Abstract
The present study examined the effects of the mGluR1 antagonist JNJ16259685 (JNJ) and the mGluR5 antagonist MPEP alone and in combination with morphine in two acute pain models (hotplate, warm water tail-withdrawal), and a persistent, inflammatory pain model (capsaicin). In the hotplate and warm water tail-withdrawal procedures, JNJ and MPEP were ineffective when administered alone. In both procedures, JNJ potentiated morphine antinociception. In the hot plate procedure, MPEP potentiated morphine antinociception at the highest dose examined, whereas in the warm water tail-withdrawal procedure MPEP attenuated morphine antinociception at a moderate dose and potentiated morphine antinociception at a high dose. For both JNJ and MPEP, the magnitude of this morphine potentiation was considerably greater in the hotplate procedure. In the capsaicin procedure, the highest dose of MPEP produced intermediate levels of antihyperalgesia and also attenuated the effects of a dose of morphine that produced intermediate levels of antihyperalgesia. In contrast, JNJ had no effect when administered alone in the capsaicin procedure and did not alter morphine-induced antihyperalgesia. The present findings suggest that the effects produced by mGluR1 and mGluR5 antagonists alone and in combination with morphine can be differentiated in models of both acute and persistent pain.
Keywords: MPEP, JNJ16259685, capsaicin, morphine, acute pain, persistent pain, metabotropic glutamate antagonists, potentiation, rat
INTRODUCTION
Evidence indicates that the excitatory amino acid glutamate is involved in both pain processing and the modulation of mu opioid-induced antinociception and antihyperalgesia (e.g., Mao, 1999; Kozela et al., 2003). Indeed, antagonists at the N-methyl-d-aspartate (NMDA) receptor site produce antihyperalgesic effects in most persistent or chronic pain models (Chaplan et al., 1997). Although NMDA antagonists do not produce antinociception in acute pain models when administered alone (Nemmani et al., 2004), they either potentiate or antagonize the antinociceptive effects of morphine and other mu opioid agonists (Allen et al., 2003; Chen et al., 2005; Fischer and Dykstra, 2006). These effects have been shown to be dependent upon the dose of morphine, dose of the NMDA antagonist, and the type of pain model (e.g., Maeda et al., 2002; Nemmani et al., 2004; Craft and Lee, 2005).
Metabotropic glutamate (mGlu) receptor sites have also been linked to pain processing, although reports describing the interaction of mGluR antagonists with mu opioid agonists are limited. However, Fischer et al., (2008b) reported that in a mouse tail-flick procedure the mGluR1 antagonist, JNJ16259685 (JNJ) and the mGlu2/3 receptor antagonist LY341495 failed to produce antinociception when administered alone, but potentiated morphine antinociception in a dose-dependent manner. Moreover, JNJ increased the antinociceptive efficacy of the partial mu opioid agonists buprenorphine and dezocine under conditions in which these opioids produced less than maximal effects (Fischer et al., 2008a). There is evidence to suggest that these effects may be indirectly mediated by activity at NMDA receptors, as activation of mGluR receptors enhances NMDA receptor activity (Kelso et al., 1992; Skeberdis et al., 2001). In contrast, the mGluR5 receptor antagonist MPEP has not been shown to produce antinociception nor does it alter morphine antinociception (Fischer et al., 2008a).
Considerably less is known about the effects of mGluR antagonists in models of persistent or chronic pain. mGluR2/3 and mGluR5 antagonists do, however, attenuate hyperalgesia induced by the administration of inflammatory agents (Simmons et al., 2002; Sevostianova and Danysz, 2006), and this finding is supported by studies indicating that mGluR receptors are present on peripheral terminals of primary sensory neurons (Walker et al., 2001b; Yang and Gereau, 2002) and are up-regulated in response to acute or persistent inflammation (Dolan et al., 2003). Moreover, in models of persistent inflammatory pain and a neuropathic pain model, some mGluR1 antagonists enhance morphine-induced antihyperalgesia (Yoon et al., 2006; Osikowicz et al., 2008). Similarly, knockdown of mGlu1 receptors increases the effectiveness of morphine in a model of neuropathic pain (Fundytus et al., 2001).
The present study was designed to examine the effects of the metabotropic glutamate antagonists JNJ and MPEP alone and in combination with morphine in rat models of both acute pain and persistent, inflammatory pain. JNJ and MPEP were selected for study as their acute effects have been examined in a number of pain models (e.g., Lea and Faden, 2006; Fischer et al., 2008a), and studies suggest that moderate doses of these compounds have minimal effects on locomotor activity (e.g., Henry et al., 2002; Popik and Wrobel, 2002; Hodgson et al., 2011). Such effects are critical when examining the effects of drugs in combination with morphine, as changes in motor behavior can interfere with the assessment of nociception.
Specifically, the effects of morphine in combination with JNJ and MPEP were examined in both hotplate and warm water tail-withdrawal procedures as well as a capsaicin procedure where persistent nociception and inflammation are induced by the administration of capsaicin locally into the tail. Comparison across acute and chronic pain models is critical, as it is well established that these models differ in terms of nociceptive duration, type of nociceptive response, nociceptive stimulus and the presence of inflammation (Le Bars et al., 2001). Moreover, the nociceptive response observed in acute and chronic pain models is mediated by distinct pain fibers and subserved by distinct excitatory amino acids and neurotransmitter systems (Le Bars et al., 2001; Kayser et al., 2007).
METHODS
Subjects
Three month old male F344 rats were obtained from Charles River Laboratories (Raleigh, NC, USA). Rats were individually housed in a colony on a 12-h/12-h light/dark cycle with unlimited access to food and water and all experiments were performed in the light phase of the animals’ light/dark cycle (between the hours of 10:00 and 16.00 h). Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of North Carolina, and the methods were in accord with the guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research, Division on Earth and Life Studies, National Research Council, 2010).
Hotplate and Warm Water Tail-withdrawal assays
Three types of sessions were conducted: warm water tail-withdrawal alone (5.6 mg/kg JNJ/morphine), hotplate alone (0.3 mg/kg JNJ/morphine), and warm water tail-withdrawal immediately followed by the hotplate (all other dose combinations of JNJ/ and MPEP/morphine). Previous studies from our laboratory (e.g., Lomas et al., 2008) indicated that baselines values and the effects produced by various drugs do not differ when hotplate and warm water tail-withdrawal procedures are conducted separately or sequentially. However, when using the procedures sequentially, testing first in the hotplate altered subsequent latencies in the warm water tail-withdrawal procedure. Consequently, in the present investigation tests were conducted first in the warm water tail-withdrawal procedure.
For each type of session, animals were tested twice under baseline conditions to yield an average baseline latency measure. A cumulative morphine dosing procedure was then used to obtain a morphine dose-effect curve. The selected dose of either JNJ or MPEP was administered once prior to obtaining each cumulative dose-effect curve, with the injections of JNJ and MPEP administered 30 min before administering the first dose of morphine, and each cumulative morphine dose administered 15 min prior to each test. Based on initial results with moderate doses of JNJ, different doses of JNJ were used in the hotplate (0.3 – 3.0 mg/kg) and warm water tail-withdrawal (1.0 – 5.6 mg/kg) procedures. Tests were also conducted with selected doses of JNJ and MPEP administered alone 15 min prior to testing, and then re-tested at 30, 45 and 60 min.
In the warm-water tail withdrawal procedure, rats were removed from their home cages and lightly restrained while the distal 7 cm of the tail was placed into a 52°C water bath. The tail withdrawal latency from the water was then recorded. A cutoff limit of 15 sec (maximal possible effect) was used to avoid tissue damage. In the hotplate procedure, rats were placed on a hotplate analgesia meter set at 52°C (Columbus Instruments, Columbus, OH). Latency to lick the hind paw or perform an escape response was recorded. A cutoff limit of 40 sec (maximal possible effect) was used to prevent tissue damage.
Capsaicin Tail Withdrawal Assay
Prior to the induction of an inflammatory state, each animal was lightly restrained and the distal 7 cm of the tail immersed in a non-noxious 45°C water bath. Animals that failed to keep their tails in the water for a maximum of 15 sec at the beginning of a session were not tested with drugs. Hyperalgesia was then induced by injecting 3.0 µg capsaicin 3.5 cm from the distal end of the tail using a protocol identical to that previously used in this laboratory (Barrett et al., 2003; Lomas et al., 2008). Animals were placed into a chamber prepared with ~1.0 mL of isoflurane and monitored for sedation. Immediately following the onset of sedation, animals were removed from the isofluorane chamber, administered capsaicin locally via s.c. injection in the tail, then placed back in the home cage. Rats recovered from the procedure within 2–3 mins. After administration of capsaicin, tail-withdrawal latencies from the 45°C water decreased from 15 sec to approximately 4 sec (data not shown).
As the antihyperalgesic effects of capsaicin peak at 15–30 min and then decline over 1 h (Barrett et al., 2003), cumulative dose testing could not be conducted. Consequently, during acute drug tests rats received varying doses of morphine either alone or in selected combinations with JNJ and MPEP. The administration of these drugs or drug combinations preceded testing by 30 min, with capsaicin administered 15 min before testing. For all tests of antihyperalgesia, a 15 s cutoff limit was used as a maximal antihyperalgesic effect (i.e., withdrawal latencies returned to pre-inflammation baseline levels). Rats were tested once per week with no more than 5 tests per animal. The number of animals tested with JNJ and MPEP in combination with the highest dose of morphine (10 mg/kg) was limited due to toxic reactions.
Drugs
Morphine sulfate was provided by the National Institute on Drug Abuse (Bethesda, MD), JNJ (Ki=0.34 nM) and MPEP (Ki=16 nM) were purchased from Tocris Biosciences (Ellisville, MO), and isoflurane from both Phoenix Pharmaceuticals (St. Joseph, MO) and Sigma-Aldrich, Inc. (St. Louis, MO). Morphine sulfate and MPEP were dissolved in a 0.9% phosphate-buffered saline solution, JNJ in 45% (w/v) 2-hydroxypropyl-β-cyclodextrin, and capsaicin in a solution of Tween 80/95% ethanol/saline in a 1/1/8 ratio. Morphine, JNJ and MPEP were all administered i.p. at a volume of 0.1 mL/100g, whereas capsaicin was administered locally in the tail at a fixed volume of 0.1 mL. In each of the procedures, testing at the higher dose combinations of JNJ, MPEP and morphine was limited due to signs of toxicity, including motor deficits and in one case death.
Data Analysis
For all tests of antinociception and antihyperalgesia, raw latency scores were converted to % maximum possible effect (%MPE) scores using the following equation:
The %MPE scores from the dose-effect curves examining morphine were used to mathematically derive the dose of the morphine required to produce a 50% effect (ED50) either alone or in combination with JNJ or MPEP. Calculation of ED50 values required the following: 1) an ascending limb of the dose effect curve comprised of at least 3 points and, 2) that the lowest mean %MPE within this limb was ~20% or lower, and the highest mean %MPE was ~80% or higher. Subsequently, relative potency estimates of morphine alone were compared to those of morphine when combined with JNJ or MPEP. For this analysis, dose ratios were calculated by comparison of the slopes of two linear regression lines representing the two dose-effect curves and the distance between those two lines determined as described by Tallarida and Murray (1987). In incidences in which the potency ratios yielded negative values, the order of dose-effect curves inputted into the Tallarida and Murray program were reversed, thus yielding positive values. All analyses of dose ratios were conducted using group data. Differences in the relative potency were considered to be significant if the 95% confidence interval did not overlap 1.0 or below. Additional analyses were conducted using isobols in which the ED50 dose (95% C.L.) of a drug combination were compared to the ED50 dose (95% C.L.) of morphine when administered alone (details are described in the Results section). In the capsaicin procedure, comparisons were made between a select dose of morphine alone and in combination with JNJ and MPEP using a one-factor ANOVA. When a significant ANOVA was obtained, a Dunnett’s multiple comparison was used to determine the statistical significance of specific dose combinations. Time course analyses for JNJ and MPEP were analyzed using separate repeated measures two-way ANOVA (RMANOVA). For all statistical tests, the alpha level was set at P=0.05.
RESULTS
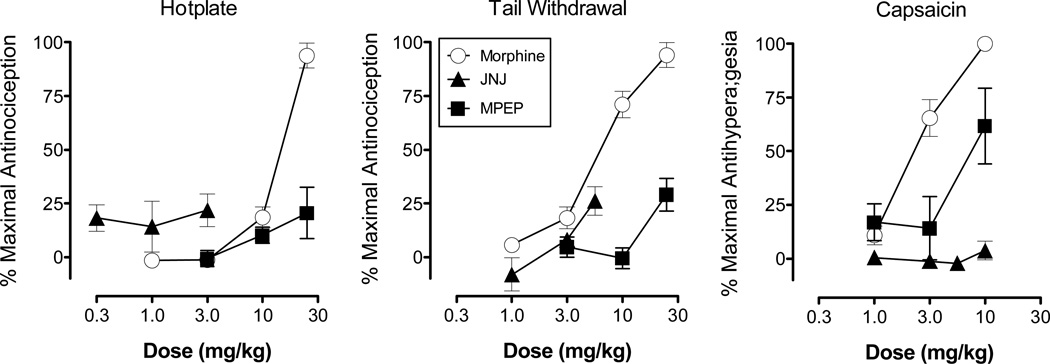
Figure 1 shows the effects of morphine, JNJ and MPEP on the hotplate (left), warm water tail-withdrawal (middle) and capsaicin (right) procedures. Morphine produced a dose-dependent increase in antinociception in both the hotplate and warm water tail-withdrawal procedures, as well as a dose-dependent increase in antihyperalgesia in the capsaicin procedure. In each of these procedures, morphine produced a maximal effect (100%). Based on the ED50 values (Table 1), morphine was most potent in the capsaicin procedure (2.51 mg/kg) and least potent in the hotplate (13.27 mg/kg) procedure.
Figure 1.
Effects of morphine, JNJ and MPEP administered systemically on the hotplate (left), warm water tail-withdrawal (center) and capsaicin procedures (right). Morphine was determined on the hotplate and warm water tail-withdrawal procedures using a cumulative dosing procedure, whereas all other tests were conducted using an acute dosing procedure in which drugs were administered 30 mins prior to testing. In the hotplate, warm water tail-withdrawal, and capsaicin procedures, morphine was tested in 16 rats, JNJ in 4–6 rats, and MPEP in 4 rats, respectively. Vertical axis: antinociception expressed as the % of maximal possible effect in the hotplate and warm water tail-withdrawal procedures, and antihyperalgesia expressed as the % of maximal possible effect in the capsaicin procedure. Horizontal axis: dose of morphine, JNJ or MPEP expressed in mg/kg. Vertical bars represent the standard error; where not indicated, the standard error fell within the data point.
TABLE 1.
ED50 values (95% confidence limits) and relative potency ratios (95% confidence limit) for morphine alone and in combination with JNJ or MPEP in the hotplate, warm water tail-withdrawal and capsaicin procedures.
| ED50 (95% C.L.) | Potency Ratioa | |
|---|---|---|
| Hotplate: | ||
| Morphine alone: | 13.27 (11.15 – 15.80) | |
| + 0.3 JNJ | 8.35 (5.59 – 12.44) | −1.59 (1.12 – 2.25)* |
| + 1.0 JNJ | 3.31 (2.18 – 5.02) | −4.27 (3.05 – 5.97)* |
| + 3.0 JNJ | 1.00 (0.58 – 1.74) | −12.52 (8.32 – 18.84)* |
| + 3.0 MPEP | 13.57 (9.79 – 18.84) | 1.04 (0.79 – 1.37) |
| + 10 MPEP | 12.89 (10.37 – 16.05) | −1.07 (0.79 – 1.44) |
| + 30 MPEP | 3.28 (1.97 – 5.45) | −4.18 (2.82 – 6.19)* |
| Warm Water Tail-Withdrawal: | ||
| Morphine alone: | 6.90 (5.55 – 8.59) | |
| + 1.0 JNJ | 3.74 (1.28 – 5.04) | −1.85 (1.28 – 2.67)* |
| + 3.0 JNJ | 4.46 (2.56 – 7.77) | −1.77 (1.10 – 2.86)* |
| + 5.6 JNJ | 4.35 (3.02 – 6.25) | −1.59 (1.01 – 2.50)* |
| + 3.0 MPEP | 8.71 (6.91 – 10.96) | 1.25 (0.88 – 1.76) |
| + 10 MPEP | 13.20 (10.61 – 16.42) | 1.88 (1.29 – 2.75)** |
| + 30 MPEP | 3.51 (2.83 – 4.35) | −1.97 (1.32 – 2.97)* |
| Capsaicin: | ||
| Morphine alone: | 2.51 (2.13 – 4.35) | |
CL, confidence limit
negative numbers indicate a leftward shift in the morphine dose-effect curve, positive numbers a rightward shift
more potent (P<0.05) than morphine alone in each respective procedure
less potent (P<0.05) than morphine alone in each respective procedure
Across the range of doses tested in the hotplate and warm water tail-withdrawal procedures, JNJ and MPEP produced only low levels of antinociception (maximum: 29%). JNJ failed to produce an antihyperalgesic effect in the capsaicin procedure, whereas at the highest dose tested MPEP produced intermediate levels of antihyperalgesia (maximum: 61%). In both the hotplate and warm water tail-withdrawal procedures, JNJ and MPEP were also evaluated 15 to 60 min after administration (data not shown). Separate RMANOVA of the effects of JNJ and MPEP in the hotplate procedure and MPEP in the warm water tail-withdrawal procedure failed to indicate a significant effect of dose, time, or a dose × time interaction. In the warm water tail-withdrawal procedure, a RMANOVA of the effects of JNJ across the different time points indicated a significant effect of dose (F2,9=5.8 P<0.05), time (F2,37=4.1 P<0.05), and dose × time interaction (F6,27=3.7 P<0.05). The maximal level of antinociception produced by JNJ, however, was only 26%.
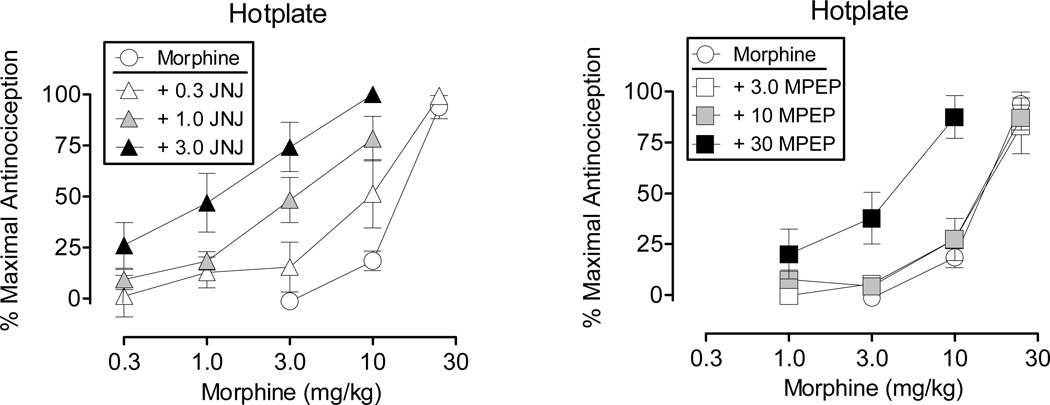
Figure 2 shows the effects of morphine alone and in combination with selected doses of JNJ (left) and MPEP (right) on the hotplate procedure. JNJ produced a dose-dependent leftward shift in the morphine dose-effect curve, with the highest dose tested decreasing the morphine ED50 value from 13.27 mg/kg to 1.00 mg/kg (Table 1). Analysis of potency ratios indicated that this dose of JNJ produced a 12.52-fold leftward shift in the morphine dose-effect curve. Whereas the two lowest doses of MPEP failed to alter the morphine dose-effect curve, the highest dose produced a 4.18-fold leftward shift (Table 1). At this dose combination of MPEP and morphine, a maximal effect was obtained at 10 mg/kg, whereas when administered alone morphine produced a maximal effect at 30 mg/kg.
Figure 2.
Effects of morphine administered alone and in combination with JNJ (left) and MPEP (right) on the hotplate procedure. Morphine alone was tested in 16 rats and in combination with the different doses of JNJ in 4–6 rats and MPEP in 4–7 rats. Vertical axis: antinociception expressed as the % of maximal possible effect. Horizontal axis: dose of morphine determined using a cumulative dosing procedure and expressed in mg/kg. Vertical bars represent the standard error; where not indicated, the standard error fell within the data point.
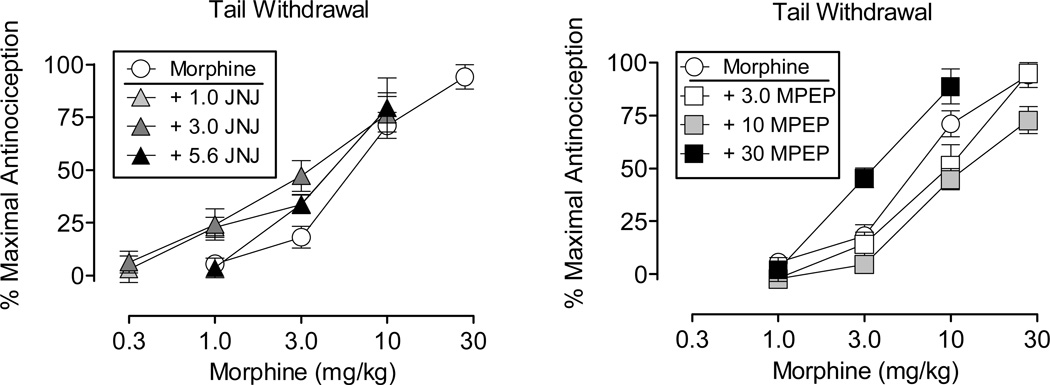
Figure 3 shows the effects of morphine alone and in combination with selected doses of JNJ (left) and MPEP (right) on the warm water tail-withdrawal procedure. At each of the doses tested, JNJ produced small, leftward shifts in the morphine dose-effect curve (Table 1). The lowest dose of MPEP failed to alter the morphine curve, the intermediate dose produced a small rightward shift in the morphine curve, and the highest dose produced a 1.97-fold leftward shift in the morphine curve. At the highest dose of MPEP, the maximal effect of morphine was obtained at a dose of 10 mg/kg, whereas when administered alone morphine produced a maximal effect at 30 mg/kg.
Figure 3.
Effects of morphine administered alone and in combination with JNJ (left) and MPEP (right) on the warm water tail-withdrawal procedure. Morphine alone was tested in 16 rats and in combination with the different doses of JNJ in 4–8 rats and MPEP in 4–7 rats. Vertical axis: antinociception expressed as the % of maximal possible effect. Abscissa: dose of morphine determined using a cumulative dosing procedure and expressed in mg/kg. Vertical bars represent the standard error; where not indicated, the standard error fell within the data point.
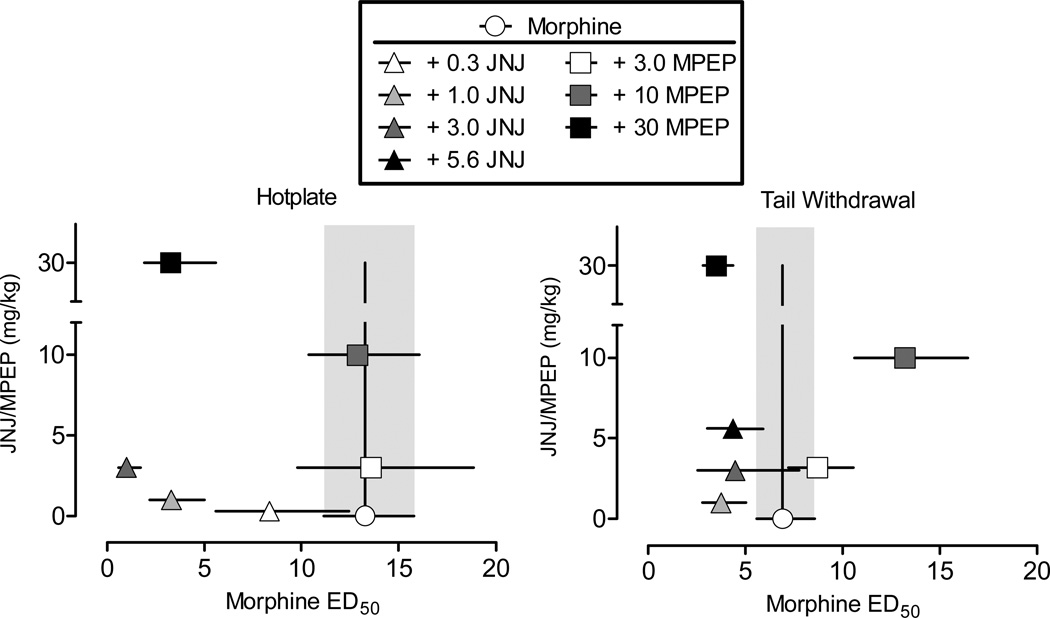
Figure 4 shows an isobolographic analysis of the effects of JNJ and MPEP administered in combination with morphine in the hotplate (left) and warm water tail-withdrawal (right) procedures. The effects of JNJ and MPEP are illustrated in both panels of the graph, as comparisons were made to the same, composite morphine dose-effect curve. As shown in the left panel, the two highest doses of JNJ (1.0 and 3.0 mg/kg) tested produced supra-additive effects with morphine, as the ED50 values (and C.L.) fell to the left of the theoretical line of additivity. As noted previously, these effects were dose-dependent with the largest effect obtained at the highest dose of JNJ. Similarly, at the lowest dose of JNJ (1.0 mg/kg) tested in the warm water tail-withdrawal procedure, the interaction with morphine was supra-additive, although the magnitude of this effect was considerably smaller than that obtained in the hotplate procedure. This graphic analysis also indicated that the interaction with morphine and the highest dose of MPEP (30 mg/kg) was supra-additive in the hotplate procedure. In the warm water-tail withdrawal procedure, the interaction at the intermediate dose of MPEP (10 mg/kg) with morphine was antagonistic (i.e., ED50 dose of morphine was to the right of the line of additivity) and at the highest dose (30 mg/kg) it was supra-additive.
Figure 4.
Isobolograms for morphine in combination with JNJ and MPEP on the hotplate (left) and warm water tail-withdrawal (right) procedures. The effects of JNJ and MPEP are illustrated in each panel of the graph, as comparisons were made to a single morphine curve in the hotplate procedure and a single morphine curve in warm water tail-withdrawal procedure. Horizontal axis: ED50 value (95% C.L.) for morphine expressed in mg/kg. The perpendicular line intersecting the morphine ED50 value represents the theoretical line of additivity. Vertical axis: dose of JNJ or MPEP, expressed in mg/kg, administered in combination with a morphine dose-effect curve. Vertical lines represent the 95% C.L. of the ED50 values for morphine: when the ED50 was to the left of the theoretical line of additivity and the C.L. lines did not overlap the shaded area, the interaction was considered to be supra-additive, whereas when the C.L. lines did overlap with the shaded area, the interaction was considered additive.
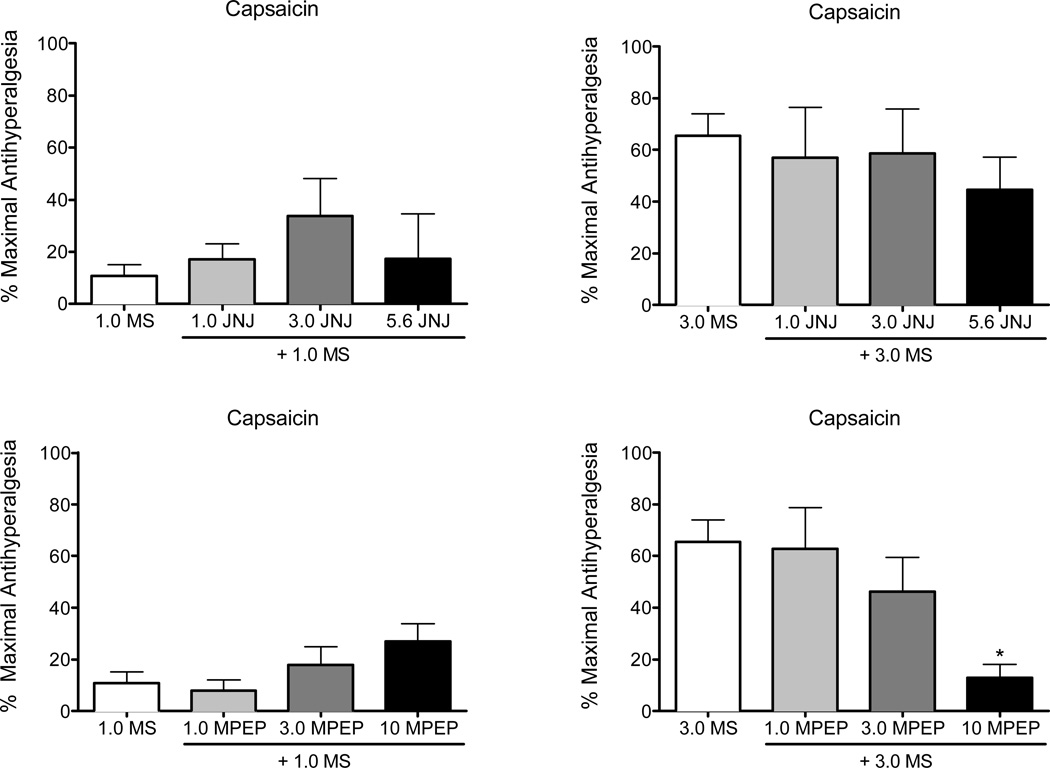
Figure 5 shows the effects of two doses of morphine alone and in combination with JNJ (top panels) and MPEP (bottom panels) in the capsaicin procedure. These two doses of morphine were selected as they produced minimal (1.0 mg/kg) and intermediate (3.0 mg/kg) levels of antihyperalgesia when administered alone, thus allowing for the identification of potential enhancement of morphine antihyperalgesia. Separate ANOVAs for 1.0 and 3.0 mg/kg morphine did not reveal an effect of JNJ on morphine antihyperalgesia. Similarly, MPEP did not alter the effects of 1.0 mg/kg morphine. In contrast, when MPEP was examined in combination with 10 mg/kg morphine it attenuated rather than potentiated morphine antihyperalgesia, with the highest dose (10 mg/kg) decreasing morphine antihyperalgesia from 65% to 12%. When 10 mg/kg MPEP was administered alone, it produced a 61% antihyperalgesic effect (see Fig. 1). An ANOVA for 3.0 mg/kg morphine confirmed a main effect of MPEP dose (F2,22=3.5, P<0.05), with a Dunnett’s multiple comparison indicating a significant (P<0.05) difference at 10 mg/kg MPEP.
Figure 5.
Effects of selected doses of morphine administered alone and in combination with JNJ (top panels) and MPEP (bottom panels) in the capsaicin procedure. These two doses of morphine were selected as they produced minimal (1.0 mg/kg: left most panels) and moderate (3.0 mg/kg: right most panels) antihyperalgesic effects when administered alone, thus allowing for the identification of potential enhancement of morphine antihyperalgesia. Morphine alone was tested in 11–13 rats, in combination with the two lower doses of JNJ and MPEP in 4–8 rats: due to toxic effects, dose combinations with the highest dose of JNJ and MPEP with morphine were limited to 2–4 rats. Vertical axis: antihyperalgesia expressed as the % of maximal possible effect. Horizontal axis: doses of morphine alone and in combination with JNJ or MPEP determined using an acute dosing procedure and expressed in mg/kg. Vertical bars represent the standard error: asterisks indicate a significant (P<0.05) difference from morphine alone.
Discussion
The present study compared the effects of the mGluR1 antagonist JNJ and the mGluR5 antagonist MPEP in rat models of acute pain (hotplate, warm water tail-withdrawal) and persistent, inflammatory pain (capsaicin). In the hotplate and warm water tail-withdrawal procedures, JNJ and MPEP were ineffective when administered alone. In both of these procedures, however, JNJ potentiated morphine antinociception. MPEP also potentiated morphine antinociception in the hotplate procedure, whereas in the warm water tail-withdrawal procedure it produced a biphasic effect, antagonizing the effects of morphine at a moderate dose and potentiating morphine antinociception at the highest dose tested. Finally, in the capsaicin procedure, the highest dose of MPEP alone produced intermediate levels of antihyperalgesia, and this dose attenuated the effects of the highest dose of morphine examined. In contrast, no dose of JNJ had an effect when administered alone in this procedure nor did JNJ alter morphine-induced antihyperalgesia.
In both of the acute pain models examined, MPEP potentiated morphine antinociception. This finding contrasts with those reported in a mice tail-flick procedure, in which MPEP had no effect on morphine antinociception (Kozela et al., 2003; Fischer et al., 2008b). As such, the present finding are the first to establish that MPEP can enhance morphine antinociception and thus extends previous studies indicating an interaction between morphine and MPEP. Indeed, MPEP has been shown to attenuate the development of tolerance to morphine antinociception (Kozela et al., 2003) and inhibit the acquisition of morphine conditioned place preference (Popik and Wrobel, 2002).
It is important to note, however, that the potentiation of morphine antinociception by MPEP in the present investigation was obtained only at a relatively high dose (30 mg/kg). Similarly, other studies report that MPEP/morphine interactions are most evident at relatively high doses of MPEP (Popik and Wrobel, 2002; Kozela et al., 2003), and there is evidence that these high doses display activity at the NMDA receptors (Cosford et al., 2003; Lea and Faden, 2006). Since NMDA receptor antagonists potentiate morphine antinociception under a range of conditions (e.g., Nemmani et al., 2004; Fischer et al., 2008a), it is possible that in the present study the effects of MPEP were mediated by activity at the NMDA receptor site.
The effects of MPEP were also examined in a rat model of persistent, inflammatory pain in which local administration of capsaicin into the tail induces a hyperalgesic response to non-noxious 45°C water. In this procedure, the highest dose MPEP produced intermediate levels of antihyperalgesia when administered alone, a finding in agreement with studies indicating that mGluR5 antagonists produce antihyperalgesic effects in a variety of inflammatory pain models (Walker et al., 2001a,b; Zhu et al., 2004; Lee et al., 2006; Sevostianova and Danysz, 2006; Jesse et al., 2008) and can reduce the development of capsaicin-induced mechanical allodynia (Soliman et al., 2004). When administered in combination with morphine in the capsaicin procedure, however, MPEP produced dose-dependent decreases in morphine antihyperalgesia. That MPEP could produce antihyperalgesia in this model and attenuate the antihyperalgesic effects of morphine suggests a mutual antagonism. Such effects have not been reported with other mGluR antagonists and morphine, thus the mechanism underlying this phenomenon has not been systematically studied.
Recent studies indicate that MPEP decreases mu opioid receptor (MOR) phosphorylation, internalization, and desensitization in HEK293 cells co-expressing mGluR5 and MOR (Schröder et al., 2009). These processes may alter the availability of MOR to its ligands, an effect which may alter the antinociceptive efficacy of mu opioid agonists. Consequently, if the number of MORs available to morphine is decreased by MPEP, then the number of MORs occupied by morphine likewise should be decreased. It is thus possible that under the inflammatory conditions, MPEP may have decreased MOR availability, thereby attenuating morphine antihyperalgesia.
In contrast to the effects produced by MPEP, JNJ produced large dose-dependent increases in morphine antinociception in the hotplate procedure and small increases in the warm water tail-withdrawal procedure. Similarly, Fischer et al., (2008b) reported that JNJ potentiated the antinociceptive effects of morphine in a mouse tail-flick procedure. As mGlu1 receptors are expressed postsynaptically on dorsal horn neurons (Jia et al., 1999; Alvarez et al., 2000) and potentiate NMDA-mediated responses in this region (Kelso et al., 1992; Skeberdis et al., 2001), it is possible that these effects are mediated by activity at the NMDA receptor site.
When administered alone, JNJ failed to attenuate antihyperalgesia in the capsaicin procedure. Whereas this finding is in agreement with studies indicating that mGluR1 antagonists are not active in some inflammatory pain models (Walker et al., 2001a; Lee et al., 2006), it contrasts with those indicating that mGluR1 antagonists produce hyperalgesia in both inflammatory and non-inflammatory pain models (Sevostianovaa and Danysz, 2006; Kohara et al., 2007; Siniscalcoa et al., 2008). For example, the non-competitive mGluR1 antagonist, A-841720, reduced thermal nociceptive responses in a persistent model of inflammatory pain induced by injections of Freund’s adjuvant as well as mechanical nociception in a neuropathic pain model (but see Fundytus et al., 2001; El-Kouhen et al., 2006). It is important to note that when antihyperalgesic effects are observed following the administration of mGluR1 antagonists, the magnitude of these effects are typically small and not always dose-dependent. Differences across persistent pain models also have been reported with NMDA antagonists (e.g., Sakurada et al., 1998; Lomas et al., 2008), and some of these discrepancies may be related to the mechanism underling the production of persistent or chronic pain. For example, nociception in the capsaicin procedure is mediated primarily by activity at neurokinin receptors (Lao et al., 2003), whereas in a number of other inflammatory (e.g., Freund, formalin) and non-inflammatory pain models (e.g., neuropathic) NMDA receptor mediation plays a prominent role (e.g., Mao et al., 1992).
In contrast to the effects obtained when JNJ was administered in combination with morphine in the hotplate and warm water-tail withdrawal procedures, JNJ had no effect on morphine antihyperalgesia when evaluated in the capsaicin procedure. As there are no reports describing the effects of JNJ or other mGluR1 antagonists on morphine antihyperalgesia in chronic pain models, the specificity of these findings remains to be determined. Nevertheless, the differences observed across pain models with JNJ clearly establish the importance of pain model when assessing the interaction between morphine and mGluR1 antagonists, and thus parallel the results obtained with MPEP and various NMDA antagonists (e.g., Nemmani et al., 2004; Lomas et al., 2008).
Recent findings suggest that combinations of morphine and NMDA antagonists may have some clinical utility, as it has been postulated that NMDA antagonists could reduce the required dose of morphine, inhibit the development of morphine tolerance, and produce fewer side effects than high dose morphine therapy. The utility of these combinations is limited, however, by findings that NMDA antagonists produce a range of side effects. Moreover, the clinical data have been conflicting, with NMDA antagonists enhancing analgesia in some studies of cancer pain (Katz, 2000) but not in studies of chronic, non-neuropathic pain (Galer et al., 2005). Since activation of mGlu receptors enhance NMDA-mediated activity (Kelso et al., 1992; Skeberdis et al., 2001), it has been proposed that mGluR antagonists might have some clinical utility in the treatment of pain as well. Evaluating this proposal has recently been initiated, with studies emphasizing involvement of mGluR subtypes and the specificity of their effects across different pain models.
One of the problems in assessing the potential clinical effectiveness of combinations of mGluR antagonist and morphine is that pain is not a unitary phenomenon and different types of pain (post-surgical, inflammatory, neuropathic) respond to different classes of drugs or drug combinations. Moreover, it is well established that pain can differ along a number of critical dimensions, including the duration of nociception, type of nociceptive stimulus, fibers underlying the nociceptive response, and mediation by distinct excitatory amino acids and neurotransmitter systems. The present findings illustrate this complexity, as the effects produced by MPEP and JNJ in a persistent pain model (capsaicin) contrast markedly with those obtained in acute pain models (hotplate, warm water tail-withdrawal). MPEP, for example, enhanced morphine antinociception in both acute pain models, but attenuated morphine antihyperalgesia in the persistent pain model. Further, a comparison across the acute pain models indicated that the effects produced by both MPEP and JNJ were considerably larger in the hotplate than the warm water tail procedure. Such findings suggest that medication development for the treatment of pain should include a diverse set of pain models, including multiple acute or chronic pain models believed to be predictors of the effectiveness of analgesics against a specific type of pain.
Acknowledgements
Support was provided by grants from the National Institute on Drug Abuse, R01-DA002749 and T32-DA00724.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Allen RM, Granger AL, Dykstra LA. The competitive N-Methyl-D-aspartate receptor antagonist (−)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid (LY235959) potentiates the antinociceptive effects of opioids that vary in efficacy at the µ-opioid receptor. J Pharmacol Exp Ther. 2003;307:785–792. doi: 10.1124/jpet.103.055319. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Carr PA, Grandes P, Somohano PM. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. J Comp Neurol. 2000;422:464–487. doi: 10.1002/1096-9861(20000703)422:3<464::aid-cne11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Smith ES, Picker MJ. Capsaicin-induced hyperalgesia and mu-opioid-induced antihyperalgesia in male and female Fischer344 rats. J Pharmacol Exp Ther. 2003;307:237–245. doi: 10.1124/jpet.103.054478. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- Chen SL, Huang EY, Chow LH, Tao PL. Dextromethorphan differentially effects opioid antinociception in rats. Br J Pharmacol. 2005;144:400–404. doi: 10.1038/sj.bjp.0706086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosford ND, Tehrani L, Roppe J. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]pyridine: A potent and highly selective metabotropic glutamate receptor subtype 5 antagonist with anxiolytic activity. J Med Chem. 2003;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- Craft RM, Lee D. NMDA antagonist modulation of morphine antinociception in female vs. male rats. Pharmacol Biochem Behav. 2005;80:639–649. doi: 10.1016/j.pbb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Dolan S, Kelly JG, Monteiro AM, Nolan AM. Up-regulation of metabotropic glutamate receptor subtypes 3 and 5 in spinal cord in a clinical model of persistent inflammation and hyperalgesia. Pain. 2003;106:501–512. doi: 10.1016/j.pain.2003.09.017. [DOI] [PubMed] [Google Scholar]
- El-Kouhen O, Lehto SG, Pan JB, Chang R, Baker SJ, Zhong C, et al. Blockade of mGluR1 receptor results in analgesia and disruption of motor and cognitive performances: effects of A-841720, a novel non-competitive mGluR1 receptor antagonist. Br J Pharmacol. 2006;149:761–774. doi: 10.1038/sj.bjp.0706877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Dykstra LA. Interactions between an NMDA antagonist and low-efficacy opioid receptor agonists in assays of schedule-controlled responding and thermal nociception. J Pharmacol Exper Ther. 2006;318:1300–1306. doi: 10.1124/jpet.106.101683. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Miller LL, Henry FE, Picker MJ, Dykstra LA. Increased efficacy of μ-opioid agonist-induced antinociception by metabotropic glutamate receptor antagonists: Comparison with (−)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid (LY235959) Psychopharmacology. 2008a;198:271–278. doi: 10.1007/s00213-008-1130-y. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Zimmerman EI, Picker MJ, Dykstra LA. Morphine in combination with metabotropic glutamate receptor antagonists on schedule-controlled responding and thermal nociception. J Pharmacol Exper Ther. 2008b;324:732–739. doi: 10.1124/jpet.107.131417. [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Yashpal K, Chabot JG, Osborne MG, Lefebvre CD, Dray A, Henry JL, Coderre TJ. Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats. Br J Pharmacol. 2001;132:354–367. doi: 10.1038/sj.bjp.0703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galer BS, Lee D, Ma T, Nagle B, Schlagheck TG. MorphiDex (morphine sulfate/dextromethorphan hydrobromide combination) in the treatment of chronic pain: three multicenter, randomized, double-blind, controlled clinical trials fail to demonstrate enhanced opioid analgesia or reduction intolerance. Pain. 2005;115:284–295. doi: 10.1016/j.pain.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Jesse CR, Jucielli S, Cristina W, Nogueira CW. Effect of a metabotropic glutamate receptor 5 antagonist, MPEP, on the nociceptive response induced by intrathecal injection of excitatory aminoacids, substance P, bradykinin or cytokines in mice. Pharmacol Biochem Behav. 2008;90:608–613. doi: 10.1016/j.pbb.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Jia H, Rustioni A, Valtschanoff JG. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J Comp Neurol. 1999;410:627–642. [PubMed] [Google Scholar]
- Katz NP. MorphiDex (MS:DM) Double-blind, multiple-dose studies in chronic pain patients. J Pain Symptom Manage. 2000;19:S37–S41. doi: 10.1016/s0885-3924(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Kayser V, Elfassi IE, Aubel B, Melfort M, Julius D, Gingrich JA. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A−/−, 5-HT1B−/−, 5-HT2A−/−, 5-HT3A−/− and 5-HTT−/−knock-out male mice. Pain. 2007;130:235–248. doi: 10.1016/j.pain.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Kelso SR, Nelson TE, Leonard JP. Protein kinase C-mediated enhancement of NMDA currents by metabotropic glutamate receptors in Xenopus oocytes. J Physio. 1992;449:705–718. doi: 10.1113/jphysiol.1992.sp019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara A, Nagakura Y, Kiso T, Toya T, Watabiki T, Tamura S, Shitaka Y, Itahana H, et al. Antinociceptive profile of a selective metabotropic glutamate receptor 1 antagonist YM-230888 in chronic pain rodent models. Eur J Pharmacol. 2007;571:8–16. doi: 10.1016/j.ejphar.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Kozela E, Pilc A, Popik P. Inhibitory effects of MPEP, an mGluR5 antagonist, and memantine, an N-methyl-D-aspartate receptor antagonist, on morphine antinociceptive tolerance in mice. Psychopharmacology. 2003;165:245–251. doi: 10.1007/s00213-002-1287-8. [DOI] [PubMed] [Google Scholar]
- Lao LJ, Song B, Marvizón JCG. Neurokinin release produced by capsaicin acting on the central terminals and axons of primary afferents: relationship with n-methyl-aspartate and gabab receptors. Neuroscience. 2003;121:667–680. doi: 10.1016/s0306-4522(03)00501-3. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Lea IV PM, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Choi HS, Ju JS, Bae YC, Kim SK, Yoon YW, Ahn DK. Peripheral mGluR5 antagonist attenuated craniofacial muscle pain and inflammation but not mGluR1 antagonist in highly anesthetized rats. Brain Res Bull. 2006;70:378–385. doi: 10.1016/j.brainresbull.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Lomas LM, Terner JM, Picker MJ. Sex differences in NMDA antagonist enhancement of morphine-antihyperalgesia in a capsaicin model of persistent pain: Comparisons to two models of acute pain. Pharmacol Biochem Behav. 2008;89:127–136. doi: 10.1016/j.pbb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kishioka S, Fan X, Fukazawa Y, Shimizu N, Ozaki M, Yamamoto H. Effects of diltiazem and MK-801 on morphine analgesia and pharmacokinetics in mice. Neurosci Lett. 2002;326:216–218. doi: 10.1016/s0304-3940(02)00350-6. [DOI] [PubMed] [Google Scholar]
- Mao J. NMDA and opioid receptors: their interactions in antinociception, tolerance and neuroplasticity. Brain Res Brain Rev. 1999;30:289–304. doi: 10.1016/s0165-0173(99)00020-x. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ, Lu J, Hayes RL. Intrathecal MK-801 and local nerve anesthesia synergistically reduce nociceptive behaviors in rats with experimental peripheral mononeuropathy. Brain Res. 1992;576:254–262. doi: 10.1016/0006-8993(92)90688-6. [DOI] [PubMed] [Google Scholar]
- Nemmani KVS, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine analgesia by site-specific N-methyl-D-aspartate receptor antagonists: Dependence on sex, site of antagonism, morphine dose, and time. Pain. 2004;109:274–283. doi: 10.1016/j.pain.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Osikowicz M, Mika J, Makuch W, Przewlocka B. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain. 2008;139:117–126. doi: 10.1016/j.pain.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M. Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology. 2002;43:1210–1217. doi: 10.1016/s0028-3908(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Sakurada R, Wako K, Sguiyama A, Sakurada C, Tan-Ho K, Kisara K. Involvement of spinal NMDA receptors in capsaicin-induced nociception. Pharmacol Biochem Behav. 1998;59:339–345. doi: 10.1016/s0091-3057(97)00423-1. [DOI] [PubMed] [Google Scholar]
- Schröder H, Wu DF, Seifert A, Rankovic M, Schulz S, Höllt V, Koch T. Allosteric modulation of metabotropic glutamate receptor 5 affects phosphorylation, internalization, and desensitization of the μ-opioid receptor. Neuropharmacology. 2009;56:768–778. doi: 10.1016/j.neuropharm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Sevostianova N, Danysz W. Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology. 2006;51:623–630. doi: 10.1016/j.neuropharm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Simmons RMA, Webster AA, Kalra AB, Iyengar S. Group II mGluR receptor agonists are effective in persistent and neuropathic pain models in rats. Pharmacol Biochem Behav. 2002;73:419–427. doi: 10.1016/s0091-3057(02)00849-3. [DOI] [PubMed] [Google Scholar]
- Siniscalcoa D, Giordanoa C, Fuccioa C, Luongoa L, Ferraracciob F, Rossia F, de Novellisa V, Rothc KA, Maionea S. Involvement of subtype 1 metabotropic glutamate receptors in apoptosis and caspase-7 over-expression in spinal cord of neuropathic rats. Pharmacol Res. 2008;57:223–233. doi: 10.1016/j.phrs.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Opitz T, Zheng X, Bennett MV, Zukin RS. mGluR1-mediated potentiation of NMDA- receptors in involves a rise in intracellular calcium and activation of protein kinase C. Neuropharmacology. 2001;40:856–865. doi: 10.1016/s0028-3908(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Soliman AC, Yu JSC, Coderre TJ. mGlu and NMDA receptor contributions to capsaicin-induced thermal and mechanical hypersensitivity. Neuropharmacology. 2004;48:325–332. doi: 10.1016/j.neuropharm.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Berlin: Springer; 1987. Manual of pharmacologic calculations with computer programs. [Google Scholar]
- Walker K, Bowes M, Pansear M, Davis A, Gentry C, Kesingland A, Gasparini F, Spooren W, Stoehr N, Pagano A. Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function I: Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology. 2001a;40:1–9. doi: 10.1016/s0028-3908(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Walker K, Reeve A, Bowes M, Winter J, Witherspoon G, Davis A, Schmid P, et al. mGlu5 receptors and nociceptive function II: mGlu5 receptors functionally expressed on peripheral sensory neurons mediate inflammatory hyperalgesia. Neuropharmacology. 2001b;40:10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- Yang D, Gereau RW. Peripheral group II metabotropic glutamate receptors (mGlu2/3) regulate prostaglandin E2-mediated sensitization of capsaicin responses and thermal nociception. J Neurosci. 2002;22:6388–6393. doi: 10.1523/JNEUROSCI.22-15-06388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MH, Choi J, Bae HB, Kim SJ, Chung ST, Jeong SW, Chung SS, et al. Antinociceptive effects and synergistic interaction with morphine of intrathecal metabotropic glutamate receptor 2/3 antagonist in the formalin test of rats. Neurosci Lett. 2006;394:222–226. doi: 10.1016/j.neulet.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Zhu CZ, Wilson SG, Mikusa JP. Assessing the role of metabotropic glutamate receptor 5 in multiple nociceptive modalities. Eur J Pharmacol. 2004;506:107–118. doi: 10.1016/j.ejphar.2004.11.005. [DOI] [PubMed] [Google Scholar]