Abstract
Purpose
Sperm flow cytometry (SFC) was used to evaluate the association of sperm chromatin condensation and ploidy with fertilization, embryo development, pregnancy and abortion rates following IVF.
Methods
Conventional semen analysis was performed in one hundred fifty men, as well as SFC analysis, after acridine orange and propidium iodide staining, for the evaluation of sperm maturity and ploidy respectively. Conventional IVF was performed in all couples.
Results
Couples with low percentages of mature spermatozoa presented with lower fertilization rates (p < 0.005), lower rates of grade A embryos (p < 0.003) and lower pregnancy rates (p < 0.006), compared to couples with high percentages of mature spermatozoa. Couples with low total aneuploidy rates presented with higher fertilization rates (p < 0.007), higher rates of grade A embryos (p < 0.004) and higher pregnancy rates (p < 0.003), compared to couples with high total aneuploidy rates.
Conclusions
Sperm chromatin condensation and ploidy constitute critical parameters for the evaluation of semen samples before IVF and for the identification of cases in need of ICSI application.
Keywords: Flow cytometry, IVF outcome, Pregnancy rate, Sperm chromatin condensation, Sperm ploidy status
Introduction
Fifteen percent of the couples worldwide face infertility problems, while half of these are affected by male factor infertility [1]. The development of assisted reproduction technologies (ART) has overcome the problem of male infertility, giving in infertile couples the possibility of a pregnancy. Sperm morphology, concentration and motility are mainly taken into account for the selection of the appropriate assisted reproduction technique. Despite normal semen analysis values, a high proportion of couples undergoing ART still fail to achieve a pregnancy. Thus, additional parameters should be used for the evaluation of sperm quality. Genetic and epigenetic sperm abnormalities such as abnormal nuclear packaging, chromosome aneuploidy, sperm DNA damage, genomic imprinting errors and centrosome malformations might contribute to poor embryogenesis [2].
The structural organization of sperm DNA has been found to be vital for the proper functioning of spermatozoa [3], necessitating the characterization of sperm chromatin condensation and stability of semen samples before an ART application. Sperm nuclear chromatin condensation involves a cascade of transcriptional alterations, topological rearrangements, nucleosomal structure loss and histones to protamines substitutions, leading to spermatozoa with almost entirely condensed chromatin in their nucleus [4]. Flaws in the mechanisms that package and protect the sperm chromatin during spermiogenesis lead to sperm chromatin condensation abnormalities [5, 6]. Chromatin condensation and stability have been proposed as reliable markers of sperm maturity [7]. Indeed, the chromatin of spermatozoa from the distal cauda epididymis is more condensed than that from spermatozoa of the proximal sites of epididymis [8].
Chromosome aneuploidies are more frequent in patients suffering from spermatogenetic failures, such as severe oligospermia or azoospermia [9]. The development of fluorescent in situ hybridization (FISH), permitting the cytogenetic analysis of large numbers of spermatozoa, has revealed significantly increased sperm aneuploidy rates in azoospermic patients compared to normozoospermic men [10, 11]. Apart from men with aberrant karyotype, who are expected to have spermatozoa with chromosomal abnormalities, men with normal somatic karyotype are often presented with aneuploid gametes [12]. The aneuploidy of these gametes may be ascribed to an altered intratesticular environment that disrupts the chromosome segregation mechanisms [13]. Many studies have reported an inverse correlation between sperm aneuploidy rates and conventional semen parameters [14–19], confirming the association of chromosomal abnormalities with poor sperm quality.
Sperm flow cytometry (SFC) after Acridine Orange (AO) and Propidium Iodide (PI) staining has been proposed as a reliable method for the evaluation of sperm chromatin condensation [20] and ploidy [21], respectively. These parameters have been recently associated with sperm morphology and motility as well as with intrauterine insemination (IUI) success rates [22]. In the current study, we used SFC analysis to investigate the putative effects of sperm nuclear chromatin condensation and ploidy on spermatozoa fertilizing capacity, embryo quality, pregnancy and spontaneous abortion rates after conventional in vitro fertilization (IVF).
Materials and methods
Subjects
The study population consisted of 150 men, aged 28–46 years, who referred to the IVF Unit of the Department of Obstetrics and Gynecology of Ioannina Medical School for conventional IVF, following three or more failed IUI attempts.
A detailed medical history was obtained from all subjects. Physical examination was performed. Men carrying microdeletions of the long arm of the Y chromosome or karyotype abnormalities, men suffering from varicocele, hydrocele, hypogonadotropic hypogonadism and obstructive syndromes of the seminal tract as well as men under spermatogenesis-impairing medication were excluded from the study. Partners of women over 37 years old, or women suffering from endometriosis and severe congenital malformations of the reproductive organs were also excluded from the study.
Semen analysis was performed according to World Health Organization [23] guidelines. Men abstained from sexual activity for 3 days prior to semen analysis. Two independent investigators performed blind semen analysis at the day of oocyte pick-up. The average values of the two investigators were calculated. In the event of inconsistency (over 10% difference) a third assessment was done. The morphology was evaluated after Papanicolaou staining.
All sperm samples were processed with sperm gradient kit and sperm preparation medium (Medicult, Jyllinge, Denmark) for the IVF procedure. All women of the study population underwent a long GnRH agonist stimulation protocol as previously described [24]. Conventional IVF was performed in all couples.
The Institutional Ethics Committee approved the study protocol in accordance to the Helsinki declaration and all participants gave informed consent.
Primary IVF outcome measures
The fertilization rate, the embryo quality and the clinical pregnancy rate were the primary outcome measures. The fertilization rate, namely the percentage of oocytes with two formed pronuclears, was recorded 24 h after oocyte retrieval. Embryo development and quality were evaluated 3 days after oocyte pick-up. The number of blastomeres and the proportion of embryo volume occupied by fragments were used for the evaluation. Embryos with <10%, <10%–20%, <20%–30% and >30% fragments were recorded as grade A,B,C and D, respectively. Three embryos with the highest blastomere number and the best morphology were transferred the third day of each cycle.
Pregnancy was diagnosed by quantitative β-hCG 2 weeks after embryo transfer. Clinical pregnancy was confirmed by observing fetal cardiac activity on transvaginal ultrasound 4 weeks after a positive pregnancy test.
Flow cytometry analysis after Acridine Orange and Propidium Iodide stainings
The methods of AO and PI stainings in combination with SFC were used to study the abnormal chromatin condensation and the ploidy of human spermatozoa, respectively, according to protocols previously described [25, 26]. Briefly, two semen samples from each patient were used for the analysis, so as to ensure reliable results. The FACScalibur Flow Cytometer and the Consort 40 (Becton Dickinson, San Jose, CA, USA) were used for the resulting data acquisition and analysis. In case of AO staining, green fluorescence (BP 530/30 filter) and red fluorescence (BP 650LP filter) were measured for 30,000 cells per sample after excitation with a 488 nm argon laser, while in case of PI staining, red fluorescence (BP 650LP filter) emitted from individual cells was recorded for 10,000 cells per sample after excitation with a 488 nm argon laser.
Statistical analysis
Statistical analysis was performed using the chi-square test. Normal distribution of continuous parameters was tested by Kolmogorov-Smirnov test. Differences in continuous parameters were assessed by using t-test for independent variables. P-value of <0.05 was set as statistically significant. All analyses used the SPSS statistical package (version 14.0, SPSS Inc, Chicago, IL, USA).
Results
Study population characteristics
Our study population consisted of 150 men from equal number of couples undergoing IVF. These men had sperm concentration 75.2 ± 32.7 × 106 spermatozoa/ml, sperm motility 54.1 ± 10.9% and normal morphology 28.3 ± 7.9%.
Sperm chromatin condensation analysis
All semen samples emitted both green and red fluorescence, at different intensities in each case, after AO staining and SFC analysis. AO staining produces green fluorescence when it binds double-stranded nucleic acids and red fluorescence when it binds single-stranded nucleic acids [27]. The intensities of green and red fluorescence were significantly increased in semen samples characterized by increased percentages of spermatozoa possessing abnormal morphology such as head defects (large, small, round, pyriform, amorphous, small acrosomal area and tapered heads), midpiece defects (thick, abnormally thin and irregular midpieces) and tail defects (short, multiple, broken, hairpin and irregular wide tails) (data not shown). In contrast, no associations were observed between these fluorescence values and sperm concentration and motility.
Taking into account that green and red fluorescent intensities are inversely correlated with sperm chromatin condensation and maturation [25], we estimated for each semen sample the percentage of cells with fully condensed chromatin, namely the percentage of mature spermatozoa. This rate was calculated as 100(WINM)/(WINT), where window M corresponded to spermatozoa with completed epididymal sperm maturation; window T to the major sperm band, excluding cell debris and satellite populations [22, 25]. The median percentage of mature spermatozoa in our study population was 65.1%, while the rates ranged from 41.3% to 83.8%.
Sperm ploidy analysis
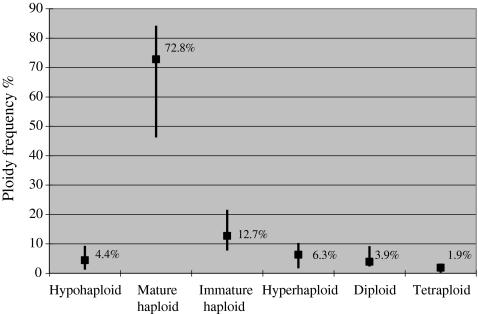
The spermatozoa ploidy analysis revealed peaks of hypohaploid, normal haploid, immature haploid, hyperhaploid, diploid, and tetraploid cells in the histograms of all samples. These values were found to differ between semen samples. The median values and ranges of every ploidy are presented in Fig. 1. The median total sperm aneuploidy rate in our study population, which was estimated excluding immature haploid cells, was 16.7% while the frequencies ranged from 5.1 to 31.1%.
Fig. 1.
The sperm ploidy status analysis of the study population. Data are shown as median value and range
Association of sperm chromatin condensation with fertilization, pregnancy rates and embryo quality
To analyze the effects of sperm chromatin condensation on IVF outcome, the study population was subdivided in subgroups using as cut off the median percentage of mature spermatozoa: men with percentage of mature spermatozoa <65.1% (group A1) and men with percentage of mature spermatozoa ≥65.1% (group A2).
Couples of group A1 presented with lower fertilization and pregnancy rates compared to couples of group A2 (Table 1). Specifically, statistical significant differences were observed in the fertilization rates between couples of group A1 and couples of group A2 (group A1: 60.7 ± 19.8% vs. group A2: 79.4 ± 19.1%, p < 0.005) and in the pregnancy rates (group A1: 19.4% vs. group A2: 39.7%, p < 0.006).
Table 1.
Association of fertilization, pregnancy, abortion rates and embryo quality with sperm chromatin condensation
| Group A1 | Group A2 | p value | |
|---|---|---|---|
| Number of patients | 72 | 78 | |
| Age (years) | 35.1 ± 6.7 | 34.4 ± 6.5 | nsa |
| Sperm concentration (×106 spermatozoa/ml) | 72.9 ± 30.8 | 71.4 ± 30.1 | nsa |
| Sperm motility (%) | 52.9 ± 8.8 | 50.2 ± 8.4 | nsa |
| Normal sperm morphology (%) | 27.2 ± 6.3 | 30.1 ± 8.7 | nsa |
| Number of oocytes | 14 ± 6.1 | 12.5 ± 5.7 | nsa |
| Fertilization rate (%) | 60.7 ± 19.8 | 79.4 ± 19.1 | 0.005a |
| Grade A embryos (%) | 19.9 ± 8.4 | 35.7 ± 10.1 | 0.003a |
| Grade B embryos (%) | 20.2 ± 9.3 | 29.5 ± 8.8 | nsa |
| Grade C embryos (%) | 38.1 ± 11.9 | 20.1 ± 9.2 | 0.002a |
| Grade D embryos (%) | 18.2 ± 6.6 | 11.3 ± 5.4 | nsa |
| Pregnancy rate (%) | 19.4 | 39.7 | 0.006b |
| Abortion rate (%) | 33.3 | 21.7 | nsb |
Group A1: percentage of mature spermatozoa <65.1%, Group A2: percentage of mature spermatozoa ≥65.1%.
at-test analysis
bChi square test analysis
In addition, couples of group A1 presented lower embryo quality compared to couples of group A2 (Table 1). Specifically, couples of group A1 had lower rates of grade A embryos than couples of group A2 (group A1: 19.9 ± 8.4% vs. group A2: 35.7 ± 10.1%; p < 0.003) and higher rates of grade C embryos (group A1: 38.1 ± 11.9% vs. group A2: 20.1 ± 9.2%; p < 0.002).
Finally, the spontaneous abortion rate, which was estimated to be 25%, differed between the two groups. In specific, couples of group A1 presented with higher spontaneous abortion rate compared to couples of group A2 (group A1: 33.3% vs. group A2: 21.7%). However, no statistical significance was observed in the above difference (Table 1).
Association of sperm ploidy with fertilization, pregnancy rates and embryo quality
To analyze the effects of sperm ploidy on IVF outcome, the study population was subdivided in subgroups using as cut off the median total aneuploidy rate: men with total aneuploidy rate <16.7% (group B1) and men with total aneuploidy rate ≥16.7% (group B2).
Couples of group B1 presented higher fertilization and pregnancy rates compared to couples of group B2 (Table 2). Specifically, statistical significant differences were observed in fertilization rates between couples of group B1 and couples of group B2 (group B1: 77.2 ± 20.9% vs. group B2: 58.7 ± 18.8%, p < 0.007) and in the respective pregnancy rates (group B1: 42.1% vs. group B2: 20.2%, p < 0.003).
Table 2.
Association of fertilization, pregnancy, abortion rates and embryo quality with sperm ploidy status
| Group B1 | Group B2 | p value | |
|---|---|---|---|
| Number of patients | 76 | 74 | |
| Age (years) | 34.1 ± 7.2 | 34.8 ± 6.9 | nsa |
| Sperm concentration (×106 spermatozoa/ml) | 69.3 ± 34.9 | 74.8 ± 33.1 | nsa |
| Sperm motility (%) | 51 ± 9.1 | 52.7 ± 8.9 | nsa |
| Normal sperm morphology (%) | 30.6 ± 5.3 | 25.2 ± 4.2 | nsa |
| Number of oocytes | 12.7 ± 5.3 | 13.8 ± 6.2 | nsa |
| Fertilization rate (%) | 77.2 ± 20.9 | 58.7 ± 18.8 | 0.007a |
| Grade A embryos (%) | 33.1 ± 11.3 | 18.3 ± 6.8 | 0.004a |
| Grade B embryos (%) | 28.9 ± 9.1 | 21.1 ± 9.8 | nsa |
| Grade C embryos (%) | 19.8 ± 9.5 | 38.4 ± 12 | 0.002a |
| Grade D embryos (%) | 10 ± 5.7 | 18.5 ± 6.9 | nsa |
| Pregnancy rate (%) | 42.1 | 20.2 | 0.003b |
| Abortion rate (%) | 24 | 28.6 | nsb |
Group B1: total sperm aneuploidy rate <16.7%, Group B2: total sperm aneuploidy rate ≥16.7%.
at-test analysis
bChi square test analysis
In addition, couples of group B1 presented higher embryo quality compared to couples of group B2 (Table 2). Specifically, couples of group B1 had higher rates of grade A embryos than couples of group B2 (group B1: 33.1 ± 11.3% vs. group B2: 18.3 ± 6.8%; p < 0.004) and lower rates of grade C embryos (group B1: 19.8 ± 9.5% vs. group B2: 38.4 ± 12%; p < 0.002).
Finally, the spontaneous abortion rate, which was estimated to be 25%, differed between the two groups. In specific, couples of group B1 presented with lower spontaneous abortion rate compared to couples of group B2 (group B1: 24% vs. group B2: 28.6%). However, no statistical significance was observed in the above difference (Table 1).
Discussion
The aim of the current study was to investigate the contribution of sperm nuclear chromatin condensation and ploidy on IVF primary outcomes. SFC analysis after AO and PI staining was used in semen samples of couples undergoing conventional IVF treatment. The fertilization rates, the embryo quality and the pregnancy rates were inversely associated with semen samples immaturity and aneuploidy rates. In contrast, no significant associations were observed between IVF primary outcomes and semen samples concentration or motility. Similar associations have been reported in a recent study of our group, examining men with “gray zone” sperm concentration undergoing IUI [22]. Namely, significantly lower pregnancy rates have been observed, when semen samples with increased aneuploidy rates and low sperm maturity have been used.
AO staining followed by SFC [25] was performed to estimate the percentage of sperm cells with complete epididymal maturation and totally condensed nuclear chromatin. The progressive chromatin packaging produces a reduction in DNA stainability relative to round spermatids and a marked decrease in AO binding to DNA as human spermatozoa traverse the epididymis [8]. Lack of appropriate sperm maturation results in an increased DNA stainability [28], frequently observed in many ejaculated spermatozoa from infertile men [6]. In our series, significant increases in green and red fluorescence values were positively correlated with increased percentages of spermatozoa with abnormal morphology due to their uncondensed nuclear chromatin. The increased intensity of green fluorescence is perhaps the result of an abnormal exchange of histone for transition proteins and protamines [9]. But an abnormal exchange would probably lead to immature spermatozoa, incapable to cause pronuclear formation after oocytes penetration. Indeed, Sakkas and co-workers [29], who examined the effects of human spermatozoan chromatin anomalies on fertilization after intracytoplasmic sperm injection (ICSI), suggested that abnormal chromatin might fail to decondense due to a physical or mechanical inability, leading to impaired fertilization. Furthermore, taking into account that protamine addition according to a specific ratio is crucial for fertilization and embryogenesis [30, 31] as well as that protamine deficiency [32] and absence [33] have been associated with significant impairments in fertilization, we could explain why semen samples with abnormal chromatin packaging and consequently decreased maturity show lower fertilization rates after conventional IVF.
Abnormal sperm nuclear chromatin condensation, apart from fertilization, has been shown to affect embryo quality and pregnancy rates following IVF. In previous studies, similar associations have been observed. Specifically, the increased DNA breaks due to faulty replacement of histones by protamines [5] have been associated with decreased embryo morphology at early cleavage stages [34], whereas the improper protamine ratios have been proposed to be responsible for poor preimplantation stage embryo morphologies [30]. These embryos have been shown to arrest at the six- to eight-cell stage, coinciding with full activation of the embryonic genome. The impaired sperm maturation has also been associated with decreased pregnancy rates [35–37], failure of embryos to progress to the blastocyst stage in culture [38] and low pregnancy rates after ICSI treatment [29]. Furthermore, the spontaneous abortion rates of our study population, which were similar with those observed in previous studies [39], were increased in cases of semen samples with low percentages of mature spermatozoa. However, no statistical significance was observed in the above association. Larger series are needed to confirm or to disprove this possible inverse association. In conclusion, we could hypothesize that the increased sperm DNA breaks and the abnormal nuclear chromatin structure, markers of an altered sperm genetic constitution, are probably responsible for the impaired embryo quality and consequently for the low implantation rates after an IVF.
On the other hand, the primary IVF outcomes have been inversely associated with sperm aneuploidy. Sperm aneuploidy arises due to nondisjunctions during metaphase I or II of meiosis. FISH development has revealed high aneuploidy rates in human spermatozoa [40], mainly in those obtained from subfertile and infertile men [15, 41], proving that sperm chromosomal abnormalities and aneuploidy rates constitute indicators of impaired sperm quality. In the current study, semen samples characterized by increased hypohaploid, hyperhaploid, diploid, and tetraploid rates led to low fertilization rates after conventional IVF. Similar results have been observed in a previous study [42], where semen samples with increased aneuploidy rates were used in ICSI. The impaired fertilization, which was observed [42], ensures the inverse association of sperm aneuploidy with fertilization.
Conventional IVF treatment of semen samples with increased aneuploidy rates also led to lower embryo quality and to decreased pregnancy rates. These results are reinforced by a previous study suggesting an association of sperm aneuploidy with an altered embryonic genetic constitution and morphology [43]. Additionally, sperm cell aneuploidy has been associated with low implantation rates [42, 44], low pregnancy rates [42, 45, 46] and increased possibilities of miscarriage in IVF cycles where the best morphologically embryos were transferred [47]. Indeed, in our study population, increased rates of spontaneous abortions were observed in cases of semen samples with high sperm cell aneuploidy. However, no statistical significance was observed in the above association. More cases are possibly needed to verify this finding. Finally, the fact that ICSI patients whose partners did not achieve a pregnancy had higher sperm aneuploidy rates compared to patients whose partners became pregnant [48], indicates that oocyte fertilization can be accomplished by aneuploid spermatozoa, but it results in lower pregnancy rates. Consequently, we could suggest that sperm aneuploidy probably does not affect the embryonic growth initiation, but seems to be responsible for a decreased implantation and live birth potential.
Taking into consideration the above analysis, we could assume that sperm nuclear chromatin condensation and ploidy constitute critical parameters for a successful conventional IVF outcome. Although swim-up and Percoll fractionation techniques generally increase the quality of semen samples used in IVF [25], a high proportion of attempts still fail to achieve a successful outcome. Given that SFC offers an aneuploidy screening of all chromosomes, while FISH technique analyses specific chromosomes overlooking a significant amount of aneuploidies [14, 49], as well as that SFC is a less time and money consuming method compared to FISH, we could suggest SFC for a better evaluation of sperm quality. This technique would help us discriminate which ART technique, conventional IVF or ICSI, is appropriate so as to increase the possibility of a successful outcome, even when sperm concentration and motility are within normal range according to WHO criteria.
This is the first study, to our knowledge, to associate simultaneously sperm chromatin condensation and ploidy with the IVF outcome. However, larger series are needed to verify our preliminary results. Whereas the construction of ‘Receiver Operating Characteristic’ curves (ROC curves) for the percentage of mature spermatozoa and for the total aneuploidy rate, individually and combined, will ensure their value in predicting the fertilization rates, the embryo quality and the pregnancy rates. SFC analysis may help in the discrimination of semen samples with high fertilizing capacity and elevated pregnancy potential after a conventional IVF as well as in the identification of the cases in need of ICSI application, reducing the number of unsuccessful assisted reproduction treatments to a minimum.
Acknowledgments
Authors have no conflicts of interest or any financial support to declare.
Footnotes
Capsule
Sperm nuclear chromatin condensation and ploidy constitute critical parameters for the evaluation of semen samples before in vitro fertilization.
References
- 1.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, Lopes P, Tabaste JM, Spira A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989) Hum Reprod. 1991;6:811–816. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 2.Emery BR, Carrell DT. The effect of epigenetic sperm abnormalities on early embryogenesis. Asian J Androl. 2006;8(2):131–142. doi: 10.1111/j.1745-7262.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 3.Ward WS, Coffey DS. DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells. Biol Reprod. 1991;44:569–574. doi: 10.1095/biolreprod44.4.569. [DOI] [PubMed] [Google Scholar]
- 4.Kramer JA, Krawetz SA. Determining the potentiative state of a chromatin domain. Biotechniques. 1997;22:879–882. doi: 10.2144/97225bm22. [DOI] [PubMed] [Google Scholar]
- 5.Manicardi GC, Bianchi PG, Pantano S, Azzoni P, Bizzaro D, Bianchi U, Sakkas D. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod. 1995;52:864–867. doi: 10.1095/biolreprod52.4.864. [DOI] [PubMed] [Google Scholar]
- 6.Sakkas D, Manicardi G, Bianchi PG, Bizzaro D, Bianchi U. Relationship between the presence of endogenous nicks and sperm chromatin packaging in maturing and fertilizing mouse spermatozoa. Biol Reprod. 1995;52(5):1149–1155. doi: 10.1095/biolreprod52.5.1149. [DOI] [PubMed] [Google Scholar]
- 7.Roux C, Dadoune JP. Use of acridine orange staining on smears of human spermatozoa after heat-treatment: evaluation of the chromatin condensation. Andrologia. 1989;21:275–281. doi: 10.1111/j.1439-0272.1989.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 8.Golan R, Cooper TG, Oschry Y, Oberpenning F, Schulze H, Shochat L, Lewin LM. Changes in chromatin condensation of human spermatozoa during epididymal transit as determined by flow cytometry. Hum Reprod. 1996;11:1457–1462. doi: 10.1093/oxfordjournals.humrep.a019419. [DOI] [PubMed] [Google Scholar]
- 9.Braekeleer M, Dao TN. Cytogenetic studies in couples experiencing repeated pregnancy losses. Hum Reprod. 1990;5(5):519–528. doi: 10.1093/oxfordjournals.humrep.a137135. [DOI] [PubMed] [Google Scholar]
- 10.Palermo GD, Colombero LT, Hariprashad JJ, Schlegel PN, Rosenwaks Z. Chromosome analysis of epididymal and testicular sperm in azoospermic patients undergoing ICSI. Hum Reprod. 2002;17:570–575. doi: 10.1093/humrep/17.3.570. [DOI] [PubMed] [Google Scholar]
- 11.Mateizel I, Verheyen G, Assche E, Tournaye H, Liebaers I, Steirteghem A. FISH analysis of chromosome X, Y and 18 abnormalities in testicular sperm from azoospermic patients. Hum Reprod. 2002;17:2249–2257. doi: 10.1093/humrep/17.9.2249. [DOI] [PubMed] [Google Scholar]
- 12.Breekeleer M, Dao TN. Cytogenetic studies in male infertility: a review. Hum Reprod. 1991;6:245–250. [PubMed] [Google Scholar]
- 13.Calogero AE, Burrello N, Palma A, Barone N, D’Agata R, Vicari E. Sperm aneuploidy in infertile men. Reprod Biomed Online. 2003;6(3):310–317. doi: 10.1016/S1472-6483(10)61850-0. [DOI] [PubMed] [Google Scholar]
- 14.Pang MG, Kim YJ, Lee SH, Kim CK. The high incidence of meiotic errors increases with decreased sperm count in severe male factor infertilities. Hum Reprod. 2005;20:1688–1694. doi: 10.1093/humrep/deh817. [DOI] [PubMed] [Google Scholar]
- 15.Calogero AE, Palma A, Grazioso C, Barone N, Romeo R, Rappazzo G, D’Agata R. Aneuploidy rate in spermatozoa of selected men with abnormal semen parameters. Hum Reprod. 2001;16:1172–1179. doi: 10.1093/humrep/16.6.1172. [DOI] [PubMed] [Google Scholar]
- 16.Vegetti W, Assche E, Frias A, Verheyen G, Bianchi MM, Bonduelle M, Liebaers I, Steirteghem A. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in situ hybridization in infertile men. Hum Reprod. 2000;15:351–365. doi: 10.1093/humrep/15.2.351. [DOI] [PubMed] [Google Scholar]
- 17.Bernardini LM, Calogero AE, Bottazzi C, Lanteri S, Venturini PL, Burrello N, Palma A, Conte N, Ragni N. Low total normal motile count values are associated with increased sperm disomy and diploidy rates in infertile patients. Int J Androl. 2005;28:328–336. doi: 10.1111/j.1365-2605.2005.00548.x. [DOI] [PubMed] [Google Scholar]
- 18.Ushijima C, Kumasako Y, Kihaile PE, Hirotsuru K, Utsunomiya T. Analysis of chromosomal abnormalities in human spermatozoa using multicolour fluorescence in-situ hybridization. Hum Reprod. 2000;15:1107–1111. doi: 10.1093/humrep/15.5.1107. [DOI] [PubMed] [Google Scholar]
- 19.Burrello N, Arcidiacono G, Vicari E, Asero P, Benedetto D, Palma A, Romeo R, D’Agata R, Calogero AE. Morphologically normal spermatozoa of patients with secretory oligo-astheno-teratozoospermia have an increased aneuploidy rate. Hum Reprod. 2004;19:2298–2302. doi: 10.1093/humrep/deh438. [DOI] [PubMed] [Google Scholar]
- 20.Evenson DP, Melamed MR. Rapid analysis of normal cell types in human semen and testis biopsies by flow cytometry. J Histochem Cytochem. 1983;31:248–253. doi: 10.1177/31.1A_Suppl.6186729. [DOI] [PubMed] [Google Scholar]
- 21.Spanò M, Evenson DP. Flow cytometric analysis for reproductive biology. Biol Cell. 1993;78:53–62. doi: 10.1016/0248-4900(93)90114-T. [DOI] [PubMed] [Google Scholar]
- 22.Lazaros L, Kaponis A, Vartholomatos G, Hatzi E, Botsari S, Plachouras N, Makrydimas G, Zikopoulos K, Sofikitis N, Georgiou I. Using semen flow cytometry to evaluate association of ploidy status and chromatin condensation of spermatozoa with conventional semen parameters: clinical application in intrauterine insemination. Fertil Steril. 2011;95(1):110–115. doi: 10.1016/j.fertnstert.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Laboratory manual for the examination of human semen and sperm–cervical mucus interaction, 4th edn. Cambridge University Press; 1999.
- 24.Hatzi E, Bouba I, Galidi A, Lazaros L, Xita N, Sakaloglou P, Kolios G, Bairaktari E, Kaponis A, Zikopoulos K, Tsatsoulis A, Georgiou I. Association of serum and follicular SHBG levels and SHBG (TAAAA)n polymorphism with follicle size in women undergoing ovarian stimulation. Gynecol Endocrinol. 2011;27(1):27–32. doi: 10.3109/09513590.2010.493961. [DOI] [PubMed] [Google Scholar]
- 25.Golan R, Shochat L, Weissenberg R, Soffer Y, Marcus Z, Oschry Y, Lewin LM. Evaluation of chromatin condensation in human spermatozoa: a flow cytometric assay using Acridine Orange staining. Mol Hum Reprod. 1997;3:47–54. doi: 10.1093/molehr/3.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Levek-Motola N, Soffer Y, Shochat L, Raziel A, Lewin LM, Golan R. Flow cytometry of human semen: a preliminary study of a non-invasive method for the detection of spermatogenetic defects. Hum Reprod. 2005;20:3469–3475. doi: 10.1093/humrep/dei247. [DOI] [PubMed] [Google Scholar]
- 27.Evenson D, Darzynkiewicz Z, Jost L, Janca F, Ballachey B. Changes in accessibility of DNA to various fluorochromes during spermatogenesis. Cytometry. 1986;7:45–53. doi: 10.1002/cyto.990070107. [DOI] [PubMed] [Google Scholar]
- 28.Evenson D, Jost L. Sperm chromatin structure assay: DNA denaturability. Methods Cell Biol. 1994;42:159–176. doi: 10.1016/S0091-679X(08)61073-0. [DOI] [PubMed] [Google Scholar]
- 29.Sakkas D, Urner F, Bianchi PG, Bizzaro D, Wagner I, Jaquenoud N, Manicardi G, Campana A. Sperm chromatin anomalies can influence decondensation after intracytoplasmic sperm injection. Hum Reprod. 1996;11:837–843. doi: 10.1093/oxfordjournals.humrep.a019263. [DOI] [PubMed] [Google Scholar]
- 30.Aoki VW, Liu L, Carrell DT. Identification and evaluation of a novel sperm protamine abnormality in a population of infertile males. Hum Reprod. 2005;20:1298–1306. doi: 10.1093/humrep/deh798. [DOI] [PubMed] [Google Scholar]
- 31.Cho C, Jung-Ha H, Willis WD, Goulding EH, Stein P, Xu Z, Schultz RM, Hecht NB, Eddy EM. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol Reprod. 2003;69:211–217. doi: 10.1095/biolreprod.102.015115. [DOI] [PubMed] [Google Scholar]
- 32.Lolis D, Georgiou I, Syrrou M, Zikopoulos K, Konstantelli M, Messinis I. Chromomycin A3-staining as an indicator of protamine deficiency and fertilization. Int J Androl. 1996;19:23–27. doi: 10.1111/j.1365-2605.1996.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 33.Carrell DT, Liu L. Altered protamine 2 expression is uncommon in donors of known fertility, but common among men with poor fertilizing capacity, and may reflect other abnormalities of spermiogenesis. J Androl. 2001;22:604–610. [PubMed] [Google Scholar]
- 34.Virant-Klun I, Tomazevic T, Meden-Vrtovec H. Sperm single-stranded DNA, detected by acridine orange staining, reduces fertilization and quality of ICSI-derived embryos. J Assist Reprod Genet. 2002;19:319–328. doi: 10.1023/A:1016006509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshi K, Katayose H, Yanagida K, Kimura Y, Sato A. The relationship between acridine orange fluorescence of sperm nuclei and the fertilizing ability of human sperm. Fertil Steril. 1996;66:634–639. doi: 10.1016/s0015-0282(16)58581-1. [DOI] [PubMed] [Google Scholar]
- 36.Esterhuizen AD, Franken DR, Lourens JG, Prinsloo E, Rooyen LH. Sperm chromatin packaging as an indicator of in-vitro fertilization rates. Hum Reprod. 2000;15:657–661. doi: 10.1093/humrep/15.3.657. [DOI] [PubMed] [Google Scholar]
- 37.Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19:1401–1408. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 38.Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82:378–383. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 39.Sala GB, Nicoli A, Villani MT, Gallinelli A, Nucera G, Blickstein I. Spontaneous embryonic loss rates in twin and singleton pregnancies after transfer of top- versus intermediate-quality embryos. Fertil Steril. 2005;84(6):1602–1605. doi: 10.1016/j.fertnstert.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 40.Spriggs EL, Rademaker AW, Martin RH. Aneuploidy in human sperm: the use of multicolor FISH to test various theories of nondisjunction. Am J Hum Genet. 1996;58:356–362. [PMC free article] [PubMed] [Google Scholar]
- 41.Martin RH, Rademaker AW, Greene C, Ko E, Hoang T, Barclay L, Chernos J. A comparison of the frequency of sperm chromosome abnormalities in men with mild, moderate, and severe oligozoospermia. Biol Reprod. 2003;69:535–539. doi: 10.1095/biolreprod.102.015149. [DOI] [PubMed] [Google Scholar]
- 42.Pang MG, Hoegerman SF, Cuticchia AJ, Moon SY, Doncel GF, Acosta AA, Kearns WG. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod. 1999;14:1266–1273. doi: 10.1093/humrep/14.5.1266. [DOI] [PubMed] [Google Scholar]
- 43.Durakbasi-Dursun HG, Zamani AG, Kutlu R, Görkemli H, Bahce M, Acar A. A new approach to chromosomal abnormalities in sperm from patients with oligoasthenoteratozoospermia: detection of double aneuploidy in addition to single aneuploidy and diploidy by five-color fluorescence in situ hybridization using one probe set. Fertil Steril. 2008;89:1709–1717. doi: 10.1016/j.fertnstert.2007.06.050. [DOI] [PubMed] [Google Scholar]
- 44.Rubio C, Gil-Salom M, Simon C, Vidal F, Rodrigo L, Minguez Y, Remohí J, Pellicer A. Incidence of sperm chromosomal abnormalities in a risk population: relationship with sperm quality and ICSI outcome. Hum Reprod. 2001;16:2084–2092. doi: 10.1093/humrep/16.10.2084. [DOI] [PubMed] [Google Scholar]
- 45.Pfeffer J, Pang MG, Hoegerman SF, Osgood CJ, Stacey MW, Mayer J, Oehninger S, Kearns WG. Aneuploidy frequencies in semen fractions from ten oligoasthenoteratozoospermic patients donating sperm for intracytoplasmic sperm injection. Fertil Steril. 1999;72:472–478. doi: 10.1016/S0015-0282(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 46.Burrello N, Vicari E, Shin P, Agarwal A, Palma A, Grazioso C, D’Agata R, Calogero AE. Lower sperm aneuploidy frequency is associated with high pregnancy rates in ICSI programmes. Hum Reprod. 2003;18:1371–1376. doi: 10.1093/humrep/deg299. [DOI] [PubMed] [Google Scholar]
- 47.Carrell DT, Liu L, Peterson CM, Jones KP, Hatasaka HH, Erickson L, Campbell B. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch Androl. 2003;49:49–55. doi: 10.1080/01485010290099390. [DOI] [PubMed] [Google Scholar]
- 48.Calogero AE, Palma A, Grazioso C, Barone N, Burrello N, Palermo I, Gulisano A, Pafumi C, D’Agata R. Sperm aneuploidy rate in unselected infertile patients and its relationship with intracytoplasmic sperm injection outcome. Hum Reprod. 2001;16:1433–1439. doi: 10.1093/humrep/16.7.1433. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigo L, Rubio C, Mateu E, Simón C, Remohí J, Pellicer A, Gil-Salom M. Analysis of chromosomal abnormalities in testicular and epididymal spermatozoa from azoospermic ICSI patients by fluorescence in-situ hybridization. Hum Reprod. 2004;19:118–123. doi: 10.1093/humrep/deh012. [DOI] [PubMed] [Google Scholar]