Abstract
Purpose
To evaluate the use of multiple displacement amplification (MDA) for preimplantation genetic diagnosis (PGD) of α- and β-double thalassemia.
Method
Whole genome of a single cell was directly amplified using MDA and its products were used as templates in fluorescent gap polymerase chain reaction (PCR) analysis of α-thalassemia and in PCR-reverse dot blot analysis ,singleplex fluorescent PCR of β-28 and CD17 mutation and HumTH01 for β-thalassemia.
Results
1) MDA from single cell could produce enough DNA templates for the detection of both α and β-thalassemia; 2) The established MDA-PGD protocol for α- and β-double thalassemia was successfully applied in PGD of six embryos, among which, three were transferred, but no pregnancy ensued.
Conclusions
The use of MDA as a universal step allows for the simultaneous diagnosis of two or more hereditary defects.
Keywords: Preimplantation genetic diagnosis, α- thalassemia, β-thalassemia, Multiple displacement amplification
Introduction
The thalassemia is a group of hereditary anaemias characterized by the reduced or absent production of one of the globin chains of hemoglobin (Hb) affecting 4.8% of the world population [1]. It is prevalent in the Mediterranean region and Southeast Asia. In Southeast China, α-thalassemia and β-thalassemia constitute the majority of monogenetic disorders, with the average carrier rates being as high as 10.3% and 8.53% for the two diseases, respectively [2, 3].
The Hb molecule is a tetramer. In human infants, the HB molecule is mainly comprised of two α globins and two γ globins. In normal adults, 95% of the circulating Hb consists of two α globins and two β globins, each containing a haem group responsible for delivering oxygen to tissues. Thus, the most common forms of thalassemia are α-thalassemia and β-thalassemia.
The α-globin gene cluster is located on chromosome 16p13.3 and comprised of embryonic γ-globin gene and two α-globin genes α2 and α1 in tandem (in cis) [4]. Homozygotes with α-thalassemia suffer from Hb Bart’s hydrops fetalis syndrome and die either in utero in late gestation or within a few minutes after birth [5]. Southeast Asia deletion (--SEA) is the most common homozygous mutation with an incidence rate ranging from 72.87% to 82.87% [6, 7].
β-thalassemias are a group of hereditary blood disorders characterized by reduced (β+) or absent (β0) β-globin chain synthesis, resulting in reduced Hb in red blood cells (RBC), decreased RBC production and anemia. They are caused by point mutations or, more rarely, deletions in the β-globin gene cluster on chromosome 11.
Infants with thalassemia major are usually diagnosed before two years old and require regular RBC transfusions to survive. For this reason, prenatal diagnosis has been advocated by the Chinese government for many years. Preimplantation genetic diagnosis (PGD) is considered as an alternative to prenatal diagnosis. PGD has been successfully applied for the detection of α-thalassemia [8–12] or β-thalassemia [10, 13–16]. Our center has also established protocols for PGD of carriers with α-thalassemia or β-thalassemia [10, 11, 15]. However, to the best of our knowledge, the application of PGD for the simultaneous diagnosis of both α- and β-thalassemia has not been reported.
Whole-genome amplification by isothermal multiple displacement amplification (MDA) provides a satisfactory solution to this problem. MDA is based on the use of φ29 DNA polymerase and random primers, which can generate large amounts of templates and offer the most complete coverage and unbiased amplification [17, 18]. To date, it has been used in PGD of many genetic diseases since 2006 [19–24].
Here, we report a novel, MDA-based PGD for both α- and β-dual thalassemia, using fluorescent gap PCR for α-thalassemia as well as PCR-RBD, fluorescent PCR, and linkage analysis with HumTH01 for β-thalassemia.
Materials and methods
Patients
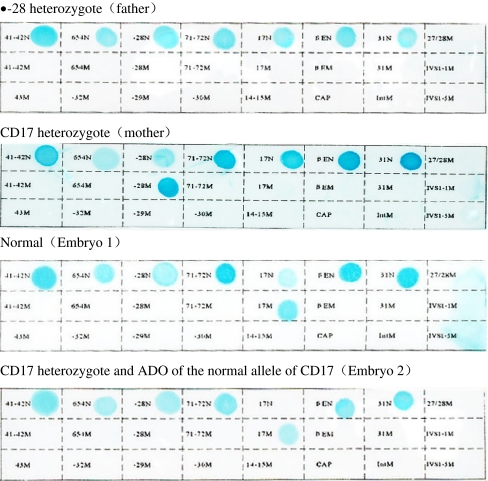
A couple aged at 41 (female) and 45 (male) were carriers of Southeast Asia deletion (--SEA) genotype (deletion of two α-globin genes in cis). In addition, the male was a heterozygote of β-thalassemia −28. The female was a heterozygote of β-thalassemia codon 17. This couple had experienced twice selective terminations due to pregnancies with Hb Bart’s hydrops fetalis. They had one daughter identified as a carrier of the --SEA mutation and β-thalassemia −28 mutation.
Written consent was obtained from the family. The study was approved by the Ethnical Board of Sun Yat-sen University.
Pedigree analysis
Genomic DNA was extracted from each member of the family using the phenol-chloroform procedure. The linkages between the β-globin gene mutations and the alleles of HumTH01 were determined by analyzing the alleles of the HumTH01 of both the parents and their daughter.
Isolation of Single Lymphocytes
Lymphocytes were isolated from EDTA-anticoagulated venous bloods using the lymphocyte segregatory fluid method as previously described [25]. Each single cell was transferred into a sterile PCR tube containing 3.5 μL PBS and used for MDA immediately. The final wash buffers for each single lymphocyte were collected and used as the negative control of the cell sorting procedure.
Cell lysates and MDA protocol
Cell lysing and MDA were performed using Repli-g kit (Qiagen, Germany). Samples and blanks were mixed with additional 3.5 μL of freshly prepared lysis buffer and incubated for 10 min at 65°C followed by addition of 3.5 μL of stop buffer. The obtained solution (10 μL) was used directly for whole-genome amplification by adding 40 μL of reaction master mix provided in the kit followed by incubation at 30°C for 8 h and consequent heat inactivation at 65°C for 3 min. The amplified DNA was subjected to PCR immediately or stored at −20°C.
Fluorescent gap PCR analysis of α-thalassemia SEA
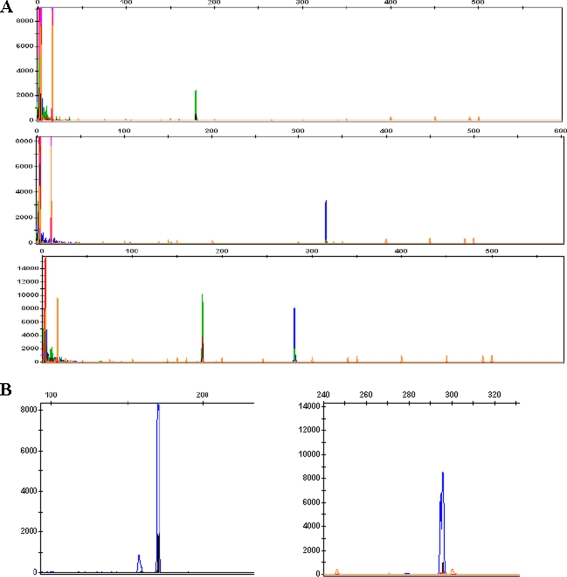
Three α-thalassemia SEA primers were used in fluorescent gap PCR analysis. The S1 and S3 primers flank the SEA deletion, whereas the S2 primer anneals within the deleted area [26]. The primer sequences and products sizes were listed in Table 1. PCR was performed in a 50 μL reaction system containing 5 μL of 1/100 diluted MDA products or genomic DNA, 4.5 μL of 10xPCR neutralizing buffer, 1.5 μL of 25 mmol/L MgCl2, 1 μL of 10 mmol/L dNTP each, 200 mM of primers S1, S2 and S3 each, and 1.5 units of AmpliTap DNA polymerase (ABI) using a Perkin Elmer Cetus 9700 PCR machine. The thermal cycling condition was an initial denaturation at 96°C for 3 min followed by 35 cycles of denaturation at 96°C for 45 s, annealing at 60°C for 1 min and elongation at 72°C for 1 min, and a final elongation at 72°C for 7 min. PCR products were then analyzed on an ABI 3100 Advant genetic analyzer (Fig. 1).
Table 1.
Primer sequences and produc sizes for detecting α- and β-thalassaemia
| Primers | Primer sequence 5′- 3′ | Product size (bp) |
|---|---|---|
| S1 | GTGTTCTCAGTATTGGAGGGAA | |
| S2 | FAM-GACACGCTTCCAATACGCTTA | Normal:S1 + S2:282 |
| S3 | HEX-CTACTGCAGCCTTGAACTCC | Abnormal:S1 + S3:178 |
| A | GGCCAATCTACTCCCAGGAG | |
| B | ACATCAAGGGTCCCATAGAC | A + B:597 |
| C | ATAACAGTGAATTTCTGG | |
| D | AAAGCGAACTTAGTGATAC | C + D:362 |
| HumTH01-F | FAM-AGGGTATCTGGGCTCTGG | |
| HumTH01-R | CTTCCGAGTGCAGGTCAC | 115–130 |
| 17 M-F | GCCGTTACTGCCCTGTGGGGCT | 17 M-F + R: 170 |
| 17 N-F | GCCGTTACTGCCCTGTGGGGCA | 17 N-F + R: 170 |
| R | FAM-ACCAATAGGCAGAGAGAGTC | |
| −28 M-F | GAGCCAGGGCTGGGCATAG | −28 M + R: 297 |
| −28 N-F | GAGCCAGGGCTGGGCATAA | −28 N + R: 297 |
Fig. 1.
Detection of the α-thalassemia SEA deletion, β-thalassemia CD17 and −28 mutations and HumTH01 by 3100 Genetic analyzer fluorescent PCR analysis. a Detection of α-thalassemia SEA deletion. The top, middle and lower lanes indicate the affected, heterozygous and normal samples, respectively. b Detection of β-thalassemia CD17(left:170 bp) and β-28(right:297 bp) mutations. c Detection of the HumTH01 locus.The first and second were of the father and mother (β-28 heterozygote and CD17 heterozygote, respectively). The third panel shows the results of the daughter (β-28 heterozygote), showing the abnormal β-28 allele from her father and normal CD17 allele from her mother. The final one was of embryo 2(β-17 heterozygote)
β-thalassemia analysis
β-thalassemia was detected using three protocols: PCR-reverse dot blot (RDB) hybridization, singleplex fluorescent PCR for −28 and CD17 mutation, and linkage analysis with HumTH01.
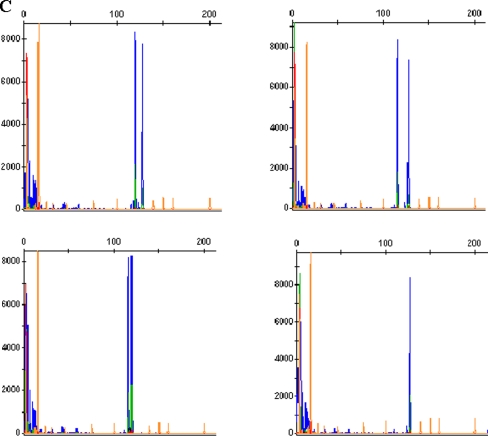
Based on an established protocol [27], we used primers A and B,C and D to detect 16 β-thalassemia mutations(CD41-42, IVS-2nt 654, CD17, −28, CD71/72, −29,βE, CD43, −32, −30, Int , CD14/15, CD27/28, CD1/1,CD1/5, and CD31) under the following conditions: an initial denaturation at 95°C for 5 min followed by 35 cycles of 95°C for 30 s, 55°C for 1 min and 72°C for 1 min, and a final extension at 72°C for 7 min. Each 25 μL of reaction contained 2.5 μLof 10xPCR buffer, 3 μL of 1/100 diluted MDA products or genomic DNA, 0.2 μM of each primer set, 200 μM dNTPS, 1.5 mM MgCl2 and 1 unit of AmpliTaq DNA polymerase (Perkin Elmer, USA). The PCR products were subsequently hybridized with oligonucleotides that were amino-modified at 5′ terminal base and specific for the 17 β-thalassemia mutations in the Chinese population. The sequences of primers and the lengths of their products are listed in Table 1. Immobilization of the oligonucleotide probes and their hybridization with amplified PCR products were performed as previously described [28] (Fig. 2).
Fig. 2.
Detection of β-thalassemia mutation by PCR-RDB. Each strip stands for hybridization from a sample. The normal and mutation probes for each mutation are indicated by the blue dots, on the first and second rows of each strip and of the same order for all the strips. The site of each mutation in the β-globin gene is indicated next to the dots
For singleplex fluorescent PCR analysis, β-thalassemia −28 and CD17 mutations were detected using MDA products as templates and specific primers designed based on amplification refractory mutation systems (ARMS) [29]. Each MDA product was amplified using both normal primer pairs and mutant primer pairs. The primer sequences used and the lengths of their products are listed in Table 1. HumTH01 is a highly polymorphic short tandem repeat (STR) markers closely linked to the β-globin gene. Its sequence was modified as previously reported and listed in Table 1 [30]. All PCR were performed following the same reaction system and PCR conditions described above for PCR-RDB analysis. All singleplex fluorescent PCR products were analyzed on an ABI 3100 Advant genetic analyzer (Fig. 1).
Preimplantation genetic diagnosis
Ovarian stimulation, oocyte retrieval, and intracytoplasmic sperm injection (ICSI) procedures were performed as previously described [11]. Embryo biopsy was carried out on the morning of Day 3 after oocyte retrieval. One blastomere was collected by aspiration from each embryo. Cell lysis, MDA amplification, and PCR analysis were performed as described for single lymphocytes.
Reanalysis of non-transferred embryos
Three embryos that were biopsied but not transferred (embryos 1, 2, and 4) were donated for research with the written consent of the couple and re-analyzed as aforementioned for blastomeres. Their genetic diagnoses were compared with those of the previous blastomere analyses.
Results
Pedigree analysis
Since the daughter was heterozygous for both the --SEA mutation and β-thalassemia −28, pedigree analysis was performed using the linkage analysis method. The results showed that she inherited her father’s abnormal β-28 chromosome and her mother’s normal β-CD17 chromosome. The HumTH01 loci were (119 bp,115 bp),(119 bp,127 bp), and ( 115 bp,127 bp)for her, her father and her mother, respectively. Therefore, the Hum TH01 linked to abnormal β-28 and CD17 was (119 bp,127 bp), and the normal one was (127 bp,115 bp).
Amplification efficiency with MDA
Ten lymphocytes from each family member and the normal control were successfully amplified by the MDA method.
PCR efficiency and allele drop-out (ADO)
ADO was defined as failed amplification for one of the two alleles at a heterozygous locus. The amplicons of MDA products from single lymphocyte of all family members were compared with those from DNAs extracted from their corresponding peripheral blood. Among the total of 160 PCR, the PCR efficiency and ADO rate for α-thalassemia were 95% (38/40) and 10.7% (3/28), respectively. Two ADO occurred at mutation loci, and one at a normal locus.
The PCR efficiency and ADO for β-thalassemia using both PCR-RBD and fluorescent PCR methods were the same, both being 100% (40/40) and 23.3% (7/30) respectively. ADO occurred with 3 at normal −28 locus, 3 at normal CD17 locus, and 1 at mutant −28 locus. For the marker HumTH01, the PCR efficiency and ADO rate were 97.5% (39/40) and 10.0% (4/40), respectively.
Clinical Preimplantation Genetic Diagnosis
Following controlled ovarian stimulation, 8 oocytes were retrieved. Among them, 7 were metaphase II oocytes. All injected oocytes were fertilized. On day 3 of the culture, 6 of the seven embryos had developed normally and were biopsied. MDA was successful in all six blastomeres.
For the detection of α-thalassemia, the PCR efficiency was 83.3% (5/6). No ADO rate was found compared with the results from the reanalysis of non-transferred embryos.
For detecting β-thalassemia, the PCR efficiency was 100% for all three different methods. The ADO rates for both PCR-RDB and fluorescent PCR were 66.7% (4/6). All ADO occurred at normal loci of heterozygotes rather than the mutated loci. For the HumTh01 loci, the PCR efficiency and ADO rate were 100% (6/6) and 25.5% (1/4), respectively. Taken the results of the three analyses together, the diagnostic rate for β--thalassemia was 100%.
Reanalysis of nontransferred embryos (E1, E4 and E5) showed that E1 was a carrier of α-thalassemia. The other results were in consistent with those of single blastomere analyses (results of genetic diagnosis were shown in Table 2).
Table 2.
Clinical preimplantation genetic diagnosis results
| Embryos | α-thalassemia | β-thalassemia | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|
| Amplified signal (bp) | Diagnosis | PCR-RDB and fluorescent PCR | HumTH01 | Diagnosis | ||||
| β-17 (mut/nor) | β-28 (mut/nor) | Pat (bp) | Mat (bp) | |||||
| E1 | 178 | Affected | NS X | NS X | 127 | 115 | Normal | NTE |
| E2 | 282 | Normal | X ADO | NS X | 127 | 127 | β-17 carrier | ET |
| E3 | 178/282 | Carrier | NS X | X ADO | 119 | 127 | β-28 carrier | ET |
| E4 | 178/282 | Carrier | X X | NS X | 127 | 127 | β-17 carrier | NTE |
| E5 | Amplification failure | NS X | NS X | 127 | 115 | Normal | NTE | |
| E6 | 282 | Normal | NS X | X ADO | 119 | ADO | β-28 carrier | ET |
ADO allele drop out; NS no signal; X visible signal; mut mutant dot;nor normal dot; pat paternal allele; mat maternal allele; ET embryo transfer; NTE nontransferred embryos.
Three embryos with normal genotype of α- and β-thalassemia were transferred on day 5 after ovulation. Unfortunately, no pregnancy was achieved.
Discussion
PGD for α-thalassemia or β-thalassemia has been widely applied in many IVF centers all over the world. However, using PGD for simultaneous detection of both α-thalassemia and β-thalassemia has not been reported. Our previous study has demonstrated as a whole-genome amplification method with extensive coverage and low offset, MDA can be used to amplify large amounts of DNA templates from single cells and therefore helps achieve simultaneous PCR detection of the gene status of two or more hereditary diseases.
In this study, we evaluated the use of MDA in the PGD for both α- and β- thalassemia. MDA efficiency was assessed using single lymphocytes. The PCR amplification efficiency and ADO rate for the detection of α-thalassemia were 95% (38/40) and 10.7% (3/28), respectively. For heterozygous embryos, ADO for the normal allele may lead to misdiagnosis as the homozygous mutant embryo; conversely, ADO of the mutant allele may cause misdiagnosis as the normal homozygote embryo. The presence of the normal allele in the transferred embryos should offset any adverse consequence due to ADO in the mutant allele in α- thalassemia. In clinical PGD, the efficiency of detecting α-thalassemia in single blastomeres was 83.3% (5/6), indicating that single cell MDA was able to cover most of the whole genome [25].
For β-thalassemia, we adopted three different methods to minimize diagnostic error. PCR-RDB and fluorescent PCR methods were used to detect mutations, while HumTh01 was used indirectly to do gene linkage analysis. PCR-RDB can simultaneously detect 17 different mutations of β-thalassemia [10], but its outcome is interpreted subjectively and associated with the time of chromogenic reaction. Inadequate or excessive chromogenic reaction may result in misdiagnosis. On the contrary, fluorescent PCR is highly specific and can be interpreted more objectively based on the height of signal value [29]. However, this method largely depends on specific primers designed for each type of mutation, which limits its application.
Our study revealed that both PCR-RDB and fluorescent PCR after MDA share the same amplification efficiencies and ADO rates. Moreover, the same ADO sites were found in both methods, indicating that ADO may be due to MDA process. Furthermore, the majority ADO sites (85.7%, 6/7) appeared in the normal loci corresponding to the mutant ones. It seems that normal loci are more prone to ADO than the mutant ones in MDA. In this case, diagnostic deficiencies will lead to a reduced number of embryos available for transfer. In the reanalysis using non-transferred embryos, we did not find any new ADO sites. Further investigation is needed to unravel the causes of this phenomenon.
It is important to note that the co-amplification of the polymorphic marker HumTh01 gene could help confirm the genetic analysis by RDB and fluorescent PCR. The analysis of HumTh01, which is located in the 5′ flanking region to the β-globin gene, can provide back-up diagnostic information for an informative family since the probability of ADO affecting both the mutant allele and the linked polymorphic marker in the same reaction is extremely low [30]. However, the genetic recombination between β-thalassemia and HumTh01 may occur due to the relative distance between the two genes. Therefore, this method only serves as a supplementary diagnostic method. In clinical PGD, the result of HumTH01 were all in consistent with those from direct genetic analyses. A combination of these three methods can improve the diagnostic accuracy of β-thalassemia and thus, greatly reduce the possibility choosing ADO-caused abnormal embryos for the implantation into the uterine.
Previous studies showed that the diagnostic efficiency of fluorescent PCR analysis of single blastomere for α-thalassemia was 76.1% (35/46) [8], 89.3–91.7% [9], 89.5% (34/38) [10], 75% (354/372) [11] and 87.3% (110/126),respectively.[12].In addition, the ADO rate was 81.6–91.7% [9], 5.9% (2/34) [10], 16.4% (8/49) [11] and 10.2% (9/88) [12], respectively. The diagnostic efficiency of traditional PGD methods for β-thalassemia was 93.9% (46/49) [10], 61% (22/36) [13], 93% [14], 89.5% (34/38)[15] and 96% (282/294) [16] ,respectively. The current findings revealed that the diagnostic efficiency of traditional PGD methods is comparable to that after MDA. Since only one cycle of PGD is used in the present study, further studies are needed to compare the diagnostic efficiencies of various methods.
Overall, our study demonstrates that MDA as a universal step to obtain large amounts of DNA templates from single cells is essential for the simultaneous genetic diagnosis of different hereditary diseases.
Acknowledgments
The study was supported by National Basic Research Program of China (973 Program) (Grant No. 2007CB948103)and China Medical Board of New York Inc (Grant No. 06840)
Footnotes
Capsule
Multiple displacement amplification method was used in preimplantation genetic diagnosis for α- and β-double thalassemia carriers.
Declaration
The authors report no financial or commercial conflicts of interest.
References
- 1.Bouljenkov V (1994) Epidemiology of haemoglobinopathies. In WHO Regional Working Group on the Prevention and Control of Thalassemia in Asia, Bangkok, Thailand
- 2.Cai R, Li L, Liang X, et al. Prevalence survey and molecular characterization of alpha and beta thalassemia in Liuzhou city of Guangxi. Zhong Hua Liu Xing Bing Xue Za Zhi. 2002;23(4):281–285. [PubMed] [Google Scholar]
- 3.Xu XM, Zhou YQ, Luo GX, et al. The prevalence and spectrum of alpha and beta thalassemia in Guangdong Province: implications for the future health burden and population screening. J Clin Pathol. 2004;57:517–519. doi: 10.1136/jcp.2003.014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forget RG. Molecular genetics of the human globin genes. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of hemoglobin: genetics, pathophysiology and clinical management. UK: Cambridge University Press; 2001. pp. 117–130. [Google Scholar]
- 5.Liang ST, Wong VCW, So WWK, Ma HK, Chan V, Todd D. Homozygous α-thalassemia: clinical presentation, diagnosis and management. A review of 46 cases. Br J Obstet Gynaecol. 1985;92:680–684. doi: 10.1111/j.1471-0528.1985.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang YJ, Ou XB, Yu YP, et al. The incidence rates and genotypes in children with α-thalassemia in Guangzhou region. Chin Pedi Hemat. 2005;10:205–208. [Google Scholar]
- 7.Duan S, Li HY, Chen Z, et al. The research on the mutation genotypes of α-thalassemia in Southern China. Zhong-Guo-Shi-Yan-Xue-Ye-Xue-Za-Zhi. 2003;11:54–60. [PubMed] [Google Scholar]
- 8.Chang MY, Soong YK, Wong ML. Preimplantation diagnosis of α-thalassemia by blastomere aspiration and polymerase chain reaction:preliminary experience. J Formos Med Assoc. 1996;95:203–208. [PubMed] [Google Scholar]
- 9.Piyamongkol W, Harper JC, Delhanty J, et al. Preimplantation genetic diangositic protocols for α- and β-thalassemias using multiplex fluorescent PCR. Prenat Diagn. 2001;21:753–759. doi: 10.1002/pd.170. [DOI] [PubMed] [Google Scholar]
- 10.Deng J, Peng WL, Li J, et al. Successful preimplantation genetic diagnosis for alpha- and beta-thalassemia in China. Prenat Diagn. 2006;26:1021–1028. doi: 10.1002/pd.1422. [DOI] [PubMed] [Google Scholar]
- 11.Xu YW, Zeng YH, Deng J, et al. Preimplantation genetic diagnosis for α-thalassemia in China. J Assist Reprod Genet. 2009;26:399–403. doi: 10.1007/s10815-009-9336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan V, Ng E, Yam I, et al. Experience in preimplantation genetic diagnosis for exclusion of homozygous α0 thalassemia. Prenat Diagn. 2006;26:1029–1036. doi: 10.1002/pd.1550. [DOI] [PubMed] [Google Scholar]
- 13.Kanavakis E, Vrettou C, Palmer G, et al. Preimplantation genetic diagnosis in 10 couples at risk for transmitting beta-thalassemia major: Clinical experience including the initiation of six singleton pregnancies. Prenat Diagn. 1999;19:1217–1222. doi: 10.1002/(SICI)1097-0223(199912)19:13<1217::AID-PD723>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Rycke M, Velde H, Sermon K, et al. Preimplantation genetic diagnosis for sickle cell anemia and for beta-thalassemia. Prenat Diagn. 2001;21:214–222. doi: 10.1002/1097-0223(200103)21:3<214::AID-PD51>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Jiao Z, Zhou C, Li J, et al. Birth of healthy children after preimplantation diagnosis of beta-thalassemia by whole genome amplification. Prenat Diagn. 2003;23:646–651. doi: 10.1002/pd.659. [DOI] [PubMed] [Google Scholar]
- 16.El-Hashemite N, Wells D, Delhanty JD. Single cell detection of beta-thalassemia mutations using silver stained SSCP analysis: an application for preimplantation diagnosis. Mol Hum Reprod. 1997;3:693–698. doi: 10.1093/molehr/3.8.693. [DOI] [PubMed] [Google Scholar]
- 17.Dean FB, Hosono S, Fang L, et al. Comprehensive human genome amplification using multiple displacement amplification. Proc Natl Acad Sci USA. 2002;99:5261–5266. doi: 10.1073/pnas.082089499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosono S, Faruqi AF, Dean FB, et al. Unbiased whole-genome amplification directly from clinical samples. Genome Res. 2003;13:954–964. doi: 10.1101/gr.816903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spits C, Caignec C, Rycke M, Liebaers I, Sermon K, et al. Whole-genome multiple displacement amplification from single cells. Nat Protoc. 2006;1(4):1965–1970. doi: 10.1038/nprot.2006.326. [DOI] [PubMed] [Google Scholar]
- 20.Ren Z, Zeng HT, Xu YW, et al. Preimplantation genetic diagnosis for Duchenne muscular dystrophy by multiple displacement amplification. Fertil Steril. 2009;91:359–364. doi: 10.1016/j.fertnstert.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Lledo B, Ten H, Galan FM, Bernabeu R. Preimplantation genetic diagnosis of Marfan syndrome using multiple displacement amplification. Fertil Steril. 2006;86:949–955. doi: 10.1016/j.fertnstert.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 22.Judy F, Chow C, William S, Yeung B, Estella Y, Lau L, et al. Singleton birth after preimplantation genetic diagnosis for Huntington disease using whole genome amplification. Fertil Steril. 2009;92:828–827. doi: 10.1016/j.fertnstert.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Renwick PL, Trussler J, Lashwood A, et al. Preimplantation genetic haplotyping: 127 diagnostic cycles demonstrating a robust, efficient alternative to direct mutation testing on single cells. Reprod Biomed Online. 2010;20:470–76. [DOI] [PubMed]
- 24.Eduardo C, Marleen M, Mark R, Ellis D, et al. Birth of a healthy infant following preimplantation PKHD1 haplotyping for autosomal recessive polycystic kidney disease using multiple displacement amplification. J Assist Reprod Genet. 2010;27:397–407. doi: 10.1007/s10815-010-9432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Z, Zhou C, Xu Y, Deng J, Zeng H, Zeng Y. Mutation and haplotype analysis for Duchenne muscular dystrophy by single cell multiple displacement amplification. Mol Hum Reprod. 2007;6:431–436. doi: 10.1093/molehr/gam020. [DOI] [PubMed] [Google Scholar]
- 26.Ko TM, Tseng LH, Hsieh FJ, et al. Carrier detection and prenatal diagnosis of alpha-thalassemia of Southest Asian deletion by polymerase chain reaction. Hum Genet. 1992;88:245–248. doi: 10.1007/BF00197254. [DOI] [PubMed] [Google Scholar]
- 27.Varawalla NY, Dokras A, Old JM, Sargent IL, Barlow DH. An approach to preimplantation diagnosis of beta-thalassemia. Prenat Diagn. 1991;11:775–785. doi: 10.1002/pd.1970111006. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JZ, Xu XM, Ma WF, Shan YX. A rapid reverse dot blot assay for all 18 beta-thalassemia in Chinese population. J Med Coll PLA. 1993;8:213–219. [Google Scholar]
- 29.Ping C, Mujun L, Linying Z, et al. Prenatal diagnosis of β-thalassemia on fetal DNA and in maternal plasma by fluorescent polymerase chain reaction. Journal of Guangxi Medical University. 2008;25:171–174. [Google Scholar]
- 30.Kuliev A, Rechitsky S, Verlinsky O, et al. Preimplantation diagnosis of thalassemia. J Assist Reprod Genet. 1998;15:219–225. doi: 10.1023/A:1022571822585. [DOI] [PMC free article] [PubMed] [Google Scholar]