Abstract
Purpose
The aim of this study was to develop and optimize a strategy for long-term cultivation of luteinizing human granulosa cells (GCs).
Methods
GCs were cultivated in DMEM/F12 medium supplemented with 2% fetal calf serum. In vitro proliferation of GCs was supported by follicular fluid as well as FSH and growth factors.
Results
The cultured GCs were maintained for 45 days with a doubling time of 159 ± 24 h. GCs initiated by the addition of follicular fluid and cultivated under low serum conditions reached 10 ± 0.7 population doublings. GCs maintain the typical phenotypic expression and the telomere length according to specific culture conditions.
Conclusion
Our present study has demonstrated that GCs can be maintained in vitro for at least 45 days and this cell model can be beneficial when studying hormonal regulation associated with follicular maturation and preparation of oocytes for fertilization.
Keywords: Cultivation, Follicular fluid, Granulosa cells, Phenotype
Introduction
Human luteinizing granulosa cells (GCs) with follicular fluid (FF) are usually harvested during in vitro fertilization (IVF) from the preovulatory follicles of hormonally stimulated women. These cells are challenged with a peak of luteinizing hormone or human chorionic gonadotrophin, which may be regarded as a signal to convert GCs to luteal cells (1, 2). Each primordial follicle contains an oocyte, arrested in meiosis and surrounded by a single layer of flattened epithelial pregranulosa cells (3). The GCs continue to divide, and as follicle maturation is reached just before ovulation, GCs division decreases and the next phase of the oocyte meiosis resumes (4). Luteinized GCs are considered to be terminally differentiated, being replaced in the midluteal phase of the menstrual cycle by small, luteinized cells originating from the surrounding theca (5).
Wen at al. examined FF and GCs production of steroid from IVF patients. Despite the size of follicle triggered after controlled luteinisation, the levels of progesterone and testosterone were maintained at relatively constant levels (median 98.1 μmol/L for progesterone, and 5.8 nanomoles/L for testosterone). However, estradiol levels were slightly lower in the larger follicles (follicular diameter 10–15 mm, median 25.3 nanomoles/L; follicles > = 15 mm, median 15.1 nanomoles/L). Absolute steroid levels are associated with follicular size, not oocyte maturation/ability to fertilize (6). Estradiol, progesterone and testosterone are the main steroid hormones that play essential roles during the follicular and luteal phases of the menstrual cycle. However other cytokines, such as inhibins, activins, insulin growth factor-2, insulin growth factor binding proteins, tumour growth factor-b and endothelial growth factor, have been measured in FF and correlated with oocyte maturation (6–8). The cellular content of FF aspirated during oocyte collection for assisted reproduction consisted of a mixture of GCs, erythrocytes and large vaginal epithelial cells (9, 10). Samples of FF used in this study were obtained from ovarian follicles of women undergoing IVF cycle in assisted reproduction centre. Only the samples of FF from cycles with mature and morfologically normal oocytes were included. Moreover, oocytes obtained in those cycles underwent IVF and/or ICSI and in all of the cycles, oocytes were fertilized and developing embryos were obtained (Table 1).
Table 1.
Summary of antibodies used for flow cytometry to their specifications. BioLegend (BL), Beckman Coulter (BC), Caltag (C), BD Pharming (BD), eBioscience (eB), Sigma-Aldrich (SA). Mesenchymal marker (MM), endothelial marker (EM), haemopoietic marker (HM), adhesion molecule (AM), proliferation marker (PM)
| Clone | Function | ||
|---|---|---|---|
| CD29 | BL | TS2/16 | MM, b1 integrin, associates with CD49a in VLA-1 integrin |
| CD31 | BL | WM59 | EM, PECAM-1, tissue regeneration and safely removing neutrophils |
| CD34 | BC | 581 | HM, HPCA1, expression on early hematopoietic and vascular-associated tissue |
| CD44 | C | MEM-85 | MM, binds hyaluronic acid, mediates adhesion of stromal cells and ECM |
| CD45 | C | HI30 | HM, protein tyrosine phosphatase, receptor type C |
| CD49d | BC | HP2/1 | AM, VLA-4, a4 integrin, associates with CD29, binds fibronectin, VCAM-1, adhesion |
| CD49e | BC | SAM-1 | AM, VLA-5, a5 integrin, associates with CD29, binds fibronectin, adhesion, apoptosis |
| CD71 | BL | MEM-75 | PM, transferrin receptor 1 controls iron uptake during cell proliferaion |
| CD73 | BD | AD2 | MM, ecto-5-nucleotidase, dephosphorylates nucleotides to allow nucleoside uptake |
| CD90 | BC | F15-42-1-5 | MM, Thy-1, possible inhibition of stem cell and neuron differentiation |
| CD105 | BL | MEM-226 | MM, EM, endoglin, protective role against pro-apoptotic factors |
| CD106 | BL | STA | EM, VCAM-1, adhesion of cells to vascular endothelium |
| CD146 | BC | TEA1/34 | EM, MCAM, mesenchymal stem cells with greater differentiation potential |
| CD166 | BC | 3A6 | MM, AM, ALCAM, ligand for CD6 |
| CD184 | C | 12 G5 | MM, HM, CXCR4, alpha-chemokine receptor specific for stromal-derived-factor-1 |
| CD197 | eB | 3D12 | Chemokine, CCR7 |
| CD222 | eB | MEM-238 | PM, receptor for insulin-like growth factor 2 |
| HLA-I | C | Tu149 | Major histocompatibility class I |
| HLA-II | C | Tu36 | Major histocompatibility class II |
| FSH-R | SA | F3929 | PM, receptor FSH, GCs are the only cell type expressing the FSH-R |
| LH-R | SA | L6792 | receptor LH, luteinization GCs |
Disruptions of hormonal stimulation and communication between GCs and oocyte represent a serious complication of assisted reproduction. An example of such complications may be the development of ovarian hyperstimulation syndrome. No animal or human cellular model is available to study these specific health problems. GCs obtained during the collection of oocytes in the IVF cycle could represent a suitable in vitro model. Luteinized GCs are believed to be terminally differentiated, which significantly complicates the establisment and cultivation of GCs primary culture, and this is not supportive for long-term cultivation. The aim of our study was to find an optimal protocol for the long-term cultivation of GCs and to characterize the GCs phenotype in vitro.
Material and methods
Isolation of human granulosa cells
Human GCs were recovered from women undergoing IVF procedures after ovarian stimulation and ovulation induction. GCs with FF were obtained from 25 patients with their consent according to the guidelines of the Assisted Reproduction Center, Prague. Cultures of GCs were established using cells recovered from the FF during oocyte retrieval procedures. The mean age of patients was 33.3 ± 4 year (range from 23–43 year), average BMI was 24.7 ± 4. Patients were stimulated by 10,000 IU Pregnyl (hCG, N.V. Organon Oss, Netherlands) 36 h prior to oocyte collection. An average of 9 ± 6 ovarian follicles were harvested together with 6 ± 4 oocytes from each patient. The mean value of FF was 17 ± 13 ml. Samples that contained high quantities of erythrocytes were excluded. The GCs suspension was trasported in FF (37°C), cells were centrifugated at 357 g for 5 min, then resuspended in cultivation medium and seeded on untreated plastic 12-well plates (TPP, Switzerland).
For GCs long-term cultivation, a new low-FCS cultivation medium was designed. Medium consisted of Dulbecco’s Modified Eagle’s Medium (DMEM/F12, Sigma-Aldrich, USA), 2% fetal calf serum FCS (PAA, Austria), 10 mg/ml ascorbic acid (Sigma-Aldrich, USA), 0.05 μM dexamethasone (Sigma-Aldrich, USA), 200 mM L-glutamine (Invitrogen, USA), 10 mg/ml gentamycine (Invitrogen, USA), 10,000 units/ml penicillin and 10,000 μg/ml streptomycin (Invitrogen, USA). Moreover we added 20 ng/ml EGF, 50 ng/ml bFGF (PeproTech, USA) and FSH (Puregon; NV Organon, Oss, the Netherlands).
On the day when the GCs were seeded, cultivation medium was supplemented with FF (2:1) and 2.5 mg/ml amphotericin (Sigma-Aldrich, USA). Cultivation medium was changed every day. Cells were cultivated at 37°C under aerobic conditions (5% CO2). Once adherent cells were more than 80% confluent, they were detached with 0.05% trypsin-EDTA (Invitrogen, USA) for 10 min and counted using a Z2 counter or cell viability analyser Vi-Cell XR 2.03 (both Beckman Coulter, USA).
Immunofluorescence
For identification of cell surface antigens, cells were fixed with 4% paraformaldehyde at 20°C for 10 min. Blocking and diluent solution consisted of phosphate-buffer saline (PBS), 5% serum (Sigma) from the same species as was the primary antibody for 30 min. The samples were then incubated with a primary antibody for 90 min. Primary antibodies used in this study were specific for the following antigens: FSHR, LHR, nestin. After washing with PBS, the antigen-binding sites were visualized with a secondary antibody conjugated to fluorochromes. Goat anti-rabbit IgG coupled to Alexa 488 (1:300, Invitrogen) was used (incubation 30 min). Cells stained with omission of a primary antibody were used as negative controls. Cell nuclei were counterstained with DAPI (4‘-6-diamidino-2-phenyindole, Sigma) for 5 min. Coverslips were mounted in Mowiol or polyvinyl/alcohol/glycerol with 1,4-diazobocyclo-[2.2.2]-octane (DABCO) as anti-fading agent. Samples were examined with a BX51 microscope (Olympus) equipped with a DP71 digital camera. The images were prepared using Adobe Photoshop software.
Doubling time and population doublings
The number of population doublings was calculated by the following formula:
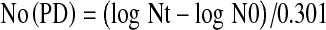 |
No (PD) [number of population doublings], Nt [number of cells after enzymatic dissociation/collection of cells], N0 [number of cells seeded]. The resulting number of population doublings is assessed individually or cumulatively during cell cultivation. Regression analysis of individual values at every passage shows the trend of proliferative potential of cell culture. The cumulation of data is important to record Hayflick‘s limit (60 doubling of the population), i.e. to show an unlimited proliferative potential (11).
Time required to double the population is expressed in the equation: DT = (t x log2)/(log Nt—log N0). DT [doubling time—time needed to double the population], t [elapsed time between seeding and cell harvesting], Nt [number of cells after enzymatic dissociation/collection of cells], N0 [number of cells seeded].
Cultivation human granulosa tumor cells COV434
COV434 cells (Sigma-Aldrich, USA) were derived from a human granulosa cell tumor (12), but possess many characteristics of normal GCs (13). The line of immortalized granulosa cells (COV434) was established from a primary human granulosa cell tumor in 1984 from a 27 year old female. The tumor granulosa cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) FCS and 2 mM L-glutamine. Cultivation medium was changed every day.
Karyotyping
Cells (subcultured in early passages and after reaching passage no. 5, 10, 15, 20 and 25) after 48 h of cultivation were subjected to a 4 h Demecolcemid (Sigma-Aldrich, USA) incubation followed by trypsin-EDTA detachment and lysis with hypotonic KCl and fixation in acid/alcohol. Metaphases were analyzed after GTG banding using the software Ikaros 5.0 (MetaSystems, USA).
Quantitative PCR—telomere length measurement
Genomic DNA was extracted from the cells using the silica-gel-membrane-based DNeasy Tissue Kit (Qiagen, Melbourne, Australia). Telomere length measurement was performed by qPCR assay according to the method described by Cawthon (14) with small modifications. Briefly, the telomere repeat copy number to single-copy gene (T/S) ratio was determined using the equation: T/S = 2−ΔCt (where ΔCt = Ct telomere−Ct single-copy gene). Then, the T/S for each sample was normalized to T/S value of a reference DNA sample to standardize between different runs, i.e.—ΔΔCt was calculated for each sample. This value is proportional to the average telomere length of the evaluated sample. 36B4, encoding acidic ribosomal phosphoprotein P0 was used as the single copy gene. Telomere and 36B4 gene PCRs were always done in separate 96-well plates with each sample run in triplicate in the same well position on an ABI 7500 HT Detection System (Applied Biosystems, USA). Each 20 μl reaction consisted of 20 ng DNA, 1 × SYBR Green master mix (Applied Biosystems), 200 nM telomere forward primer (CGG TTT GTT TGG GTT TGG GTT TGG GTT TGG GTT TGG GTT) and 200 nM telomere reverse primer (GGC TTG CCT TAC CCT TAC CCT TAC CCT TAC CCT TAC CCT). For the 36B4 gene PCR the following primer pairs were used: 36B4u, CAGCAAGTGGGAAGGTGTAATCC; 36B4d, CCCATTCTATCATCAACGGGTACAA. The DNA quantity standards were derived from serial dilutions of a reference DNA sample to produce three final concentrations (0.02, 0.2, and 2.0 ng/µL). In each run, a standard curve and a negative control (water) were included. Cycling conditions (for both telomere and 36B4 products) were 10 min at 95°C, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. Following amplification, a dissociation curve was done to confirm the specificity of the reaction. Standard and dissociation curves were generated with the ABI Prism 7500 SDS software. R2 for each standard curve was >0.98.
The Du145 prostate cancer cells were cultured as described previously (15) and served as a control for the comparison of the length of short telomeres with the relative T/S value of GCs.
Flow cytometric analysis
A total of 100 000 cells in 100 μL PBS (Invitrogen, USA) per well of 96-well plate (TPP, Switzerland) were incubated with 1 μg antibody for 30 min in the dark. Anti-human FSHR and LHR polyclonal antibodies (both Sigma-Aldrich, USA) were conjugated using a Lightning-LinkTM Fluorescein Conjugation Kit (Innova Biosciences Ltd, UK) before use (according to the instructions of the manufacturer). Control samples included an autofluorescence control and an isotype (IgG1, IgG2a, IgG2b) immunoglobulin control conjugated with FITC and PE. Then all samples were analyzed using a flow cytometer (Cell Lab QuantaTMSC, Beckman Counter, USA). Data analysis was performed using CXP analytic software (Beckman Counter, USA). The percentage of positive cells was determined as a percentage of cells with a higher fluorescence intensity than the upper isotype immunoglobulin control of 0.5%. Classification criteria: <10% no expression, 10–40% low expression, 40–70% moderate expression and >70% high expression.
Statistical analysis
The relationship between the average telomere length and the selected parameters was performed by correlation analysis using the statistic software GraphPad Prism 5.01 (San Diego, USA). A value of p < 0.05 was considered statistically significant.
Results
Culture of human GCs in medium with and without FF
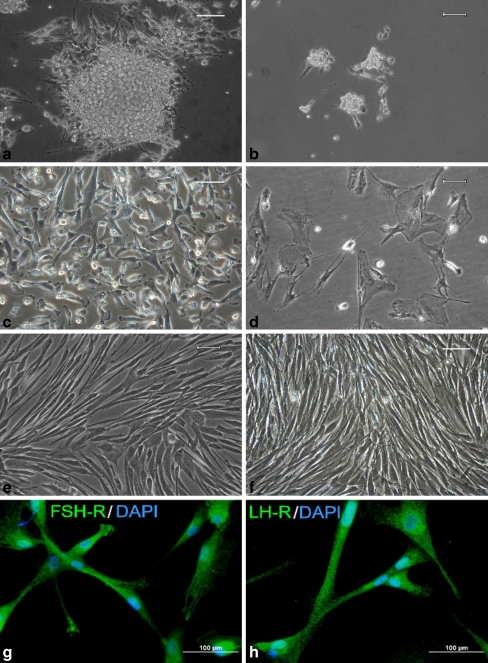
We evaluated the effect of follicular fluid (FF) on the prolonged survival of GCs in vitro. Isolated luteinizing GCs from each patient were splitted into two groups and cultured separately. One group was initially cultured in a medium supplemented with FF (Fig. 1a, c), whereas the other group was cultivated without FF (Fig. 1b, d). The luteinizing GCs cultured without FF consistently died within 2 weeks (Fig. 1d), whereas those initially cultivated in medium supplemented with FF remained viable for up to 1.5 months and could be passaged (Fig. 1e). In one sample, cells remained viable for up to 3.5 months (passage no. 25), Fig. 1f. These unique long-term proliferating cells were labeled as the GC8 line and became the subject of our interest. Cultivated GCs were characterized by immunocytochemistry as luteinizing GCs through their expression of both FSHR (Fig. 1g) and LHR (Fig. 1h).
Fig. 1.
Representative figures of human granulosa cells (GCs) grown in 2% FCS containing medium supplemented with EGF, bFGF and FSH. a GCs was initiated by the addition of folicular fluid into the cultivation medium (day 1). The follicular fluid created a protein surface on the botton of the cultivation flask that facilitated cell adhesion. b Morphology of GCs cultivated without follicular fluid (day 1). c Figure shows a small, rapidly dividing GCs with high proliferative potential, which were initially cultivated with follicular fluid (day 10). d Culture of GCs cultivated without follicular fluid and undergoing the cell degradation process, containing fewer dividing cells (day 10). e Typical spindle-shaped morphology of human GCs initially cultivated with follicular fluid (day 31). f Spindle-shaped cells were not morphologically changed during long-term cultivation; granulosa cells (GC8 line) initially cultivated in media with follicular fluid (day 99). Scale bar 50 μm. g Immunocytochemical analysis of GCs for FSHR (day 69) and h LHR after initial cultivation with FF (day 69). Cells stained with the omission of a primary antibody were used as negative controls. Scale bar 100 μm
FF was added for the initial day of in vitro cultivation only. Following the addition of FF, GCs retained their morphology, established intercellular connections, and became strongly attached to the culture dish. Most of the erythrocytes were very gently washed from the protein layer of FF with PBS and large vaginal epithelial cells were not observed after trypsinization in the subculture.
The average cell viability of proliferating cells was 90 ± 5%. After 15 days (passage no. 3), the GCs became spindle-shaped (fibroblast-like cells). The proliferative activity of these cells was different for each patient. After 25–45 days, cells entered to either senescence or irreversible apoptotic changes leading to cell death. In contrast, GCs cultivated in the absence of FF became less viable (46 ± 8%) after 7 days and lost intercellular connections.
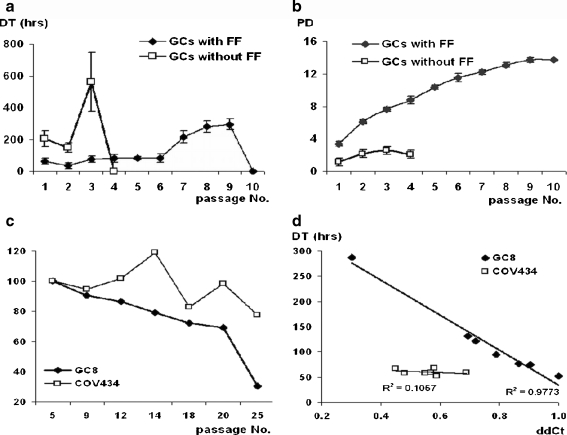
An effect of FF on doubling time (DT) and population doublings (PD) of GCs in vitro is shown in Fig. 2a, b. The average DT of GCs initially cultured with FF was 71 ± 15 h, whereas DT of GCs cultivated without FF was 305 ± 81 h. GCs cultured without FF died after 3 passages, therefore, cumulative PD decreased, whereas those cultivated in medium with FF remained proliferating for up to the ninth passage.
Fig. 2.
a, b GCs cultivated in medium with FF were viable for up to the ninth passage, demonstrated lower DT and a higher number of PDs. In contrast, GCs cultured in the absence of FF rapidly increased DT, slightly increased the number of PDs (cumulative data), cell died after the third passage. c Telomere length measurement (y-axis;%) in long-term growing cells (GC8 line) and GC-derived tumor cell line COV434. The GC8 line showed a significant decrease in the relative telomere length (y-axis;%) with each increasing passage number (P < 0.01). d Changes in doubling time (DT) versus relative telomere length (ddCt) were significant (P < 0.0001)
Doubling time (DT) and population doublings (PD) of GCs, GC8 and tumor granulosa cells COV434
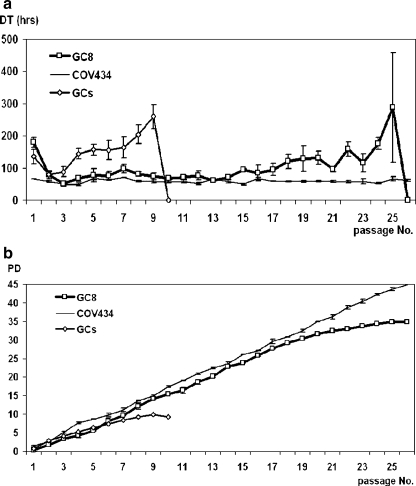
GCs initially stimulated by FF were used for DT and PD analyses. The average DT of GCs was 159 ± 24 h (mean ± SD, n = 24). All GCs demonstrated similar proliferation potential until the third passage. Thereafter, progressive prolongation of DT was seen in the majority of GCs (24 out of 25). After the ninth passage, DT of GCs increased to 260 h and cells died. In contrast, the long-term growing GCs (GC8) were maintained until the twenty-fifth passage as presented in Fig. 3a. The average DT of the GC8 line was 105 ± 21 h (mean ± SD, n = 3) which is significantly shorter than the DT of other GCs but longer than the DT of tumor cells COV434 where the DT was 59 ± 3 h.
Fig. 3.
a The comparison of the average doubling time (DT) of GCs (n = 24), the GC-derived tumor cell line COV434 (experiment carried in triplicates) and the long-term growing granulosa cell line GC8 (experiment carried in triplicates) that were isolated and cultivated in the same way as other GC lines but presented a different characteristics. b Comparison of the average cumulative population doublings (PD) of GCs (n = 24), GC8 (n = 3) and the GC-derived permanent tumor cell line COV434—cumulative data
Similarly, the cumulative PD was highest (44 ± 0.3) in the immortalized tumor line COV434. GCs lines increased the PD until the ninth passage, cumulative PD was 10 ± 0.7. Contrary to this, the unique GC8 line grew to the twentieth passage, cells reached 35 ± 0.3 population doubling (Fig. 3b).
In both graphs (Fig. 3a and b), we can compare the growth patterns of long-term proliferating cells (GC8) resembling the tumor cells (COV434) rather than GCs.
Karyotyping
All cultured GCs had normal karyotype in early passages. Long-term growing cells (GC8) were found to be cytogenetically stable even after 25 passages.
Telomere length measurement in GC8 and COV434
GC8 and COV434 included in this analysis were harvested from passages no. 5 to no. 25, i.e. they reached 4 to 40 population doublings. The GC8 line showed an overall and significant decrease in relative telomere length with each increasing passage number (P < 0.01). Also, changes in DT versus relative telomere length (ddCt) were significant (P < 0.0001) which demonstrated the prolongation of DT in cells with shortened telomere length (Fig. 2c, d).
Flow cytometry analysis
GCs primed with FF were used for flow cytometric analyses. In the absence of FF, the cell loss was very high and there were not enough GCs left for analysis. A phenotype of GCs lines was analyzed (n = 25) in passage no. 5. A phenotype of long-term growing cell line—GC8 was assessed in passages no. 5, 9, 12, 15 and 17. For a comparison, granulosa tumor cell line COV434 was also analyzed in passages no. 5, 9, 12, 15 and 17.
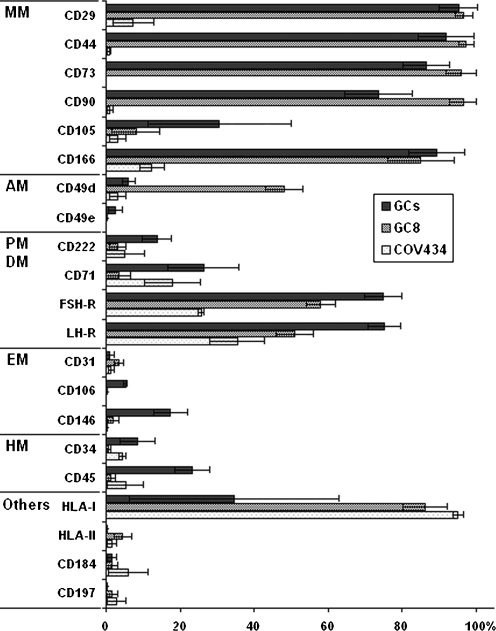
The GCs resulting data from flow cytometric analysis were as follows (Fig. 4): high expression of mesenchymal markers CD29, CD44, CD73, CD90, CD166 and gonadotropin receptors LHR and FSHR. Surface cell markers CD31, CD34, CD49d, CD49e, CD106, CD184, CD197, HLA-II were not expressed and a low expression was observed in CD45, CD71, CD105, CD146, CD222, HLA-I.
Fig. 4.
Phenotypic analysis of GCs, the long-term growing granulosa cell line GC8 and the GC-derived tumor cell line COV434. Classification: <10% no expression; 10–40% low expression; 40–70% moderate expression; >70% high expression. Percentage of the population GCs (mean ± SD, n = 24); GC8 (mean ± SD, n = 3), COV434 (mean ± SD, n = 3). Mesenchymal markers (MM), adhesion molecules (AM), proliferation markers (PM), differentiation markers (DM), endothelial markers (EM) and haemopoietic markers (HM)
Flow cytometric analysis revealed that long-term proliferating cells GC8 (in passages no. 5, 9, 12, 15 and 17) did not express either haemopoietic markers CD34, CD45, CD184 or endothelial cell markers CD31, CD106, CD146; a low expression of CD105 was observed. Contrary to this, the cell line GC8 expressed high levels of mesenchymal markers (CD29, CD44, CD73, CD90, CD166) and HLA-I. Adhesion molecules CD49e, CD222; proliferation marker CD71; chemokine CD197 and HLA-II were not expressed and a moderate expression was observed in alfa4 integrin CD49d. A moderate expression of specific markers for GCs FSHR and LHR was observed. The exception was found in passage no. 5, where the GC8 line expresed CD71 (++), CD105 (+) and CD222 (+) and within the following passages (no. 9, 12, 15 and 17) the expression of these surface markers was lost.
The resulting data of COV434 were as follows: high expression of HLA-I, low expression of FSHR, LHR, CD71 and CD166 and no expression of CD29, CD31, CD34, CD44, CD45, CD49d, CD49e, CD73, CD90, CD105, CD106, CD146, CD184, CD197, CD222, HLA-II.
Surprisingly, the GC8 cell line expressed high levels of HLA-I, similarly to COV34 but in contrast to other GC lines. The unique feature of the GC8 cell line was an elevated expression of the adhesion molecule CD49d.
Discussion
In this work, a long-term cultivation protocol of the luteinizing granulosa cell line has been established and tested. The protocol utilises our previous pilot study (16) where the addition of FF to GC improves their attachment and proliferation of primary culture.
The development of GCs is uniquely controlled by follicle stimulating hormone (FSH) during folliculogenesis, causing them to proliferate and subsequently differentiate and contribute to the formation of the follicular antrum, secreting fluids, ions, and proteins characteristic of the FF (17). FSH and LH are the primary survival factors for ovarian follicles, the antiapoptotic effects of these gonadotropins are probably mediated by the production of ovarian growth factors (18). Some of growth factors such as fibroblast (bFGF) and epidermal growth factor (EGF) prevent apoptosis in antral follicles (19) and have potent mitogenic effects on human GCs (20). Therefore GCs were cultivated in DMEM/F12 medium supplemented with 2% fetal calf serum (FCS). In vitro proliferation of GCs was supported by FF as well as FSH and growth factors (bFGF and EGF).
Some authors used media containing 10% (or higher) concentrations of FCS for the cultivation of GCs (2, 21–23). Cultures of GCs grew to confluence through four passages but grew slowly, taking approximately 2 weeks to double in number (2). GCs plated on tissue culture plastic become apoptotic and/or undergo rapid cell death, whereas GCs plated on ECM (extracellular matrix) survive for several days in culture (24, 25). We were able to cultivate for up to the ninth passage (45 days), taking approximately 6 days to double in number. Serum contains multiple factors (polypeptides, hormones, growth factors and cytokines, or binding proteins soluble receptors) that might influence cell function (1) and GC luteinization is accelerated in medium supplemented with serum (26). FF is as poorly defined as any FCS used in cultivation media. However samples of FF were obtained from ovarian follicles together with GCs always from same patient. FF is the intercellular enviroment crucial for fertilization and early embryonic development, as the follicle enlarges a fluid-filled antrum forms due to the replication of the GCs (3).
The crucial difference between our approach and other approaches was the application of follicular fluid. FF facilitates the communication of the oocyte with the surrounding environment leading to oocyte maturation accompanied by a differentiation and proliferation of GCs in vivo. It is an indicator of secretory activities and metabolism of GCs (27). Pioneering work suggested that GCs of mammalian follicles synthesize glycosaminoglycans at the time of antrum formation and secrete them into the FF (28). These glycosaminoglycans were identified as chondroitin sulphate, dermatan sulphate, hyaluronan and a heparin. GCs cultured in vitro synthesize and secrete proteoglycans with chemical and physical properties very similar to those isolated from FF. Proteoglycans are macromolecules formed by a protein backbone to which one or more glycosaminoglycan side chains are co-valently attached. They are involved in matrix formation, cell-cell and cell-matrix adhesion, cell proliferation and migration, and show co-receptor activity for growth factors (29). Our results suggest that the nutrients in a medium with addition of FF are essential to maintain the proliferative potential of primary culture of GCs.
Several studies have shown that the in vitro culture of GCs plated on matrices of ECM proteins of various types and densities leads to changes in cell adhesion and shape (25). Normal GC morphology in vivo is round, yet GCs seeded onto uncoated tissue culture plastic resemble fibroblast-like shape, flattened in a monolayer and spread with little interaction between neighboring cells (24, 25, 30, 31). When plated on type I collagen gels, isolated basal lamina, or Matrigel (BD, USA), however, GCs retain their spherical, epithelioid shape (25, 30, 31). Our results suggest that adhesion of individual cells and also clumps of GCs were initiated by the addition of FF into the cultivation medium (Fig. 1a,c). FF partially precipitated and created a thin layer on the botton of the cultivation plate which facilitated the cell adhesion. GCs retained their round morphology, constructed intercellular connections, and became strongly attached to the culture dish. We observed that cells were in clumps of small rounded cells (Fig. 1a), after trypsinization they took an epithelial-like appearance (Fig. 1c). After approximately 7–15 days (Fig. 1e), GCs became spindle-shaped (fibroblast-like cells) because GCs were seeded onto uncoated tissue culture plastic without FF. Similar changes in morphology cultivated GCs were observed in other works (2, 32). Kossowa-Tomaszczuk cultivated GCs in monolayers, cells invariably became luteinized and converted from epithelial-like into a fibroblast-like morphology, explaining why the latter morphology became dominant during prolonged culture (32). Gutiérrez et al. published that the spindle-shaped GCs may have lost aromatase activity, cytoplasmic changes compatible with luteinization (33).
The addition of FF in culture medium showed an overall and significant increase in GC proliferation (Fig. 2a, b), viability and cell survival. Our work brings the first evidence of the determination of population doublings (PD) and doubling time (DT) of prolongated cultivated GCs initiated by the addition of FF. The progressive prolongation of DT was seen in the majority of GCs until the ninth passage (days 25–45). In contrast, unique long-term growing cells (GC8) isolated from the patient remained viable for up to passage no. 25 (day 102, Fig. 1f).
The average DT of all GCs was 159 ± 24 h versus the DT of the long-term growing GC8 where DT was lower—105 ± 21 h. All GCs demonstrated a similar PD trend until the ninth passage. All GCs lines stopped growing in the ninth passage because cells died. Only the long-term growing line GC8 increased its PD in the twenty-fifth passage (Fig. 3a, b). The total cumulative PD of all GCs was 10 ± 0.7 while the GC8 line reached 35 ± 0.3 PD. Immunofluorescent microscopy showed that all cultured granulosa cells (GC8 also) retain their FSH receptor expression through all passages (Fig. 1g, h).
The population of GCs in a healthy follicle is not uniform but rather consists of subpopulations of differentiated and less differentiated cells, the latter being more capable of mitosis (10). We suggest that we isolated a population of granulosa cells (GC8), which contained a subpopulation of less differentiated cells. Immunofluorescence microscopy showed that these cells also express stem cell marker nestin (results not shown). For the first time in 2009, Kossowa-Tomaszczuk at al. demostrated that prolonged culture of luteinizing GCs in medium supplemented with LIF (leukemia-inhibiting factor) allows the selection of less differentiated GCs, which exhibited a certain degree of plasticity and multipotency. FF contains a high concentration of LIF which stimulates GCs through LIF-R physiologically in mature follicles (32). FF was added for the initial day of in vitro cultivation however its influence was evident during the whole cultivation. There is the possibility of an alternative mechanism, i.e. de-differentitation of granulosa cells (GC8) in vitro. Currently we have no evidence for this mechanism.
A futher explanation for the long growing GC8 could be the neoplastic transformation within in vitro cultivation. This was the reason for using the standard tumor cell line COV434 to compare with GCs. Zhang et al. published that during 38 passages the properties of the COV434 tumor granulosa cells did not undergo significant changes (13). Throughout the in vitro cultivation of tumor cells COV434, stable DT in every passage was observed. The gradually increasing PD was the highest in tumor line COV434 where growth continued even after 25 passages. Growth patterns of the GC8 line were closer to tumor cells COV434 than to other GCs (Fig. 3a, b). Therefore, karyotype was analyzed to confirm GCs chromosomal stability and to exclude neoplasm transformation. All cultivated GCs as well as the long-term growing cells GC8 showed normal karyotype and chromosomal stability in early passages (GC8 also after reaching twenty-fifth passage).
GCs proliferation potential was furthermore analyzed by the measurement of the length of the telomere repeat sequence. Expression of telomerase, the enzyme that maintains telomere length, is repressed in most adult differentiated somatic cells, although it is active in human ovaries and testes (34), in the endometrium (35), in stem cells present in the bone marrow and blood cells (36), in the liver (37), in the epidermis (38), and all structures characterized by an intense proliferative status. In fact, the introduction of telomerase into normal human somatic cells has been shown to extend normal cell life by ~20 doublings (39). Thus, telomerase activity is present in regenerative tissues with high proliferative need, where telomere loss would deplete the stem cell populations (3). We compared the length of telomeres of long-term growing cells (GC8) and immortal tumor granulosa cells COV434, the maintenance of the telomere length seems to guarantee cell immortality. We found that changes in doubling time (DT) versus relative telomere length (ddCt) of the GC8 line were significant (Fig. 2d) which demonstrated the prolongation of DT in cells with a shortened telomere length.
Moreover, GCs were characterized by the determination of the phenotype and its comparison with the GCs-derived tumor cell line COV434 (Fig. 4). Based on current knowledge of human stem cell phenotype, twenty-one surface markers were chosen and determined by flow cytometry. GCs originate from mesoderm, therefore especially mesenchymal markers were examined. Analysis revealed that GCs (in passage no. 5, n = 24) expressed high levels of the mesenchymal markers CD29, CD44, CD73, CD90, CD166 and low levels of the marker CD105. Kossowa-Tomaszczuk et al. published similar results—CD29, CD44, CD90, CD105, CD166, but not CD73, were expressed by substantial subpopulations of the freshly collected GCs (32). Several recent studies, using techniques of molecular biology, confirmed that the GCs are the only cell type expressing the FSH-R, thus expression of the FSH-R is strictly gonad- and highly cell specific (40). LH-R expression can be demostrated in a variety of organs and tissues (41). These markers, FSH-R and LH-R were highly expressed on GCs in vitro. The typical marker for haematopoietic cells, CD45, was present in low concentration probably due to contamination with blood cells in early passages, CD34 and CD184 were not expressed.
Moreover, due to a different phenotypic pattern, we suggest the presence of several subpopulations of GCs as in preovulatory follicles, each expressing different markers according to their degree of differentiation. For example, the GC8 line remained viable for up to the twenty-fifth passage and presented an increased expression of adhesion marker integrin CD49d and HLA-1, cells lost expression of markers CD45, CD71, CD105, CD146, CD222 and also decreased the expression of markers (FSH-R, LH-R) during in vitro cultivation. It is noteworthy that the granulosa cell (GC8) showed elevated expression of the alpha4 integrin subunit (CD49d). Integrins comprise a family of cell surface receptors that mediate cell-ECM and cell-cell adhesions by interacting with ECM proteins such as fibronectin, laminin, collagen and vitronectin, as well as with counterreceptors of the immunoglobulin superfamily of cell adhesion molecules (42). At a cellular level, integrins are involved in a number of cellular processes such as cell proliferation and differentiation, cell migration, cytoskeletal organization and cell polarization. (43).
We hypothesize that long-term proliferating cells (GC8) expressing different markers were less differentiated subpopulation isolated from ovarian follicle. Moreover, the expression of the proliferation marker CD71 (Transferrin-R) was higher in the GC8’s fifth passage together with the expression of the mesenchymal marker CD105 and CD222 (IGF-II R). It is possible that these three markers could be expressed differently in several subpopulations of GCs in preovulatory follicles.
Isolated GCs lines with well defined biological characteristics may be used for the development of in vitro maturation of immature oocytes. We characterized the long-term cultivated GCs obtained from patients and we compared them with GCs-derived permanent tumor cell line COV434. The results of the proliferation and phenotype analyses demonstrated that there is a fundamental difference between the GCs from patients and the GCs-derived cell line COV434. Significantly different phenotypes of GCs-derived tumor cell line COV434 was determined and we found no expression of mesenchymal markers CD29, CD44, CD73, CD90, CD105, CD166 and low expression of FSHR, LHR, CD166 (Fig. 4). Thus the in vitro model of GCs-derived permanent tumor cell line COV434 can be inconvenient because cells were neoplasm transformed. On the other hand, GCs obtained during the collection of oocytes from patients could represent a suitable In vitro model.
Our research has shown that some cells of ovarian follicle origin possess stem cell-like characteristics of the mesenchymal lineage. Since these cells present the FSH-R on their surface, it is likely that they are derived from the GCs. These ovarian stem cells can be cultured over prolonged time period while maintaining the biological characteristics of GCs. The long-term culture of those GCs can be used in studies of oocyte in vitro maturation, in differentiation studies of embryonic stem cells co-cultured with GCs or simply to study the endocrine functions of GCs in vitro.
Conclusions
Our cultivation protocol enables GCs to proliferate for 45 days and up to 100 days in the case of the GC8 cell line. This new approach was due to the addition of follicular fluid to the cultivation medium with a low serum concentration. Doubling time and population doublings assesment demonstrated the possibility of “long-term” cultivation of isolated GCs. Primary culture of human GCs initiated by the addition of FF could represent a suitable in vitro model to study disruptions of hormonal stimulation and serious complications of assisted reproduction protocols.
Acknowledgments
The authors wish to acknowledge MSMT 0021620820, MSMT 0021627502 and IGA MZ NS/9781-3 for financial support of research program. The authors would like to acknowledge Ms. Rückerova for help in the tissue cultures laboratory.
Abbreviations
- bFGF
basic fibroblast growth factor
- COV434
cell line derived from a human granulosa cell tumor (Sigma-Aldrich)
- DMEM/F12
dulbecco’s modified eagle’s medium
- DT
doubling time
- EGF
epidermal growth factor
- FCS
fetal calf serum
- FF
follicular fluid
- FSH
follicle stimulating hormone
- GCs
luteinizing human granulosa cells
- GC8
one of the isolated GCs from patient with different characters
- IVF
in vitro fertilization
- LH
luteinizing hormone
- PD
the number of population doublings
Footnotes
Capsule
This paper is especially focused on the improvement of the cultivation protocol enabling granulosa cells to proliferate and maintain the typical phenotypic expression.
References
- 1.Figenschau Y, Sundsfjord JA, Yousef MI, Fuskevag OM, Sveinbjornsson B, Bertheussen K. A simplified serum-free method for preparation and cultivation of human granulosa-luteal cells. Hum Reprod. 1997;12:523–531. doi: 10.1093/humrep/12.3.523. [DOI] [PubMed] [Google Scholar]
- 2.Quinn MCJ, McGregor SB, Stanton JL, Hessian PA, Gillett WR, Gren DPL. Purification of granulosa cells from human ovarian follicular fluid using granulosa cell aggregates. Reprod Fertil Dev. 2006;18:501–508. doi: 10.1071/RD05051. [DOI] [PubMed] [Google Scholar]
- 3.Lavranos TC, Mathis JM, Latham SE, Kalionis B, Shay JW, Rodgers RJ. Evidence for ovarian granulosa stem cells: telomerase activity and localization of the telomerase ribonucleic acid component in bovine ovarian follicles. Biol Reprod. 1999;61:358–366. doi: 10.1095/biolreprod61.2.358. [DOI] [PubMed] [Google Scholar]
- 4.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 5.Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and the life span of the corpus luteum. Physiol Rev. 2000;80:1–24. doi: 10.1152/physrev.2000.80.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Wen X, Li D, Tozer AJ, Docherty SM, Iles RK. Estradiol, progesterone, testosterone profiles in human follicular fluid and cultured granulosa cells from luteinized pre-ovulatory follicles. Reprod Biol Endocrinol. 2010;8:117–127. doi: 10.1186/1477-7827-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez E, Tarín JJ, Pellicer A. Oocytes maturation in humans: the role of gonadotrophins and growth factors. Fertil Steril. 1993;60:40–46. [PubMed] [Google Scholar]
- 8.Muttukrishna S, Groome N, Ledger W. Gonadotropic control of secretion of dimeric inhibins and activin A by human granulosa-luteal cells in vitro. J Assist Reprod Genet. 1997;14:566–574. doi: 10.1023/A:1022524516824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HC, Lee SW, Lee KW, Cha KY, Kim KH, Lee S. Identification of new proteins in follicular fluid from mature human follicles by direct sample rehydration method of two-dimensional polyacrylamide gel electrophoresis. J Korean Med Sci. 2005;20:456–460. doi: 10.3346/jkms.2005.20.3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82:1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 11.Shay JW, Wright WE. Hayflick, his limit and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 12.Berg-Bakker CA, Hagemeijer A, Franken EM. Establishment and characterization of 7 ovarian carcinoma cell lines and one granulosa tumor cell line: growth features and cytogenetics. Int J Cancer. 1993;53:613–620. doi: 10.1002/ijc.2910530415. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Vollmer M, Geyter M, Litzistorf Y, Ladewig A, Dürrenberger M, Guggenheim R, Miny P, Holzgreve W, Geyter C. Characterization of an immortalized human granulosa cells cell line (COV434) Mol Hum Reprod. 2000;6:146–153. doi: 10.1093/molehr/6.2.146. [DOI] [PubMed] [Google Scholar]
- 14.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asai A, Oshima Y, Yamamoto Y, Uochi TA, Kusaka H, Akinaga S, Yamashita Y, Pongracz K, Pruzan R, Wunder E, Piatyszek M, Shihong L, Chin AC, Harley CB, Gryaznov S. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res. 2003;63:3931–3939. [PubMed] [Google Scholar]
- 16.Bruckova L, Soukup T, Moos J, Moosova M, Pavelkova J, Rezabek K, Visek B, Mokry J. The cultivation of human granulosa cells. Acta Medica (Hradec Kralove) 2008;51:165–172. doi: 10.14712/18059694.2017.19. [DOI] [PubMed] [Google Scholar]
- 17.Sasson R, Dantes A, Tajima K, Amsterdam A. Novel genes modulated by FSH in normal and immortalized FSH responsive cells: new insights into the mechanism of FSH action. FASEB J. 2003;17:1256–1266. doi: 10.1096/fj.02-0740com. [DOI] [PubMed] [Google Scholar]
- 18.Lima-Verde IB, Matos MH, Saraiva MV, Bruno JB, Tenorio SB, Martins FS, Rossetto R, Cunha LD, Name KP, Bao SN, Campello CC, Figueiredo JR. Interaction between estradiol and follicle-stimulating hormone promotes in vitro survival and development of caprine preantral follicles. Cells Tissues Organs. 2010;191:240–247. doi: 10.1159/000231484. [DOI] [PubMed] [Google Scholar]
- 19.Parborell F, Pecci A, Gonzalez O, Vitale A, Tesone A. Effects of a gonadotropin releasing hormone agonist on rat ovarian follicle apoptosis: regulation by epidermal growth factor and the expression of Bcl-2-related genes. Biol Repro. 2002;67:481–486. doi: 10.1095/biolreprod67.2.481. [DOI] [PubMed] [Google Scholar]
- 20.Matos MH, Hurk R, Lima-Verde IB, Luque MC, Santos KD, Martins FS, Báo SN, Lucci CM, Figueiredo JR. Effects of fibroblast growth factor-2 on the in vitro culture of caprine preantral follicles. Cells Tissues Organs. 2007;186:112–120. doi: 10.1159/000103016. [DOI] [PubMed] [Google Scholar]
- 21.Adachi T, Iwashita M, Kuroshima A, Takeda Y. Regulation of IGF binding proteins by FSH in human luteinizing granulosa cells. J Assist Reprod Genet. 1995;12:639–643. doi: 10.1007/BF02212589. [DOI] [PubMed] [Google Scholar]
- 22.Wakim AN, Polizotto SL, Burholt DR. Influence of thyroxine on human granulosa cell steroidogenesis in vitro. J Assist Reprod Genet. 1995;12:274–277. doi: 10.1007/BF02212931. [DOI] [PubMed] [Google Scholar]
- 23.Lobb DK, Younglai EV. A simplified method for preparing IVF granulosa cells for culture. J Assist Reprod Genet. 2006;23:93–95. doi: 10.1007/s10815-006-9025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang DH, Kee SH, Kim K, Cheong KS, Yoo YB, Lee BL. Role of reconstituted basement membrane in human granulosa cell culture. Endocr J. 2000;47:177–183. doi: 10.1507/endocrj.47.177. [DOI] [PubMed] [Google Scholar]
- 25.Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 2007;4:6–10. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holst N, Bertheussen K, Burhol PG, Forsdahl F. Medium-associated luteinization expressed as progesterone release in granulosa-luteal cells and isolated from patients undergoing in-vitro fertilization. Hum Reprod. 1991;6:1343–1348. doi: 10.1093/oxfordjournals.humrep.a137266. [DOI] [PubMed] [Google Scholar]
- 27.Hanrieder J, Nyakas A, Naessén T, Bergquist J. Proteomic analysis of human follicular fluid using an alternative bottom-op approach. J Proteome Res. 2008;7:443–449. doi: 10.1021/pr070277z. [DOI] [PubMed] [Google Scholar]
- 28.Zachariae F. Studies on the mechanism of ovulation. Permeability of the blood-liquir barrier. Acta Endocrinol. 1958;27:339–342. doi: 10.1530/acta.0.0270339. [DOI] [PubMed] [Google Scholar]
- 29.Salustri A, Camaioni A, Giacomo M, Fulop C, Hascall VC. Hyaluronan and proteoglycans in ovarian follicles. Hum Reprod. 1999;4:293–301. doi: 10.1093/humupd/5.4.293. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Ze’ev A, Amsterdam A. Regulation of cytoskeletal proteins involved in cell contact formation during differentiation of granulosa cells on extracellular matrix. Proc Natl Acad Sci U SA. 1986;83:2894–2898. doi: 10.1073/pnas.83.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asem EK, Feng S, Stingley-Salazar SR, Turek JJ, Peter AT, Robinson JP. Basal lamina of avian ovarian follicle: influence on morphology of granulose cells in-vitro. Comp Biochem Physiol Part C. 2000;125:189–201. doi: 10.1016/s0742-8413(99)00100-0. [DOI] [PubMed] [Google Scholar]
- 32.Kossowska-Tomaszczuk K, Geyter C, Geyter M, Martin I, Holzgreve W, Scherberich A, Zhang H. The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells. 2009;27:210–219. doi: 10.1634/stemcells.2008-0233. [DOI] [PubMed] [Google Scholar]
- 33.Gutiérrez CG, Glazyrin AN, Robertson GW, Campbell BK, Gong JG, Bramley TA, Webb R. Ultra-structural characteristics of bovine granulosa cells associated with maintenance of oestradiol production in vitro. Mol Cell Endocrinol. 1997;134:51–58. doi: 10.1016/S0303-7207(97)00171-8. [DOI] [PubMed] [Google Scholar]
- 34.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Kyo S, Takakura M, Kohama T, Inoue M. Telomerase activity in human endometrium. Cancer Res. 1997;57:610–614. [PubMed] [Google Scholar]
- 36.Broccoli D, Young JW, Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burger AM, Bibby MC, Double JA. Telomerase activity in normal and malignant mammalian tissues: feasibility of telomerase as a target for cancer chemotherapy. Br J Cancer. 1997;75:516–522. doi: 10.1038/bjc.1997.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis in human skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci USA. 1996;93:6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 40.Camp TA, Rahal JO, Mayo KE. Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Mol Endocrinol. 1991;5:1405–1417. doi: 10.1210/mend-5-10-1405. [DOI] [PubMed] [Google Scholar]
- 41.Lei ZM, Rao CV. Novel presence of luteinizing hormone/human chorionic gonadotropin (hCG) receptors and the downregulating action of hCG on gonadotropin releasing hormone gene expression in immortalized hypothalamic GT1–7 neurons. Mol Endocrinol. 1994;8:1111–1121. doi: 10.1210/me.8.8.1111. [DOI] [PubMed] [Google Scholar]
- 42.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-S. [DOI] [PubMed] [Google Scholar]
- 43.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by a4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]