponding regions from young and old lenses can be compared directly because there is no compression of tissue in the centre of adult human lenses as the lens continues to grow throughout life (Fagerholm et al. 1981; Heys et al. 2008). The outer region contains the most recently synthesised proteins.
Membrane fractions were isolated from each region following extraction with 4 M urea, then 7 M urea, as described for the 4-plex iTRAQ experiment. Under these conditions only those proteins that are very tightly associated with lens membranes are retained. Proteins from each lens region were digested with trypsin then labelled with a specific iTRAQ reagent. The labelled peptides from each of the 8 digests were then mixed together prior to analysis by LC/mass spectrometry. Relative changes between regions of a single lens were mapped using the barrier region of the young lens as the reference. Relative changes between barrier regions of the two lenses were determined using the young barrier region as the reference in order to compare the 8-plex results with the 4-plex results. Likewise, binary comparisons between the same region from young and old lenses were made by using the young lens region as the reference. Since the 8-plex data enables comparison of different regions within a single lens, it permits detection of membrane processes that are involved in differentiation of young fibre cells as well as those that take place over a period of decades in the one lens region, e.g. protein detachment or proteolysis and protein binding in older cells.
In Figs. 3 and 4, we have used a format that shows both significant differences in protein abundance between groups (age) and across the lens. The graphs are designed to illustrate (1) abundance gradients across the lens which vary by age group (the solid lines connecting the central point of the error bar for each region), (2) uncertainty in the fold change estimate derived from the proteomic experiment (error bars, peptide-level fold change point plots, and kernel density estimates of the fold change) and (3) differences in age within a region (horizontal separation of error bars).
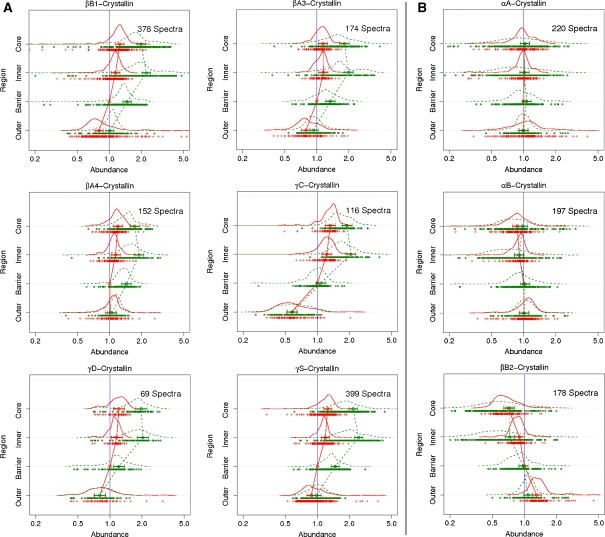
Fig. 3.
A Membrane-bound proteins that increase with age. B Membrane-bound proteins that showed little or no significant change with age. 8-plex iTRAQ analysis was used to generate the data sets. Four geometric regions were dissected from two lenses aged 20 and 52. These regions are denoted as the core, inner, barrier and outer. The 8-plex results were normalised for each protein to the value of the young lens barrier region to allow direct comparison with the 4-plex data. Each curve green (old lens) and red (young lens) represents the frequency of peptides at given expression fold changes from that protein. The dots represent individual data points. The circles represent the average change in abundance for that particular region, with horizontal lines representing credible intervals
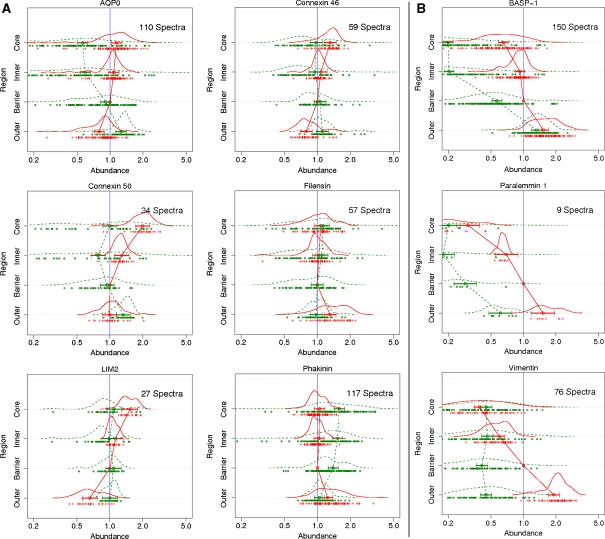
Fig. 4.
A Integral membrane and cytoskeletal protein changes with age. B Membrane-bound proteins that decrease in abundance in the older fibre cells. 8-plex iTRAQ analysis was used to generate the data sets with the same samples and analyses as for Fig. 3
The 8-plex results shown in Figs. 3 and 4 demonstrate some clear trends. In almost every case the inner region was very similar to the lens core region. This was predicted, since both are synthesised in utero. Since the barrier region was created post-natally it might be expected that if there were any age-dependent effects, that these should be less pronounced in this region. In nearly all cases where an effect was observed, this was indeed the case.
It should be noted that the crystallin composition of the human lens alters to some degree around the time of birth. Gamma crystallin synthesis ceases post-natally and appears to be replaced by gamma S crystallin (Thomson and Augusteyn 1985) whereas the other alpha and beta crystallin subunits continue to be formed throughout life. This compositional change could affect the results of comparisons between the different lens regions (Figs. 3 and 4)
Crystallins that increased in older lens regions
As was observed in the barrier region by 4-plex iTRAQ experiments, many crystallins exhibited relative increases in membrane binding to the older lens barrier region using 8-plex analysis (Fig. 3A) as evidenced by green curves shifted to higher expression fold changes. In addition, as one moved further into the lens from the barrier to the inner region, the relative degree of binding for each protein increased. This was true for βA3-, βA4-, βΒ1-, γS-, γC- and γD-crystallin in both the young and the old lenses. In each case the relative increase in abundance was significantly greater in the older lens, being approximately 2-fold compared with the barrier region of the 20-year-old lens. Increases in the inner and core regions of the young lens were less than 1.5-fold. These changes are summarised in Fig. 3A. In each case, there was very little change in the relative abundance in the inner region compared with the core region. Two other less abundant crystallins, γB and βΒ3 were also detected with similar patterns; however, the number of spectra obtained (12 and 17, respectively) were small, and therefore they were not included in this figure.
Crystallins that decreased, or were unchanged, in older lens regions
By contrast to the crystallins listed above, three crystallins, αA-, αB- and βΒ2-crystallin, did not increase in the extent of membrane binding in the barrier region with age (Fig. 3B). This is in agreement with 4-plex data (Fig. 2). In addition, neither αA-, nor αB-crystallin showed any significant increase in membrane binding in the older lens regions compared with the youngest part of the lens. The appearance of the profile for βΒ2-crystallin was slightly different from that of the α-crystallins. It showed a slight decrease in relative abundance as one moved from the outer (recently synthesised) to the core region of the lens.
Integral membrane proteins and intermediate filament proteins
Interpretation of changes in the integral membrane proteins is more of a challenge due to the fact that few tryptic peptides are typically detected, the potential for age-related truncation causing loss of signal, and due to the fact that, with age, their overall contribution to the membrane fraction is diminished due to soluble crystallin binding. As shown in Fig. 4A, the relative quantity of the major lens fibre cell integral membrane proteins connnexin 46, connnexin 50 and aquaporin 0 (AQP0) did not change significantly with age in the barrier region.
In the young lens there was little change in AQP0 across the four lens regions. In the older lens an almost threefold decrease of AQP0 in the inner and core was observed from the outer region to the core. This might be attributed to known age-related truncation of AQP0, e.g. at the C terminus (Ball et al. 2004; Korlimbinis et al. 2008). The lens-specific cytoskeletal component filensin also did not change significantly with fibre cell age in either the young or the older lens; however, phakinin the other beaded chain intermediate filament protein (Merdes et al. 1993) increased its membrane association nearly 2-fold in comparison to the younger lens. The relative quantity of LIM 2 showed no clear trend although this is on the basis of only 27 spectra. The relative amounts of vimentin decreased approximately 2-fold in going from the outer to the core region of the young lens (Fig. 4B). It is likely that this is a reflection of extensive proteolysis of this cytoskeletal component which occurs quite early in fibre cell development (Prescott et al. 1996). Two proteins displayed dramatic decreases in expression change with age; brain abundant membrane-associated signal protein 1 and paralemmin 1.
As summarised above, major lens proteins such as crystallins and cytoskeletal proteins become tightly bound to membranes of older fibre cells. Other less abundant lens proteins, such as the enzymes aldehyde dehydrogenase and carbonyl reductase, were also found to be associated with membranes. In most cases there was little or no change in the binding of these enzymes between the two age groups studied. This observation may however, reflect the fact that fewer than 30 spectra were used for quantification. The results for these, and other proteins, are summarised in a heat map displayed in the Electronic supplementary material (Fig. 1) showing the relative change in each region between the young and old lenses.
Discussion
This investigation has demonstrated that some proteins become very tightly bound to cell membranes with age. Moreover, there is pronounced selectivity in terms of which of the formerly soluble polypeptides become membrane associated with age.
Foremost amongst the proteins that did not increase their age-dependent binding, were the two α-crystallin subunits, which are chaperones. One other soluble protein, βB2-crystallin, also did not increase, and this crystallin is known to be very thermally stable in that it can be selectively purified from the supernatant of a crystallin mixture after boiling (Feng et al. 2000) and it has also been proposed to aid solubility of proteins (Zhang et al. 2001). These findings suggest that resistance to denaturation may be one mechanism that could inhibit strong interaction with the membranes. Therefore, by implication, age-dependent denaturation, in particular thermal denaturation, of formerly soluble proteins may be a prime driving force of this process. It should be emphasised that iTRAQ technology, whilst very selective and sensitive in detecting proteins on the basis of numerous tryptic peptides, can provide only relative quantification of proteins when used without internal standards.
The precise mechanism for the observed tight membrane binding of selected crystallins with fibre cell age is unknown. A covalent component cannot be ruled out, although sodium dodecyl sulfate (SDS)-PAGE did not show an increase of crosslinked polypeptides in the older membrane fractions (data not shown). It is also possible that some of the tight binding of proteins to membranes we observed could be due to disulfide bonding. Artefactual disulfide bond formation in normal lenses is considered unlikely because proteins were extracted initially with buffer and the lens contains high levels of glutathione (GSH). Endogenous GSH in the extract would prevent protein–protein disulfide bond formation. In relation to protein–protein disulfides between crystallins and membrane-associated proteins in the lens, there is little data to support an increase in disulfide bond formation in normal lenses with age (Lou 2000; Truscott 2005) and intrinsic membrane proteins typically contain few thiol groups (Garner and Spector 1980).
More likely, the results reflect strong non-covalent interactions involving regions of the denatured proteins within the lens cells, with membrane lipids or integral membrane proteins. Such a mechanism could involve partial insertion of the proteins into the membrane itself as has been shown for several peptides (Wong et al. 2009). It is of interest that a protein permeability pathway that enables movement of large molecules between cells (Shestopalov and Bassnett 2003), arises in older fibre cells and this may be a function of cell membrane fusion. Protein interactions with membranes of the type documented here may play a role in this process that leads to facilitated, but unregulated, cell-to-cell communication.
The 8-plex data agreed closely with 4-plex experiments on the barrier region, which were carried out using different young and old lenses. In addition the 8-plex revealed that similar protein–membrane interactions take place in all regions of the lens and that they differ primarily in the extent of the interaction (Figs. 3 and 4). In almost all cases, the degree of binding was similar in the inner and core regions, but was significantly less in the barrier region. This is consistent with the developmental stages of lens growth and the ages of the proteins within the cells. The core region, also known as the embryonic nucleus, is formed early in utero (Kuszak 1995) with the inner region being laid down later in the months prior to birth (Kuszak 1995). By contrast, the barrier region is synthesised in childhood (Kuszak 1995).
Are there any functional consequences for the lens of this age-dependent increase in protein attachment? It might be expected that access to cell membrane pores would be significantly compromised by such age-dependent attachment of denatured proteins. Interestingly, it has been found that a barrier to the diffusion of both water (Moffat et al. 1999) and small molecules such as glutathione (Sweeney and Truscott 1998), becomes evident in the lens at middle age. Protein binding to cell membranes could explain the formation of the barrier via partial blocking of both of these types of membrane pores, and in this way cell-to-cell diffusion would be restricted.
Our 8-plex data suggest that the membrane association may not be specific to the barrier region, but rather is a general process that occurs throughout the central region of the lens and is driven by age-related denaturation of formerly soluble proteins. The barrier could simply be a reflection of the need for a certain extent of binding of denatured proteins to the gap junctions and AQP0 channels of the fibre cells, before such a phenomenon became evident.
In the years prior to 45, as proteins in lens fibre cells denature, they complex with the small heat shock protein, alpha crystallin, to form large soluble aggregates (Roy and Spector 1976). After middle age, when the supply of alpha crystallin has been consumed (Roy and Spector 1976; McFall-Ngai et al. 1985), we postulate that denatured proteins may increasingly bind to membranes (Friedrich and Truscott 2009). Another feature that may be implicated in the increased binding of proteins to cell membranes with age is the phospholipid composition of the fibre cell membranes. We (manuscript in preparation) and others (Borchman et al. 1994) have shown that there are significant age-dependent changes in human lens membranes. Other alterations, such as the continuous age-dependent truncation of the cytosolic C-terminal tail of AQP0 (Ball et al. 2004; Korlimbinis et al. 2008), could also play a role in permitting closer access of protein aggregates to the cell membranes of older cells.
In the comparisons shown between the four regions of the individual lenses, other factors can be detected using the iTRAQ methodology. For example, if a protein were a significant component involved in the synthesis and elongation of fibre cells, a process that takes place only in the outer cortex, but was removed from the membranes in mature fibre cells, it would be predicted that it should show a relative decrease in going from the outer to the barrier and then to the inner/core regions. Two proteins that showed such a clear pattern, were brain abundant signal protein 1 (BASP1) and paralemmin 1 (Fig. 4B); both of which have been reported to be present in the lens (Bagchi et al. 2003; Castellini et al. 2005; Bagchi et al. 2008). BASP1 belongs to a family of growth-associated proteins that modulate nerve sprouting, and are enriched in both the brain and nerve endings (Mosevitskya et al. 1997; Mosevitsky 2005). The number of spectra obtained, suggest it may also be an abundant protein in the lens. Interestingly, BASP1 is anchored to membrane lipids by N-myristoylation (Mosevitsky et al. 1997). Though de-myristoylation of BASP1 has not been studied in the lens, unmyristoylated BASP1 has been found in the brain (Zakharov et al. 2003), suggesting a possible mechanism for its removal in mature lens fibre cells. Since the levels within the core and inner regions of the old and young lens differed significantly, but were more similar in the barrier region, there may be a slow detachment of BASP from cell membranes over a period of years. Alterations in membrane lipids could also contribute to the observed loss of BASP1 with fibre age. Paralemmin 1 is a prenyl-palmitoyl-anchored membrane protein involved in neuronal membrane and process formation and is hypothesised to play a role in lens membrane and interdigitation formation (Bagchi et al. 2003; Castellini et al. 2005).
It should be emphasised that the proteins identified in this study represent only those that are very tightly bound to lens cell membranes. With age, a large proportion of formerly soluble crystallins become associated with membranes and can be isolated using sucrose gradient centrifugation (Chandrasekher and Cenedella 1995; Friedrich and Truscott 2009). Most of these are readily stripped from the membranes using urea or SDS. This initial association of crystallins with older membranes may be an important intermediate step in the tight binding of some of the proteins. The relative abundances of the individual crystallins in the tightly and loosely bound crystallins are not the same, indicating that the processes involved in these two binding phases may be fundamentally different.
It is possible, but not yet proven, that similar processes to those documented here for the human lens may also take place in other long-lived cells, for example, those present in the frontal cortex of the human brain (Spalding et al. 2005; Bhardwaj et al. 2006; Nowakowski 2006), adipocytes (Spalding et al. 2008) or cardiomyocytes (Bergmann et al. 2009). In the case of the nervous system, communication between neurons and non-neural (glial) cells is essential for axonal conduction and synaptic transmission, (Fields and Stevens-Graham 2002) and, if this were compromised by the binding of aggregates to membranes, normal functioning may be disrupted.
On the basis of our data, we hypothesise that proteins which are more susceptible to denaturation may interact to the greatest extent with cell membranes, particularly if the cellular processes for clearance of such denatured aggregates are compromised in older cells. In the next phase, we aim to use similar iTRAQ approaches to those used here, to examine such systems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
A heat map showing relative changes in major proteins that were tightly bound to lens membranes. Four geometric regions were dissected from each of two lenses aged 20 and 52. The average abundance in the young lens for each region was compared with the same region of the older lens. Red represents an overall relative protein decrease, blue an overall increase and white no change between the two age groups. Proteins with a spectral count less than 15 were not included in the data set. (GIF 94 kb)
Acknowledgements
The authors acknowledge financial support from the NIH EY-013570 (RJWT) and NIH R24 EY14793 as well as support from the MUSC Mass Spectrometry Facility Mr Raj Devasahayam and Meidong Zhu from Sydney Lions Eye bank are thanked for providing the human lenses.
References
- Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Investig Ophthalmol Vis Sci. 2003;44:5287–5292. doi: 10.1167/iovs.03-0573. [DOI] [PubMed] [Google Scholar]
- Aisenbrey C, Borowik T, Byström R, Bokvist M, Lindström F, Misiak H, Sani M-A, Gröbner G. How is protein aggregation in amyloidogenic diseases modulated by biological membranes? Eur Biophys J. 2008;37:247–255. doi: 10.1007/s00249-007-0237-0. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Katar M, Lo WK, Maisel H. Paralemnin of the lens. J Cell Biochem. 2003;89:917–921. doi: 10.1002/jcb.10574. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Kousis S, Maisel H. BASP1 in the lens. J Cell Biochem. 2008;105:699–702. doi: 10.1002/jcb.21863. [DOI] [PubMed] [Google Scholar]
- Ball L, Garland D, Crouch R, Schey K. Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: spatial and temporal occurence. Biochemistry. 2004;43:9856–9865. doi: 10.1021/bi0496034. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisen J. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H, Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Borchman D, Byrdwell WC, Yappert MC. Regional and age-dependent differences in the phospholipid composition of human lens membranes. Investig Ophthalmol Vis Sci. 1994;35:3938–3942. [PubMed] [Google Scholar]
- Bron AJ, Vrensenb GFJM, Koretzc J, Marainid G, Hardinga JJ. The Ageing lens. Ophthalmologica. 2000;214:86–104. doi: 10.1159/000027475. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Petropoulos I, Friguet B. Age-related alterations of proteasome structure and function in aging epidermis. Exp Gerontol. 2000;35:767–777. doi: 10.1016/S0531-5565(00)00136-4. [DOI] [PubMed] [Google Scholar]
- Castellini M, Wolf L, Chauhan B, Galileo D, Kilimann M, Cvekl A, Duncan M. Palm is expressed in both developing and adult mouse lens and retina. BMC Ophthalmol. 2005;5:14. doi: 10.1186/1471-2415-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekher G, Cenedella RJ. Protein associated with human lens ‘native’ membrane during aging and cataract formation. Exp Eye Res. 1995;60:707–717. doi: 10.1016/S0014-4835(05)80012-0. [DOI] [PubMed] [Google Scholar]
- Derham BK, Harding JJ. Effect of aging on the chaperone-like function of human alpha-crystallin assessed by three methods. Biochem J. 1997;328(Pt3):763–768. doi: 10.1042/bj3280763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CM. The structural basis of protein folding and its links with human disease. Philos Trans R Soc Lond B Biol Sci. 2001;356:133–145. doi: 10.1098/rstb.2000.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm PP, Philipson BT, Lindstrom B. Normal human lens—the distribution of protein. Exp Eye Res. 1981;33:615–620. doi: 10.1016/S0014-4835(81)80101-7. [DOI] [PubMed] [Google Scholar]
- Fan X, Zhang J, Theves M, Strauch C, Nemet I, Liu X, Qian J, Giblin FJ, Monnier VM. Mechanism of lysine oxidation in human lens crystallins during aging and in diabetes. J Biol Chem. 2009;284:34618–34627. doi: 10.1074/jbc.M109.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Smith DL, Smith JB. Human lens beta-crystallin solubility. J Biol Chem. 2000;275:11585–11590. doi: 10.1074/jbc.275.16.11585. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci. 1996;93:4765. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Truscott RJW. Membrane association of proteins in the aging human lens: profound changes take place in the 5th decade of life. Investig Ophthalmol Vis Sci. 2009;50:4786–4793. doi: 10.1167/iovs.09-3588. [DOI] [PubMed] [Google Scholar]
- Gao J, Yin D, Yao Y, Williams TD, Squier TC. Progressive decline in the ability of calmodulin isolated from aged brain to activate the plasma membrane Ca-ATPase. Biochem Cell Biol. 1998;30:9536. doi: 10.1021/bi9803877. [DOI] [PubMed] [Google Scholar]
- Garner MH, Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci USA. 1980;77:1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys K, Friedrich M, Truscott RJW. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- Heys KR, Friedrich MG, Truscott RJW. Free and bound water in normal and cataractous human lenses. Investig Ophthalmol Vis Sci. 2008;49:1991–1997. doi: 10.1167/iovs.07-1151. [DOI] [PubMed] [Google Scholar]
- Hill EG, Schwacke JH, Comte-Walters S, Slate EH, Oberg AL, Eckel-Passow JE, Therneau TM, Schey KL. A statistical model for iTRAQ data analysis. J Proteome Res. 2008;7:3091–3101. doi: 10.1021/pr070520u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/S0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Ji S-R, Wu Y, Sui S-f. Cholesterol is an important factor affecting the membrane insertion of beta-amyloid peptide (A-beta 1-40) which may potentially inhibit the fibril formation. J Biol Chem. 2001;277:6273–6279. doi: 10.1074/jbc.M104146200. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Cook CA, Kuszak JR. The zones of discontinuity in the human lens: development and distribution with age. Vis Res. 1994;34:2955–2962. doi: 10.1016/0042-6989(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Korlimbinis A, Berry Y, Thibault D, Schey KL, Truscott RJW. Protein aging: truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp Eye Res. 2008;88:966–973. doi: 10.1016/j.exer.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszak JR. The development of lens sutures. Prog Retin Eye Res. 1995;14:567–591. doi: 10.1016/1350-9462(94)00019-C. [DOI] [Google Scholar]
- Lampi KJ, Ma Z, Shih M, Shearer TR, Smith JB, Smith DL, David LL. Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens. J Biol Chem. 1997;272:2268–2275. doi: 10.1074/jbc.272.4.2268. [DOI] [PubMed] [Google Scholar]
- Lou MF. Thiol regulation in the lens. J Ocul Pharmacol Ther. 2000;16:137–148. doi: 10.1089/jop.2000.16.137. [DOI] [PubMed] [Google Scholar]
- Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS ONE. 2008;3:e1529. doi: 10.1371/journal.pone.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall-Ngai MJ, Ding L-L, Takemoto LJ, Horwitz J. Spatial and temporal mapping of the age-related changes in human lens crystallins. Exp Eye Res. 1985;41:745–758. doi: 10.1016/0014-4835(85)90183-6. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Chakrabartty A. Membrane disruption by alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem. 1996;271:26482–26489. doi: 10.1074/jbc.271.43.26482. [DOI] [PubMed] [Google Scholar]
- Merdes A, Gounari F, Georgatos SD. The 47-kD lens-specific protein phakinin is a tailless intermediate filament protein and an assembly partner of filensin. J Cell Biol. 1993;123:1507–1516. doi: 10.1083/jcb.123.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis BDJ, Schöneich C, Williams TD, Ramonda L, Yin D, Hühmer AFR, Yao Y, Gao J, Squier C. Decreased plasma membrane calcium transport activity in aging brain. Life Sci. 1996;59:405–412. doi: 10.1016/0024-3205(96)00319-0. [DOI] [PubMed] [Google Scholar]
- Michels A, Joisher JN, Hagen TM. Age-related decline of sodium-dependent ascorbic acid transport in isolated rat hepatocytes. Arch Biochem Biophys. 2003;410:112–120. doi: 10.1016/S0003-9861(02)00678-1. [DOI] [PubMed] [Google Scholar]
- Moffat BA, Landman KA, Truscott RJ, Sweeney MH, Pope JM. Age-related changes in the kinetics of water transport in normal human lenses. Exp Eye Res. 1999;69:663–669. doi: 10.1006/exer.1999.0747. [DOI] [PubMed] [Google Scholar]
- Mosevitsky MI (2005) Nerve ending “Signal” proteins GAP-43, MARCKS, and BASP1. In: Kwang WJ (ed) A survey of cell biology. Academic, pp 245–325 [DOI] [PubMed]
- Mosevitsky MI, Capony JP, Skladchikova GY, Novitskaya VA, Plekhanov AY, Zakharov VV. The BASP1 family of myristoylated proteins abundant in axonal termini. Primary structure analysis and physico-chemical properties. Biochimie. 1997;79:373–384. doi: 10.1016/S0300-9084(97)80032-6. [DOI] [PubMed] [Google Scholar]
- Mosevitskya MI, Caponyb JP, Skladchikovaa GY, Novitskayaa VA, Yu PA, Zakharov VV. The BASP1 family of myristoylated proteins abundant in axonal termini. Primary structure analysis and physico-chemical properties. Biochimie. 1997;79:373–384. doi: 10.1016/S0300-9084(97)80032-6. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS. Stable neuron numbers from cradle to grave. Proc Natl Acad Sci. 2006;103:12219–12220. doi: 10.1073/pnas.0605605103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott AR, Sandilands A, Hutcheson AM, Carter JM, Quinlan RA. The intermediate filament cytoskeleton of the lens: an ever changing network through development and differentiation. A minireview. Ophthalmic Res. 1996;28(Suppl 1):58–61. doi: 10.1159/000267946. [DOI] [PubMed] [Google Scholar]
- Roy D, Spector A. Absence of low-molecular-weight alpha crystallin in nuclear region of old human lenses. Proc Natl Acad Sci USA. 1976;73:3484–3487. doi: 10.1073/pnas.73.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke J, Hill E, Krug E, Comte-Walters S, Schey K. iQuantitator: a tool for protein expression inference using iTRAQ. BMC Bioinform. 2009;10:342. doi: 10.1186/1471-2105-10-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalov VI, Bassnett S. Development of a macromolecular diffusion pathway in the lens. J Cell Sci. 2003;116:4191–4199. doi: 10.1242/jcs.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H. Retrospective birth dating of cells in humans. Cell (Cambridge, Mass.) 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PlO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature (London) 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Sweeney MH, Truscott RJ. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp Eye Res. 1998;67:587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Augusteyn RC. Ontogeny of human lens crystallins. Exp Eye Res. 1985;40:393–410. doi: 10.1016/0014-4835(85)90152-6. [DOI] [PubMed] [Google Scholar]
- Truscott RJW. Age-related nuclear cataract—oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Wong PT, Schauerte JA, Wisser KC, Ding H, Lee EL, Steel DG, Ari G. Amyloid-beta membrane binding and permeabilization are distinct processes influenced separately by membrane charge and fluidity. J Mol Biol. 2009;386:81–96. doi: 10.1016/j.jmb.2008.11.060. [DOI] [PubMed] [Google Scholar]
- Zakharov VV, Capony J-P, Derancourt J, Kropolova ES, Novitskaya VA, Bogdanova MN, Mosevitsky MI. Natural N-terminal fragments of brain abundant myristoylated protein BASP1. Biochim Biophys Acta. 2003;1622:14–19. doi: 10.1016/S0304-4165(03)00099-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, David LL, Smith DL, Smith JB. Resistance of human beta B2-crystallin to in vivo modification. Exp Eye Res. 2001;73:203–211. doi: 10.1006/exer.2001.1023. [DOI] [PubMed] [Google Scholar]
- Zieske LR. A perspective on the use of iTRAQ™ reagent technology for protein complex and profiling studies. J Exp Bot. 2006;57:1501–1508. doi: 10.1093/jxb/erj168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
A heat map showing relative changes in major proteins that were tightly bound to lens membranes. Four geometric regions were dissected from each of two lenses aged 20 and 52. The average abundance in the young lens for each region was compared with the same region of the older lens. Red represents an overall relative protein decrease, blue an overall increase and white no change between the two age groups. Proteins with a spectral count less than 15 were not included in the data set. (GIF 94 kb)