Abstract
Addition chemistries are widely used in preparing biological conjugates, and in particular, maleimide-thiol adducts have been widely employed. Here we show that the resulting succinimide thioether formed by a Michael type addition of a thiol to N-ethylmaleimide (NEM), generally accepted as stable, can in fact undergo retro and exchange reactions in the presence of other thiol compounds at physiological pH and temperature, offering a novel strategy for controlled release. Model studies (1H NMR, HPLC) of NEM conjugated to 4-mercaptophenylacetic acid (MPA), N-acetylcysteine, or 3-mercaptopropionic acid (MP) incubated with glutathione showed half lives of conversion from 20–80 hrs, with extents of conversion from 20–90% for MPA and N-acetylcysteine conjugates. Ring-opened the resultant succinimide thioether as well as any MP adduct did not show retro and exchange reactions. The kinetics of the retro reactions can be modulated by the Michael donor’s reactivity; therefore the degradation of maleimide-thiol adducts could be tuned for controlled release of drugs or degradation of materials at timescales different than those currently possible via disulfide-mediated release. Such approaches may find a new niche for controlled release in reducing environments relevant in chemotherapy and sub-cellular trafficking.
Introduction
Controlled release of drugs has been a key area of research in the field of polymeric biomaterials, owing to the need to sustain release in order to expand the therapeutic window and efficacy of known drugs. Many chemical degradation approaches have been used to control materials-based drug delivery including chemical hydrolysis,1 enzymatic degradation2 and disulfide exchange.3 The rate of non-specific chemical hydrolysis depends mainly on aqueous pH and temperature, as well as on the hydrophobicity of the environment around the hydrolytically labile group. Enzymatic degradation, in contrast, occurs specifically at enzyme-recognized peptide sequences, although rates of degradation are dependent on the local enzyme concentration, activity and accessibility to the substrate. Disulfide exchange is sensitive to reducing environments and has found use in drug delivery systems due to the weak reducing capacity of blood (ca. 2–20μM glutathione) compared with that of reductive cellular compartments or highly reductive and hypoxic tumor tissues (ca. 0.5–10mM glutathione).4–9 Disulfide bonds have relatively short half-lives (<1hr) in highly reductive environments, while maintaining a degree of stability in circulation.5,8 Although control of the cleavage kinetics is possible with variations in reducing agent concentration, only limited control of kinetics has been achieved by varying the disulfide’s neighboring chemical substituents (half-lives ranging from 8 to 45 minutes).8
In contrast to disulfide-based drug conjugation and polymer network formation strategies, thiol-based Michael-type addition reactions have emerged as a widely employed strategy for covalent conjugation of proteins, peptides, and drugs (to various polymers and other molecules) by the reaction of free cysteine or thiols with acrylamides, acrylates, vinyl sulfones and maleimides.10 Maleimides have been commonly employed due to their specificity to thiols, fast aqueous reaction kinetics, lack of byproducts, and the stability of the thioether addition product.11 Maleimides have thus been utilized in homobifunctional crosslinkers,12 heterobifunctional crosslinkers,13 fluorescent labels,14,15 as well as in PEGylation reagents16 and crosslinking of hydrogels.17
While the wide use of maleimide-thiol conjugation reactions have been motivated by the product stability, there have been limited reports indicating that select succinimide thioethers can undergo retro reactions at high temperatures (>300°C)18 and in some aqueous environments19–23 although the mechanisms and exact solution conditions for these retro reactions were not elucidated in great detail. Such reports are very limited, however, despite the fact that the reversibility of adducts of thiols with α,β unsaturated carbonyls such as ethacrynic acid and 4-hydroxyalkenals has been long reported.24–27 We have thus recently explored the reversibility of maleimide-thiol conjugation reactions as a potential controlled degradation mechanism, which may have similar and complementary applications to the disulfide-mediated release of drugs in reducing environments. Scheme 1 illustrates the likely mechanism for the covalent bond transfer from the initial succinimide thioether compound (1) to a stable glutathione-conjugate (3) in the presence of excess reductant. We have found that the rate and extent of this exchange, which is governed by the rate of the retro-addition, can be modulated by increasing or decreasing the Michael donor’s reactivity.
Scheme 1.
Proposed exchange of synthesized maleimide thiol adducts (1) with glutathione in aqueous solutions.
Experimental Procedures
Chemicals and General Methods
N-ethylmaleimide (2), 4-mercaptophenylacetic acid (MPA), N-acetyl-L-cysteine (AcCys), 3-mercaptopropionic acid (MP), and glycine were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification. All other reagents including glutathione (GSH) and oxidized glutathione (GSSG) were purchased from Fisher Scientific (Pittsburgh, PA). Crude reactions were purified via reverse-phase chromatography on a Delta600 HPLC (Waters, Milford, MA) equipped with analytical (3.5μm particle size, 4.6 × 75mm) and preparative (5μm particle size, 19 × 150 mm) Waters Symmetry300 C18 columns. Analytical linear gradients from 0% to 75% of solvent B were run over 20 min at 1 mL/min, where solvent A is 0.1% trifluoroacetic acid (TFA) in water and solvent B is 0.1% TFA in acetonitrile. Preparative-scale experiments utilized similar elution profiles determined from the analytical experiments, although with flow rates of 5ml/min. Peaks were collected and analyzed via ESI-MS and 1H-NMR. 1H NMR spectra were acquired under standard quantitative conditions at ambient temperature on a Bruker DRX-400 NMR spectrometer (Billerica, MA). The spectra of all purified compounds were recorded in either deuterated methanol or deuterium oxide.
Synthesis of Succinimide Thioether Compounds
Methanol-soluble mercapto-acids, MPA and MP (conjugates 1a and 1c), were dissolved to a concentration of 100mg/ml in methanol and reacted with molar equivalents of 2. A catalytic amount of triethylamine (0.01x) was added to the reaction mixture. The reaction was stirred for 30 minutes at room temperature. The crude product was diluted to 20ml with solvent A and purified using RP-HPLC. Water-soluble thiol compounds AcCys and GSH (conjugates 1b and 3) were dissolved to a concentration of 50mg/ml in de-ionized water. A molar equivalent of 2 was dissolved in MeOH (75mg/ml) and added to the thiol solution. The un-buffered MeOH/aqueous solution had a final pH of ~4, retarding the maleimide-thiol addition reaction; therefore, the solution was stirred overnight at room temperature. The crude product was diluted to 5ml with solvent A and purified using RP-HPLC. Purified fractions were collected and freeze-dried, with approximately 90% yield for all reactions. 1a (Figure S1) 1H-NMR (MeOD): δ 0.96 (t, 3H), 2.63–2.69 (dd, 1H), 3.17–3.24 (dd, 1H), 3.39 (q, 2H), 3.61 (s, 2H), 4.13–4.17 (dd, 1H), 7.29 (d, 2H), 7.48 (d, 2H). ESI-MS: 338.1 (M-1H+2Na)+, 338.0 calc’d (C14H14NO4SNa2+). 1b (Figure S2) 1H-NMR (MeOD): δ 1.16 (t, 3H), 2.03 (d, 3H), 2.42–2.55 (m, 1H), 2.93–2.99 (m, 0.5H), 3.15–3.28 (m, 2H), 3.52–3.58 (m, 2.5H), 3.96–4.00 (dd, 1H), 4.67–4.75 (m, 1H). ESI-MS: 289.1 (M+H)+, 289.1 calc’d (C11H17N2O5S+). 1c (Figure S3) 1H-NMR (MeOD): δ 1.14 (t, 3H), 2.44–2.2.50 (dd, 1H), 2.69 (t, 2H), 2.91–2.99 (m, 1H), 3.09- 3.23 (m, 2H), 3.53 (q, 2H), 3.91–3.94 (dd, 1H). ESI-MS: 276.0 (M-1H+2Na)+, 276.0 9 calc’d (C9H12NO4SNa2+). 3 (Figure S4) 1H-NMR (D2O): δ 1.03 (t, 3H), 2.14 (m, 2H), 2.49 (m, 2H), 2.56–2.64 (m, 1H), 2.91–2.97 (dd, 0.5H), 3.06–3.28 (m, 2.5H), 3.45 (q, 2H), 3.89 (t, 1H), 3.93 (s, 2H), 3.94–4.00 (td, 1H), 4.60 (dt, 1H). ESI-MS: 455.2 (M+Na)+, 455.1 calc’d (C16H24N4O8SNa+).
NMR analysis of MPA-NEM retro reactions
1H-NMR spectroscopy with W5 water suppression28 was used to monitor retro reactions of maleimide conjugates (Scheme 1). Samples of MPA were dissolved to a concentration of 3mg/ml in 0.2M phosphate buffer pH 7.4 with 10% D2O. High buffer concentrations were needed (relative to those employed in the HPLC experiments below) in order to maintain a constant pH throughout the experiment, owing to the high concentration of MPA necessary for NMR investigation. After addition of compounds the pH was adjusted to 7.4 if necessary. A molar equivalent of 2 was added to each NMR tube and the spectrum was recorded. Samples were ring-opened by incubation at pH 8.0 @ 37°C until ring opening was complete (~5days). Molar equivalents of GSH or glycine were added to ring-opened and non-ring-opened samples. Samples were incubated at 37°C and spectra were recorded at time zero and at 24hrs.
HPLC evaluation of reactions kinetics
Synthesized conjugates (1) were dissolved at a concentration of 0.1mM in 50mM phosphate buffers (pH 7.4) containing 10mM GSH (and 5.0mM, 0.5mM and 0.05mM for GSSG). Lower buffer concentrations were employed to permit quantitative detection by HPLC, and these lower concentrations were sufficient to maintain a constant pH throughout the experiment. The kinetics of succinimide ring-opening were measured by monitoring reactions incubated without reductant. The pH of all samples were verified and adjusted to 7.4 if needed before incubation at 37°C. 150μl samples were collected periodically and added to 150μl of 0.5% formic acid solution to reduce the pH and quench the retro and ring-opening reactions. Samples were stored at −20°C until analyzed. RP-HPLC injections were done using above defined conditions and areas of peaks were integrated to calculate conversion curves. The identities of the compounds present in each peak were determined using LC-MS or MS.
Results and Discussion
NMR analysis of MPA-NEM retro reactions
Our first experiments sought to validate that retro Michael-type additions were in fact a primary reaction route for select succinimide thioethers under reducing conditions. These experiments involved 1H-NMR analysis of the reaction of 4-mercaptophenylacetic acid (MPA) with N-ethylmaleimide (NEM, 2) to yield (1a), with subsequent incubation with glutathione (GSH). The formation of the Michael-type adduct and its degradation via retro reactions were easily observed in the NMR experiment. Figure 1A shows the 1H-NMR spectrum for MPA in solution; this spectrum presents chemical shifts centered at 6.88 and 7.18ppm, which result from the reduced MPA, as well as minor contributions from the oxidized form centered at 7.14 and 7.41ppm. The chemical shifts of the thiophenyl aromatic protons are sensitive to the identity of the thiol substituents and thus provided a facile means to follow the addition and retro reactions (Figure 1B and 1C). A molar equivalent of 2 was added to the solution of MPA; upon addition of 2, the aromatic protons shift downfield (to peaks centered at 7.18 and 7.36ppm), indicating the production of the conjugate 1a (Figure 1B). The resonances resulting from the small fraction of oxidized MPA (centered at 7.14 and 7.41ppm) remained unchanged. A molar equivalent of GSH was added, resulting in immediate reduction of the unreacted MPA, indicated by a shift in the aromatic protons from 7.14 and 7.41ppm (oxidized) to 6.88 and 7.18ppm (Figure 1C). No other changes in the spectrum for conjugate 1a were immediately apparent. After 24 hours of incubation at 37°C, however, MPA was liberated from 1a as indicated by the increase in intensity of the MPA aromatic protons (centered at 6.88 and 7.18ppm, Figure 1D). Under these conditions a minor amount of ring opening of the 1a also occurred (resonances centered at 7.14 and 7.32ppm), but the low intensity of these resonances indicates this occurs at a significantly slower rate than the retro reaction. Hydrolysis of the succinimide ring could occur on either carbonyl with respect to the thioether; however, the thiophenyl protons of either species were not distinct. Hydrolysis of the succinimide ring before incubation with GSH in these NMR experiments hindered the retro reaction as no exchange was observed over seven days incubation (data not shown). Furthermore, incubation of conjugate 1a in the absence of reducing agents or in the presence of other nucleophiles such as glycine yielded only the ring-opened substituent (data not shown), clearly indicating that the equilibrium lies toward the Michael adduct and that formation of detectable quantities of free MPA only occurred when exogenous thiols were added to the reaction. Therefore, succinimide thioethers should be sensitive to variations in reducing environments near physiological conditions.
Figure 1.
1H-NMR of aromatic protons of (A) MPA, (B) after addition of NEM to MPA, (C) immediately after addition of GSH to (NEM+MPA) and (D) after incubation of (C) @ 37°C for 24hrs. Evident in (D) are free MPA and small amounts of ring-opened 1a (7.14, 7.32ppm) and GS-MPA mixed disulfide (7.45, 7.18ppm).
HPLC evaluation of reactions kinetics
We next sought to determine the selectivity of these retro and thiol-exchange reactions, as well as to obtain a quantitative measure of the timescales of these reactions, at biologically relevant concentrations of reductant. Such analysis required the quantification of small quantities of multiple compounds; HPLC and LC-MS were thus used to permit both quantification and identification of liberated compounds. Various addition products were synthesized by reacting 2 with MPA (1a), N-acetylcysteine (1b) and 3-mercaptopropionic acid (1c). These thio-acids, which exhibit different thiol pKa values (6.6,29 9.5,30 10.331 respectively), were selected to determine how the rate of exchange may vary with the pKa of the thio-acid. 0.1mM of 1 was incubated in 10mM GSH or 5.0mM, 0.5mM and 0.05mM GSSG (see below) in phosphate buffer at pH 7.4 and 37°C; succinimide ring-opening hydrolysis rates were determined from solutions of 1 in buffer without addition of GSH. Rates of formation of the product 3 (Scheme 1), under these various conditions, were determined by monitoring changes of peak area in the HPLC experiment; the identity of the chemical species present in each fraction was confirmed by LC-MS (data not shown).
Figure 2 shows a typical set of traces obtained upon incubation of 1a with excess GSSG, showing the location of all peaks and expanded regions highlighting relevant peaks. Arrows indicate the direction of peak growth or recession with increasing time. At time zero, a single peak was observed for compound 1a. Over time, the peak for 1a decreased in intensity, while peaks for compounds 3, GS-MPA mixed disulfide, and 1aRO (ring-opened 1a) all increased in intensity. The loss of 1 occurred concomitantly with the generation of 3. Peaks for 3 and 1aRO are split into two equal peaks. Peak 3 is split as a result of two diastereomers formed from the Michael addition of GSH to 232 (verified by the equivalence of masses determined from LC-MS of the two different peaks), while 1aRO is split depending on the side of succinimide ring opening in relation to the thioether (also verified by the equivalence of masses determined from LC-MS of the two different peaks). Scheme 2 illustrates the likely reaction cycle for an initial conjugate 1 in the presence of excess thiols, showing ring-opening of conjugates as well as the exchange with GSH or GSSG. Either GSH or GSSG causes exchange, with 5.0mM GSSG yielding identical results as 10mM GSH (Supporting Information, Figure S5). This observation suggests that the formation of 2 is the rate-limiting step in the overall reaction to 3 and is significantly slower than the disulfide exchange of the free thio-acid with excess GSSG; therefore the oxidation state of GSH does not significantly impact the rate of formation of 3.
Figure 2.
HPLC traces of degradation of 1a in 50mM phosphate buffer with 0.5mM GSSG pH 7.4 @ 37°C over a period of 6days, masses by LC-MS and displayed for (M+H)+. The peak area for 1a decreases with time as peak areas for 3, GS-MPA (mixed disulfide), and 1aRO increase with time, indicating the occurrence of the retro Michael-type reaction. Time arrows indicate the direction of peak area growth or decline with time. Peak 3 consists of two equal peaks representing two diastereomers while the two peaks of 1aRO evolves from ring opening either side of the succinimide with respect to the thioether.
Scheme 2.
Complete lifecycle of 1 in solution with oxidized or reduced thiols.
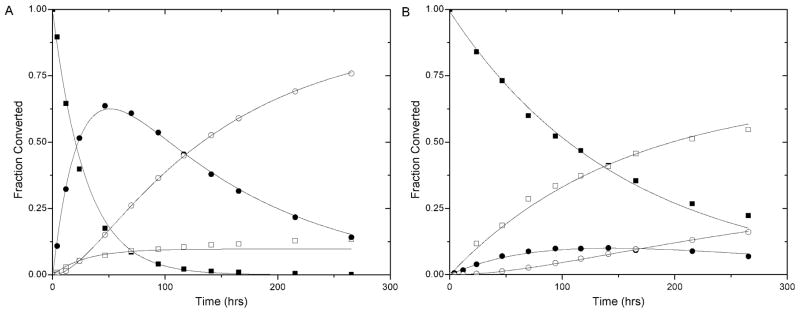
Throughout these experiments, no measurable amount of 2 was detected, confirming the widely known fact that the equilibrium greatly favors the succinimide thioether under the experimental conditions. Thus, by neglecting the kinetics of formation of 2 (and the thiol-exchange of GSSG to yield GSH as our above experiments validate), Scheme 2 can be simplified to the combination of a consecutive and parallel reaction (Scheme 3). Thus, pseudo first-order rate constants, k1 and k3, can be defined for the ring-opening of 1 and 3, and k2 can be defined as the pseudo first-order rate constant for the retro and exchange reaction of 1 (to yield 3) in the presence of a large excess of a thiol compound (10mM GSH or 5mM GSSG). Using the simplified Scheme 3, reaction rate equations (eq 1–4) can be defined and converted to integrated rate laws (eq 5–8). Fractional concentrations of 1, 1RO, 3 and 3RO measured by HPLC (relative to the initial concentration) were plotted as a function of time for 1a (Figure 3A) and 1b (Figure 3B). Equation 7 was employed to fit data for 3 (converted from 1a and 1b (R2 > 0.98)), yielding k1, k2 and k3 with values shown in Table I, with their corresponding half-lives. Standard deviations for all values were calculated from the standard error from fit.
Scheme 3.

Simplified kinetics negating products that are not measureable during experiment
Figure 3.
Relative HPLC measured concentrations for 1a (panel A) and 1b (panel B) over time, with constructed curves using derived rate constants and equations 5–8 for (■) 1 (a or b) (□) 1RO (●) 3 and (○) 3RO.
Table I.
Pseudo-first order rate constants and respective half lives for retro and ring openening reactions. Standard deviations for all values were calculated from the standard error from fit.
| Conjugate | Retro Reaction | Ring-Opening | ||
|---|---|---|---|---|
| k2 (hr−1) | Half-Life (hr) | k1 or k3 (hr−1) | Half-Life (hr) | |
| 1a | 0.0371±0.0038 | 19±2 | 0.0033±0.0002 (k1) | 211±15 |
| 1b | 0.00207±0.0002 | 337±27 | 0.0044±0.0003 (k1) | 157±12 |
| 1c | n/a | n/a | 0.0032±0.0002 (k1) | 215±11 |
| 3 | -- | -- | 0.0076±0.0007 (k3) | 92±2 |
Curves to plot the fractional concentrations of the other compounds as a function of time were constructed, as shown in Figure 3, by input of the appropriate rate constants into equation 5–8. As clearly illustrated in the figure, the fits show exceptional agreement with collected data for all compounds. 1c did not exhibit measurable retro and exchange reactions under these experimental conditions, therefore only k3 was determined. The fractional concentrations of 1 and 1RO as a function of time decreased and increased, respectively, as exponential decay functions with respect to k1 and k2, as shown in Figure 3. The dependence of the fractional concentrations of 3 and 3RO as a function of time were more complex, owing to the consecutive reaction mechanism, as shown by an initial increase and subsequent decrease in the fractional concentration of 3; the fractional concentration of 3RO increased over time with a delayed onset. The extent of formation of 1RO and 3RO was directly related to the relative reaction rates of k1 and k2. In the reactions of 1a, k2>k1, therefore the major product at equilibrium is 3RO; conversely for 1b k1>k2, therefore 1RO is the major product. Retro and exchange rates for 1a were an order of magnitude greater than those for 1b, with rate constants of 0.0371±0.0038 versus 0.00207±0.0002hr−1 and respective half-lives of 19±2 versus 337±27hrs while half-lives for ring-opening reactions of compounds 1 were the same order of magnitude (ca. 102) for all compounds. In comparison, half-lives for the glutathione-mediated cleavage of disulfide bonds under similar conditions range from 8 to 45mins (with pseudo first-order rate constants of 5 to 0.9hr−1),8 indicating that the use of maleimide-thiol adducts rather than disulfides may have advantages for producing more stable, yet degradable bioconjugates. Indeed, in vivo blood circulation stability has been shown to be as little as 4hrs for disulfide-based immunotoxin-antibody conjugates, with circulation stability directly dependent on glutathione reduction kinetics.7,33,34 Accordingly, the decreased rate of the retro reaction of the maleimide-thiol adduct with glutathione would increase compound circulation stability while maintaining cleavage sensitivity in highly reducing environments.
The release data from HPLC experiments illustrates the extent of turnover of the initial succinimide thioether under various reducing conditions relevant to the use of these strategies for the liberation of drug conjugates or materials degradation. Thus using these treatments, variations in the overall generation of 3 as a function of time (for different compounds and reductant concentrations) are presented in Figure 4 (calculations from obtained data are shown in the Supporting Information, Figures S6-S8). 1a supported the greatest rate of conversion of the initial adduct, with conversion of nearly 85% after 70 hours, followed by 1b with much slower kinetics and substantially lower conversion, and then by 1c, for which no measurable conversion was observed. The rate of exchange of the initial adduct is clearly impacted by the thiol pKa, with higher pKa decreasing the rate (Figure 4); use of a Michael donor with sufficiently high pKa (~10.3) can eliminate any impact of the retro reaction, as illustrated by the lack of conversion of 1c.
Figure 4.
Fraction of 3 generated from (■) 1a (●) 1b (▲) 1c (at the baseline) in the presence of (−) 10mM, (--) 1.0mM, and (··) 0.1mM GSH.
Two other observations recommending the use of these strategies for tailoring the degradation of succinimide thioethers are indicated from these data. Importantly, the data in Figure 4 illustrate that the rate of thiol exchange varies with the concentration of reductant. 1a showed the greatest dependence on reductant concentration with the total production of 3 ranging from 30% to 90% under these conditions. In contrast, conversion of 1b showed little dependence, and that of 1c showed no dependence, on reductant concentration; Michael donors can thus be selected for application on the basis of desired sensitivity to reductant. Additionally, the conversion to 3 can be impacted by the inactivation of 1 by ring opening of certain adducts. The minimum half-lives for the retro reaction were approximately 19hrs for 1a and 337hrs for 1b while those for inactivation of 1 by ring-opening were approximately 200hrs. Thus, the inactivation by ring opening became significant and limited the overall conversion to 3 for conjugates 1b and 1c (evidenced by the reduction in turnover of these compounds at later time points), which could be used to tailor initial release and long-term stability for specific conjugates.
This observed and selective retro Michael-type addition has very limited precedent in literature, and there are no previous reports to our knowledge that seek to selectively utilize the retro reaction for a specific use. In studies by Lewis et al, a bis-maleimide was used to conjugate a chelating agent to a monoclonal antibody, it was determined that the resulting adduct was cleaved to some extent when exposed to fresh human serum.19 Later, Alley et al. described mass spectrometry experiments that illustrated that an antibody-drug conjugate linked by maleimide-thiol chemistries had exchanged with rat serum albumin in vivo to yield albumin-drug adduct.20 Concurrently Lin et al. described studies indicating that maleimide-cysteine adducts disappeared in cells while similar iodoacetate adducts were stable.21 The commonality in these reports stems from the use of the resulting succinimide thioether in environments containing reduced or oxidized thiol species, and although the mechanism underlying these previous observations was not discussed or determined, the reaction mechanism could proceed as suggested in Scheme 1, as supported by our experiments. More recently, Baker and coworkers have described the bromination of maleimides for reversible conjugation of thiols as a bioconjugate technique.35–37 The process of bromination and subsequent elimination of HBr by the addition of a protected cysteine was found to be more rapid than the addition to maleimides. This bromomaleimide conjugate was found to be reversible by adding reducing agents such as GSH36 or TCEP37 with complete conversion within 4hrs for incubation with excess GSH. In contrast, 70hrs was required for 85% conversion of 2a, indicating that the reduced reactivity of maleimides compared with bromomaleimides correlates with the rate of the retro reaction.
Conclusions
We have confirmed that succinimide thioethers undergo reversible addition in solutions and that exchange with nearby free thiols or disulfides can be manipulated under relevant physiological conditions. Reverse reactions of these kind have not been reported for other Michael-type addition products such as thiol-acrylate conjugates, most likely due to the reduced Michael acceptor reactivity of acrylates compared to maleimides.11 Glycine, substituted for GSH in control experiments, did not induce the reverse reaction under these conditions, although it has been shown that maleimides can be Michael acceptors for amine donors;38,39 these observations suggest that the retro reaction may not be substantially affected by amine-bearing compounds present in vivo. Our data also indicate that ring opening of the succinimide before the addition of GSH stabilizes the conjugate and will inhibit the liberation of free thio-acid and conversion to 3. Hence purposely ring opening a succinimide thioether will stabilize bioconjugates for in vivo or in vitro assays if retro reactions are not desired, and ring-opening that would occur in vivo could be employed to dictate a lifetime over which a conjugated drug may be cleaved by GSH.
Importantly, thiophenyl conjugates were also sensitive to the reducing environment, with only approximately 30% converted at low reductant concentration within three days, versus 90% at high reductant concentration, potentially allowing for targeted release/delivery. The slower reaction rates for the retro reaction relative to disulfide-mediated release (e.g., half lives >20hrs for succinimide thioethers compared to minutes for disulfide-mediated release) may also allow for longer-term delivery of drugs in reducing environments. We note that the exchange and retro reactions kinetics discussed here were modulated by altering the thiol serving as the Michael donor; in many bioconjugates such alteration of the thiol reactivity would not be possible, particularly given that alkylthiols (i.e., cysteine) of natural proteins and peptides are common Michael donors in bioconjugation reactions. In cases in which Michael donor reactivity on proteins/peptides would be desirable, post-translational modification of natural proteins or peptides or non-natural amino acid incorporation could be employed for the addition of suitable thiols.40 Other possibilities not investigated here rely on modulating the maleimide stability and reactivity as has been accomplished by addition of cyclohexyl or benzyl moieties to the nitrogen group to reduce the susceptibility of the maleimide ring to hydrolysis prior to addition reactions.41 Albeit there are many different possibilities for tailoring retro reactions for use as delivery mechanisms, our observations expressed here could be exploited for both systemic and local administration of bioconjugated drugs or for imparting degradation sites in polymeric backbones or crosslinked biomaterials. Studies to test these potential opportunities are underway.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
Supplementary Material
Acknowledgments
This work was supported in part by the Nemours Foundation, the National Science Foundation (DGE-0221651) and the National Institutes of Health (5-P20-RR016472-10). The contents of the manuscript are the sole responsibility of the authors and do not necessarily reflect the official views of the National Institutes of Health nor of the National Center for Research Resources. The authors would like to thank Professor J.M. Fox and research group members for assistance with LC-MS experiments, as well as Dr. S. Bai for assistance with water suppression 1H-NMR techniques.
Footnotes
Associated Content
Supporting Information Available: Experimental procedures, characterization details, and data analysis are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric Systems for Controlled Drug Release. Chem Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 2.Sakiyama-Elbert SE, Panitch A, Hubbell JA. Development of growth factor fusion proteins for cell-triggered drug delivery. The FASEB Journal. 2001;15:1300–1302. doi: 10.1096/fj.00-0564fje. [DOI] [PubMed] [Google Scholar]
- 3.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Del Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 4.Kuppusamy P, Afeworki M, Shankar RA, Coffin D, Krishna MC, Hahn SM, Mitchell JB, Zweier JL. In Vivo Electron Paramagnetic Resonance Imaging of Tumor Heterogeneity and Oxygenation in a Murine Model. Cancer Res. 1998;58:1562–1568. [PubMed] [Google Scholar]
- 5.Bulmus V, Woodward M, Lin L, Murthy N, Stayton P, Hoffman A. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J Controlled Release. 2003;93:105–120. doi: 10.1016/j.jconrel.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Senter PD, Pearce WE, Greenfield RS. Development of a drug-release strategy based on the reductive fragmentation of benzyl carbamate disulfides. The Journal of Organic Chemistry. 1990;55:2975–2978. [Google Scholar]
- 7.Thorpe PE, Wallace PM, Knowles PP, Relf MG, Brown ANF, Watson GJ, Knyba RE, Wawrzynczak EJ, Blakey DC. New Coupling Agents for the Synthesis of Immunotoxins Containing a Hindered Disulfide Bond with Improved Stability in Vivo. Cancer Res. 1987;47:5924–5931. [PubMed] [Google Scholar]
- 8.Trimble SP, Marquardt D, Anderson DC. Use of Designed Peptide Linkers and Recombinant Hemoglobin Mutants for Drug Delivery: In Vitro Release of an Angiotensin II Analog and Kinetic Modeling of Delivery. Bioconj Chem. 1997;8:416–423. doi: 10.1021/bc970037h. [DOI] [PubMed] [Google Scholar]
- 9.Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30:2180–2198. doi: 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Mather BD, Viswanathan K, Miller KM, Long TE. Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci. 2006;31:487–531. [Google Scholar]
- 11.Hermanson GT. Bioconjugate techniques. 2. Academic Press; San Diego, California: 2008. [Google Scholar]
- 12.Moore JE, Ward WH. Cross-linking of Bovine Plasma Albumin and Wool Keratin. J Am Chem Soc. 1956;78:2414–2418. [Google Scholar]
- 13.Yoshitake S, Imagawa M, Ishikawa E, Niitsu Y, Urushizaki I, Nishiura M, Kanazawa R, Kurosaki H, Tachibana S, Nakazawa N, Ogawa H. Mild and Efficient Conjugation of Rabbit Fab’ and Horseradish Peroxidase Using a Maleimide Compound and Its Use for Enzyme Immunoassay. J Biochem. 1982;92:1413–1424. doi: 10.1093/oxfordjournals.jbchem.a134065. [DOI] [PubMed] [Google Scholar]
- 14.Curtis SK, Cowden RR. Demonstration of sulfhydryl and disulfide groups by a fluorescent maleimide procedure. Histochem Cell Biol. 1980;68:23–28. doi: 10.1007/BF00498497. [DOI] [PubMed] [Google Scholar]
- 15.Sekine T, Ando K, Machida M, Kanaoka Y. Fluorescent thiol reagents: V. Microfluorometry of thiol compounds with a fluorescent-labeled maleimide. Anal Biochem. 1972;48:557–568. doi: 10.1016/0003-2697(72)90111-x. [DOI] [PubMed] [Google Scholar]
- 16.Veronese FM. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials. 2001;22:405–417. doi: 10.1016/s0142-9612(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 17.Nie T, Baldwin A, Yamaguchi N, Kiick KL. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J Controlled Release. 2007;122:287–296. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White JE, Snider DA, Scaia MD. Synthesis and properties of some new polyimidosulfides with highly mobile backbones. Journal of Polymer Science: Polymer Chemistry Edition. 1984;22:589–596. [Google Scholar]
- 19.Lewis MR, Shively JE. Maleimidocysteineamido-DOTA Derivatives: New Reagents for Radiometal Chelate Conjugation to Antibody Sulfhydryl Groups Undergo pH-Dependent Cleavage Reactions. Bioconj Chem. 1998;9:72–86. doi: 10.1021/bc970136v. [DOI] [PubMed] [Google Scholar]
- 20.Alley SC, Benjamin DR, Jeffrey SC, Okeley NM, Meyer DL, Sanderson RJ, Senter PD. Contribution of Linker Stability to the Activities of Anticancer Immunoconjugates. Bioconj Chem. 2008;19:759–765. doi: 10.1021/bc7004329. [DOI] [PubMed] [Google Scholar]
- 21.Lin D, Saleh S, Liebler DC. Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity. Chem Res Toxicol. 2008;21:2361–2369. doi: 10.1021/tx800248x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nampalli S, McDougall MG, Lavrenov K, Xiao H, Kumar S. Utility of Thiol-Cross-Linked Fluorescent Dye Labeled Terminators for DNA Sequencing. Bioconj Chem. 2002;13:468–473. doi: 10.1021/bc015591c. [DOI] [PubMed] [Google Scholar]
- 23.Wan L, Zhang X, Gunaseelan S, Pooyan S, Debrah O, Leibowitz M, Rabson A, Stein S, Sinko P. Novel multi-component nanopharmaceuticals derived from poly(ethylene) glycol, retro-inverso-Tat nonapeptide and saquinavir demonstrate combined anti-HIV effects. AIDS Research and Therapy. 2006;3:1–15. doi: 10.1186/1742-6405-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 25.Koechel DA, Cafruny EJ. Synthesis and structure-activity relationship of some thiol adducts of ethacrynic acid. J Med Chem. 1973;16:1147–1152. doi: 10.1021/jm00268a018. [DOI] [PubMed] [Google Scholar]
- 26.Ploemen JHTM, Van Schanke A, Van Ommen B, Van Bladeren PJ. Reversible Conjugation of Ethacrynic Acid with Glutathione and Human Glutathione S-Transferase P1-1. Cancer Res. 1994;54:915–919. [PubMed] [Google Scholar]
- 27.van Iersel MLPS, Ploemen JPHTM, Lo Bello M, Federici G, van Bladeren PJ. Interactions of α,β-unsaturated aldehydes and ketones with human glutathione S-transferase P1-1. Chem-Biol Interact. 1997;108:67–78. doi: 10.1016/s0009-2797(97)00096-3. [DOI] [PubMed] [Google Scholar]
- 28.Liu M, Mao X-a, Ye C, Huang H, Nicholson JK, Lindon JC. Improved WATERGATE Pulse Sequences for Solvent Suppression in NMR Spectroscopy. J Magn Reson. 1998;132:125–129. [Google Scholar]
- 29.DeCollo TV, Lees WJ. Effects of Aromatic Thiols on Thiol-Disulfide Interchange Reactions That Occur during Protein Folding. The Journal of Organic Chemistry. 2001;66:4244–4249. doi: 10.1021/jo015600a. [DOI] [PubMed] [Google Scholar]
- 30.Harman B, Sóvágó I. Metal complexes of sulphur-containing ligands. V Interactions of cobalt(II) ion with L-cysteine and its derivatives. Inorg Chim Acta. 1983;80:75–83. [Google Scholar]
- 31.Danehy JP, Noel CJ. The Relative Nucleophilic Character of Several Mercaptans toward Ethylene Oxide. J Am Chem Soc. 1960;82:2511–2515. [Google Scholar]
- 32.Jemal M, Hawthorne D. High performance liquid chromatography/ionspray mass spectrometry of N-ethylmaleimide and acrylic acid ester derivatives for bioanalysis of thiol compounds. Rapid Commun Mass Spectrom. 1994;8:854–857. [Google Scholar]
- 33.Arpicco S, Dosio F, Brusa P, Crosasso P, Cattel L. New Coupling Reagents for the Preparation of Disulfide Cross-Linked Conjugates with Increased Stability. Bioconj Chem. 1997;8:327–337. doi: 10.1021/bc970025w. [DOI] [PubMed] [Google Scholar]
- 34.Dosio F, Arpicco S, Adobati E, Canevari S, Brusa P, De Santis R, Parente D, Pignanelli P, Negri DRM, Colnaghi MI, Cattel L. Role of Cross-Linking Agents in Determining the Biochemical and Pharmacokinetic Properties of Mgr6-Clavin Immunotoxins. Bioconj Chem. 1998;9:372–381. doi: 10.1021/bc970192w. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher FF, Nobles M, Ryan CP, Smith MEB, Tinker A, Caddick S, Baker JR. In Situ Maleimide Bridging of Disulfides and a New Approach to Protein PEGylation. Bioconj Chem. 2011;22:132–136. doi: 10.1021/bc1004685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith MEB, Schumacher FF, Ryan CP, Tedaldi LM, Papaioannou D, Waksman G, Caddick S, Baker JR. Protein Modification, Bioconjugation, and Disulfide Bridging Using Bromomaleimides. J Am Chem Soc. 2010;132:1960–1965. doi: 10.1021/ja908610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tedaldi LM, Smith MEB, Nathani RI, Baker JR. Bromomaleimides: new reagents for the selective and reversible modification of cysteine. Chem Commun. 2009:6583–6585. doi: 10.1039/b915136b. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa H, Suzuki T, Hayashi Y. High-Yielding Synthesis of the Anti-Influenza Neuramidase Inhibitor (−)-Oseltamivir by Three “One-Pot” Operations. Angew Chem. 2009;121:1330–1333. doi: 10.1002/anie.200804883. [DOI] [PubMed] [Google Scholar]
- 39.White JE, Scaia MD. Polymerization of N,N′-bismaleimido-4,4′-diphenylmethane with arenedithiols. Synthesis of some new polyimidosulphides. Polymer. 1984;25:850–854. [Google Scholar]
- 40.Hohsaka T, Sisido M. Incorporation of non-natural amino acids into proteins. Curr Opin Chem Biol. 2002;6:809–815. doi: 10.1016/s1367-5931(02)00376-9. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa E, Kawai T, Miyai K. A more stable maleimide N-(4-carboxycyclohexylmethyl)maleimide, for enzyme labeling. Igaku-Shoin; 1981. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.