Abstract
Objective
To evaluate the relationship between different hCG priming-to-oocyte retrieval intervals and assisted reproductive technology (ART) outcome.
Methods
We systematically searched PubMed, EMBASE, the Cochrane Library, Science Citation Index, Chinese biomedicine (CBM) literature database, and Chinese Journal Full-text Database for randomized controlled trials (RCTs) published up to November 2010. Data was extracted from the studies by two independent reviewers. Statistical analysis was performed with Cochrane Collaboration’s Review Manager (RevMan) 5.0.2. From extracted data, Risk Ratio (RR) with 95% confidence interval (CI) was calculated.
Results
5 RCTs totaling 895 participants were included. Oocyte maturation rate was higher in the long interval group compared with short interval group (RR, 0.67; 95% CI, 0.62–0.73). There were no significant difference between the two groups with regard to fertilization rate (RR, 0.99; 95% CI, 0.94–1.04), implantation rate (RR, 0.91; 95% CI, 0.40–2.04), and pregnancy rate (RR, 0.79; 95% CI, 0.58–1.08).
Conclusion
The percentage of mature (MII) oocytes can be increased by prolonging the interval between hCG priming and oocyte retrieval. The prolonged interval could not increase the fertilization rate, implantation rate, and pregnancy rate. Although there was evidence to confirm the results, they still need to be confirmed by large-sample, multicenter, randomized controlled trials. The time interval dependent mechanisms responsible for ART performance need to be elucidated.
Keywords: Human chorionic gonadotropin, Oocyte retrieval, Time interval, Infertility, Assisted reproductive technology, Meta-analysis
Introduction
During controlled ovarian hyperstimulation (COH), the natural endogenous Luteinizing Hormone (LH) surge often does not appear, or has appeared with improper timing and magnitude. Therefore, exogenous gonadotropin is needed to replace the endogenous LH surge. The most commonly used exogenous LH is human chorionic gonadotropin (hCG), which simulates the physiologic effects of LH, and is used to trigger the final follicular maturation before oocyte retrieval in ART program [1]. The interval between hCG priming and oocyte retrieval is very important, because a series of crucial processes, such as the start of luteinization, expansion of cumulus cells, and the resumption of oocyte meiosis are accomplished in the interval [2]. Many factors, such as the final products of renin angiotensin system, angiotensin II, vascular endothelial growth factor (VEGF), interleukin I (IL-1), IL-6, IL-8, angiopoietin, insulin-like growth factor (IGF), basic fibroblast growth factor, and endothelin are also involved in follicular development, oocyte maturation, fertilization, and embryo development. All of these substances have an effect in a time-dependent manner after hCG priming [3–5]. For example, VEGF can increase follicular vascularization and thus optimize dissolved oxygen content in follicle and consequently increase follicular maturation and oocyte quality. The follicular fluid VEGF level was significantly higher in the hCG +38 h oocyte retrieval group compared with the hCG +34 h oocyte retrieval group (2276.0 ± 790.1 versus 1946.6 ± 954.5 pg/ml, P < 0.001) [5]. On the day of hCG administration, serum IL-6 concentration was 8.40 ± 6.68 pg/ml, and 7.10 ± 0.68 pg/ml on the day of oocyte retrieval [6]. So the optimal time interval between hCG priming and oocyte retrieval has been encountered controversy, whether prolonged interval (>36 h) has any influence on the ART outcome, such as oocyte maturation rate, fertilization rate, implantation rate, and pregnancy rate.
Physiologic studies suggest that ovulation occurs anytime from 24 to 56 h after the onset of LH surge, with a mean time of 32 h [7]. Nader et al. [8] studied the pharmacokinetics of hCG and its relation to ovulation and concluded that ovulation may occur earlier than 36 h in some women, they advised aiming for a <35 h interval if ovulation is to be avoided. In most in vitro fertilization (IVF) programs, the commonly practiced interval was 32 to 36 h that was derived from the studies on patients who used Clomiphene Citrate (CC) and/or human menopausal gonadotropin (hMG) for ovulation induction [9–11]. However, several studies [2, 12–14] had shown that ideal ART performance can be obtained when oocyte retrieval was done more than 36 h (up to 39 h) after hCG priming. Successful fertilization in vitro of oocytes retrieved 60 h after hCG injection was reported in 1998 [15]. The prolonged luteinization-to-oocyte retrieval increased the production of oocytes with fully expanded cumulus, which may reflect oocyte maturation, then researchers presumed that the subsequent proportion of oocytes proceeding to fertilization and cleavage also increased, implying that a longer interval may improve gamete quality by allowing more optimal in vivo maturation. Significantly more high quality cleaving embryos had been reported to be obtained when the interval was 38 h rather than 36 h [12]. Meanwhile, there were studies [16, 17] indicated that there was no significant difference on the outcome of IVF treatment cycles among the interval prolonged. Therefore, we carry out a meta-analysis to determine whether a prolonged hCG-to-oocyte retrieval interval is beneficial to ART outcome.
Methods
Inclusion criteria
We included randomized and quasi-randomized controlled trials that provided original analyses on the relationship between different hCG priming-to-oocyte retrieval intervals and ART outcome. Infertility patients presented with indications, such as primary infertility, tubal factor, male factor, or unexplained indication, underwent their ART treatment cycles and reported the outcome. We included participants from the general population were in good general health, besides infertility, without other diseases. The COH protocols were performed by combined therapy of gonadotropic hormone releasing hormone analogue (GnRH-a) and hMG or follicle-stimulating hormone (FSH).
We excluded studies in which the hCG was added to the culture medium rather than injection for patients. Studies in which the outcome data were not clearly defined were excluded. Reviews letters, commentaries, case reports, and editorials were also excluded if they did not contain original data. If multiple published reports from the same study were available, only the one with the most detailed information for original data and outcome was included.
Literature search
We systematically searched PubMed, EMBASE, Cochrane Library, Science Citation Index, Chinese biomedicine (CBM) literature database, and Chinese Journal Full-text Database (from their inception to November 2010) for randomized controlled trials (RCTs), using the text and key words in combination both as MeSH (Medical Subject Headings) terms and text words. The search strategy used the following main search terms: “human chorionic gonadotropin” in combination with “interval”. We hand-searched reference lists of every retrieved study and reviewed relevant studies for additional publications. We searched the National Institute of Health, National Research Register, Current Controlled Trials, and Trials Central for unpublished studies and those in progress. We also used search engine such as Google™ to search for related references on the Internet. The search was restricted to human studies and without language restriction. Two reviewers (Wei Wang and Ya-Li Liu) independently assessed the titles and abstracts of all identified studies to confirm fulfillment of inclusion criteria; disagreements were resolved in consultation with a third reviewer (Ke-Hu Yang).
Types of intervention
Types of intervention were short time interval (<36 h) between hCG priming and oocyte retrieval versus long time interval (>36 h) between hCG priming and oocyte retrieval in the ART treatment cycles.
Types of outcome measures
The outcome measures of ART program were: oocyte maturation rate, defined as percentage of mature (MII) oocytes, metaphase II oocytes were defined by the presence of first polar body and round ooplasm. Fertilization rate, defined as the mean number of two-pronuclear (2PN) zygotes divided by MII-aspirated oocytes. Implantation rate, defined as percentage of transferred embryos of all embryos available for transfer. Pregnancy rate was calculated by considering clinical pregnancy, determined by the visualization of a viable gestational sac within the uterine cavity by ultrasound 3–4 weeks after embryo transfer.
Quality assessment
The methodological quality of included studies was assessed independently by two reviewers (Wei Wang and Ya-Li Liu) according to the criteria stated in The Cochrane Collaboration Handbook [18]. For each study, the risk of bias assessed and tabulated for each of the following items: sequence generation, allocation concealment, blinding (participants, personnel, outcome assessors, and data analysts), incomplete outcome data, selective outcome reporting, and other sources of bias. Judgement of ‘Yes’ indicates low risk of bias, ‘No’ indicates high risk of bias, and ‘Unclear’ indicates unclear or unknown risk of bias. Disagreements were resolved by discussion with a third reviewer (Ke-Hu Yang).
Data extraction
Two reviewers, Wei Wang and Ya-Li Liu extracted data independently from full text papers. Differences were resolved by discussion with a third reviewer (Ke-Hu Yang). For each study, we extracted the following information: first author, year of publication, location of study, sample size, patients’ characteristics, such as mean age, duration of infertility, causes of infertility, usage and dose of hCG, and different methods of ART.
Statistical analysis
Data was analyzed by using the Cochrane Review Manager (RevMan) 5.0.2 to mix the extracted data for summary effect estimates and generate forest plots. Results for dichotomous variables would be expressed as Risk Ratio (RR) with 95% confidence interval (CI). We used fixed-effect model for calculations of summary estimates and their 95% CIs unless there was significant heterogeneity, in which result was confirmed by using the random-effect model. For the summary of each total or subtotal, we provided the χ2-test statistic for heterogeneity across studies with its degree of freedom and P value, the statistic I2 that measured the extent of inconsistency in results, and the Z statistic with P value for overall effect. Generally, values of I2 above 50% are deemed to suggest large heterogeneity, values of 25–50% are deemed to show modest heterogeneity, and values below 25% are deemed to represent low heterogeneity. However, these estimates can have large uncertainty, especially in the presence of few trials, and should be interpreted with caution [19].
Results
Literature search results
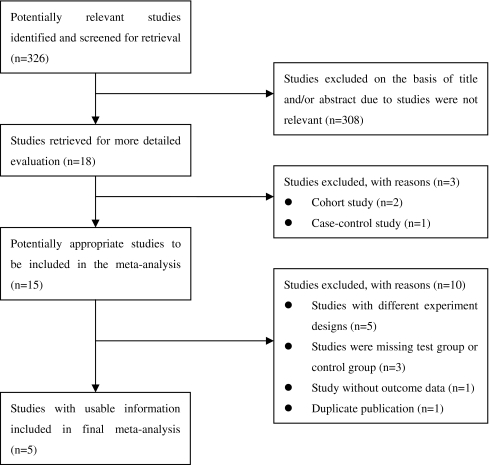
Detailed search procedures were summarized in the flow diagram (Fig. 1) illustrating the mechanisms of exclusion for certain studies in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement [20]. The search strategy retrieved 326 potentially eligible studies and subsequently excluded 321 studies with the following reasons: 308 were not relevant to the contents we interested; two were cohort studies and 1 used case-control design; five were with different experiment designs [21–25], the time interval referred to hCG-to-intrauterine insemination (IUI) or hMG-to-hCG rather than hCG-to-oocyte retrieval; three were missing test group or control group [12, 26, 27]; one was missing available outcome data [10]; one was duplicate publication [17]. Finally, 5 RCTs [13, 16, 17, 28, 29] totaling 895 participants were included in this meta-analysis.
Fig. 1.
Flow diagram of search strategy
The methodological quality of included studies
One trial [28] described odd or even IVF identity numbers of patients were used for the allocation of patients, this trial was known as quasi-randomized trial. Of the five included studies, only 1 trial [13] mentioned blinding: the gynecologist who performed the oocyte aspiration was blinded to the time interval, and only 1 trial [17] had adequate allocation concealment described: randomization was performed by drawing of sealed envelopes by a nurse who was not an active participant in the study. There was no losing of follow-up, the outcome data was complete, and without selective reporting bias. The methodological quality of included studies was summarized in Table 1.
Table 1.
Methodological quality assessment of included studies
| Study | Sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting bias | Other bias |
|---|---|---|---|---|---|---|
| Raziel A (2006) [28] | IVF identity number | Unclear | Unclear | Yes | Yes | Unclear |
| Nargund G (2001) [16] | Unclear | Unclear | Unclear | Yes | Yes | Unclear |
| Bjercke S (2000) [17] | Unclear | Yes | Unclear | Yes | Yes | Unclear |
| Mansour RT (1994) [13] | Unclear | Unclear | Yes | Yes | Yes | Unclear |
| Jamieson ME (1991) [29] | Unclear | Unclear | Unclear | Yes | Yes | Unclear |
IVF in vitro fertilization
The characteristics of included studies
The retrieved five trials were done in Israel, England, Norway, Egypt, and Scotland respectively from 1991 to 2006. One trial described the ethical approval that was obtained from the local research ethics committee [16]. Three trials applied IVF treatment cycles and two trials applied intracytoplasmic sperm injection (ICSI) treatment cycles. Two trials reported the ART outcome oocyte maturation rate, four trials reported the fertilization rate, two trials reported the implantation rate, and four trials reported the pregnancy rate. The COH protocols of the five studies were performed by combined therapy of GnRH-a and hMG or FSH (Table 2). The characteristics of included studies were summarized in Table 3.
Table 2.
COH protocols of ART treatment cycles
| Study | Controlled Ovarian Hyperstimulation (COH) |
|---|---|
| Raziel A (2006) [28] | Triptorelin or Nafarelin + hMG or recombinant preparations |
| Nargund G (2001) [16] | GnRH-a Buserelin acetate + hMG |
| Bjercke S (2000) [17] | GnRH-a Suprefact + FSH |
| Mansour RT (1994) [13] | GnRH-a Buserelin acetate + hMG |
| Jamieson ME (1991) [29] | GnRH-a Buserelin acetate + hMG |
Table 3.
COH protocols of ART treatment cycles
| Study | Controlled Ovarian Hyperstimulation (COH) |
|---|---|
| Raziel A (2006) [28] | Triptorelin or Nafarelin + hMG or recombinant preparations |
| Nargund G (2001) [16] | GnRH-a Buserelin acetate + hMG |
| Bjercke S (2000) [17] | GnRH-a Suprefact + FSH |
| Mansour RT (1994) [13] | GnRH-a Buserelin acetate + hMG |
| Jamieson ME (1991) [29] | GnRH-a Buserelin acetate + hMG |
Meta-analysis results
The meta-analysis results of outcome measures were shown in Table 4.
Table 4.
Meta-analysis results of ART outcome measures
| Outcome measures | No. of trials | Heterogeneity (I2, %) | Statistical model | Effect size | 95% CI | P value |
|---|---|---|---|---|---|---|
| Oocyte maturation rate | 2 | 33 | Fixed | RR | 0.67(0.62–0.73) | <0.00001 |
| Fertilization rate | 4 | 47 | Fixed | RR | 0.99(0.94–1.04) | 0.64 |
| Implantation rate | 2 | 58 | Random | RR | 0.91(0.40–2.04) | 0.81 |
| Pregnancy rate | 4 | 18 | Fixed | RR | 0.79(0.58–1.08) | 0.13 |
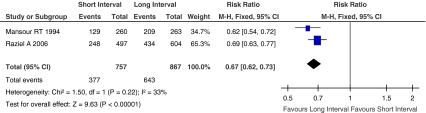
Oocyte maturation rate, defined as percentage of mature (MII) oocytes, MII oocytes were defined by the presence of first polar body and round ooplasm. Figure 2 showed the Risk Ratio with 95% CI of oocyte maturation rate associated with interval between hCG priming and oocyte retrieval in randomized studies, a total of 1624 oocytes were retrieved in two eligible studies [13, 28], there were 1,020 oocytes reached MII. The oocyte maturation rate was significantly higher when oocyte retrieval >36 h after hCG injection compared with oocyte retrieval <36 h after hCG injection (RR, 0.67; 95% CI, 0.62–0.73; I2 = 33%).
Fig. 2.
Oocyte maturation rate of short interval versus long interval in ART program
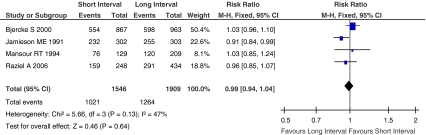
Fertilization rate, defined as the mean number of 2PN zygotes divided by MII-aspirated oocytes. Figure 3 showed the Risk Ratio with 95% CI of fertilization rate associated with interval between hCG priming and oocyte retrieval in randomized studies, a total of 3455 MII oocytes were retrieved in four eligible studies [13, 17, 28, 29], there were 2285 MII oocytes fertilized into 2PN zygotes. The fertilization rate did not differ significantly between short interval group and long interval group (RR, 0.99; 95% CI, 0.94–1.04; I2 = 47%).
Fig. 3.
Fertilization rate of short interval versus long interval in ART program
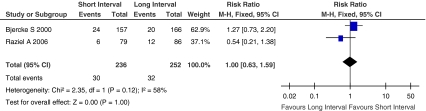
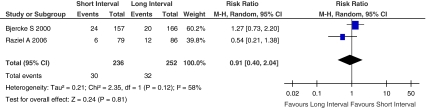
Implantation rate, defined as percentage of transferred embryos of all embryos available for transfer. Figure 4 showed the Risk Ratio with 95% CI of implantation rate associated with interval between hCG priming and oocyte retrieval in randomized studies, a total of 488 embryos available for transfer in two eligible studies [17, 28], there were 62 embryos transferred on the ET (embryo transfer) day. Since the implantation rate was heterogeneous (I2 = 58%) among the included studies, random-effect model was used to confirm the result (Fig. 5). There was no statistically significant difference between short interval group and long interval group (RR, 0.91; 95% CI, 0.40–2.04; I2 = 58%).
Fig. 4.
Implantation rate of short interval versus long interval in ART program (Fixed-effect model)
Fig. 5.
Implantation rate of short interval versus long interval in ART program (Random-effect model)
There were no differences in the pooled results of the two studies between fixed effect model and random effect model, although the weighted proportion for each study was changed. So the tendency of implantation rate was not changed.
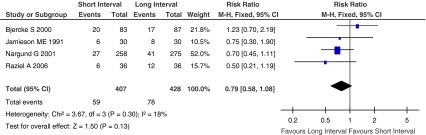
Pregnancy rate was calculated by considering only clinical pregnancy, determined by the visualization of a viable gestational sac within the uterine cavity by ultrasound 3–4 weeks after embryo transfer. Figure 6 showed the Risk Ratio with 95% CI of pregnancy rate associated with interval between hCG priming and oocyte retrieval in randomized studies, a total of 835 patients were included in four eligible studies [16, 17, 28, 29], and 137 patients become pregnant after embryo transfer. The pregnancy rate did not differ significantly between the two groups (RR, 0.79; 95% CI, 0.58–1.08; I2 = 18%).
Fig. 6.
Pregnancy rate of short interval versus long interval in ART program
Discussion
The number of included studies is relatively small. The small number of participants and included studies, as well as the low quality of most studies, might not allow for a reliable conclusion. Adequate randomization can prevent selection bias in allocating interventions to participants. However, proper randomization is not always possible in the clinical context of infertility, as patients’ choices have to be taken into account such as work and travel arrangements. Future study should describe how to generate the randomized allocation sequence. For example, opaque, sealed envelopes mentioned herein were used to conceal allocation. Blinding participants to the time interval might reduce the impact of subjective psychological factors. Blinding the doctors who perform oocyte retrieval, outcome assessors, and data analysts to the time interval could ensure that the compared groups receive similar attention, treatment and assessment. Without effective blinding might result in performance bias and measurement bias. In this meta-analysis, the follow-up time just was the interval between hCG priming and oocyte retrieval. Most infertility patients were willing to cooperate with doctors with better compliance, there was no losing of follow-up unless suffered spontaneous ovulation to cancel the treatment cycle, thus the outcome data was relatively complete. Further research should pay attention to the impact of geographic differences. We still need more high-quality, multicenter, randomized controlled trials from other countries and regions.
There were two kinds of hCG dose used in the five studies, two studies used 5000 IU and three studies used 10000 IU. The hCG dose was determined according to the patients’ responding to COH, levels of estradiol. An hCG dose of 5000 IU can give similar results compared to a dose of 10000 IU in terms of oocyte maturation rate, fertilization rate, and pregnancy rate, regardless of the route of administration. So the differences between hCG dose does not affect the ART outcome [30, 31]. The causes of infertility showed in the five studies were primary infertility, tubal factor, male factor, or unexplained infertility. These causes above are the very indications for IVF or ICSI, and their differences dose not interfere with the outcome of ART. Only one study described the duration of infertility, further study should describe the information of patients in detail with similar demographics to compare the baseline conditions roundly.
The timing of oocyte maturation in vivo after an injection of hCG was first estimated by Jagiello [32]. Of the five retrieved studies, the comparison was based on the short interval versus long interval: 35.3 ± 0.7 versus 38.6 ± 1.2 h, 33 versus 41 h, 34 versus 38 h, 35 versus 37 h, and 34 versus 39 h. The short interval varied from 33 to 36 h, while the long interval varied from 36 to 41 h. Van Steirteghem et al. reported that of the oocytes retrieved 33–36 h after hCG priming, 85% were in the MII stage [33]. Steptoe and Edwards reported that the preovulatory oocytes were in the predicted stage about 35 to 36 h after hCG injection [34]. In stimulated cycles with CC or hMG, ovulation can occur between 32 h and 36 h [35]. Mansour et al. [13] concluded that oocyte maturity was attained 36 h after hCG injection, and therefore oocyte recovery should not be performed before 36 h. Fertilization rate and cleavage rate were significantly higher in oocytes retrieved >36 h after luteinizing stimulus than in those <36 h after luteinizing stimulus. So we defined the 36 h as the demarcation to determine short interval group and long interval group.
Natural ovulation after LH surge may occur between 30 h and 36 h, whereas ovulation after hCG injection may occur between 36 h and 40 h in superovulation cycles without the use of GnRH-a [10]. Nevertheless, in the five included studies, the COH protocols were performed by combined therapy of GnRH-a and hMG or FSH. The use of COH protocols is intended to produce large cohorts of follicles and oocytes by creating continuous pituitary suppression and eliminate endogenous gonadotropin fluctuations. It is well known that with GnRH-a COH there is an implicit LH suppression and a longer in vivo maturation time than is possible without the use of GnRH-a. By producing a hypogonadotropic condition it is possible to control the follicular development more readily and reduce the variations in response to hCG.
The hCG was administered when a sufficient number of follicles had developed, typically more than three follicles ≥17 mm in mean diameter, and the transvaginal ultrasound (TUS) guided approach was used for oocyte retrieval.
One of our concerns was the possibility of spontaneous ovulation before the scheduled time for oocyte retrieval as a result of prolonged exposure to hCG. Andersen et al. [36] found a mean time interval of 38.3 h from hCG injection to first follicular rupture. Nargund et al. concluded that no women ovulated up to 41 h from hCG administration. Fleming et al. reported that no ovulation occurs within a delay of less than 39.5 h [14]. Gudmundsson et al. [2] described 39 h as the critical time to avoid spontaneous ovulation. Of the five included studies, a patient from 38 h group [17] encountered spontaneous ovulation. We should control the interval properly to avoid cancelling the treatment cycles as a result of spontaneous ovulation.
Since the hours after luteinizing stimulus is a period of intense nuclear and cytoplasmic activity in human oocytes, so the interval between hCG priming and oocyte retrieval probably determines the degree of cellular and cytogenetic maturation. A prolonged interval would have an increased intrafollicular influence, yield oocytes in which all processes are completed, allow more in vivo maturation, and therefore more likely to develop into 2PN zygotes and high quality normal cleaving embryos. The incidence of fully expanded cumulus cells is increased in the patients with longer interval (>36 h), and this apparent benefit is reflected in higher fertilization rate and cleavage rate. Immature oocytes are firmly attached to the follicular wall and not easy to aspirate; when oocytes reach maturity, this attachment becomes loose and oocytes are easily retrieved [37]. The procedure becomes easier and faster, flushing of oocytes with its potential disadvantages is avoided. In addition, extension of the in vivo maturation might reduce the incidence of polyspermy.
Oocyte meiotic maturation is an important factor for achieving fertilization and developing high quality embryos. Delaying oocyte retrieval to a longer interval is helpful to improve oocyte maturity, while there is no significant improvement in fertilization rate, implantation rate, and pregnancy rate. Son WY et al. obtained the similar results in their programmed in vitro maturation (IVM) cycles [38]. Apart from the time required for hCG to become available after administration analyzed herein, ART outcome is also associated with other factors as follows. The age of patient: the oocytes quality would become poorer with increased age of women. The number of mitochondria in the cytoplasm is reduced, incidence of DNA mutation is increased, adenosine triphosphate (ATP) is reduced, active oxygen is increased, and aneuploidy is increased, thus the developmental potential of oocytes and embryos are decreased [39]. The number of transferred embryos: although more embryos transfer could increase pregnancy rate, multiple pregnancy is also increased [40]. When implanted ≥3 embryos, the pregnancy rate has not increased any more, the risk of multiple pregnancy is increased. In women under 35 years of age, transferring double high-quality embryos can help maximize clinical pregnancy rate while minimizing multiple pregnancy rate. Moreover, elective single embryo transfer with an acceptable pregnancy rate might be considered if a top quality embryo is available. The pregnancy rate of once-transfer was 40%, and the cumulative pregnancy rate was 60%, equal to even higher than the cumulative pregnancy rate of double-embryo transfer [41–43]. The quality of embryos (cleavage speed and morphologic distribution): embryos that absence of multinucleated blastomeres, four or five blastomeres on day 2, seven or more cells on day 3, and ≤20% anucleated fragments are considered as top quality embryos [44]. The majority of embryos selected for transfer were at the 4-cell stage on day 2 at 42 h post-insemination. It is clear that 4-cell embryos on day 2 are more likely to be good grade embryos on day 3 and that 3-cell and greater than 4-cell embryos have poor developmental potential. Considering cell number at a set time (42–44 h post-hCG), even (two and four cells) rather than uneven (3, 5 and greater) cell numbers were significant in positive pregnancy events. The transition from 2-cell to 4-cell requires that the embryo proceeds through a 3-cell phase first and then rapidly cleaves again to form a 4-cell tetrahedron embryo [45–47]. If an embryo is cleaving too fast, relevant to all other embryos, it is most likely abnormal or has a cytokinetic abnormality [48, 49]. Endocrine profile: during COH, E2 increased too much is not good for embryo nidation and increased LH might produce granulosa cells that prematurely luteinize, affecting the quality of embryos and pregnancy outcome. Immunity factors: presence of various antibodies, such as anti-sperm antibody, anti-endometrial antibody, anti-ovarian antibody, anti-cardiolipin antibody, anti-human chorionic gonadotropin antibody and so on, binding with corresponding antigens could result in antigen-antibody reactions, activate complement system, induce autoimmunity reaction, finally influence the pregnancy events. So we must take these factors into consideration sufficiently when carrying out ARTs.
Oocyte retrieval after hCG priming can be done at any time within a relatively broad interval. This flexibility of hCG-to-oocyte retrieval provides more convenience both for patients and physicians. The meta-analysis results also suggest that the temporal relationship between LH surge, follicle rupture, oocyte retrieval, and the potential for successful pregnancy during natural and artificial assisted menstrual cycles is needed to be learned.
Conclusion
The results indicate that the percentage of mature oocytes can be increased by prolonging the interval between hCG priming and oocyte retrieval. The fertilization rate, implantation rate, and pregnancy rate do not differ significantly between oocyte retrieval <36 h after hCG injection and oocyte retrieval >36 h after hCG injection. Although there is evidence to confirm the results, the results still need to be confirmed by large-sample, multicenter, randomized, controlled trials from other countries and regions.
Acknowledgments
The authors would like to thank Jin-Hui Tian, Bin Ma, Lei Jiang, Wen-Qin Jia, Kang Yi, and Lun Li (Evidence-Based Medicine Center of Lanzhou University, Lanzhou, China) for advice on conducting the meta-analysis and writing the article.
Conflicts of interest statement The authors declared no conflicts of interest related to this study.
Abbreviations
- ART
Assisted reproductive technology
- ATP
Adenosine triphosphate
- CC
Clomiphene citrate
- COH
Controlled ovarian hyperstimulation
- ET
Embryo transfer
- FSH
Follicle-stimulating hormone
- GnRH-a
Gonadotropic hormone releasing hormone analogue
- hCG
Human chorionic gonadotropin
- hMG
Human menopausal gonadotropin
- ICSI
Intracytoplasmic sperm injection
- IGF
Insulin-like growth factor
- IL
Interleukin
- IM
Intramuscular injection
- IUI
Intrauterine insemination
- IVF
In vitro fertilization
- IVM
In vitro maturation
- LH
Luteinizing hormone
- LI
Long interval
- MII
Metaphase II
- NA
Not available
- RCT
Randomized controlled trial
- RevMan
Review Manager
- RR
Risk ratio
- SI
Short interval
- TUS
Transvaginal ultrasound
- VEGF
Vascular endothelial growth factor
- 2PN
Two-pronuclear
- 95% CI
95% confidence interval
Footnotes
Capsule In ART treatment cycles, the chances to achieve a pregnancy are critically dependent on the retrieval of a suitable number of high quality oocytes and embryos. Angiotensin II, vascular endothelial growth factor (VEGF), interleukin I (IL-1), IL-6, IL-8, angiopoietin, insulin-like growth factor (IGF), basic fibroblast growth factor (bFGF), and endothelin levels appear able to identify women candidate for an ART treatment from whom a suitable number of high quality oocytes may be retrieved.
Contributor Information
Xue-Hong Zhang, Email: zhang.xuehong@yahoo.com.cn.
Wei-Hua Wang, Email: wangweihua11@yahoo.com.
References
- 1.Griesinger G, Kolibianakis EM, Papanikolaou EG, Diedrich K, Steirteghem A, Devroey P, et al. Triggering of final oocyte maturation with gonadotropin-releasing hormone agonist or human chorionic gonadotropin. Live birth after frozen-thawed embryo replacement cycles. Fertil Steril. 2007;88:616–621. doi: 10.1016/j.fertnstert.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Gudmundsson J, Fleming R, Jamieson ME, McQueen D, Coutts JRT. Luteinization to oocyte retrieval delay in women in whom multiple follicular growth was induced as part of an in vitro fertilization/gamete intrafallopian transfer program. Fertil Steril. 1990;53:735–737. doi: 10.1016/s0015-0282(16)53474-8. [DOI] [PubMed] [Google Scholar]
- 3.Kuo TC, Endo K, Dharmarajan AM, Miyazaki T, Atlas SJ, Wallach EE. Direct effect of angiotensin II on in-vitro perfused rabbit ovary. J Reprod Fertil. 1991;92:469–474. doi: 10.1530/jrf.0.0920469. [DOI] [PubMed] [Google Scholar]
- 4.Artini PG, Fasciani A, Monti M, Luisi S, D’Ambrogio G, Genazzani AR. Changes in vascular endothelial growth factor levels and the risk of ovarian hyperstimulation syndrome in women enrolled in an in vitro fertilization program. Fertil Steril. 1998;70:560–564. doi: 10.1016/S0015-0282(98)00221-0. [DOI] [PubMed] [Google Scholar]
- 5.Bokal EV, Vrtovec HM, Virant Klun I, Verdenik I. Prolonged HCG action affects angiogenic substances and improves follicular maturation, oocyte quality and fertilization competence in patients with polycystic ovarian syndrome. Hum Reprod. 2005;20:1562–1568. doi: 10.1093/humrep/deh789. [DOI] [PubMed] [Google Scholar]
- 6.Artini PG, Monti M, Fasciani A, Battaglia C, D’Ambrogio G, Genazzani AR. Vascular endothelial growth factor, interleukin-6 and interleukin-2 in serum and follicular fluid of patients with ovarian hyperstimulation syndrome. Eur J Obstet Gynecol Reprod Biol. 2002;101:169–174. doi: 10.1016/S0301-2115(01)00568-1. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Temporal relationships between ovulation and defined changes in the concentration of plasma oE2–17β, luteinizing hormone, follicle stimulating hormone and progesterone. Am J Obstet Gynecol. 1980;138:383–390. [PubMed] [Google Scholar]
- 8.Nader S, Berkowitz AS. Study of the pharmacokinetics of human chorionic gonadotropin and its relation to ovulation. In Vitro Fert Embryo Transfer. 1990;7:114–118. doi: 10.1007/BF01135585. [DOI] [PubMed] [Google Scholar]
- 9.Edwards RG, Steptoe PC. Induction of follicular growth, ovulation and luteinization in the human ovary. J Reprod Fertil. 1975;22(Suppl):121–163. [PubMed] [Google Scholar]
- 10.Testart J, Frydman R. Minimum time lapse between luteinizing hormone surge or human chorionic gonadotropin administration and follicular rupture. Fertil Steril. 1982;37:50–53. doi: 10.1016/s0015-0282(16)45976-5. [DOI] [PubMed] [Google Scholar]
- 11.Trounson AO, Leeton JF, Wood C. In vitro fertilization and embryo transfer in the human. In: Rolland R, van Hall EV, Hillier SG, Mcnatty KP, Schoemaker J, editors. Follicular maturation and ovulation. Amsterdam, Exerpta Medica 1982:313
- 12.Vits A, Gerris J, Joostens M, Aytos A.Comparison between two hCG-to-oocyte aspiration intervals (36 versus 38) on the outcome of in-vitro fertilization Hum Reprod 19949Suppl12–15.7962457 [Google Scholar]
- 13.Mansour RT, Aboulghar MA, Serour GI. Study of the optimum time for human chorionic gonadotropin-ovum pickup interval in in vitro fertilization. J Assist Reprod Genet. 1994;11:478–481. doi: 10.1007/BF02215712. [DOI] [PubMed] [Google Scholar]
- 14.Fleming R, Coutts JRT. Induction of multiple follicular development for IVF. Brit Med Bull. 1990;46:596–615. doi: 10.1093/oxfordjournals.bmb.a072419. [DOI] [PubMed] [Google Scholar]
- 15.Al-Mizyen ES, Balet R, Lower AM, Wilson C, McClure AF, Shawaf T, et al. Unexpected successful fertilization in vitro of oocytes retrieved 60 hours after human chorionic gonadotrophin injection. Hum Reprod. 1998;13:1020–1021. doi: 10.1093/humrep/13.4.1020. [DOI] [PubMed] [Google Scholar]
- 16.Nargund G, Reid F, Parsons J. Human chorionic gonadotropin-to-oocyte collection interval in a superovulation IVF program. A prospective study. J Assist Reprod Genet. 2001;18:87–90. doi: 10.1023/A:1026530624575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjercke S, Tanbo T, Dale PO, Abyholm T. Comparison between two hCG-to-oocyte aspiration intervals on the outcome of in vitro fertilization. J Assist Reprod Genet. 2000;17:319–322. doi: 10.1023/A:1009401027251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions 5.0.0 [updated February 2008]. In: The Cochrane collaboration. Wiley 2008.
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claman P, Wilkie V, Collins D. A short (335 h) compared with a long (395 h) interval between HCG injection and intrauterine insemination after superovulation therapy. Hum Reprod. 2000;15:6–7. [Google Scholar]
- 22.Claman P, Wilkie V, Collins D. Timing intrauterine insemination either 33 or 39 hours after administration of human chorionic gonadotropin yields the same pregnancy rates as after superovulation therapy. Fertil Steril. 2004;82:13–16. doi: 10.1016/j.fertnstert.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 23.Fischer RA, Nakajima ST, Gibson M, Brumsted JR. Ovulation after intravenous and intramuscular human chorionic gonadotropin. Fertil Steril. 1993;60:418–422. [PubMed] [Google Scholar]
- 24.Howles CM, Macnamee MC, Edwards RG. Progesterone supplementation in the late follicular phase of an in-vitro fertilization cycle: a ‘natural’ way to time oocyte recovery? Hum Reprod. 1988;3:409–412. doi: 10.1093/oxfordjournals.humrep.a136718. [DOI] [PubMed] [Google Scholar]
- 25.Laufer N, DeCherney AH, Tarlatzis BC, Zuckerman AL, Polan ML, Dlugi AM, et al. Delaying human chorionic gonadotropin administration in human menopausal gonadotropin-induced cycles decreases successful in vitro fertilization of human oocytes. Fertil Steril. 1984;42:198–203. doi: 10.1016/s0015-0282(16)48013-1. [DOI] [PubMed] [Google Scholar]
- 26.Templeton AA, Look P, Angell RE, et al. Oocyte recovery and fertilization rates in women at various times after the administration of hCG. J Reprod Fertil. 1986;76:771–778. doi: 10.1530/jrf.0.0760771. [DOI] [PubMed] [Google Scholar]
- 27.Schachter M, Friedler S, Ron-El R, Zimmerman AL, Strassburger D, Bern O, et al. Can pregnancy rate be improved in gonadotropin-releasing hormone (GnRH) antagonist cycles by administering GnRH agonist before oocyte retrieval? A prospective, randomized study. Fertil Steril. 2008;90:1087–1093. doi: 10.1016/j.fertnstert.2007.07.1316. [DOI] [PubMed] [Google Scholar]
- 28.Raziel A, Schachter M, Strassburger D, Kasterstein E, Ron-El R, Friedler S. In vivo maturation of oocytes by extending the interval between human chorionic gonadotropin administration and oocyte retrieval. Fertil Steril. 2006;86:583–587. doi: 10.1016/j.fertnstert.2006.02.091. [DOI] [PubMed] [Google Scholar]
- 29.Jamieson ME, Fleming R, Kader S, Ross KS, Yates RW, Coutts JR. In vivo and in vitro maturation of human oocytes: effects on embryo development and polyspermic fertilization. Fertil Steril. 1991;56:93–97. doi: 10.1016/s0015-0282(16)54424-0. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt DW, Maier DB, Nulsen JC, Benadiva CA. Reducing the dose of human chorionic gonadotropin in high responders does not affect the outcomes of in vitro fertilization. Fertil Steril. 2004;82:841–846. doi: 10.1016/j.fertnstert.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 31.Isik AZ, Vicdan K. Combined approach as an effective method in the prevention of severe ovarian hyperstimulation syndrome. Eur J Obstet Gynecol Reprod Biol. 2001;97:208–212. doi: 10.1016/S0301-2115(00)00539-X. [DOI] [PubMed] [Google Scholar]
- 32.Jagiello G, Karniki J, Ryan R. Superovulaton with pituitary gonadotropin methods for obtaining meiotic metaphase figures in human ova. Lancet. 1968;1:178–180. doi: 10.1016/S0140-6736(68)92566-X. [DOI] [Google Scholar]
- 33.Steirteghem AC, Liu J, Joris H, Nagy Z, Janssenswillen C, Tournaye H, et al. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Hum Reprod. 1993;8:1055–1060. doi: 10.1093/oxfordjournals.humrep.a138191. [DOI] [PubMed] [Google Scholar]
- 34.Steptoe PC, Edwards RG. Laparoscopic recovery of preovulatory human oocytes after priming of ovaries with gonadotrophins. Lancet. 1970;1:683–689. doi: 10.1016/S0140-6736(70)90923-2. [DOI] [PubMed] [Google Scholar]
- 35.Edwards RG, Steptoe PC. Control of human ovulation, fertilization and implantation. Proc R Soc Med. 1974;67:932–936. [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen AG, Als-Nielsen B, Hornnes PJ, Franch Andersen L. Time interval from human chorionic gonadotrophin (HCG) injection to follicular rupture. Hum Reprod. 1995;10:3202–3205. doi: 10.1093/oxfordjournals.humrep.a135888. [DOI] [PubMed] [Google Scholar]
- 37.Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril. 1994;62:353–362. doi: 10.1016/s0015-0282(16)56891-5. [DOI] [PubMed] [Google Scholar]
- 38.Son WY, Chung JT, Chian RC, Herrero B, Demirtas E, Elizur S, et al. A 38 h interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Hum Reprod. 2008;23:2010–2016. doi: 10.1093/humrep/den210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hull MG, Fleming CF, Hughes AO, McDermott A. The age-related decline in female fecundity: a quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil Steril. 1996;65:783–790. doi: 10.1016/s0015-0282(16)58214-4. [DOI] [PubMed] [Google Scholar]
- 40.Schnorr JA, Doviak MJ, Muasher SJ, Jones HW., Jr Impact of a cryopreservation program on the multiple pregnancy rate associated with assisted reproductive technologies. Fertil Steril. 2001;75:147–151. doi: 10.1016/S0015-0282(00)01661-7. [DOI] [PubMed] [Google Scholar]
- 41.Thurin A, Hausken J, Hillensjo T, Jablonowska B, Pinborg A, Strandell A, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351:2392–2402. doi: 10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- 42.Lukassen HGM, Braat DD, Wetzels AMM, Zielhuis GA, Adang EMM, Scheenjes E, et al. Two cycles with single embryo transfer versus one cycle with double embryo transfer: a randomized controlled trial. Hum Reprod. 2005;20:702–708. doi: 10.1093/humrep/deh672. [DOI] [PubMed] [Google Scholar]
- 43.Veleva Z, Vilska S, Hyden-Granskog C, Tiitinen A, Tapanainen JS, Martikainen H. Elective single embryo transfer in women aged 36–39 years. Hum Reprod. 2006;21:2098–2102. doi: 10.1093/humrep/del137. [DOI] [PubMed] [Google Scholar]
- 44.Royen E, Mangelschots K, Neubourg D, Valkenburg M, Meerssche M, Ryckaert G, et al. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum Reprod. 1999;14:2345–2349. doi: 10.1093/humrep/14.9.2345. [DOI] [PubMed] [Google Scholar]
- 45.Roux C, Joanne C, Agnani G, Fromm M, Clavequin MC, Bresson JL. Morphometric parameters of living human in-vitro fertilization embryos; importance of the asynchronous division process. Hum Reprod. 1995;10:1201–1207. doi: 10.1093/oxfordjournals.humrep.a136119. [DOI] [PubMed] [Google Scholar]
- 46.Hiiragi T, Solter D. Mechanism of first cleavage specification in the mouse egg: is our body plan set at day 0? Cell Cycle. 2005;4:661–664. doi: 10.4161/cc.4.5.1680. [DOI] [PubMed] [Google Scholar]
- 47.Gardner RL, Davies TJ. An investigation of the origin and significance of bilateral symmetry of the pronuclear zygote in the mouse. Hum Reprod. 2006;21:492–502. doi: 10.1093/humrep/dei318. [DOI] [PubMed] [Google Scholar]
- 48.Racowsky C, Jackson KV, Cekleniak NA, Fox JH, Hornstein MD, Ginsburg ES. The number of eight-cell embryos is a key determinant for selecting day 3 or day 5 transfer. Fertil Steril. 2000;73:558–564. doi: 10.1016/S0015-0282(99)00565-8. [DOI] [PubMed] [Google Scholar]
- 49.Scott L, Finn A, O’Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod. 2007;22:230–240. doi: 10.1093/humrep/del358. [DOI] [PubMed] [Google Scholar]