Background: Specific activators turn on Mec1/ATR kinase during DNA damage or replication stress, mediating cell cycle arrest.
Results: We have separated the replication function of multifunctional Dpb11 from its checkpoint activation function.
Conclusion: Dpb11 functions primarily during the G2/M phase.
Significance: Our results suggest that each phase of the cell cycle may use specific Mec1 activators.
Keywords: Cell Cycle, Checkpoint Control, DNA Damage Response, DNA Repair, DNA Replication, Protein Kinases, Yeast Genetics, ATR Kinase, Unstructured Domains
Abstract
Budding yeast Dpb11 (human TopBP1, fission yeast Cut5) is an essential protein required for replisome assembly and for the DNA damage checkpoint. Previous studies with the temperature-sensitive dpb11–1 allele, truncated at amino acid 583 of the 764-amino acid protein, have suggested the model that Dpb11 couples DNA replication to the replication checkpoint. However, the dpb11–1 allele shows distinct replication defects even at permissive temperatures. Here, we determine that the 1–600-amino acid domain of DPB11 is both required and sufficient for full replication function of Dpb11 but that this domain is defective for activation of the principal checkpoint kinase Mec1 (human ataxia telangiectasia and Rad3-related) in vitro and in vivo. Remarkably, mutants of DPB11 that leave its replication function intact but abrogate its ability to activate Mec1 are proficient for the replication checkpoint, but they are compromised for the G2/M DNA damage checkpoint. These data suggest that replication checkpoint defects may result indirectly from defects in replisome assembly. Two conserved aromatic amino acids in the C terminus of Dpb11 are critical for Mec1 activation in vitro and for the G2/M checkpoint in yeast. Together with aromatic motifs identified previously in the Ddc1 subunit of 9-1-1, another activator of Mec1 kinase, they define a consensus structure for Mec1 activation.
Introduction
The coordination of DNA replication and DNA damage repair with cell cycle progression is vital in maintaining genome integrity. The DNA checkpoint machinery regulates cell cycle progression in response to DNA damage or DNA replication stress. It also promotes DNA repair and the stabilization of stalled replication forks (1). In Saccharomyces cerevisiae, Mec1 is the PI3K-like kinase that initiates the signal transduction network leading to checkpoint activation, cell cycle arrest, and the up-regulation of DNA repair (2, 3). Mec1 forms a constitutive heterodimer with its regulatory subunit Ddc2. The generation of single-stranded DNA coated with the single-stranded binding protein RPA3 is a critical step in the recruitment of Mec1 to sites of damage, which is mediated through Ddc2-RPA interactions (4, 5). One or more Mec1 activators are also recruited to these sites, and the juxtaposition on chromatin of a given activator with Mec1 results in the activation of Mec1 protein kinase activity (6) and the phosphorylation of a large number of proteins, including RPA, subunits of the 9-1-1 checkpoint clamp, mediator proteins, and downstream effector kinases such as Rad53.
The heterotrimeric checkpoint clamp 9-1-1 (S. cerevisiae Ddc1-Mec3-Rad17, human Rad9-Rad1-Hus1) and the DNA replication initiation factor Dpb11 (human TopBP1, Schizosaccharomyces pombe Cut5) are the two known activators of Mec1 kinase in S. cerevisiae (7–9). The 9-1-1 clamp functions in checkpoint activation in the G1 and G2 phases of the cell cycle (10). The Ddc1 (human Rad9) subunit of the clamp directly activates Mec1 in G1 phase, an activity identified so far only in S. cerevisiae (11). However, 9-1-1 performs an additional function in the DNA damage checkpoint pathway. That is, chromatin association of Dpb11 depends on interaction with Ddc1, and this mode of chromatin association is conserved. Efficient recruitment of Dpb11/TopBP1/Cut5 requires prior phosphorylation of Ddc1/Rad9 in its C-terminal tail in response to DNA damage (12, 13). Therefore, mutants in DDC1 that are defective both for activation of Mec1 and for recruitment of Dpb11 show a complete G2/M DNA damage checkpoint defect.
The replication machinery plays a role in activation of the checkpoint and is also the target of the checkpoint. The slowing of DNA replication because of DNA damage or depletion of dNTPs (in the presence of the ribonucleotide reductase inhibitor hydroxyurea) activates the replication checkpoint. Mutants that show defects in replisome assembly are often defective in checkpoint activation after replication stress (14–19). On the basis of these type of data, a model has been proposed of a threshold level of damage sensing in S phase. Normal replication forks contain stretches of RPA-coated single-stranded DNA that may conceivably signal to the Mec1-dependent checkpoint machinery. To prevent unwarranted checkpoint activation by the presence of these normal replication forks during regular S-phase progression, there may exist an S-phase-specific threshold level of damage, and checkpoint activation during S occurs only above this threshold level (20). However, a consequence of this threshold model would be that cells containing less replication forks than normal because of initiation defects may not reach this threshold level for checkpoint activation, even if these replication forks stall. This model has been elaborated experimentally for an orc2–1 mutant that causes the assembly of fewer replication forks (18). However, this model could explain why other replication initiation mutants with defects that may cause a decrease in the number of replication forks during S phase would also be defective in the replication checkpoint after nucleotide depletion, e.g. by hydroxyurea. These considerations are particularly pertinent to the case of DPB11 because Dpb11 is both actively involved in replisome assembly and in checkpoint activation.
Dpb11 is required for the initiation of DNA replication. It has four BRCA1 C-terminal (BRCT) domains, the first two involved in the interaction with Sld3 and the last two with Sld2. These interactions are regulated by the S phase cyclin-dependent kinase and are crucial for replisome assembly (21, 22). Loading of DNA polymerases α and ϵ at replication origins is dependent on Dpb11 (23). A temperature-sensitive mutant, dpb11-1, codes for a protein with a premature stop codon at amino acid 583, just after the last BRCT domain, and most genetic studies in yeast have been carried out with this allele (14). Dpb11-1 is synthetically lethal with mutations in POL2 and SLD2 suggesting that this mutant is defective for physical interactions with its key replication partners (14) Indeed, yeast two-hybrid and coimmunoprecipitation studies have confirmed the defective interactions between Dpb11-1 and Sld2 (24). The dpb11-1 mutant is also sensitive to hydroxyurea treatment and shows decreased checkpoint activation in response to hydroxyurea, as measured by Rad53 phosphorylation (17). However, whether this checkpoint defect is caused directly by the inability of Dpb11-1 to activate Mec1 in S phase, or is an indirect effect caused by defective replisome assembly in dpb11-1 remains to be established.
In this study, we have identified the motifs in Dpb11 that are important for the activation of Mec1 kinase activity, and we have generated domain mutants that separate the replication function of Dpb11 from its checkpoint activation function. Using these genetic tools, we show that mutants of Dpb11 that are proficient for DNA replication but deficient for Mec1 activation have no effect on the replication checkpoint. Rather these DPB11 mutants show a defect for the G2/M checkpoint in response to DNA-damaging agents. We conclude from our studies that the replication checkpoint defects observed previously with the dpb11-1 allele are an indirect effect of defects in replisome assembly that in itself leads to an inefficient replication checkpoint response.
EXPERIMENTAL PROCEDURES
Plasmids, DNA Substrates, and Yeast Strains
Plasmid series pBL512 contains full-length DPB11 (or point mutants) cloned into multicopy vector pRS426-GALGST (2 mm origin, URA3, GAL1–10, GST). The DPB11 gene was fused to the GST gene via an intervening rhinoviral 3C protease site. Plasmid series pBL527 contains the 571-764 domain of DPB11 (or point mutants) fused via an intervening rhinoviral 3C protease site to the GST domain of pGEX-6P1 (GE Healthcare). Plasmid series pBL530 contains the DPB11 gene (or point mutants) together with regulatory sequences (from 820 nt upstream of start to 505 nt downstream of stop) cloned into centromere vector pRS314. Truncation mutant plasmids pBL530–583 (dpb11-1) and pBL530–601 (dpb11–601) were made by the insertion of two tandem stop codons. Plasmids are listed in supplemental Tables S1–S3. Plasmids and their sequences are available upon request. Deca-primed single-stranded DNA was made by annealing single-stranded bluescript SKII+ DNA with ten 28-mer primers spaced approximately equally around the single-stranded DNA. The yeast strains used are listed in supplemental Table S4.
Proteins
Mec1-Ddc2, Rad53-kd, RPA, and Dpb11 were purified as described earlier (7, 8). Mutants of full-length Dpb11 were overproduced and purified from yeast similar to the wild-type protein.
To purify individual Dpb11-truncated proteins, the pBL527 series of plasmids was transformed in Escherichia coli BL21 (DE3) cells and selected on LB + Amp plates. A few colonies were used to inoculate a 50-ml culture of LB broth and grown overnight at 37 °C with vigorous shaking (300 rpm). A 10-ml inoculum was used to inoculate 1 liter of LB broth (A600 = 0.01), and the flask was incubated at 30 °C with vigorous shaking (250 rpm) to an A600 = 0.5. The cells were then induced with 1 mm isopropyl β-d-thiogalactopyranoside, and shaking was continued for an additional 4 h. The cells were harvested and resuspended in buffer A (50 mm Hepes (pH 7.8), 1 mm EDTA, 0.1% Tween 20, 0.01% Nonidet P-40, 150 mm NaCl, 175 mm ammonium sulfate, 1 mm DTT, 10% glycerol) and stored frozen at −70° C. Purification was carried out at 0–4 °C. Protease inhibitor mixture was added to the thawed cells, and they were lysed by sonication. Cell debris was removed by centrifugation at 18,000 rpm for 30 min. The cleared lysate was kept for batch binding onto 1 ml of glutathione beads for 2 h on a rocker. After batch binding, the beads were subjected to four consecutive 50-ml washes in buffer B (50 mm Hepes (pH 7.8), 100 mm NaCl, 10% glycerol, 0.1% Tween 20, 0.01% Nonidet P-40, 1 mm EDTA). The first 50-ml wash was supplemented with protease inhibitor mixture (2 mm pepstatin A, 2 mm leupeptin, 10 mm NaHSO3, 1 mm benzamidine) and 1 mm phenylmethylsulfonyl fluoride, and the third wash was supplemented with 1 mm ATP and 10 mm MgCl2. The GST-tagged protein was eluted with reduced glutathione in buffer B. The GST tag was removed by incubating the eluted protein with rhinoviral 3C protease overnight at 4 °C. The resulting protein was stored at −70 °C.
Protein Phosphorylation Assays
Standard 20-μl phosphorylation assays contained 25 mm Hepes-NaOH (pH 7.8), 5 mm MgCl2, 100 μm unlabeled ATP, and 0.5 μCi [γ-32P]ATP, 125 mm NaCl, 1 mm DTT, 100 μg/ml BSA, 1 nm deca-primed single-stranded DNA, 150 nm RPA, 100 nm Rad53-kd, and the indicated concentrations of (mutant) Dpb11. Reactions were preheated for 1 min at 30 °C and initiated by the addition of 5 nm Mec1/Ddc2 kinase. After 10 min at 30 °C or the indicated times, the assays were stopped with 5 μl of 5× SDS-PAGE gel loading dye. The samples were run on 10% SDS-PAGE, dried, and exposed to a phosphor screen (GE Healthcare). Protein phosphorylation was quantified using Imagequant software.
Western Blot Analysis of Rad53 Phosphorylation
Yeast cells were grown in 5 ml of selective media to A660 = 0.5. They were then arrested in G1 phase by α factor (20 μg/ml for 2 h) or in G2 phase with nocodazole (20 μg/ml for 2 h) in 1% dimethyl sulfoxide-containing media. G1-arrested cells were treated with 4NQO (2 μg/ml) for 30 min at 30 °C or released into S phase with or without 200 mm hydroxyurea. G2-arrested cells were treated with 4NQO (2 μg/ml) for 30 min at the indicated temperature. Protein extracts were prepared by trichloroacetic acid precipitation. The Rad53 YC-19 antibody (Santa Cruz Biotechnology, Inc.) was used at a dilution of 1:1000 in TBS containing 5% milk and 0.1% Tween 20. Alkaline phosphatase-conjugated anti-goat secondary antibody (Sigma) was used at a dilution of 1:5000 in TBS containing 5% milk and 0.1% Tween 20. Rad53 was detected using a fluorescent substrate (GE Healthcare) and scanned using a Typhoon scanner (GE Healthcare). All experiments were performed two to four times. The blots were quantified using ImageJ software, and overlapping peaks were deconvoluted using the IGOR-Pro software package. In our analysis, the result obtained with wild-type yeast and no damage was set to 0% Rad53 phosphorylation. Data are mean ± S.E.
DNA Damage Sensitivity Assays
10-fold serial dilutions of cells were spotted on YPD (yeast extract/peptone/dextrose) plates or YPD plates containing 10 mg/ml camptothecin or 75 mm hydroxyurea. After incubation at specified temperatures for 2–3 days, the plates were photographed.
RESULTS
A Bipartite Mec1 Activation Motif in the Unstructured C-terminal Tail of Dpb11
To further understand the mechanism of Mec1 kinase activation by Dpb11 and to separate the checkpoint function of Dpb11 from its replication function, we carried out a domain analysis of the protein, which was followed by site-directed point mutagenesis (Fig. 1). Our assay contained single-stranded DNA coated with RPA and Rad53, Mec1-Ddc2, and Dpb11 or mutants thereof. Both the Rpa1 and Rpa2 subunits of RPA and Rad53 are substrates for Mec1 kinase, and the presence of Dpb11 as an activator enhances phosphorylation of these substrates 10- to 30-fold (8). A kinase-dead version of Rad53 was used to avoid complications in the analysis because of Rad53 kinase activity. A domain analysis showed that full Mec1 activation resides in the C-terminal domain (amino acids 571–764), whereas the N-terminal region (amino acids 1–600) containing the four BRCT domains was inactive (Fig. 1B and supplemental Fig. 1A), consistent with previous studies by us and others (8, 9).
FIGURE 1.
Aromatic amino acids in Dpb11 are required for Mec1 activation. A, domain map of Dpb11 and sequence alignment of the C-terminal domains from selected fungi. The four BRCT repeats and their role in binding Sld2 and Sld3 are indicated. Selected mutants used in this study are indicated. dpb11-1 and dpb11–601 are truncations, and dpb11-3A is a triple point mutant. B, Mec1 kinase assays were as described under “Experimental Procedures” using the indicated concentrations of either full-length Dpb11 or its domains. C, as in B, with mutant forms of full-length Dpb11. D, quantification of the data in B and C. Shown is fold stimulation of Mec1 activity at 75 nm (mutant) Dpb11. Three or four independent assays were performed for each mutant, and data are mean ± S.E.
We then proceeded to determine specific motifs and amino acids critical of Mec1 activation. A multiple sequence alignment of Dpb11 and its fungal homologs revealed that the N-terminal region with four BRCT repeats was highly conserved but that the unstructured C-terminal tail containing the activity of interest was very poorly conserved (Fig. 1A). In the C-terminal region, we found three very small stretches of conserved sequences, each containing both an aromatic residue and a hydrophobic residue. One stretch at Trp-583, one at Trp-700, and a third at Tyr-735. This last conserved sequence also contains Thr-731, which in earlier work was shown to be significant for Mec1 activation (9). The conserved aromatic residues in the C-terminal domain of Dpb11 drew our attention because a previous analysis of the Ddc1 subunit of 9-1-1 had identified two aromatic amino acids in the unstructured tail of Ddc1 as critical for Mec1 activation (11). In addition, even though the domain of metazoan TopBP1 responsible for activation of ATR kinase activity bears no detectable sequence homology to the C-terminal domain of its ortholog Dpb11, mutation of a conserved Trp in the activation domain of TopBP1 abrogated its function (25). We tested the Mec1 activation activity of single and combinatorial mutations in Dpb11 overexpressed and purified from yeast (supplemental Fig. 1A). Only about 10 or 5% of wild-type activity was found for Dpb11-W700A or for Dpb11-Y735A, respectively, whereas Dpb11-T731A still showed 30–60% activity (Fig. 1, C and D). The double mutant Dpb11-WY700,735AA and the triple mutant Dpb11-WTY700,731,735AAA were completely deficient for activation of Mec1.
Unfortunately, we were unable to purify the Dpb11-W583A mutant protein from yeast as it was unstable and readily proteolyzed. Therefore, we switched to an E. coli overexpression system of a fragment comprising the C-terminal region 571–764 (supplemental Fig. 1B). The wild-type domain isolated from E. coli had the same Mec1 stimulatory activity as full-length Dpb11 isolated from yeast (supplemental Fig. 1C). Several mutants were made and tested for activity. Like with full-length Dpb11, the W700A mutation in the 571–764 domain resulted in loss of activity (supplemental Fig. 1D). However, mutant domains containing T731A and W583A showed considerable residual activity. From these data we conclude that neither W583A nor Thr-731 is essential for Mec1 stimulatory activity. Hence, W700 and Y735 are the two aromatic residues that critically contribute to Mec1 activation, and the sequence alignment with other fungal species indicates that they constitute two different conserved motifs (Fig. 1A). These results show that like Ddc1, Dpb11 has a bipartite Mec1 activation sequence with two aromatic residues, Trp-700 and Tyr-735, critical for activation.
The basal protein kinase activity of metazoan ATR is activated by an approximately 200-amino acid unstructured domain (AAD) that localizes between BRCT6 and BRCT7 of the eight BRCT domain-containing TopBP1 ortholog of Dpb11 (25). Although there is no sequence conservation between the metazoan AAD and the fungal Dpb11 C-terminal domain, the similarity between the two activation modes, i.e. the presence of essential aromatic amino acids in the disordered region, was striking to us. Indeed, Xenopus AAD was able to activate Mec1 protein kinase, although much higher concentrations of AAD were required compared with Dpb11, and full activation was not achieved (supplemental Fig. 1E). Thus, both yeast Dpb11 and metazoan TopBP1 may share a common mechanism of Mec1/ATR activation.
The Unstructured C-terminal Tail of Dpb11 Is Dispensable for DNA Replication
Having identified the motifs that are essential for Mec1 activation in vitro, we next asked which region of Dpb11 was required to permit full replication initiation function in yeast. The four N-terminal BRCT domains of Dpb11 are essential for interaction with the key replication initiation proteins Sld3 and Sld2, via domains 1 and 2 and domains 3 and 4, respectively (Fig. 1A) (21, 22). The dpb11-1 mutant, encoding a protein with a stop codon at amino acid 583 (Fig. 1A), has been used extensively in both replication and checkpoint studies since its initial isolation in 1995 (9, 11, 12, 14, 17, 23, 24, 26–28). The dpb11-1 mutant is temperature sensitive for growth, has slow progression of DNA replication, and shows defects in both the S phase and G2/M checkpoint. The fourth BRCT repeat is involved in binding Sld2, and a two-hybrid analysis showed a strong reduction in the interaction signal between Sld2 and mutant Dpb11-1 compared with the wild type (29). The minimal fourth BRCT domain ends at Leu-566, however, extensive sequence conservation continues past amino acid 583, the point of truncation in dpb11-1, until position 586 (Fig. 1A). We asked whether this small conserved sequence, which was deleted from Dpb11-1, was important for replication by generating a truncation at a more C-terminal position (amino acid 601), in a region that lacks any sequence conservation, even in highly related Saccharomyces species.
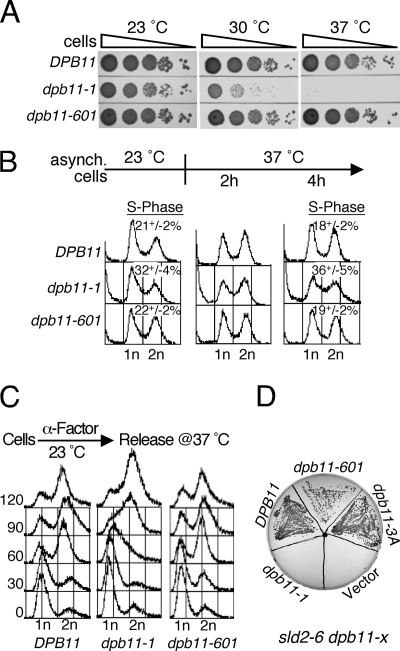
We asked if dpb11–601, which lacks all determinants for the activation of Mec1 kinase activity in vitro, can support DNA replication like the wild type and confer robust growth. We compared the growth of dpb11-1 and dpb11–601 with that of the wild type at different temperatures. As shown earlier, dpb11-1 was permissive for growth at 23 °C, semipermissive at 30 °C, and non-permissive at 37 °C (14). However, dpb11–601 showed no temperature sensitivity for growth (Fig. 2A). We then carried out a comparative FACS analysis of dpb11-1 and dpb11–601. Asynchronously growing dpb11-1 cells showed a larger fraction of S-phase cells at 23 °C compared with the wild type, and they also accumulated more dead cells (Fig. 2B). Upon a shift to 37 °C, both of these phenotypes were exacerbated. In contrast, dpb11–601 cells exhibited FACS profiles that were identical to the wild type at both temperatures. We also tested whether S phase progression in dpb11–601 was similar to that of the wild type (Fig. 2C). Cells were arrested in G1 phase at 23 °C with α factor, released from arrest at 37 °C, and S phase progression was monitored using FACS analysis (Fig. 2C). Within 60 min, most wild-type and dpb11–601 cells had entered G2 phase, and their FACS profiles were indistinguishable. In contrast, severe defects in S phase progression were detected for the dpb11-1 mutant. These data indicate that even at the permissive temperature, replication defects exist in dpb11-1, but none could be detected in dpb11–601.
FIGURE 2.
Comparison of replication and interaction defects of the dpb11-1 and dpb11–601 mutants. A, serial dilutions of wild-type and the indicated dpb11 mutants were tested for temperature sensitivity. The plates were incubated at indicated temperatures for 2 days and photographed. B, FACS profiles of asynchronously growing cells before and after the shift from 23 °C to 37 °C. The percentage of S phase cells (with S.E.) of asynchronous cells at 23 °C (three experiments), and after shift to 37 °C (three experiments for 4 h shift) is shown. C, cells were grown and synchronized in G1 phase using α mating factor at 23 °C for 3 h and released into fresh media at 37 °C containing 0.1 mg/ml Pronase. Cells were collected at the indicated time points at 37 °C and subjected to FACS analysis. D, strain PY264 (sld2–6, dpb11-Δ) containing plasmid pBL514 (URA3 DPB11) was transformed at 23 °C with the pLB530 series of plasmids (HIS3, wild-type or mutant DPB11) or with empty vector. Transformants were grown on synthetic complete His− plates and then streaked onto a 5-fluoro orotic acid containing plate at 23 °C. dpb11–3A, dpb11-WTY700,731,735AAA.
The replication initiator Sld2 binds to the BRCT3-BRCT4 motifs of Dpb11, and this binding is decreased in the dpb11-1 mutant (29). The temperature-sensitive sld2–6 allele shows a synthetic lethality phenotype with dpb11-1 (24). We asked whether extending the BRCT4 motif with the extra 18 amino acids present in dpb11–601 alleviated this replication initiation defect. A sld2–6 dpb11-Δ mutant, which was kept alive by the presence of DPB11 on a URA3 centromere plasmid, was transformed with a pBL530-series plasmid containing either DPB11, dpb11-1, dpb11–601, dpb11-3A (WTY700,731,735AAA), or an empty vector as a negative control, and the URA3-DPB11 plasmid was evicted at 23 °C on 5-fluoroorotic acid-containing media. Although no growth was observed with dpb11-1, reiterating the previously observed synthetic lethality with sld2–6, robust growth was observed with dpb11–601 and dpb11-3A (Fig. 2D). These data indicate that the synthetic lethality between dpb11-1 and sld2–6 was caused by the replication defect and not by the checkpoint defect in this DPB11 allele.
The C-terminal Tail of Dpb11 Is Dispensable for the Replication Checkpoint and the G1 DNA Damage Checkpoint
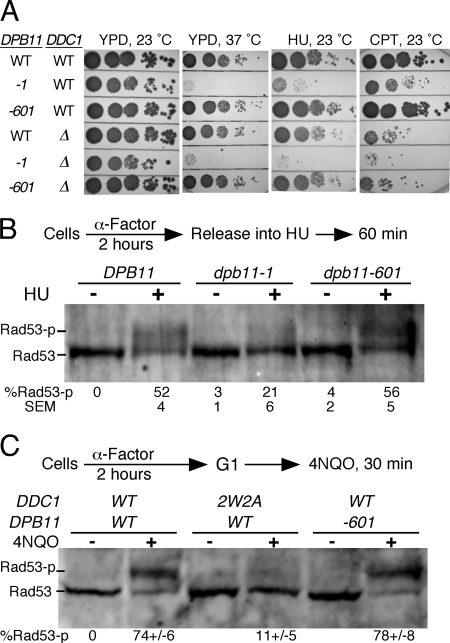
Now that we have been able to separate the replication domain of Dpb11 from its Mec1 activation domain, we wanted to reinvestigate the role of Dpb11 in checkpoint function during the different phases of the yeast cell cycle. The dpb11-1 mutant is extremely sensitive to the replication inhibitor hydroxyurea, and this sensitivity has been attributed in part to the replication checkpoint defect of this mutant (26, 28). We designed a set of six isogenic strains with either wild-type DPB11 or the dpb11-1 or dpb11–601 alleles, with either DDC1 or ddc1-Δ, the subunit of 9-1-1 responsible for Mec1 activation in G1 and G2/M (2). Growth at 37 °C showed that the dpb11–601 allele did not become temperature-sensitive when combined with ddc1-Δ (Fig. 3A). All damage survival assays were carried out at 23 °C.
FIGURE 3.
The Dpb11 C-terminal tail is dispensable for the replication checkpoint. A, serial dilutions of the wild type and the indicated mutants were tested for sensitivity to growth on hydroxyurea (HU)- (75 mm) and camptothecin (CPT)- (10 μg/ml) containing YPD plates. The plates were incubated at 23 °C for 2 days and photographed. B, cells were synchronized in G1 phase using α mating factor at 23 °C for 3 h and released into fresh media containing 0.1 mg/ml Pronase at 37 °C. The cells were allowed to grow for 30 min, and then hydroxyurea (0.2 m) was added and incubation continued for 1 h. C, cells were synchronized in G1 phase using α mating factor at 30 °C for 2 h, and treated with 4NQO for 30 min. Extracts were subjected to Western blot analysis using anti Rad53 antibody. Mean ± S.E. for four (B) and two (C) independent experiments is shown.
Growth of dpb11-1 on hydroxyurea-containing media was strongly inhibited, and this sensitivity was further enhanced in the double mutant with ddc1-Δ (Fig. 3A). Surprisingly, the dpb11–601 allele was not at all sensitive to hydroxyurea, either alone or when combined with ddc1-Δ (some minor hydroxyurea sensitivity is associated with the DDC1 deletion). Therefore, if defects in the replication checkpoint are associated with an increase in hydroxyurea sensitivity, one can conclude that the dpb11–601 mutant, although deficient for Mec1 activation in vitro, is proficient for the replication checkpoint. Camptothecin is a DNA topoisomerase inhibitor that induces stalling of replication forks and the generation of double-stranded breaks. Defects in the S phase damage and G2/M checkpoints are associated with camptothecin sensitivity. The ddc1-Δ mutant is sensitive to camptothecin because of its demonstrated defect in the G2/M DNA damage checkpoint. However, this sensitivity is not increased in the double mutant ddc1-Δ dpb11–601, nor does the single dpb11–601 mutant show an increased sensitivity to camptothecin compared with the wild type (Fig. 3A, right panel). Therefore, both the increased hydroxyurea and camptothecin sensitivity observed in the dpb11-1 mutant is most likely caused by replication-related defects and not by checkpoint-related defects.
Mec1-dependent initiation of cell cycle checkpoints that result from DNA damage or the stalling of replication forks proceeds through phosphorylation of the effector kinase Rad53, which in turn phosphorylates downstream targets that propagate these signal transduction pathways. Therefore, Rad53 phosphorylation has been used as a diagnostic probe for an active checkpoint that is associated with cell cycle arrest, in G1 or G2/M, or cell cycle slowdown in S phase (30–36). Here, we tested whether dpb11–601 is defective in S phase checkpoint activation by monitoring Rad53 phosphorylation upon hydroxyurea treatment of cells. As anticipated from the growth data, Rad53 phosphorylation after hydroxyurea treatment of dpb11–601 cells was similar to the wild type (Fig. 3B). These data suggest that dpb11–601 is proficient in both DNA replication and in the activation of the replication checkpoint in response to hydroxyurea. However, the dpb11–1 mutant showed a strong defect in the phosphorylation of Rad53 upon growth on hydroxyurea. Considering the disparate results that we obtained for dpb11-1 and dpb11–601, it is likely that the replication checkpoint defect observed in dpb11-1 is a consequence of its replication initiation defect.
We determined previously that DNA damage checkpoint activation during the G1 phase of the cell cycle is uniquely dependent on the capacity of the Ddc1 subunit of 9-1-1 to activate Mec1 kinase activity but not on the activation properties of Dpb11 (11). However, as these studies were carried out with the dpb11-1 mutant, which has a mixed replication/checkpoint phenotype, we repeated them with the well defined dpb11–601 mutant. Analogous results were obtained. The G1 checkpoint was abrogated in ddc1–2W2A (a double point mutant that is defective for Mec1 activation) but not in the dpb11–601 mutant (Fig. 3C).
The C-terminal Tail of Dpb11 Is Required for Robust G2 Checkpoint Activation
We have shown earlier that the 9-1-1 clamp contributes to G2 checkpoint activation in response to the UV-mimetic damaging agent 4NQO (4-nitroquinoline 1-oxide) by two mechanisms (11). One mechanism involves direct activation of the Mec1 kinase. Conserved aromatic amino acids in the unstructured C-terminal tail of Ddc1 directly mediate activation of Mec1, and a double point mutant, ddc1–2W2A (ddc1-WW352,544AA), is completely defective. The other mechanism involves the recruitment of Dpb11 by the clamp via the unstructured C-terminal tail of Ddc1, followed by Dpb11-mediated Mec1 activation. Phosphorylation of Tyr-602 in the Ddc1 tail is required for recruitment, and this recruitment mechanism is evolutionary conserved (37). We previously studied the role of Dpb11 in the G2 checkpoint using the dpb11-1 mutant prior to recognizing the severe replication defects associated with this allele (11). Here, we have reinvestigated the roles of Ddc1 and Dpb11 in checkpoint function using both the dpb11–601 truncation allele as well as single and multiple point mutants in the aromatic residues required for Mec1 activation in vitro.
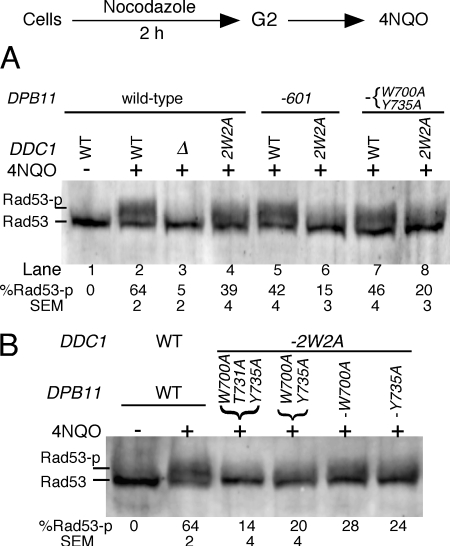
Cells were arrested in the G2/M phase of the cell cycle with nocodazole, treated with the UV-mimetic agent 4NQO, and the efficiency of DNA damage checkpoint activation was assessed by measuring the extent of Rad53 phosphorylation. Activation was abrogated in a ddc1-Δ strain (Fig. 4A, lane 3), as shown previously (10, 11, 38). Previously, we engineered the ddc1–2W2A mutant, which is defective for the activation of Mec1 kinase activity in vitro but not for recruitment of Dpb11 (11). This mutant showed a partial defect in Rad53 phosphorylation in G2 phase after 4NQO treatment (Fig. 4A, lane 4). Dpb11–601, which is also defective for the activation of Mec1 kinase activity in vitro (Fig. 1A), similarly showed a minor but significant defect in Rad53 phosphorylation (Fig. 4A, lane 5). However, when ddc1–2W2A was combined with dpb11–601, thereby eliminating Mec1 activation by both factors, the G2 checkpoint was almost completely abrogated (Fig. 4A, lane 6). Similarly, a substantial defect in Rad53 phosphorylation was observed when ddc1–2W2A was combined with the double point mutant dpb11-WY700,735AA (Fig. 4A, lane 8) or the triple mutant dpb11-WTY700,731,735AAA (B). Single point mutations that retained residual Mec1 activation activity in vitro (Fig. 1C) showed only partial defects in the G2 checkpoint (Fig. 4B). Therefore, when both Ddc1 and Dpb11 are unable to activate Mec1 kinase in vitro, the G2/M checkpoint is almost completely defective, as assessed by the lack of phosphorylation of Rad53.
FIGURE 4.
The Dpb11 C-terminal tail is required for robust G2 checkpoint activation. Log phase cells at 30 °C were arrested in G2 phase with nocodazole and then treated with 4NQO (see “Experimental Procedures”). Extracts were subjected to Western blot analysis using anti Rad53 antibody. A, analysis of DDC1 or ddc1–2W2A with the indicated DPB11 mutants (mean ± S.E. for three experiments). B, analysis of DPB11 point mutants in a ddc1–2W2A background.
These varying checkpoint defects in the various mutants are reflected in the sensitivity they show to damaging agents, in particular to the topoisomerase drug camptothecin, which elicits a strong G2/M checkpoint response. Single mutants of DDC1 (supplemental Fig. 2, row 2) or of DPB11 (supplemental Fig. 2, rows 4 and 7) that are individually defective for activation of Mec1 showed no sensitivity to camptothecin, whereas the double mutants (supplemental Fig. 2, rows 5 and 8) showed moderate sensitivity to camptothecin. The phenotype of the triple point mutant in DPB11 (supplemental Fig. 2, row 5) was indistinguishable from that of its C-terminal truncation dpb11–601 (supplemental Fig. 2, row 8). As noted before, the ddc1-Δ mutant shows greater damage sensitivity than the combined mutants that abrogate Mec1 activation by both Dpb11 and Ddc1 (supplemental Fig. 2A, compare ddc1–2W2A dpb11–601 (row 5) or ddc1–2W2A dpb11–3A (row 8) with ddc1-Δ in rows 3, 6, or 9), indicative of additional functions of the 9-1-1 complex in DNA damage response and DNA repair (2). In agreement with the sensitivity data in Fig. 3A discussed above, none of the DPB11 tail mutants showed sensitivity to hydroxyurea (supplemental Fig. 2, center panel), consistent with our conclusion that the replication functions of DPB11 stayed intact in these mutants.
DISCUSSION
A Bipartite Mec1 Activation Motif in the Dpb11 C-terminal Tail Contributes to G2/M Checkpoint Activation
Our analysis of the motifs in Dpb11 that are required to activate Mec1 kinase activity in vitro has revealed a strong similarity between this activator and the Ddc1 subunit of the 9-1-1 checkpoint clamp. Both Mec1 activators have a C-terminal tail that is intrinsically disordered (Fig. 5A). One of the two motifs in Ddc1 that are essential for activation is localized near the end of the PCNA-like domain and the second motif approximately 200 amino acids further down in the disordered region. For Dpb11, both motifs essential for activation are localized in the disordered region, separated by approximately 30 amino acids. An analysis of these motifs in ten fungal species, from S. cerevisiae to S. pombe, shows very little conservation for each motif and no conservation in primary sequence distance between the two motifs beyond the presence of essential aromatic amino acids and one or two adjacent hydrophobic/aromatic residues, as well as a prevalent glycine residue (Fig. 5B).
FIGURE 5.
Ddc1 and Dpb11 activate Mec1 by a similar mechanism. A, predicted intrinsically unstructured regions using IUPred and curve smoothing. Scores > 0.5 are considered to be structurally disordered. The critical aromatic amino acids for Mec1 activation are indicated by arrows and also in the WEBLogo of ten fungal sequences in B. C, model for Mec1 activation in the different phases of the cell cycle.
The importance of these motifs is validated by our genetic analysis. In S. cerevisiae, activation of the DNA damage checkpoint in G1 phase is completely dependent on Mec1 activation by the 9-1-1 clamp, and the checkpoint is abrogated in a ddc1–2W2A mutant with the two critical tryptophans at 352 and 544 mutated to alanines (11) (Figs. 3C and 5C). A contribution of Dpb11 to the G1 checkpoint has not been apparent in our studies. However, when yeast cells are deleted for the histone H3 methylase gene DOT1, which strongly reduces the localization of checkpoint factors to damaged chromatin, an additional deleterious effect of the dpb11-1 mutation on the G1 damage checkpoint has been observed (12). The DNA damage checkpoint in G2/M is dependent on Mec1 activation by 9-1-1 and by Dpb11, and the contribution by Dpb11 is abrogated in point mutants in which both motifs in the unstructured C terminus have been mutated (Fig. 4). Their phenotypes are comparable with that of dpb11–601, in which the entire 174-amino acid unstructured C-terminal tail has been deleted. Currently it is not clear what factors regulate the relative contributions of Dpb11 to either the G1 or G2 checkpoint.
The Mec1-activating Function of Dpb11 Is Not Essential for the Replication Checkpoint
DNA replication and the replication checkpoint are intimately connected (14, 16, 39, 40). The establishment of replication forks is required for checkpoint activation in S phase (19). Intriguingly, the robustness of the replication checkpoint is reduced or abrogated in yeast replication initiation mutants that cause the establishment of fewer replication forks during S phase (18). Therefore, a critical number of active DNA replication forks may exist, below which threshold the checkpoint machinery of the cell is unresponsive to imposed replication stress. Indeed, many replication initiation mutants show a defect in the replication checkpoint (14–17, 19), but with the exception of one study with an orc2–1 mutant (18), the inverse correlation between replication fork number and checkpoint function has not been addressed.
In this study, we have investigated the role of Dpb11 in checkpoint function during the different phases of the cell cycle. An incisive study of Dpb11 in particular has been complicated because this protein is both a replication initiation factor and a bona fide checkpoint protein through its Mec1 activation function. The establishment of separation of function mutants of DPB11 was essential to unraveling the contributions made directly by Dpb11 through activation of Mec1 from those made indirectly through its role in the assembly of replication forks. Our studies are generally in agreement with studies of Xenopus TopBP1, in which the replication function of this protein was assigned to the N-terminal half with BRCT domains 1–5, which are homologous to S. cerevisiae BRCT domains 1–4, and the checkpoint function was assigned to the C-terminal half, which contains the ATR-activation domain AAD (25, 41).
We have made S. cerevisiae mutant dpb11–601 (truncated at amino acid 601) that is fully proficient for replication assembly but defective for Mec1 kinase activation. The phenotypes of dpb11–601 are the same as those of a triple point mutant (dpb11-WTY700,731,735AAA), specifically mutated in the amino acids essential for Mec1 activation. The dpb11–601 mutant is neither temperature-sensitive for growth, nor synthetically lethal with sld2–6, nor sensitive to hydroxyurea, and the mutant is not defective for the replication checkpoint in response to hydroxyurea. The classical and often used dpb11-1 mutant (truncated at amino acid 583) displays all of these phenotypes. We conclude that the known checkpoint function of Dpb11, i.e. the activation of Mec1 kinase activity, is not essential for a functional replication checkpoint. Consequently, the replication checkpoint defect observed in dpb11-1 cannot be the result of a failure by the mutant protein to activate Mec1 but is likely caused indirectly by the replication initiation defect of this allele. We note that the dpb11-1 mutant accumulates S phase cells and dead cells even at 23 °C (Fig. 2B). Interestingly, a temperature-sensitive allele of SLD2 had also been named drc1-1, designating a mutant defective for the DNA replication checkpoint (17). In addition, mutations in genes coding for the leading strand polymerase complex DNA polymerase e also show defects in the replication checkpoint (16, 28). The genetic and biochemical connectivity between Dpb11, Sld2, and Pol e suggests a model in which defects in this complex, which is required both for replisome assembly and for leading strand replication, are associated with replication checkpoint defects that potentially result from a decrease in the number of active replication forks.
Our conclusion that, paradoxically, defects in the replication function rather than the checkpoint function of DPB11 mutants determine the efficiency of the replication checkpoint may have implications for analogous pathways in other organisms. In S. pombe, most checkpoint studies have been carried out with the temperature-sensitive rad4–116 allele of Rad4/Cut5, the ortholog of S. cerevisiae Dpb11 and metazoan TopBP1. The central importance attributed to Rad4 in the intra S phase checkpoint in S. pombe could possibly in part be a consequence of defective replisome assembly, which may cause indirectly a defect in activation of Rad3 (ortholog of Mec1) (42). And although several other Rad4 mutants have been studied with various defects in DNA replication and checkpoint function (43), to our knowledge no Rad4/Cut5 mutants have been isolated that affect the C-terminal domain in which the Rad3-activation domain likely resides.
Our analysis of Ddc1 and Dpb11 has generated mutants that are specifically defective for the activation of Mec1. With these mutants, we have shown that the replication checkpoint is still functional. These data suggest that additional activator(s) of Mec1 must exist that are specific for the function of the replication checkpoint (Fig. 5C). Our analysis does not allow us to conclude that Dpb11 and 9-1-1 do not function during the replication checkpoint in response to stalling of the replication fork. However, if these activators do function, they do so redundantly with additional activator(s). The increased insight into the required structural motifs for Mec1 activation gained from the current study may allow us to apply bioinformatics to identify additional potential activators of Mec1 kinase.
Supplementary Material
Acknowledgments
We thank Bonnie Yoder and Carrie Stith for strain construction and protein purification, Lynn White and Helen Piwnica-Worms for help with FACS analysis, and John Majors for critical discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants GM032431 and GM083970.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” Figs. 1 and 2, and Tables S1–S4.
- RPA
- replication protein A
- ATR
- ataxia telangiectasia and Rad3-related
- 9-1-1
- Ddc1-Rad17-Mec3 checkpoint complex
- AAD
- ATR activation domain
- 4NQO
- 4-nitroquinoline 1-oxide
- BRCT
- BRCA1 C-terminal.
REFERENCES
- 1. Nyberg K. A., Michelson R. J., Putnam C. W., Weinert T. A. (2002) Annu. Rev. Genet. 36, 617–656 [DOI] [PubMed] [Google Scholar]
- 2. Navadgi-Patil V. M., Burgers P. M. (2009) DNA Repair 8, 996–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedel A. M., Pike B. L., Gasser S. M. (2009) Curr. Opin. Cell Biol. 21, 237–244 [DOI] [PubMed] [Google Scholar]
- 4. Rouse J., Jackson S. P. (2002) Mol. Cell 9, 857–869 [DOI] [PubMed] [Google Scholar]
- 5. Zou L., Elledge S. J. (2003) Science 300, 1542–1548 [DOI] [PubMed] [Google Scholar]
- 6. Bonilla C. Y., Melo J. A., Toczyski D. P. (2008) Mol. Cell 30, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Majka J., Niedziela-Majka A., Burgers P. M. (2006) Mol. Cell 24, 891–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Navadgi-Patil V. M., Burgers P. M. (2008) J. Biol. Chem. 283, 35853–35859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mordes D. A., Nam E. A., Cortez D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 18730–18734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Longhese M. P., Paciotti V., Fraschini R., Zaccarini R., Plevani P., Lucchini G. (1997) EMBO J. 16, 5216–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Navadgi-Patil V. M., Burgers P. M. (2009) Mol. Cell 36, 743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Puddu F., Granata M., Di Nola L., Balestrini A., Piergiovanni G., Lazzaro F., Giannattasio M., Plevani P., Muzi-Falconi M. (2008) Mol. Cell. Biol. 28, 4782–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furuya K., Poitelea M., Guo L., Caspari T., Carr A. M. (2004) Genes Dev. 18, 1154–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Araki H., Leem S. H., Phongdara A., Sugino A. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 11791–11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D'Urso G., Grallert B., Nurse P. (1995) J. Cell Sci. 108, 3109–3118 [DOI] [PubMed] [Google Scholar]
- 16. Navas T. A., Zhou Z., Elledge S. J. (1995) Cell 80, 29–39 [DOI] [PubMed] [Google Scholar]
- 17. Wang H., Elledge S. J. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3824–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimada K., Pasero P., Gasser S. M. (2002) Genes Dev. 16, 3236–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tercero J. A., Longhese M. P., Diffley J. F. (2003) Mol. Cell 11, 1323–1336 [DOI] [PubMed] [Google Scholar]
- 20. Cobb J. A., Shimada K., Gasser S. M. (2004) Curr. Opin. Genet. Dev. 14, 292–300 [DOI] [PubMed] [Google Scholar]
- 21. Zegerman P., Diffley J. F. (2007) Nature 445, 281–285 [DOI] [PubMed] [Google Scholar]
- 22. Tanaka S., Umemori T., Hirai K., Muramatsu S., Kamimura Y., Araki H. (2007) Nature 445, 328–332 [DOI] [PubMed] [Google Scholar]
- 23. Masumoto H., Sugino A., Araki H. (2000) Mol. Cell. Biol. 20, 2809–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kamimura Y., Masumoto H., Sugino A., Araki H. (1998) Mol. Cell. Biol. 18, 6102–6109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumagai A., Lee J., Yoo H. Y., Dunphy W. G. (2006) Cell 124, 943–955 [DOI] [PubMed] [Google Scholar]
- 26. Wang H., Elledge S. J. (2002) Genetics 160, 1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogiwara H., Ui A., Onoda F., Tada S., Enomoto T., Seki M. (2006) Nucleic Acids Res. 34, 3389–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puddu F., Piergiovanni G., Plevani P., Muzi-Falconi M. (2011) PLoS Genet. 7, e1002022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tak Y. S., Tanaka Y., Endo S., Kamimura Y., Araki H. (2006) EMBO J. 25, 1987–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allen J. B., Zhou Z., Siede W., Friedberg E. C., Elledge S. J. (1994) Genes Dev. 8, 2401–2415 [DOI] [PubMed] [Google Scholar]
- 31. Weinert T. A., Kiser G. L., Hartwell L. H. (1994) Genes Dev. 8, 652–665 [DOI] [PubMed] [Google Scholar]
- 32. Paulovich A. G., Hartwell L. H. (1995) Cell 82, 841–847 [DOI] [PubMed] [Google Scholar]
- 33. Sun Z., Fay D. S., Marini F., Foiani M., Stern D. F. (1996) Genes Dev. 10, 395–406 [DOI] [PubMed] [Google Scholar]
- 34. Sanchez Y., Desany B. A., Jones W. J., Liu Q., Wang B., Elledge S. J. (1996) Science 271, 357–360 [DOI] [PubMed] [Google Scholar]
- 35. Sugimoto K., Ando S., Shimomura T., Matsumoto K. (1997) Mol. Cell. Biol. 17, 5905–5914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pellicioli A., Lucca C., Liberi G., Marini F., Lopes M., Plevani P., Romano A., Di Fiore P. P., Foiani M. (1999) EMBO J. 18, 6561–6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Navadgi-Patil V. M., Burgers P. M. (2011) Biochem. Soc. Trans. 39, 600–605 [DOI] [PubMed] [Google Scholar]
- 38. Kondo T., Matsumoto K., Sugimoto K. (1999) Mol. Cell. Biol. 19, 1136–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alcasabas A. A., Osborn A. J., Bachant J., Hu F., Werler P. J., Bousset K., Furuya K., Diffley J. F., Carr A. M., Elledge S. J. (2001) Nat. Cell Biol. 3, 958–965 [DOI] [PubMed] [Google Scholar]
- 40. Lou H., Komata M., Katou Y., Guan Z., Reis C. C., Budd M., Shirahige K., Campbell J. L. (2008) Mol. Cell 32, 106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hashimoto Y., Tsujimura T., Sugino A., Takisawa H. (2006) Genes Cells 11, 993–1007 [DOI] [PubMed] [Google Scholar]
- 42. Marchetti M. A., Kumar S., Hartsuiker E., Maftahi M., Carr A. M., Freyer G. A., Burhans W. C., Huberman J. A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 7472–7477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taricani L., Wang T. S. (2006) Mol. Biol. Cell 17, 3456–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.