Abstract
Purpose
To determine common molecular markers between endometriosis and ovarian cancer.
Methods
Patients included women who underwent laparoscopic excision of ovarian endometriotic lesions (n = 7), healthy non-pregnant women with normal pelvises, who underwent excision of normal peritoneum (n = 7). Two epithelial ovarian cancer (EOC) cell lines were also utilized. Expression of transforming growth factor (TGF)-β1, cyclooxygenase (COX)-2, vascular endothelial growth factor (VEGF), estrogen receptor (ER)-1α, progesterone receptor (PR), androgen receptor (AR), and aromatase was evaluated by real-time RT-PCR.
Results
Endometriosis and EOC cells manifested significantly higher mRNA levels of TGF-β1, COX-2, VEGF, ER-1α, AR, and aromatase, while they expressed significantly lower mRNA levels of PR.
Conclusions
Increased TGF-β1, COX-2, VEGF, ER-1α, AR, and aromatase and decreased PR in endometriotic as well as EOC cells suggests a potential association between these two disease processes. This association is important, as it may reveal common mechanisms for both diseases.
Keywords: Endometriosis, Ovarian cancer, Hormone receptors, TGF-β1, COX-2, VEGF, Aromatase
Introduction
Endometriosis, a significant cause of both infertility and pelvic pain, is defined pathologically as the ectopic location and growth of endometrial glands and stroma [1, 2]. Implants of endometriosis have been demonstrated to contain estrogen (ER), progesterone (PR), and androgen receptors (AR), usually at lower concentrations than eutopic endometrium [3]. Estrogens stimulate proliferation of endometrium and endometriotic implants, while androgens induce atrophy and regression. Progestins oppose the growth-promoting effects of estrogen on the proliferation of endometriosis. Sex steroids such as androgens and progestins, and gonadotrophin-releasing hormone (GnRH) and its analogs, such as nafarelin, have been used to treat endometriosis [3, 4]. While endometriosis is an estrogen-dependent disease occurring primarily in reproductive age women, postmenopausal hormone replacement therapy (HRT) stimulation of the growth of endometriosis has been described with estrogen only therapy (ET) [5], and with combined estrogen-progesterone therapy (HT) [6–8]. Endometriosis can, however, also occur in postmenopausal women not receiving exogenous hormones [9–16]. Importantly, patients with endometriosis have an increased risk of ovarian cancer and other malignancies and the risk of malignant transformation is increased in patients receiving ET [17–22].
Chronic inflammation has been implicated in a variety of cancers [23–30]. It is known that colon carcinoma is associated with inflammatory bowel disease (chronic ulcerative colitis and Crohn’s disease), esophageal adenocarcinoma is associated with reflux esophagitis (Barrett’s esophagus), hepatitis predisposes individuals to liver cancer, schistosomiasis causes an increased risk of bladder and colon carcinomas, and chronic Helicobacter infection leads to cancer of the stomach [31]. Thus, the association between endometriosis and ovarian cancer may be due to the enhancement of aberrant inflammatory and hormonal mediators.
In this study, we investigated the potential association between endometriosis and ovarian cancer by studying the expression of selected biomarkers known to play a vital role in the pathogenesis of each disease. These biomarkers included transforming growth factor (TGF)-β1, cyclooxygenase (COX)-2, vascular endothelial growth factor (VEGF), ER-1α, PR, AR, and aromatase. Additionally, the results of this study may provide evidence to elucidate whether endometriosis promotes alterations in sex steroid hormones and inflammatory mediators that contribute to the development ovarian cancer.
Materials and methods
Patients
Two groups of women, average age 25–45 years old, were included in the study. Patients did not receive antibiotics in the time leading up to surgery. The first group consisted of seven patients with ovarian endometriosis who underwent surgery for pelvic pain and had laparoscopic excision of ovarian endometriotic cysts. The cyst diameter was measured by ultrasound and found to be in the range of 3–10 cm. All patients were classified as having stage III or IV endometriosis according to the revised American Fertility Society classification of endometriosis [32]. The control group consisted of seven healthy, non-pregnant women with normal pelvises who underwent surgery for appendicitis and had excision of normal peritoneum. The controls were strictly selected based on no history of gynecologic surgery, infertility, and no pelvic pain.
Tissue specimens were collected and further confirmed by ultrasound following established histological criteria [33, 34]. Samples were immediately frozen and stored at −80°C. Exclusion criteria included patients who received steroid treatment during the previous 6 months, or those with pituitary, thyroid, or adrenal disorders. Study design was approved by the Ethics Board of the Department of Obstetrics and Gynecology at Qianfo Shan Hospital, Shandong University, Jinan, Shandong, P.R. China, and written informed consent was obtained from all patients before enrollment in the study.
Epithelial ovarian cancer cells
Epithelial ovarian cancer (EOC) cell lines, OVCAR-3 and Caov-3 (ATCC, Manassas, VA), were cultured in DMEM and RPMI 1640 (Invitrogen, Beijing, China) containing 10% fetal bovine serum, 1% antibiotic-antimycotic solution, respectively. Each cell line was maintained in a humidified incubator (5% CO2, 37°C). Cells (2.5 × 105) were plated in 12-well dish for 24 h, collected, and stored at −80°C.
Quantitative RT-PCR
Total RNA extraction
Tissue samples were homogenized with the use of Mixer Mill MM 300 (Qiagen, Beijing, China). Cells were homogenized with lysis buffer provided in the PAREx Kit, according to the manufacturer’s protocol (Takara Bio Inc., Takara, Japan). Total RNA was extracted from tissues and cells with the PAREx Kit, according to the manufacturer’s protocol, and was quantified by ultraviolet absorption at 260 nm.
Complimentary DNA (cDNA) synthesis
The reverse-transcription reaction was performed with a PrimeScript™ RT reagent kit, as described by the manufacturer’s protocol (Takara Bio Inc.).
Quantitative real-time RT-PCR reaction
To quantify each target transcript, a standard curve was constructed with a tenfold dilution series of standard plasmid glyceraldehyde-3-phosphate dehydrogenase (GAPDH). mRNA expression levels in the control and study groups were estimated by quantitative real-time RT-PCR. The three-step PCR reaction was performed with a SYBR Premix ExTaq kit (Takara Bio Inc.) and a Thermal Cycler Dice™ Real-time System (Takara Bio Inc.) under the following conditions: Each reaction contained 12.5 μL of 2 X SYBR® Premix Ex Taq™, 0.2 μM each of target-specific primer designed to amplify the gene fragment of interest, 1 μL of cDNA template, and 9.5 μL of ddH2O in a 25-μL total volume.
Cycling conditions applied for TGF-β1, COX-2, VEGF, ER-1α, PR, AR, and aromatase reactions were: 95°C for 10 min, followed by 45 cycles at 95°C for 15 s, 60°C (TGF-β1, COX-2, VEGF, GAPDH, and aromatase) or 65°C (ER-1α, PR, and AR) for 1 min, and 72°C for 30 s. The specific primers used to amplify cDNA fragments corresponding to TGF-β1, COX-2, VEGF, ER-1α, PR, AR, aromatase and GAPDH are listed in Table 1. Computer analysis, performed to compare the synthesized oligomers with the human sequences in the Medline gene database, revealed no significant homology to other genes or pseudogenes. Following real-time RT-PCR, a melting curve analysis was performed to demonstrate the specificity of the PCR product as a single peak. A control, which contained all the reaction components except for the template, was included in all experiments.
Table 1.
Oligonucleotide primer sequences for real-time RT-PR
| Gene | Primer | Sequence | Product size (bp) |
|---|---|---|---|
| GAPDH | Forward | 5′-CCACCCAGAAGACTGTGGAT-3′ | 127 |
| Reverse | 5′-TTCAGCTCAGGG ATGACCTT-3′ | ||
| TGF-β1 | Forward | 5′-AAGGGCTACCATGCCAACTTC-3′ | 62 |
| Reverse | 5′-TGCGTGTCCAGGCTCCA-3′ | ||
| COX-2 | Forward | 5′-CAGCACTTCACGCATCAGTT-3′ | 127 |
| Reverse | 5′-CGCAGTTTACGCTGTCTAGC-3′ | ||
| VEGF | Forward | 5′-ATGACGAGGGCCTGGAGTGTG-3′ | 91 |
| Reverse | 5′-CCTATGTGCTGGCCTTGG TGAG-3′ | ||
| ER-1α | Forward | 5′-CCACCAACCAGTGCACCATT-3′ | 108 |
| Reverse | 5′-GGTCTTTTCGTATCCCACCTTTC-3′ | ||
| PR | Forward | 5′-CGCGCTCTACCCTGCACTC-3′ | 121 |
| Reverse | 5′-TGAATCCGGCCTCAGGTAGTT-3′ | ||
| AR | Forward | 5′-CCTGGCTTCCGCAACTTACAC-3′ | 168 |
| Reverse | 5′-GGACTTGTGCATGCGGTACTCA-3′ | ||
| Aromatase (CYP19) | Forward | 5′-CTAAATTGCCCCCTCTGAGGT-3′ | 150 |
| Reverse | 5′-CCACACCAAGAGAAAAAGGCC-3′ |
Amplification products (10 μL) were confirmed on a 2% agarose gel stained with 5% ethidium bromide under ultraviolet light. The relative amounts of TGF-β1, COX-2, VEGF, ER-1α, PR, AR, and aromatase mRNAs were normalized as ratios to GAPDH mRNA. Data were processed with Rotor-Gene version 6 software and were given a threshold cycle (CT) corresponding to the PCR cycle at which an increase in reporter fluorescence above a baseline signal can first be detected. Plasmid DNAs containing target gene sequences were used to generate the standard curves. The CT was converted to number of copies, and the values for each sample were calculated as the ratio of the number of copies of the target gene to the number of copies of GAPDH, and were expressed as arbitrary units.
Statistical analysis
For each study group, the outcome measurements were summarized using mean and standard deviation. Statistical significance of the data was determined by the two-tailed Student’s t test. P values less than 0.05 were considered significant.
Results
Data are expressed as interquartile ranges and are presented as box plots, in which the boxes represent the first and third quartiles, and the lines outside the box represent the spread of values. P values indicate the comparison to normal peritoneum.
TGF-β1 gene expression
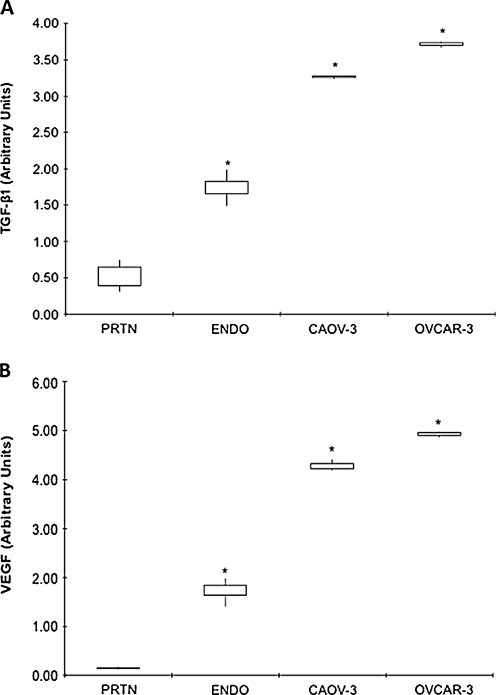
TGF-β1 mRNA levels were significantly lower in normal peritoneum (0.53 ± 0.17) as compared to EOC cell lines (Caov-3, 3.26 ± 0.02; OVCAR-3, 3.71 ± 0.04, P < 0.0001) and ovarian endometriosis tissue (1.74 ± 0.16, P = 0.0009) (Fig. 1a). TFG-β1 mRNA levels were significantly higher in EOC cells as compared to ovarian endometriosis tissues (Fig. 1a).
Fig. 1.
a TGF-β1 and b VEGF gene expression in different tissues and cells. mRNA levels, as determined by real-time RT-PCR, in normal peritoneum (PRTN) (n = 7), ovarian endometriosis (ENDO) (n = 7) amd EOC cells Caov-3 (n = 3) and OVCAR-3 (n = 3). Data are expressed as interquartile ranges and are presented as box plots, in which the boxes represent the first and third quartiles, and the lines outside the box represent the spread of values. *P < 0.001, P values indicate the comparison to normal peritoneum
VEGF gene expression
VEGF mRNA levels were significantly lower in normal peritoneum (0.15 ± 0.02) as compared to both EOC cells (Caov-3, 4.28 ± 0.12; OVCAR-3, 4.92 ± 0.06, P < 0.0001) and ovarian endometriosis tissue (1.74 ± 0.19, P < 0.0001) (Fig. 1b). VEGF mRNA levels were significantly higher in EOC cells as compared to ovarian endometriosis tissues (Fig. 1b).
ER-1α gene expression
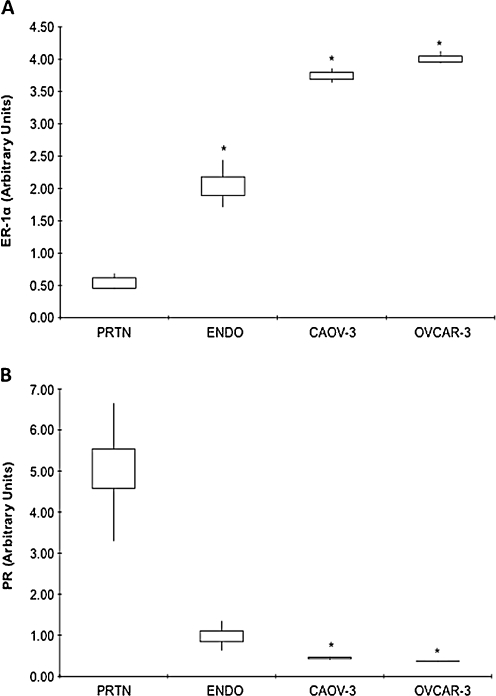
ER-1α mRNA levels were significantly lower in normal peritoneum (0.54 ± 0.10) as compared to both EOC cell lines (Caov-3, 3.75 ± 0.11; OVCAR-3, 4.01 ± 0.10, P < 0.0001) and ovarian endometriosis tissue (2.04 ± 0.24, P = 0.0006) (Fig. 2a). ER-1α mRNA levels were significantly higher in EOC cells as compared to ovarian endometriosis tissues (Fig. 2a).
Fig. 2.
a ER-1α and b PR gene expression in different tissues and cells. mRNA levels, as determined by real-time RT-PCR, in normal peritoneum (PRTN) (n = 7), ovarian endometriotis (ENDO) (n = 7) and EOC cells Caov-3 (n = 3) and OVCAR-3 (n = 3). Data are expressed as interquartile ranges and are presented as box plots, in which the boxes represent the first and third quartiles, and the lines outside the box represent the spread of values. *P < 0.001, P values indicate the comparison to normal peritoneum
PR gene expression
PR mRNA levels were significantly higher in normal peritoneum (5.05 ± 1.07) as compared to both EOC cell lines (Caov-3, 0.44 ± 0.03; OVCAR-3, 0.37 ± 0.01, P < 0.002) and ovarian endometriosis tissue (0.99 ± 0.24, P = 0.003) (Fig. 2b). PR mRNA levels were significantly lower in EOC cells as compared to ovarian endometriosis tissues (Fig. 2b).
AR gene expression
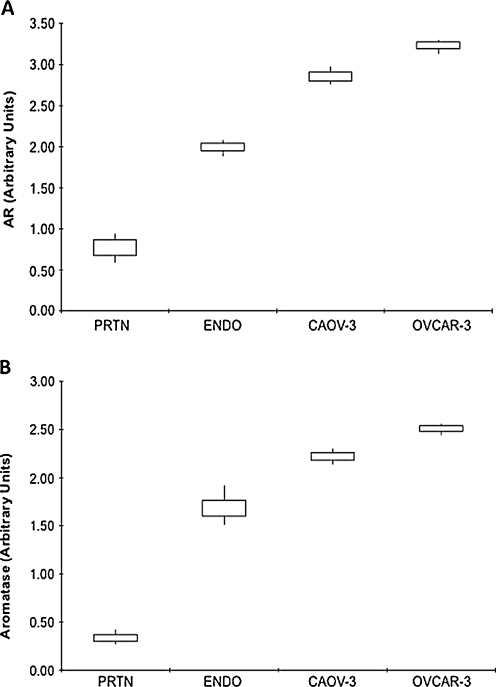
AR mRNA levels were significantly lower in normal peritoneum (0.77 ± 0.13) as compared to both EOC cell lines (Caov-3, 2.86 ± 0.11; OVCAR-3, 3.23 ± 0.09, P < 0.0001) and ovarian endometriosis tissue (1.99 ± 0.071, P = 0.0002) (Fig. 3a). AR mRNA levels were significantly higher in EOC cells as compared to ovarian endometriosis tissues (Fig. 3a).
Fig. 3.
Androgen (AR) and aromatase gene expression in different tissues and cells. mRNA levels, as determined by real-time RT-PCR, in normal peritoneum (PRTN) (n = 7), ovarian endometriotis (ENDO) (n = 7) and EOC cells Caov-3 (n = 3) and OVCAR-3 (n = 3). Data are expressed as interquartile ranges and are presented as box plots, in which the boxes represent the first and third quartiles, and the lines outside the box represent the spread of values. *P < 0.001, P values indicate the comparison to normal peritoneum
Aromatase gene expression
Aromatase mRNA levels were significantly lower in normal peritoneum (0.34 ± 0.06) as compared to both EOC cell lines (Caov-3, 2.22 ± 0.08; OVCAR-3, 2.51 ± 0.06, P < 0.0001) and ovarian endometriosis tissue (1.70 ± 0.14, P = 0.0001) (Fig. 3b). Aromatase mRNA levels were significantly higher in EOC cells as compared to ovarian endometriosis tissues (Fig. 3b).
COX-2 gene expression
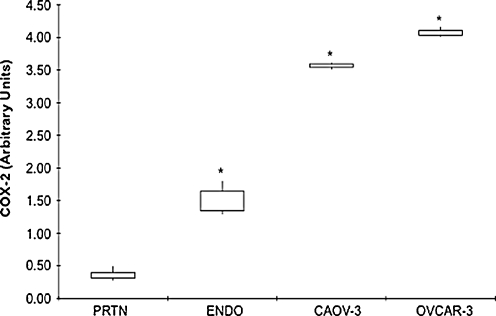
COX-2 mRNA levels were significantly lower in normal peritoneum (0.36 ± 0.076) as compared to EOC cell lines (Caov-3, 3.56 ± 0.05; OVCAR-3, 4.08 ± 0.08, P < 0.0001) and ovarian endometriosis tissue (1.50 ± 0.20, P = 0.0008) (Fig. 4). COX-2 mRNA levels were significantly higher in EOC cells as compared to ovarian endometriosis tissues (Fig. 4).
Fig. 4.
COX-2 gene expression in different tissues and cells. mRNA levels, as determined by real-time RT-PCR, in normal peritoneum (PRTN) (n = 7), ovarian endometriosis (ENDO) (n = 7) and EOC cells Caov-3 (n = 3) and OVCAR-3 (n = 3). Data are expressed as interquartile ranges and are presented as box plots, in which the boxes represent the first and third quartiles, and the lines outside the box represent the spread of values. *P < 0.001, P values indicate the comparison to normal peritoneum
Discussion
Although there is compelling epidemiological and clinical data linking endometriosis and ovarian cancer [28, 29, 35, 36], this link remains controversial [37]. Malignant ovarian tumors have been shown to arise from approximately 1% of cases with ovarian endometriosis [38–41]. Furthermore, in a previous study of women with endometriosis, the risk of ovarian malignant transformation was initially increased 2-fold, and after a follow-up period over 10 years, the risk was further increased 4.2-fold [42]. Another study including patients undergoing surgery for ovarian malignancies found endometriosis in 26.3% of patients with endometrioid malignancies and 21.2% of patients with clear cell malignancies [43]. In a more recent retrospective cohort study, a strong relationship between endometriosis, resulting in infertility, and ovarian cancer has been demonstrated [35]. Yet another recent health survey had also reported a 5-fold increase in ovarian cancer as compared to expected rates among women in the general population [44, 45]. Collectively, these studies provide strong support for an association between endometriosis and ovarian cancer.
Pro-inflammatory cytokines are a hallmark of inflammation and malignancies [46]. Overexpression of TGF-β1, COX-2 and VEGF have been implicated in the pathogenesis of various human malignancies, including ovarian cancer [47, 48]. Upregulation of COX-2, the enzyme that catalyzes the synthesis of prostaglandins, is known to decrease cell differentiation, inhibit apoptosis, increase tumor cell proliferation, and induce angiogenesis via VEGF [49, 50]. Therefore, the role of TGF-β1, VEGF, and COX-2 are thought to be critical to implantation and infiltration of ectopic endometrium, as well as the invasion and metastasis of ovarian tumor cells [45, 48].
The results of this study, utilizing biomarkers common between the two diseases, further supports the previous studies. Ovarian endometriosis tissues and EOC cells exhibited markedly increased basal expression levels of TGF-β1, COX-2, VEGF, ER-1α, AR, and aromatase, and significantly decreased basal expression levels of PR, as compared to normal peritoneum. The similar expression pattern observed in both ovarian endometriosis tissues and EOC cells may indicate a common molecular mechanism between the two diseases. Indeed, patients with endometriosis who develop endometriosis-associated ovarian carcinoma have a distinct clinical profile. The overall frequency of malignant transformation was estimated to be 0.3–0.8% [37, 45, 51]. More recent, larger pathology series (up to 1000 cases) have found ovarian cancer in 5–10% of ovarian endometriotic lesions [45].
The presence of sex steroid hormone receptors for estrogens, androgens, and progesterone may influence the risk for ovarian cancer. This hypothesis is further supported by the fact that increased estrogen and progestin during both pregnancy and oral contraceptive use are known to be protective against ovarian cancer whereas, early menarche and late menopause increase ovarian cancer risk [45]. Additionally, progestin-only oral contraceptives are also protective, however, estrogen–only or sequential estrogen plus progestin (wherein most of the month involves ingestion of estrogen alone) increases the risk of ovarian cancer during hormone therapy [45]. Furthermore, a previous prospective study found significantly higher levels of androgens in the patients’s serum of those with polycystic ovarian syndrome to be associated with ovarian cancer [52, 53]. Thus, androgenic agents used to treat endometriosis may also increase the risk of ovarian cancer, possibly through the conversion of androgen to estrogen via aromatase, however, such a risk may be reduced by androgen inhibition of follicular development [54]. Collectively, these observations suggest a role for sex hormones, particularly androgens and estrogen, in the development of ovarian cancers, whereas progesterone is protective.
A mechanism involving steroid hormones and inflammation in promotion of endometriosis and its transformation to ovarian cancer has been previously reported [48]. Systemic estrogens and androgens increase the concentration of estradiol and estrone through aromatase within endometriotic implants. Estrone is subsequently converted to the more potent estradiol by 17β-hydroxysteroid dehydrogenase (17β-HSD)-1 [55]. Normal endometrium glandular cells express large amounts of progesterone-induced 17β-HSD-2, which converts estradiol to estrone [55]. However, there is no intrinsic activity of 17β-HSD-2 in ectopic endometrium, which leads to increased levels of estradiol [55]. In regard to inflammation, estrogen induces prostaglandins (PGE2), via increased COX-2 expression, which is known to stimulate aromatase, the enzyme that converts androstenedione to estrone and estradiol. This interplay between PGE2 and aromatase creates a positive feedback loop within ectopic endometrium, resulting in local elevations in both pro-inflammatory PGE2 and estrogens, and thus, endometriotic foci enrich the estrogen in their local environment.
Systemic inflammatory cells, including macrophages and T cells, generally act in an immunoreceptive fashion, producing a predominance of anti-inflammatory type 2 helper T-cell cytokines, as well as TGF-β1, VEGF, and MMPs [56]. Progesterone counteracts both steroid hormone and immune effects on growth and invasion of endometriosis by promoting conversion of estradiol to estrone and by blocking MMP expression [57]. Thus, in endometriosis, a relatively well-understood inflammatory-hormonal modulation has the potential to promote tumor development.
In conclusion, utilizing biomarkers that link inflammation, endometriosis and ovarian cancer, we have further demonstrated an association between the two diseases at the molecular level. Although there were a limited number of patients used for this study, due to the availability of specimens, the relationship between endometriosis and ovarian cancer deserves further evaluation. They are both progressive, estrogen-dependent diseases also associated with early menarche and late menopause, infertility, and nulliparity [58, 59]. Protection against both conditions can be achieved through tubal ligation, oral contraceptives, hysterectomy, and treatment with progesterone [58, 59]. Additionally, endometriosis is characterized by a chronic inflammatory state, which leads to the release of cytokines that promote the growth of tumors by causing unregulated mitotic division, growth, and differentiation.
Footnotes
Capsule
The observations of higher mRNA levels of TGF-β, COX-2, VEGF, ER-α1, AR, and aromatase in endometriosis and ovarian cancer samples supports an association between them.
References
- 1.Diamond M, Osteen K. Adhesion development after endometriosis surgery. Endometrium and Endometriosis: Wiley-Blackwell; 1997
- 2.Hammoud A, Gago LA, Diamond MP. Adhesions in patients with chronic pelvic pain: a role for adhesiolysis? Fertil Steril. 2004;82(6):1483–91. doi: 10.1016/j.fertnstert.2004.07.948. [DOI] [PubMed] [Google Scholar]
- 3.Diamond M, Osteen K. Hormonal (non-danazol and non-GnRH) modulation of endometriosis. Endometrium and Endometriosis: Blackwell Sciences; 1997
- 4.Diamond MP, DeCherney AH. Nafarelin for treatment of endometriosis. N Engl J Med. 1988;319(8):519–20. doi: 10.1056/NEJM198808253190814. [DOI] [PubMed] [Google Scholar]
- 5.Schenken R. Endometriosis. 1989
- 6.Hulka BS. Links between hormone replacement therapy and neoplasia. Fertil Steril. 1994;62(6 Suppl 2):168S–75. [PubMed] [Google Scholar]
- 7.Kempers RD, Dockerty MB, Hunt AB, Symmonds RE. Significant postmenopausal endometriosis. Surg Gynecol Obstet. 1960;111:348–56. [PubMed] [Google Scholar]
- 8.Rattanachaiyanont M, Tanmahasamut P, Angsuwatthana S, Techatraisak K, Inthawiwat S, Leerasiri P. Hormonal replacement therapy in surgical menopause with underlying endometriosis. J Med Assoc Thai. 2003;86(8):702–7. [PubMed] [Google Scholar]
- 9.Barbieri RL. Etiology and epidemiology of endometriosis. Am J Obstet Gynecol. 1990;162(2):565–7. doi: 10.1016/0002-9378(90)90430-f. [DOI] [PubMed] [Google Scholar]
- 10.Deval B, Rafii A, Felce Dachez M, Kermanash R, Levardon M. Sigmoid endometriosis in a postmenopausal woman. Am J Obstet Gynecol. 2002;187(6):1723–5. doi: 10.1067/mob.2002.128394. [DOI] [PubMed] [Google Scholar]
- 11.Djursing H, Petersen K, Weberg E. Symptomatic postmenopausal endometriosis. Acta Obstet Gynecol Scand. 1981;60(5):529–30. doi: 10.3109/00016348109155479. [DOI] [PubMed] [Google Scholar]
- 12.Habuchi T, Okagaki T, Miyakawa M. Endometriosis of bladder after menopause. J Urol. 1991;145(2):361–3. doi: 10.1016/s0022-5347(17)38341-6. [DOI] [PubMed] [Google Scholar]
- 13.Kurioka H, Takahashi K, Okada M, Ozaki T, Miyazaki K, Maruyama R, et al. A case of postmenopausal endometriosis unrelated to neoplasm. Int J Fertil Womens Med. 1999;44(3):160–2. [PubMed] [Google Scholar]
- 14.Punnonen R, Klemi PJ, Nikkanen V. Postmenopausal endometriosis. Eur J Obstet Gynecol Reprod Biol. 1980;11(3):195–200. doi: 10.1016/0028-2243(80)90069-6. [DOI] [PubMed] [Google Scholar]
- 15.Toki T, Horiuchi A, Li SF, Nakayama K, Silverberg SG, Fujii S. Proliferative activity of postmenopausal endometriosis: a histopathologic and immunocytochemical study. Int J Gynecol Pathol. 1996;15(1):45–53. doi: 10.1097/00004347-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Vorstman B, Lynne C, Politano VA. Postmenopausal vesical endometriosis. Urology. 1983;22(5):540–2. doi: 10.1016/0090-4295(83)90238-8. [DOI] [PubMed] [Google Scholar]
- 17.Stern RC, Dash R, Bentley RC, Snyder MJ, Haney AF, Robboy SJ. Malignancy in endometriosis: frequency and comparison of ovarian and extraovarian types. Int J Gynecol Pathol. 2001;20(2):133–9. doi: 10.1097/00004347-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Purdie DM, Bain CJ, Siskind V, Russell P, Hacker NF, Ward BG, et al. Hormone replacement therapy and risk of epithelial ovarian cancer. Br J Cancer. 1999;81(3):559–63. doi: 10.1038/sj.bjc.6690731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oxholm D, Knudsen UB, Kryger-Baggesen N, Ravn P. Postmenopausal endometriosis. Acta Obstet Gynecol Scand. 2007:1–7 [DOI] [PubMed]
- 20.Reimnitz C, Brand E, Nieberg RK, Hacker NF. Malignancy arising in endometriosis associated with unopposed estrogen replacement. Obstet Gynecol. 1988;71(3 Pt 2):444–7. [PubMed] [Google Scholar]
- 21.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Hormone replacement therapy and the risk of invasive epithelial ovarian cancer in Swedish women. J Natl Cancer Inst. 2002;94(7):497–504. doi: 10.1093/jnci/94.7.497. [DOI] [PubMed] [Google Scholar]
- 22.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(Suppl 2):S19–32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 23.Carlson JA, Ambros R, Malfetano J, Ross J, Grabowski R, Lamb P, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29(9):932–48. doi: 10.1016/S0046-8177(98)90198-8. [DOI] [PubMed] [Google Scholar]
- 24.Correa P. Helicobacter pylori as a pathogen and carcinogen. J Physiol Pharmacol. 1997;48(Suppl 4):19–24. [PubMed] [Google Scholar]
- 25.Deeb ZE, Fox LA, deFries HO. The association of chronic inflammatory disease in lichen planus with cancer of the oral cavity. Am J Otolaryngol. 1989;10(5):314–6. doi: 10.1016/0196-0709(89)90105-1. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi PH, Zeldis JB. Molecular biology of viral hepatitis and hepatocellular carcinoma. Compr Ther. 1993;19(5):188–96. [PubMed] [Google Scholar]
- 27.Hogg RP, Ayshford C, Watkinson JC. Parotid duct carcinoma arising in bilateral chronic sialadenitis. J Laryngol Otol. 1999;113(7):686–8. doi: 10.1017/S002221510014486X. [DOI] [PubMed] [Google Scholar]
- 28.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91(17):1459–67. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 29.Risch HA, Howe GR. Pelvic inflammatory disease and the risk of epithelial ovarian cancer. Cancer Epidemiol Biomark Prev. 1995;4(5):447–51. [PubMed] [Google Scholar]
- 30.Rosin MP, Anwar WA, Ward AJ. Inflammation, chromosomal instability, and cancer: the schistosomiasis model. Cancer Res. 1994;54(7 Suppl):1929s–33. [PubMed] [Google Scholar]
- 31.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16(2):217–26. [PubMed] [Google Scholar]
- 32.Society TAF. Revised American fertility society classification of endometriosis: 1985. Fertil Steril. 1985;43(3):351–2. doi: 10.1016/s0015-0282(16)48430-x. [DOI] [PubMed] [Google Scholar]
- 33.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262–3. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 34.Severi FM, Bocchi C, Florio P, Cobellis L, Ignacchiti E, Petraglia F. Transvaginal ultrasonography in women receiving emergency contraception. Fertil Steril. 2003;79(5):1074–7. doi: 10.1016/S0015-0282(02)04962-2. [DOI] [PubMed] [Google Scholar]
- 35.Brinton LA, Lamb EJ, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, et al. Ovarian cancer risk associated with varying causes of infertility. Fertil Steril. 2004;82(2):405–14. doi: 10.1016/j.fertnstert.2004.02.109. [DOI] [PubMed] [Google Scholar]
- 36.Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, et al. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2002;155(3):217–24. doi: 10.1093/aje/155.3.217. [DOI] [PubMed] [Google Scholar]
- 37.Sampson J. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg. 1925;10(1):1–72. [Google Scholar]
- 38.Diamond M, Osteen K. Malignant transformation of endometriosis. Endometrium and Endometriosis: Blackwell Science; 1997
- 39.Mostoufizadeh M, Scully RE. Malignant tumors arising in endometriosis. Clin Obstet Gynecol. 1980;23(3):951–63. doi: 10.1097/00003081-198009000-00024. [DOI] [PubMed] [Google Scholar]
- 40.Seidman JD. Prognostic importance of hyperplasia and atypia in endometriosis. Int J Gynecol Pathol. 1996;15(1):1–9. doi: 10.1097/00004347-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Swiersz LM. Role of endometriosis in cancer and tumor development. Ann NY Acad Sci. 2002;955:281–92. doi: 10.1111/j.1749-6632.2002.tb02788.x. [DOI] [PubMed] [Google Scholar]
- 42.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176(3):572–9. doi: 10.1016/S0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 43.Vercellini P, Parazzini F, Bolis G, Carinelli S, Dindelli M, Vendola N, et al. Endometriosis and ovarian cancer. Am J Obstet Gynecol. 1993;169(1):181–2. doi: 10.1016/0002-9378(93)90159-g. [DOI] [PubMed] [Google Scholar]
- 44.Duczman L, Ballweg M. Endometriosis and cancer: what is the connection?; 1999 Contract No.: Document Number|
- 45.Ness RB, Modugno F. Endometriosis as a model for inflammation-hormone interactions in ovarian and breast cancers. Eur J Cancer. 2006;42(6):691–703. doi: 10.1016/j.ejca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, Wright KA, et al. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995;87(7):506–16. doi: 10.1093/jnci/87.7.506. [DOI] [PubMed] [Google Scholar]
- 48.Ness RB. Endometriosis and ovarian cancer: thoughts on shared pathophysiology. Am J Obstet Gynecol. 2003;189(1):280–94. doi: 10.1067/mob.2003.408. [DOI] [PubMed] [Google Scholar]
- 49.Munkarah AR, Ali-Fehmi R, Jiang JZ, Elhammady E, Malone JM, Jr, Saed GM. The effects of combining docetaxel and cyclooxygenase-2 inhibitors on proliferation and apoptosis in epithelial ovarian cancer. Anticancer Drugs. 2007;18(8):889–96. doi: 10.1097/CAD.0b013e3280cc2b46. [DOI] [PubMed] [Google Scholar]
- 50.Munkarah AR, Morris R, Baumann P, Deppe G, Malone J, Diamond MP, et al. Effects of prostaglandin E(2) on proliferation and apoptosis of epithelial ovarian cancer cells. J Soc Gynecol Investig. 2002;9(3):168–73. doi: 10.1016/S1071-5576(02)00141-7. [DOI] [PubMed] [Google Scholar]
- 51.Heaps JM, Nieberg RK, Berek JS. Malignant neoplasms arising in endometriosis. Obstet Gynecol. 1990;75(6):1023–8. [PubMed] [Google Scholar]
- 52.Helzlsouer KJ, Alberg AJ, Gordon GB, Longcope C, Bush TL, Hoffman SC, et al. Serum gonadotropins and steroid hormones and the development of ovarian cancer. JAMA. 1995;274(24):1926–30. doi: 10.1001/jama.274.24.1926. [DOI] [PubMed] [Google Scholar]
- 53.Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88(4 Pt 1):554–9. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- 54.Cottreau CM, Ness RB, Modugno F, Allen GO, Goodman MT. Endometriosis and its treatment with danazol or lupron in relation to ovarian cancer. Clin Cancer Res. 2003;9(14):5142–4. [PubMed] [Google Scholar]
- 55.Carrell D. Reproductive endocrinology and infertility: integrating modern clinical and laboratory practices: Springer; 2010
- 56.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Hampton AL, Nie G, Salamonsen LA. Progesterone inhibits activation of latent matrix metalloproteinase (MMP)-2 by membrane-type 1 MMP: enzymes coordinately expressed in human endometrium. Biol Reprod. 2000;62(1):85–94. doi: 10.1095/biolreprod62.1.85. [DOI] [PubMed] [Google Scholar]
- 58.Kashyap S, Davis OK. Ovarian cancer and fertility medications: a critical appraisal. Semin Reprod Med. 2003;21(1):65–71. doi: 10.1055/s-2003-39996. [DOI] [PubMed] [Google Scholar]
- 59.Moen MH, Schei B. Epidemiology of endometriosis in a Norwegian county. Acta Obstet Gynecol Scand. 1997;76(6):559–62. doi: 10.3109/00016349709024584. [DOI] [PubMed] [Google Scholar]