Abstract
Purpose
To evaluate the frequency of sperm nuclei disomy for chromosomes 8, X, and Y in patients with severe non-obstructive oligozoospermia and to assess possible correlations between sperm nuclei aneuploidy and semen parameters or a particular clinical phenotype.
Materials and methods
The sperm aneuploidy rate for chromosomes X, Y, and 8 were assessed in 16 infertile men with severe non-obstructive oligozoospermia and 7 healthy men with normal semen parameters. The frequency of sperm aneuploidy was compared between several patients groups according to their clinical and biological factors.
Results
The total rate of chromosomally abnormal spermatozoa was significantly higher in patients with severe oligozoospermia compared to control group (P < 0.001). A significant relationship was found between the age of patients, sperm concentration, and morphology and the mean rate of sex chromosomes disomy. In addition to the low sperm count (<5 × 106/ml), an elevated FSH level and an exposed to an elevated temperature are two major predictive factors leading to the production of higher numbers of chromosomally abnormal gametes.
Conclusion
Patients with severe oligozoospermia, who are potential candidates for assisted reproduction technology, presented a high level of sex numerical chromosome abnormalities, and consequently are at high risk of chromosome abnormalities in their offspring.
Keywords: Aneuploidy, FISH, Predictive factors, Severe oligozoospermia
Introduction
Intracytoplasmic sperm injection (ICSI) has become the method of choice for the treatment of severe male factor infertility. Because this method avoids natural sperm selection, it is important to investigate the aneuploidy sperm rate of ICSI-treated patients. Recent studies in infertile men have demonstrated an increased frequency of synaptic disruptions in both the autosomes and the sex chromosomes. Pairing abnormalities observed during meiosis in infertile men can lead to meiotic arrest or the production of chromosomal abnormalities, depending on the ability of the affected cell to complete meiosis; this most often results in oligozoospermia or azoospermia [1]. Indeed, severe quantitative impairments of spermatogenesis can be associated with qualitative alterations in the process of meiotic chromosome recombination and segregation, resulting in increased numbers of aneuploïd spermatozoa [2]. Several studies on aneuploidy rate in spermatozoa from infertile men using fluorescence in situ hybridization (FISH) have been reported [3–6]. Most of these studies showed increased rates of aneuploïd spermatozoa, especially of the sex chromosomes, in the population of infertile men presenting moderate to severe oligozoospermia compared with the control population [5–12]. However, when we would like to compare the incidence of disomy of several chromosomes in the sperm nuclei of infertile males, we show that the results are heterogenic. The heterogeneity of the results could be explained by the heterogeneity of the population itself. In the most of the studies, the seminal parameters of the individuals included in each study were heterogeneous. Moreover, in the several of the studies, the karyotype of infertile men was not analysed; some of the subjects might thus have had an abnormal karyotype, e.g. 46,XY/47,XXY mosaïcism, that might have led to a high frequency of XY disomy in spermatozoa. So in order to classify the population analysed with greater accuracy, our patients were included in the present study after having a clinical urological examination, a lymphocyte karyotype, a Y microdeletion analysis, a dosage of hormonal of FSH, LH and testosterone, and dosage of α-glucosidase. In addition, the risk of a higher sperm aneuploidy rate associated with specific clinical and/or biological parameters has not yet been clearly assessed. Hence, the aim of the present study was to evaluate the frequency of sperm nuclei disomy for chromosomes 8, X, and Y in patients with severe non-obstructive oligozoospermia and to establish possible correlations between sperm nuclei aneuploidy and semen parameters or a particular clinical phenotype.
Materials and methods
Selection criteria for semen samples
Semen samples were collected from patients undergoing standard semen analysis in our laboratory of Cytogenetics and reproductive biology Farhat Hached university hospital. Only patients with severe oligozoospermia (<5 × 106/ml) and normal sperm morphology (abnormal forms <30%) were first selected. In order to eliminate an excretory origin of oligozoospermia, among these patients only those with normal level of α-glucosidase (> 60 mUI) and normal somatic karyotype were included. At the end we have obtained 16 men with idiopathic severe oligozoospermia with normal karyotype and normal level of α-glucosidase. In addition, we have recruited 7 healthy fertile men with normal peripheral blood karyotype and normal semen analysis, served as control group.
This protocol was approved by the local ethics committee and all patients and controls had previously given informed consent for the study.
Semen analysis
Semen samples were collected by masturbation in to sterile cups following 3 days of sexual abstinence and semen analysis was performed after semen liquefaction for 30 min at room temperature. Basic semen parameters (volume, vitality, concentration, and total motility) were assessed according to the World Health Organization guidelines [13]. Sperm were stained using Diff-Quik (Dade Behring, Netwark, Delaware). Sperm morphology was assessed using David’s classification [14]. A total of 100 sperm were graded at each evaluation and percentage normal forms determined. Sperm morphology grading was performed by one of the two andrology technicians, and uniformity of grading between the two andrology technicians was routinely monitored.
Semen preparation
The sperm samples were prepared using a PureSperm discontinuous density gradient (Nidacon, Gothemberg, Sweden). This discontinuous density gradient consisted of three 1 mL layers of Pure Sperm: 90%, 70%, and 50%. On the 50% layer, 1 mL of semen was deposited. The gradient was then centrifuged at 300 g for 20 min. Spermatozoa retrieved in the 90% fraction were rinsed in 5 mL of RPMI (GIBCOTM, Grand Island, USA) and centrifuged for 10 min at 650 g. The sperm pellet obtained was fixed with 8 ml of Acetic Acid/Methanol mixture.
Aneuploidy analysis
Fluorescence in situ hybridization, which employs sequence-specific DNA probes incorporated with fluorescently labeled nucleotides, was carried out on each patient and control, using alpha centromeric probes for chromosomes 8, X, and Y. The probes were provided by the University of Bari (Bari, Italy).
Sperm head decondensation In order to render the sperm chromatin accessible to DNA probes, slides were incubated in NaOH 1 N, at room temperature for 2 min. The slides were distilled-water washed, dehydrated through an ethanol series (70–90–100%) and air-dried.
DNA probes The probe mixture for triple FISH consisted of a repetitive DNA sequence of centromeric probes for chromosome X (pDMX1) labeled Rhodamine, for chromosome Y (pLAY5.5) labeled fluorescein isothiocyanate (FITC) and for chromosome 8 (pZ8.4) labeled FITC and Rhodamine. The use of an autosomal probe, in addition to X and Y probes, allowed the distinction between disomy and diploidy.
Hybridization procedure Slides were incubated in a denaturation solution of 70% formamide, 20X standard saline citrate (SSC) (pH 5,3) and distilled water at 72°C for 2 min. Slides were snap-cooled in 70% ethanol at −20°C for 2 min and then dehydrated through an ethanol series (90–100%) at room temperature. The probes, precipitated and denatured at 72°C for 8 min, were applied directly to the slides which were then covered with a cover slip and sealed with rubber cement. Slides were hybridized for 2 h in a dark humidified chamber at 37°C. Finally slides were washed in 1x SSC, counterstained with 4’,6-diamidino-2-pheneylindole (DAPI) and stored in the dark at 4°C prior to carrying out microscopic observation.
Scoring criteria The slides were observed using an Axioplan epifluorescence microscope (Leica, Wetzlar, Germany) with the appropriate set of filters: single band DAPI, FITC, and Rhodamine. For each probe a minimum of 1,000 spermatozoa were counted per patient. Only intact spermatozoa bearing a similar degree of decondensation and clear hybridization signals were scored; disrupted or overlapping spermatozoa were excluded from analysis.
Statistical analysis Statistical analysis was performed using SPSS10 (SPSS, Inc., Chicago, IL, USA). The comparisons between controls and patients were calculated using Mann–Whitney U-test. Spearman's correlation coefficients were also calculated. A statistical difference was considered as significant when the p-value was <0.05.
Results
General characteristics of study group
The mean age of our patients was 33.8 ± 2.32 years. 87.5% of the patients presented a primary infertility with a mean duration of 4 years.
Among the 16 patients, 2 patients had a previous history of urological infections disease, 8 patients had been exposed to an elevated temperature (>50°C) in their work (driver, Smith, worker in a brickyard…) with a minimum of 8 h per day, 12 patients were smokers, 6 patients were alcoholic, and 10 patients had no previous medical history. Following clinical examination, only one patient had a clinical unilateral varicocele, and the rest of patients had a normal clinical examination.
According to the results of hormonal dosage, 10 patients presented normal concentrations of FSH, LH, and testosterone, 3 patients had hypergonadotropic hypogonadism and 3 other patients had hypogonadotropic hypogonadism.
The results of semen analysis for our patients are shown in Table 1. The mean sperm concentration of our patients was 2.04 ± 2.5 × 106/ml (ranged from 0.64–4.45 × 106/ml), the mean motility (a + b) was 20.13 ± 2% (ranged from 0–49%) and the rate of morphologically abnormal spermatozoa was 56.69 ± 5.3% (ranged from 44–69%). Of the 16 patients, 6 showed only severe oligospermia and 10 severe oligoasthenospermia.
Table 1.
Semen parameters of the 16 infertile patients with severe oligozoospermia and controls
| Patient number | Volume (ml) | Sperm count (X106/ml) | Total motility (%) | Atypical forms (%) |
|---|---|---|---|---|
| 1 | 3 | 1.24 | 30 | 46 |
| 2 | 5 | 1 | 9 | 64 |
| 3 | 2.5 | 2.34 | 10 | 51 |
| 4 | 4 | 2.82 | 5 | 68 |
| 5 | 3.8 | 2.30 | 7 | 45 |
| 6 | 2.8 | 0.64 | 22 | 54 |
| 7 | 2 | 0.78 | 0 | 66 |
| 8 | 5 | 1 | 30 | 45 |
| 9 | 4 | 4.32 | 44 | 58 |
| 10 | 4.8 | 2.12 | 40 | 45 |
| 11 | 2 | 4.45 | 5 | 66 |
| 12 | 2 | 1.08 | 8 | 49 |
| 13 | 6 | 1.44 | 11 | 69 |
| 14 | 4.5 | 2.74 | 46 | 68 |
| 15 | 3.3 | 2.74 | 6 | 69 |
| 16 | 5 | 1.74 | 49 | 44 |
| Patients Mean±SD | 3.73 ± 1.26 | 2.04 ± 1.16 | 20.13 ± 17.04 | 56.69 ± 10.23 |
| Controls Mean±SD | 03.20 ± 1.43 | 139 ± 59.95 | 55.51 ± 3.49 | 54.33 ± 10.65 |
SD: Standard Deviation
FISH analysis
Using a triple colour FISH for chromosomes 8, X and Y, a total of 20,000 sperm from the 16 oligozoospermic group and 8,000 sperm from 7 normal control men were scored.
The number of spermatozoa scored, the nullisomy, disomy, and diploidy rates for patient and control were reported in Table 2.
Table 2.
Results of chromosomal abnormalities in controls and oligozoospermic men
| Controls Mean±SD | Oligozoospermia Mean±SD | P-Value | |
|---|---|---|---|
| Sperm nuclei scored | 1,141 ± 109.77 | 1,083 ± 83.85 | 0.345 |
| Disomy X (%) | 0.19 ± 0.10 | 0.69 ± 0.28 | 0.001* |
| Disomy Y (%) | 0.24 ± 0.04 | 0.96 ± 0.67 | 0.001* |
| Disomy XY (%) | 0.31 ± 0.12 | 1.18 ± 0.65 | 0.001* |
| Total Disomy of sex chromosomes (%) | 0.76 ± 0.06 | 2.83 ± 0.70 | 0.001* |
| Nullisomy of sex chromosomes (%) | 0.25 ± 0.09 | 2.06 ± 2.50 | 0.001* |
| Disomy 8 (%) | 0.08 ± 0.07 | 012 ± 0.09 | 0.341 |
| Nullisomy 8 (%) | 0.21 ± 0.06 | 0.25 ± 0.09 | 0.198 |
| Diploidy (%) | 0.20 ± 0.10 | 0.53 ± 0.28 | 0.01* |
SD Standard Deviation
*p < 0.05
Among the normal spermatozoa, the percentages of X- and Y- bearing spermatozoa was respectively 47.22% and 46.94% in oligozoospermic group and 49.62% and 48.85% in control group. Statistical analysis demonstrated that the proportion of X- and Y- bearing spermatozoa was not significantly different from the 1:1 ratio expected in all patients and controls.
The total rate of chromosomally abnormal spermatozoa was 5.83% in patients with severe oligozoospermia and 1.53% in the control group, with a statistically significant difference (P < 0.001). All oligozoospermic subjects showed a significant increase in the rates of sex chromosomes disomy (XX, YY, XY) and nullisomy compared to the control group (p < 0.001). However, no statistically significant difference was observed in the frequency of disomy and nullisomy of chromosome 8 compared with control (P > 0.05).
In addition, we did not find a statistically significant difference between the results of non-disjunction at the first meiotic division (XY) and second meiotic division (XX and YY) (p > 0.05).
The diploidy rate was significantly higher in patients with severe oligozoospermia (0.53%) compared to controls (0.20%; P < 0.01).
Correlation between clinical phenotype, semen parameters and aneuploidy rate
A significant relationship was found between the age of patients and the mean rate of sex chromosomes disomy (r = 0.58; p = 0.017). In addition, an inverse relationship was shown between sperm concentration and the mean frequency of sex chromosomes disomy (r = −0.55; p = 0.006).
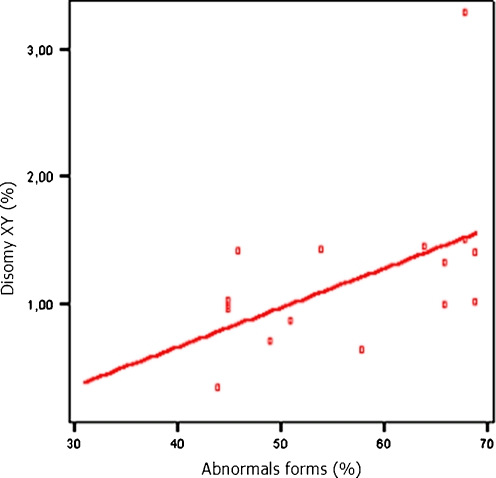
A significant correlation was established among the morphological abnormalities and the disomy XY (r = 0.54; p < 0.05) (Fig. 1). The correlation analyses of the chromosomal abnormalities were compared with the specific types of morphological abnormalities and a significant correlation was found between macrocephalic head and disomy XY (r = 0.52; p = 0.03). In addition microcephalic head were correlated positively to disomy 8 (r = 0.56; p = 0.02) and to total rate of sex chromosomes aneuploidy (r = 0.51; p = 0.04). However, sperm motility was not significantly correlated with sperm aneuploidy.
Fig. 1.
Correlation between the percentage of spermatozoa with abnormal sperm morphology and the rate of disomy XY (r = 0.54; p < 0.05)
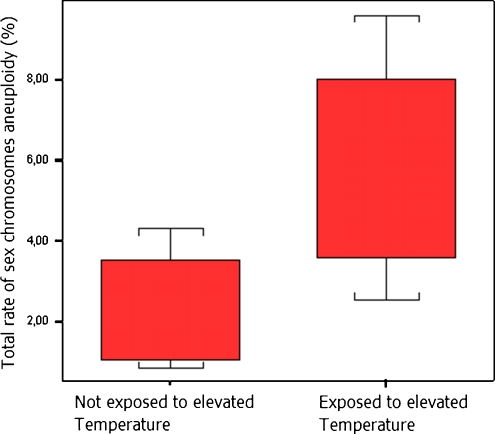
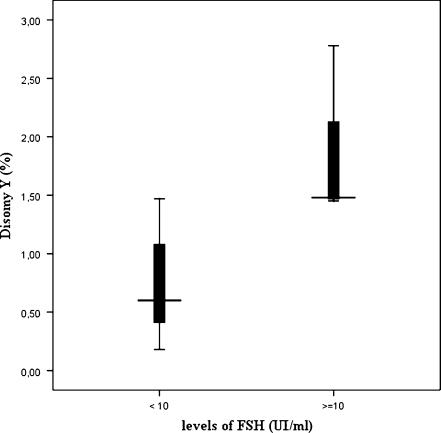
In order to correlate the sperm aneuploidy rates observed in the patients with their clinical and biological records, the following subgroups of patients were defined: patients with an exposure to an elevated temperature (n = 8); patients with an exposure to cigarette smoking (n = 12); patients with an FSH level higher than 10 IU/ml (n = 3); patients exposure to alcohol drink (n = 6). No statistically difference was observed in the group of patients with an exposure to cigarette smoking or alcohol drink (p > 0.05). However, the total rate of sex chromosomes aneuploidy was significantly higher in the group of patients exposed to an elevated temperature than in non exposed patients (p < 0.01) (Fig. 2). In addition, in patients with an elevated FSH (>10UI/ml), significantly higher rate of chromosome Y disomy was observed (Fig. 3).
Fig. 2.
Variations of total rate of sex chromosomes aneuploidy according to the exposure to elevated temperature
Fig. 3.
Variation of disomy Y according to the levels of FSH. Values are medians (─) with interquartile ranges (▌25–75%) and minimum-maximum values (Ι)
Discussion
Using a triple colour FISH for chromosomes X, Y, and 8, our results show significantly higher frequencies of sex chromosomes disomies and diploidy in the group of patients with severe oligozoospermia compared with a control group. However no statistically significant difference was observed in the frequency of disomy and nullisomy of chromosome 8. The rate of XY-bearing spermatozoa in our study was significantly higher in the sperm nuclei of infertile males with severe oligozoospermia. Our results are consistent with those of several similar studies performed in severe oligozoospermic men [10, 15, 16]. In fact, Ohashi et al. [15] studied chromosomes 18, X and Y in 20 oligozoospermic men, 10 with sperm concentration of <5 × 106 sperm/ml and 10 with 5–20 × 106 sperm/ml. They found a significant increase in the frequency of XY disomy and diploidy for the group with severe oligozoospermia. In addition, Faure et al. [16] had assessed the sperm aneuploidy rate of chromosomes X, Y, 13, 18 and 21 in 31 infertile men with severe oligozoospermia (< 5 × 106 sperm/ml) and in a population of control men with proven fertility. Nearly half of the oligozoospermic males (15/31) had a significantly increased disomy rate for at least one of the five chromosome compared with that observed in the control population. Martin et al. [10] have studied the frequencies of sperm chromosome abnormalities in over 600.000 sperm from infertile patients with mild, moderate and severe oligozoospermia. They found that the frequencies of sperm chromosome abnormalities increased with decreased sperm concentration and this was particularly marked for the sex chromosomes and diploidy. The mean frequencies of abnormalities observed in men with severe oligozoospermia were more dramatic, with 3–4 times the mean frequencies observed in normal men [7, 10, 17].
In addition to the high frequency of XY disomy in our oligozoospermic men, the rate of YY and XX-bearing spermatozoa were also increased compared to control group. However we did not show a statistically significant difference between the results of non-disjunction at the first meiotic division (XY) and second meiotic division (XX and YY). Our results suggested that nondisjunction for chromosomes X and Y can occur at the first and the second meiotic division with the same frequency. Our findings were in discordance with the data of Schmid et al. [11] and Ohashi et al. [15] who did not show a statistically significant difference in the disomy XX and YY in oligozoospermic men compared to control group. They suggested that chromosomal non disjunction occurs mainly during the first meiotic division [11, 15]. In the present study, we did not found an increase in autosomal disomy of chromosome 8. This is consistent with the data of Schmid et al. (2003, 2004) who found that aneuploidy among infertile oligozoospermic men occurred in the gonosomes and not autosomes [11, 18]. This difference in chromosomal susceptibility may be related to the small pseudoautosomal region, which may lead to a greater chance of non-disjunction than in larger homologous autosomes [11, 18, 19]. Despite that our data showed an increased rate of aneuploïd spermatozoa in patients with non obstructive severe oligozoospermia, the small sample size of our study group can be considered as a limitation of our research. For more accurate results, others studies with more number of patients should be done in future.
A significant relationship was found between the age of our patients and the mean rate of sex chromosomes disomy. Our data are in discordance with the data found by Morel et al. [20] and Rives et al. [21]. Indeed, Morel et al. [20] have conducted a study on human semen samples randomly selected from a population of men undergoing an infertility work up and did not established a correlation between subject age and disomy frequencies. Rives et al. [21] also confirmed that the disomy frequency is not correlated to subject age.
Many studies have investigated the relationship between semen parameters and chromosomes abnormalities. The association between sperm concentration and sperm aneuploidy has probably been the most extensively studied. As shown in our study, inverse relationships between sperm concentration and the mean frequency of disomy of sex chromosome and diploidy were found. Our results are in accordance with the data of many other studies that reported significant negative correlations between sperm concentration and the mean frequency of disomy, especially for the sex chromosomes [6, 10, 15, 21, 22]. However, other studies did not show a significant correlation between sex disomy rates and sperm concentration [3, 20, 23, 24]. The disparate results of these studies might be related to their investigation of different types of male infertility: in the majority of cases oligozoospermia was associated with one or more abnormal other parameters. In addition the classification criteria of individuals appeared to be subjective and no mention was made of the characteristics of the sperm in the study of Miharu et al. [3].
For sperm motility, we did not find a significant correlation between this parameter and disomy frequency of sex and autosomes chromosomes, in accordance with the results of literature. Indeed, the most studies comparing disomy frequency and sperm motility showed very consistent results with the absence of any significant association between disomy frequency and sperm motility [19]. Despite that all our patients presented a normal range of abnormal morphology according to David classification, our data showed that the incidence of sex chromosomes abnormalities increased significantly with an increase in the percentage of morphologically abnormal spermatozoa, and this association seems to be related to head abnormalities especially macrocephalic and microcephalic heads. Studies carried out to investigate the correlation between chromosome aneuploidy and morphologically abnormal spermatozoa by FISH have produced no consistent data. Some studies did not found a statistically correlations between the frequency of disomy for all the chromosomes analysed and the sperm morphology [6, 21]. However other studies show a statistically significant correlation between aneuploidy and poor spermatozoa morphology [9, 22]. Increased disomy frequencies could be associated with a restricted category of sperm morphological abnormalities, and in particular abnormal sperm heads (including large sperm heads) [25, 26].
One of our major objectives was to determine if the disomy frequency in our patients with severe oligozoospermia was correlated with a particular clinical phenotype. Our study shows that an FSH concentration higher than 10 UI/ml was a predictive factor of meiotic abnormalities. Our data are in accordance with the data of Vendrell et al. [27] and Faure et al. [16] who showed that an elevated FSH level is significantly correlated with higher risk of high sperm disomy rates. In addition the total rate of sex chromosomes aneuploidy was found to be significantly increased in patients exposed to an elevated temperature. The deleterious effects of hyperthermia on spermatogenesis are well recognized. It was shown that a small increase in testicular temperature during waking hours of man leads to qualitative and quantitative alterations of spermatogenesis. Indeed, prolonged heating of the testes above 37°C causes a severe slowdown in spermatogenesis caused mainly by the disruption of meiosis [28].
A significant increase in sperm disomy rates was observed among smoking patients compared with non smoking patients [16, 29], and in XY hyperhaploidy in sperm of alcohol drinkers compared with non-drinkers [29]. However our results did not show any correlation between these two parameters and high risk of increased sperm aneuploidies rates. Due to the small size of our study group, a link between these parameters and sperm chromosomes anomalies cannot be ruled out.
In conclusion our study show that patients with severe oligozoospermia, who are potential candidates for assisted reproduction technology, presented a high level of sex numerical chromosome abnormalities, consequently are at high risk of chromosome abnormalities (including klinefelter syndrome) in their offsprings. In addition to the low sperm count (<5 × 106/ml), an elevated FSH level and an exposed to an elevated temperature are two major predictive factors leading to the production of higher numbers of chromosomally abnormal gametes. According to our results, sperm FISH analysis should be systematically recommended to patients presenting a severe oligozoospermia associated with one or more biological predictive factors, particularly if they want to attempt an ICSI program. However, the small sample size of our study group can be considered as a limitation of our research. For more accurate results, additional studies with more patients should be done in the future.
Footnotes
Soumaya Mougou-Zerelli and Sonia Brahem equally contribute to the work.
Capsule
Patients with severe oligozoospermia presented a high level of sex numerical chromosome abnormalities, and consequently are at high risk of chromosome abnormalities in their offspring.
References
- 1.Egozcue J, Templado C, Vidal F, Navarro J, Morer-Fargas F, Marina S. Meiotic studies in a series of 1100 infertile and sterile males. Hum Genet. 1983;65:185–188. doi: 10.1007/BF00286660. [DOI] [PubMed] [Google Scholar]
- 2.Egozcue J, Sarrate Z, Codina-Pascual M, Egozcue S, Oliver- Bonet M, Blanco J, Navarro J, Benet J, Vidal F. Meiotic abnormalities in infertile males. Cytogenet Genome Res. 2005;111:337–342. doi: 10.1159/000086907. [DOI] [PubMed] [Google Scholar]
- 3.Miharu N, Best RG, Young SR. Numerical chromosome abnormalities in spermatozoa of fertile and infertile men detected by fluorescence in situ hybridization. Hum Genet. 1994;93:502–506. doi: 10.1007/BF00202812. [DOI] [PubMed] [Google Scholar]
- 4.Guttenbach M, Martinez-Exposito MJ, Michelmann HW, Engel W, Schmid M. Incidence of diploid and disomic sperm nuclei in 45 infertile men. Hum Reprod. 1997;12:468–473. doi: 10.1093/humrep/12.3.468. [DOI] [PubMed] [Google Scholar]
- 5.Pang MG, Hoegerman SF, Cuticchia AJ, Moon SY, Doncel GF, Acosta AA, Kearns WG. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmatic sperm injection. Hum Reprod. 1999;14:1266–1273. doi: 10.1093/humrep/14.5.1266. [DOI] [PubMed] [Google Scholar]
- 6.Vegetti W, VanAssche E, Frias A, Verheyen G, Bianchi MM, Bonduelle M, Liebaers I, VanSteirteghem A. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum Reprod. 2000;15:351–365. doi: 10.1093/humrep/15.2.351. [DOI] [PubMed] [Google Scholar]
- 7.McInnes B, Rademaker A, Greene CA, Ko E, Barclay L, Martin RH. Abnormalities for chromosomes 13 and 21 detected in spermatozoa from infertile men. Hum Reprod. 1998;13:2787–2790. doi: 10.1093/humrep/13.9.2489. [DOI] [PubMed] [Google Scholar]
- 8.Bernadini L, Martini E, Geraedts JPM, Hopman AHN, Lanteri S, Conte N, Capitanio GL. Comparison of gonosomal aneuploidy in spermatozoa of normal fertile men and those with severe male factor detected by in-situ hybridization. Mol Hum Reprod. 1997;3:431–438. doi: 10.1093/molehr/3.5.431. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa N, Murakami I, Ikuta K, Suzumori K. Sex chromosomal analysis of spermatozoa from infertile men using fluorescence in situ hybridization. J Assist Reprod Genet. 2000;17:97–102. doi: 10.1023/A:1009413916753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RH, Rademaker AW, Greene C, Ko E, Hoang T, Barclay L, Chernos J. A comparison of the frequency of sperm chromosome abnormalities in men with mild, moderate, and severe oligozoospermia. Biol Reprod. 2003;69:535–539. doi: 10.1095/biolreprod.102.015149. [DOI] [PubMed] [Google Scholar]
- 11.Schmid TE, Kamischke A, Bollwein H, Nieschlag E, Brinkworth MH. Genetic damage in oligozoospermic patients detected by FISH, iRSM, SCSA and the Comet assay. Hum Reprod. 2003;18:1474–1486. doi: 10.1093/humrep/deg259. [DOI] [PubMed] [Google Scholar]
- 12.Mehdi M, Smatti B, Saad A, Guerin JF, Benchaib M. Analysis by fluorescence in situ hybridization (FISH) of the relationship between gonosomic aneuploidy and the results of assisted reproduction in men with severe oligozoospermia. Andrologia. 2006;38:137–141. doi: 10.1111/j.1439-0272.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 13.Laboratory manual for the examination of human semen and semen-cervical mucus interaction. New York: Cambridge University Press; 1992. [Google Scholar]
- 14.David G. Editorial: sperm banks in France. Arch Fr Pediatr. 1975;5:401–404. [PubMed] [Google Scholar]
- 15.Ohashi Y, Miharu N, Honda H, Samura O, Ohama K. High frequency of XY disomy in spermatozoa of severe oligozoospermic men. Hum Reprod. 2001;16:703–708. doi: 10.1093/humrep/16.4.703. [DOI] [PubMed] [Google Scholar]
- 16.Faure AK, Aknin-Seifer I, Frérot G, Pelletier R, Robertis C, Cans C, Levy R, Jimenez C, Lejeune H, Terrier N, Bergues U, Hennebicq S, Rousseaux S. Predictive factors for an increased risk of sperm aneuploidies in oligo-astheno-teratozoospermic males. Int J Androl. 2007;30:153–162. doi: 10.1111/j.1365-2605.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- 17.Kinakin B, Rademaker A, Martin R. Paternal age effect of YY aneuploidy in human sperm, as assessed by fluorescence in situ hybridization. Cytogenet Cell Genet. 1997;78:116–119. doi: 10.1159/000134641. [DOI] [PubMed] [Google Scholar]
- 18.Schmid TE, Brinkworth MH, Hill F, Sloter E, Kamischke A, Marchetti F, Nieschlag E, Wyrobek AJ. Detection of structural and numerical chromosomal abnormalities by ACM-FISH analysis in sperm of oligozoospermic infertility patients. Hum Reprod. 2004;19:1395–1400. doi: 10.1093/humrep/deh278. [DOI] [PubMed] [Google Scholar]
- 19.Shi Q. Martin RH:Aneuploidy in human spermatozoa: FISH analysis in men with constitutional chromosomal abnormalities, and in infertile men. Reproduction. 2001;121:655–666. doi: 10.1530/rep.0.1210655. [DOI] [PubMed] [Google Scholar]
- 20.Morel F, Mercier S, Roux C, Elmrini T, Clavequin MC, Bresson JL. Interindividual variations in the disomy frequencies of human spermatozoa and their correlation with nuclear maturity as evaluated by aniline blue staining. Fertil Steril. 1998;69:1122–1127. doi: 10.1016/S0015-0282(98)00058-2. [DOI] [PubMed] [Google Scholar]
- 21.Rives N, SaintClair A, Mazurier S, Sibert L, Simeon N, Joly G, Mace B. Relationship between clinical phenotype, semen parameters and aneuploidy frequency in sperm nuclei of 50 infertile males. Hum Genet. 1999;105:266–272. doi: 10.1007/s004390051100. [DOI] [PubMed] [Google Scholar]
- 22.Calogero AE, Palma A, Grazioso C, Barone N, Romeo R, Rappazzo G, D’Agata R. Aneuploidy rate in spermatozoa of selected men with abnormal semen parameters. Hum Reprod. 2001;16:1172–1179. doi: 10.1093/humrep/16.6.1172. [DOI] [PubMed] [Google Scholar]
- 23.Damri LE, Vutyavanich T, Fishel S. Comparison of sex chromosome aneuploidy in spermatozoa of fertile men and those requiring ICSI treatment detected by fluorescence in situ hybridization. J Obstet Gynaecol Res. 2000;26:181–188. doi: 10.1111/j.1447-0756.2000.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 24.Schultz H, Mennicke K, Schlieker H, AlHasani S, BalsPratsch M, Diedrich K, Schwinger E. Comparative study of disomy and diploidy rates in spermatozoa of fertile and infertile men: a donor-adapted protocol for multi-colour fluorescence in situ hybridization (FISH) Int J Androl. 2000;23:300–308. doi: 10.1046/j.1365-2605.2000.00241.x. [DOI] [PubMed] [Google Scholar]
- 25.Machev N, Gosset P, Viville S. Chromosome abnormalities in sperm from infertile men with normal somatic karyotypes: teratozoospermia. Cytogenet Genome Res. 2005;111:352–357. doi: 10.1159/000086910. [DOI] [PubMed] [Google Scholar]
- 26.Sun F, Ko E, Martin RH. Is there a relationship between sperm chromosome abnormalities and sperm morphology? Reprod Biol Endocrinol. 2006;4:1–5. doi: 10.1186/1477-7827-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vendrell JM, Garcia F, Veiga A, Calderon G, Egozcue S, Egozcue J, Barri PN. Meiotic abnormalities and spermatogenic parameters in severe oligoasthenozoospermia. Hum Reprod. 1999;14:375–378. doi: 10.1093/humrep/14.2.375. [DOI] [PubMed] [Google Scholar]
- 28.Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a review. Int J Androl. 1995;18:169–184. doi: 10.1111/j.1365-2605.1995.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 29.Robbins WA, Elashoff DA, Xun L, Jia J, Li N, Wu G, Wei F. Effect of lifestyle exposures on sperm aneuploidy. Cytogenet Genome Res. 2005;111:371–377. doi: 10.1159/000086914. [DOI] [PubMed] [Google Scholar]