Background: Transport of GABA into astrocytes is crucial for excitability control.
Results: The neurotrophin BDNF, through TrkB-t receptor activation, enhances GABA transport into astrocytes, which requires adenosine A2A receptor signaling.
Conclusion: BDNF plays an active role in the synaptic clearance of GABA.
Significance: This new regulatory role for TrkB-t receptors discloses their relevance for excitability control at the tripartite synapse.
Keywords: MAP Kinases (MAPKs), Neurotransmitter Transport, Neurotrophic Factor, Phospholipase C, Tyrosine Protein Kinase (Tyrosine Kinase), Adenosine Receptors, Astrocytes, GABA, GAT-1 Transporter, TrkB Receptor
Abstract
The γ-aminobutyric acid (GABA) transporters (GATs) are located in the plasma membrane of neurons and astrocytes and are responsible for termination of GABAergic transmission. It has previously been shown that brain derived neurotrophic factor (BDNF) modulates GAT-1-mediated GABA transport in nerve terminals and neuronal cultures. We now report that BDNF enhances GAT-1-mediated GABA transport in cultured astrocytes, an effect mostly due to an increase in the Vmax kinetic constant. This action involves the truncated form of the TrkB receptor (TrkB-t) coupled to a non-classic PLC-γ/PKC-δ and ERK/MAPK pathway and requires active adenosine A2A receptors. Transport through GAT-3 is not affected by BDNF. To elucidate if BDNF affects trafficking of GAT-1 in astrocytes, we generated and infected astrocytes with a functional mutant of the rat GAT-1 (rGAT-1) in which the hemagglutinin (HA) epitope was incorporated into the second extracellular loop. An increase in plasma membrane of HA-rGAT-1 as well as of rGAT-1 was observed when both HA-GAT-1-transduced astrocytes and rGAT-1-overexpressing astrocytes were treated with BDNF. The effect of BDNF results from inhibition of dynamin/clathrin-dependent constitutive internalization of GAT-1 rather than from facilitation of the monensin-sensitive recycling of GAT-1 molecules back to the plasma membrane. We therefore conclude that BDNF enhances the time span of GAT-1 molecules at the plasma membrane of astrocytes. BDNF may thus play an active role in the clearance of GABA from synaptic and extrasynaptic sites and in this way influence neuronal excitability.
Introduction
γ-Aminobutyric acid (GABA) is the predominant inhibitory neurotransmitter in the central nervous system. Its activity at the synapse is terminated by reuptake into nerve terminals and astrocytes, through membrane-located GABA transporters (GATs),2 which therefore shape GABAergic transmission. There are three main high affinity subtypes of GATs, GAT-1, GAT-2, and GAT-3, and a low affinity one, the betaine transporter. GAT-1 is the predominant GABA transporter in the brain and is expressed in both neurons and astrocytes; GAT-3 is predominantly, if not exclusively, expressed in astrocytes (for a review, see Ref. 1). Interestingly, patients with temporal lobe epilepsy have an enhanced astrocytic expression of GATs (2), and inhibitors of GABA transport or GABA metabolism in astrocytes are efficient antiepileptic drugs (3, 4), highlighting the relevant role of glial GATs in the control of excitability.
The regulation of the continuous traffic of GATs to and from the neuronal plasma membrane can occur through changes in the endocytosis and exocytosis rates and/or the number of transporters available for recycling (5). Surface expression of GAT-1 in cultured neurons (6, 7) and isolated nerve terminals (8) is decreased by protein kinase C (PKC)-dependent phosphorylation. In contrast, surface expression of GAT-1 in neurons is enhanced by brain-derived neurotrophic factor (BDNF)-mediated tyrosine kinase-dependent phosphorylation (9, 10). BDNF, however, inhibits GAT-1-mediated GABA transport at the isolated nerve endings (11), suggesting that this neurotrophin operates in a very localized way, so that it may retard GABA uptake by the nerve terminal, enhancing synaptic actions of GABA, and accelerate its reuptake at extracellular neuronal areas, allowing replenishment of neuronal pools of GABA. In nerve terminals, the regulation of GAT-1 by BDNF is modulated by activation of adenosine A2A receptors (11), as it is observed for other fast BDNF actions (for a review, see Ref. 12).
Astrocytes, the major class of glial cells in the mammalian brain, play a relevant role in synaptic transmission and contribute to information processing because they can control the ionic environment of the neuropil and control the supply of several neurotransmitters to synapses (13–15) as well as modulating cell-to-cell communication (16). Astrocytes play an important role in the regulation of extracellular GABA levels (17), but surprisingly little is known about how GABA transporters are controlled in these cells. We now report the action of BDNF upon the GAT-1- and GAT-3-mediated GABA transport in astrocytes and the underlying mechanisms for its action.
EXPERIMENTAL PROCEDURES
Rat Astrocyte Cell Cultures
Cultures of astrocytes from rat cerebral cortex were prepared as reported previously (18). In brief, rat brains were dissected out of newborn Wistar rats (0–2 days old). The cortex was dissected in cold PBS solution (140 mm NaCl, 2.7 mm KCl, 1.5 mm KH2PO4, and 8.1 mm NaHPO4, pH 7.40) and was dissociated gently by trituration in 4.5 g/liter glucose Dulbecco's modified Eagle's medium (DMEM; Invitrogen). Next, the dissociated cells were filtered through meshes of 230 μm and centrifuged at 200 × g for 10 min at room temperature. The pellet was resuspended in 4.5 g/liter glucose DMEM, passed through meshes of 70 μm (BD Falcon, Erembodegem, Belgium), and centrifuged at 200 × g for 10 min at room temperature. Cells were seeded into 24-well plates for uptake experiments, 6-well plates for biotinylation experiments, and 96-well plates for ELISA experiments. Cultures were maintained for 3 weeks in 4.5 g/liter glucose DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 0.01% antibiotic/antimycotic (Sigma) at 37 °C in a humidified atmosphere with 5% CO2.
GABA Uptake Mediated by GABA Transporters in Rat Astrocytes
For determination of GABA uptake, astrocytes were preincubated for 3 h at 37 °C in serum-free 1 g/liter glucose DMEM (Invitrogen). Following preincubation, the medium was then exchanged with control DMEM or drug-containing DMEM. The transport was initiated by the addition of 30 μm [3H]GABA (specific activity 0.141 Ci/mmol) (PerkinElmer Life Sciences) in a transport buffer (KHR) composed of 137 mm NaCl, 5.4 mm KCl, 1.8 mm CaCl2·2H2O, 1.2 mm MgSO4, and 10 mm HEPES, pH adjusted with Tris-base to 7.40. For determination of saturation curves of GABA transporters, GABA uptake assays were performed for 1 min at 37 °C using 8.62 nm [3H]GABA and increasing amounts of unlabeled GABA (final concentrations 1–50 μm). Transport was stopped 1 min after [3H]GABA addition by rapidly washing the cells twice with ice-cold stop buffer (137 mm NaCl and 10 mm HEPES, pH adjusted with Tris-base to 7.40) followed by solubilization with lysis buffer (100 mm NaOH and 0.1% SDS) at 37 °C for 1 h. The amount of [3H]GABA taken up by astrocytes was quantified by liquid scintillation counting. GAT-1- and GAT-3-mediated GABA uptake was taken as the difference between the [3H]GABA uptake in the absence and in the presence of the GAT-1 blocker, SKF 89976a (20 μm), and the GAT-3 blocker, SNAP 5114 (20 μm), respectively.
Except when otherwise indicated, BDNF was added to astrocytes 10 min before the addition of [3H]GABA, and the effect of BDNF was expressed relative (in percentage) to the uptake of GABA in the absence of BDNF in the same experiments and under the same experimental conditions. Whenever the influence of any drug on the effect of BDNF was tested, that drug was incubated with the cells for 20 min before the addition of BDNF except when otherwise indicated; the effect of BDNF in the presence of these drugs was expressed relative (in percentage) to the uptake of GABA in the presence of the same drugs but in the absence of BDNF. GAT-1 and GAT-3 blockers were added to astrocytes at the same time as other drugs used. For protein determination, the Bio-Rad Dc protein assay was used.
Plasmid Construction
To generate an HA-tagged GAT-1, the sequence of YPYDVPDYA (HA epitope) was inserted in extracellular loop 2 (EL2) of rat GAT-1-pRc/CMV as described previously (19). The HA tag insertion was made using a Stratagene (La Jolla, CA) QuikChange mutagenesis kit according to the manufacturer's protocol. The designed primers for HA tag insertion in EL2 of rat GAT-1 were as follows: forward, CTGGTCAACACCACCTACCCCTACGACGTCCCCGATTACGCCTCACTCAACATGACCAGTGCC; reverse, GGCACTCATGTTGAGTGAGGCGTAATCGGGGACGTCGTAGGGGTAGGTGGTGTTGCCAG. The HA tag insertion was verified by automatic dideoxynucleotide sequencing.
To insert GAT-1 and HA-tagged GAT-1 into the lentiviral transfer vector pHsCXW (20), a forward primer (GCATAAGCTTCTAGACATGGCGACTGACAACAGC) containing an XbaI digestion site and a reverse primer (CAACTAGAAGGCACAGTCGAG) annealing downstream from the XbaI restriction site in pRc/CMV were used to amplify the rat GAT-1 sequence by PCR using Phusion polymerase. Both PCR fragments, GAT-1 and HA-GAT-1, were cloned into pHsCXW vector, using XbaI restriction enzyme.
Lentivirus Production and Transduction
Lentiviral vectors were produced according to procedures modified from Ref. 21. HEK293T packaging cells (ATCC number CRL-11268) were plated on polylysine-coated 175-cm2 flasks and transiently triple-transfected with the following: 1) 18 μg of a packaging plasmid encoding viral structure proteins (pBRΔ8.91) (22); 2) 12 μg of an envelope plasmid encoding the envelope protein VSV-G (pMD.G) (23); and 3) 18 μg of the transfer plasmid containing the gene of interest (pHsCXW-rGAT-1 or pHsCXW-HA-rGAT-1). Transfection was performed in DMEM supplemented with 10% FBS using calcium phosphate precipitation. Medium was replaced with fresh medium after 5 h. Approximately 48 and 72 h after transfection, medium containing lentivirus was collected, centrifuged at 900 × g for 5 min to remove cellular debris, filtered through a 0.45-μm filter, and concentrated by ultracentrifugation at 50,000 × g for 1.5 h at 4 °C. The virus-containing pellet was resuspended in minimum essential medium (Sigma) at of the original volume and stored in aliquots at −80 °C. The astrocyte cultures were incubated with concentrated lentivirus on days 13–15 in vitro, and experiments were performed 6–8 days after infection.
HEK293 Cell Culturing and Transfection
HEK293 cells (ATCC number CRL-1573) were grown in DMEM supplemented with 10% FBS and gentamicin (10 μg/ml) at 37 °C in a humidified incubator with 5% CO2. Transfection was carried out using Lipofectamine 2000 (Invitrogen). For uptake experiments, 1 μg of plasmid encoding the cDNA of interest was used for transient transfection of cells in a 75-cm2 culture flask. Cells were assayed 48–72 h after transfection.
Biotinylation Experiments
Astrocytes transduced with rGAT-1 were used for the biotinylation assays. After a starvation period of 3 h, cells were treated with BDNF for 10 min. The cells were rinsed twice with 4 °C PBS/Ca2+/Mg2+ (138 mm NaCl, 2.7 mm KCl, 1.5 mm KH2PO4, 9.6 mm Na2HPO4, 1 mm MgCl2, 0.1 mm CaCl2, pH 7.3). The cells were next incubated with a solution containing 1.2 mg/ml sulfo-NHS biotin (Pierce) in PBS/Ca2+/Mg2+ for 40 min at 4 °C. The biotinylation solution was removed by two washes in PBS/Ca2+/Mg2+ plus 100 mm glycine. The cells were rinsed twice with 4 °C PBS/Ca2+/Mg2+. The cells were solubilized with lysis buffer (25 mm Tris-base, pH 7.5, 150 mm NaCl, 5 mm N-ethylmaleimide, 1 mm EDTA, 1% Triton X-100, 0.2 mm phenylmethanesulfonyl fluoride (PMSF), and protease inhibitor mixture from Roche Applied Science) at 4 °C and incubated with rotation for 20 min at 4 °C. The cell lysates were centrifuged at 16,000 × g at 4 °C for 15 min. The supernatant fractions (300 μg of protein) were incubated with avidin-agarose resin (Pierce) at room temperature for 60 min. The beads were washed four times with lysis buffer, and adsorbed proteins were eluted with 5× SDS sample buffer (50 mm Tris-Cl, pH 6.8, 2% SDS, 100 mm dithiothreitol (DTT), 0.1% bromphenol blue, 10% glycerol) at 37 °C for 30 min. The supernatant was collected (surface membrane expression) for Western blot analysis. Astrocyte lysates were also denaturated with 5× SDS sample buffer, and an equal quantity (30 μg) were used for Western blot analysis. Protein determination was made using the Bio-Rad Dc protein assay.
Western Blot Assays
Both the extracts from biotinylated fractions and the astrocyte lysates were run on a 7.5% SDS-polyacrylamide gel. Protein was transferred to a PDVF membrane (Millipore, Bedford, MA) by electroblotting and blocked for 1 h at room temperature with 5% nonfat milk in PBS with 0.05% Tween 20 (PBS-T). Incubations with primary antibodies were performed overnight at 4 °C, all of them diluted in 3% BSA in PBS-T and 0.02% sodium azide. HRP-coupled secondary antibodies were diluted in blocking buffer and incubated for 1 h at room temperature. Detection of proteins was made with ECL plus Western blotting detection (Amersham Biosciences).
Kinetic Analysis of rGAT-1 and HA-rGAT-1 in HEK293 Cells
The HEK293 cells were grown in 24-well plates for 2 days and transfected with rGAT-1 or HA-rGAT-1 using Lipofectamine 2000. Cells were assayed in the transport buffer, KHR (137 mm NaCl, 5.4 mm KCl, 1.8 mm CaCl2, 1.2 mm MgSO4, 10 mm HEPES, pH-adjusted with Tris-base to 7.40). Assays included 8.62 nm [3H]GABA and increasing concentrations of unlabeled GABA (1, 2.5, 4, 5, 7.5, 15, 25, and 50 μm). Nonspecific [3H]GABA accumulation was determined in the presence of 20 μm SKF 89976a. After 1 min of incubation with [3H]GABA at room temperature, uptake was terminated by quickly washing the cells two times with ice-cold stop solution (137 mm NaCl and 10 mm HEPES, pH 7.4). Cells were then solubilized in 1% SDS for 60 min with gentle shaking. Accumulated [3H]GABA was determined by liquid scintillation counting.
Affinity Screening by ELISA
Astrocytes transduced with HA-rGAT-1 were used for ELISA experiments. After a starvation period of 3 h, astrocytes were treated with different concentrations of BDNF for 10 minutes, as indicated. After BDNF incubation, cells were fixed in 4% PFA in PBS/Ca2+/Mg2+ for 20 min on ice, washed twice in PBS/Ca2+/Mg2+, blocked in 5% goat serum in PBS/Ca2+/Mg2+ for 30 min, and incubated with HA.11 antibody (1:1000) in 5% goat serum in PBS/Ca2+/Mg2+ for 60 min. Following four washes in PBS/Ca2+/Mg2+, cells were then incubated with HRP-conjugated goat anti-mouse antibody (1:1000; Pierce) for 30 min and subsequently washed four additional times in PBS/Ca2+/Mg2+. The HRP activity was detected and quantified instantaneously by chemiluminescence using Supersignal ELISA Femto maximum sensitivity substrate (Pierce) and a Wallac Victor 2 luminescence counter (PerkinElmer Life Sciences).
Reagents
GABA was purchased from Sigma, and [3H]GABA (4-amino-n-[2,3–3H]butyric acid, specific activity 92.0 Ci/mmol) was from PerkinElmer Life Sciences. Stock solutions of BDNF (kindly supplied by Regeneron Pharmaceuticals (Tarrytown, NY)) were in PBS at a final concentration of 1 mg/ml. Adenosine deaminase (EC 3.5.4.4, 200 units/mg in 50% glycerol (v/v), 10 mm potassium phosphate) was purchased from Roche Applied Science. CGS 21680, SCH 58261, U73122, GF109203x, SKF 89976a, and SNAP 5114 were purchased from Tocris (Bristol, UK). K252a was acquired from Calbiochem. LY 294002 and U0126 were acquired from Ascent (Weston-Super-Mare, UK). H-89 dihydrochloride hydrate, forskolin, dynasore hydrate, and monensin sodium salt were obtained from Sigma.
The following stock solutions were prepared in dimethyl sulfoxide: CGS 21680 (5 mm), SCH 58261 (5 mm), U73122 (5 mm), H-89 (5 mm), forskolin (5 mm), K252a (1 mm), LY 294002 (10 mm), U0126 (10 mm), GF109203x (10 mm), dynasore hydrate (10 mm), and monensin sodium salt (10 mm). SKF 89976a (10 mm) and SNAP 5114 (10 mm) were prepared in water. All aliquots were kept frozen at −20 °C until used; appropriate dilutions in incubation buffer were prepared daily.
Antibodies were purchased from the following sources: rabbit polyclonal antibody to GAT-1 (AB1570W) from Millipore (Bedford, MA); rabbit anti-phospho-Trk (pTyr-490), rabbit anti-phospho-Akt, rabbit anti-PLC-γ1, rabbit anti-phospho-p44/p42 MAPK, and rabbit anti-p44/p42 MAPK from Cell Signaling (Boston, MA); mouse monoclonal antibody to Akt1 and mouse monoclonal antibody to PLC-γ1 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); mouse monoclonal antibody HA11 from Covance (Princeton, NJ); rabbit polyclonal antibody to β-actin from Abcam (Cambridge, MA); mouse IgG1 antibody to TrkB (tyrosine kinase B receptor) from BD Biosciences; goat anti-mouse antibody conjugated with HRP from Pierce; goat anti-mouse antibody conjugated with HRP from Santa Cruz Biotechnology, Inc.; and goat anti-rabbit antibody conjugated with HRP from Santa Cruz Biotechnology, Inc.
Statistics
Non-linear regression analysis for calculation of Vmax and Km values and all statistical analysis were performed using GraphPad (San Diego, CA) Prism software. Two-sample comparisons were made using t tests; multiple comparisons were made using one-way analysis of variance (ANOVA) followed by Bonferroni correction post-test.
RESULTS
BDNF Increases GAT-1-mediated GABA Uptake by Increasing Vmax of the Transporter
We characterized GABA transport into the astrocytic culture by evaluating the maximum velocity (Vmax) and affinity constant (Km) for GAT-1 transport and the relative contribution of GAT-1 and GAT-3 for total GABA transport. The Km value obtained for GABA uptake in astrocytes was around 30 μm (data not shown), a value similar to the one already reported by others (24). When we isolated GAT-1-mediated GABA transport (see “Experimental Procedures”), the obtained Km value was 4.7 ± 1.89 μm (n = 6, Fig. 1A, open circles), a value also of the same magnitude as the one previously reported in relation to GAT-1 (24, 25).
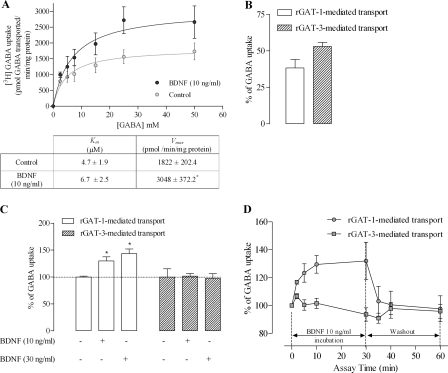
FIGURE 1.
BDNF enhances GAT-1 GABA transport in astrocyte primary cultures. A, saturation analysis of GAT-1-mediated GABA transport. Cells were incubated in the absence (open circles) or presence of BDNF (filled circles) before the addition of [3H]GABA at the concentrations indicated on the abscissa; data shown in the upper panel represent the mean values from six experiments performed in quadruplicate (four wells per GABA concentration), the averaged Km and Vmax values being shown in the lower panel; *, p < 0.05 (Student's t test, as compared with control). B, percentage of GABA uptake that occurs through GAT-1 (open bar) and GAT-3 (filled bar) GABA transporters. C, influence of BDNF upon GAT-1-mediated (open bars) or GAT-3-mediated (filled bars) GABA transport; *, p < 0.05 (one-way ANOVA followed by Bonferroni's post-test, as compared with control). D, time course of the BDNF effect and wash-out. In A, C, and D, the ordinates represent the [3H]GABA uptake relative to uptake in the absence of BDNF (100%) in the same experiments. The results are expressed as mean ± S.E. (error bars) from six (A), five (B and D), or seven (C) independent experiments. BDNF was incubated with astrocytes for 10 min, except for the time course experiments (C), where the BDNF incubation times are indicated below each time point.
The uptake of GABA in the presence of the selective GAT-1 inhibitor, SKF 89976a (20 μm), was reduced by 38 ± 5.8% (n = 5) of total uptake; when GAT-3 was blocked with SNAP 5114 (20 μm), a selective inhibitor of GAT-3 transporter, GABA transport was reduced by 53 ± 2.8% (n = 5). These data indicate that nearly 55% of total GABA transport into astrocytes occurs through GAT-3, the remaining 40% being through GAT-1 (Fig. 1B). From now on, when we refer to GAT-1-mediated GABA transport, we are reporting data from experiments where GAT-1 had been blocked with a supramaximal concentration (20 μm) of SKF 89976a (26), being the transport mediated by GAT-1 calculated by the difference between total transport (absence of GAT-1 blocker) and the transport measured in the presence of GAT-1 blocker in the same experiment. Conversely, when we refer to GAT-3-mediated GABA transport, we are referring to experiments where GAT-3 had been blocked with a supramaximal concentration (20 μm) of SNAP 5114 (27), the GAT-3-mediated transport being calculated by the difference between total transport and transport in the presence of the GAT-3 blocker in the same experiment.
As can be observed in Fig. 1C, BDNF (10–30 ng/ml) caused a concentration-dependent increase in GAT-1-mediated GABA transport in astrocytes, representing the effect of 10 ng/ml BDNF of 30 ± 8.0% (n = 7, p < 0.05) increase. GAT-3-mediated transport remained, however, unaffected.
Changes in the uptake of neurotransmitters induced by transporter-interacting compounds can result from alterations in the number of functional transporters at the cytoplasmatic membrane, or from changes in the transport capacity of individual transporters. Data obtained from saturation experiments are often used to distinguish between these two possibilities because changes in the maximum velocity of transport (Vmax) are indicative of changes in the number of transporter binding sites which are correlated with translocation of transporter to or from plasma membrane, and changes in affinity (Km) are indicative of changes in the function of individual transporters. Michaelis-Menten fitting of saturation curves for GAT-1-mediated GABA transport into astrocytes reveals a significant (p < 0.05, n = 6) increase in Vmax in the presence of BDNF (10 ng/ml) compared with untreated cells from the same culture (Fig. 1A). Km values were not significantly (p > 0.05) affected by BDNF (Fig. 1A).
From the time course of the effect of BDNF (10 ng/ml) upon GAT-1-mediated GABA transport (Fig. 1D), it is clear that the effect of BDNF occurs within minutes after its application, with the half-maximal effect being observed after about 2 min and the maximal effect more than 10 min after its application. Increasing the incubation time up to 30 min did not alter the effect of BDNF as compared with 10 min of incubation. Wash-out experiments performed after a 30-min incubation period showed that the effect of BDNF was reversible (Fig. 1D), with GABA uptake values being back to control levels 30 min after removing the neurotrophin from the incubation medium. In the remaining experiments, a 10-min incubation period with BDNF was used.
Modulation of GAT-1 by BDNF Occurs through the Truncated TrkB Receptor Isoform
BDNF operates through high affinity receptor tyrosine kinase B, TrkB, which exists in at least two isoforms, a full-length isoform (TrkB-fl) and a truncated isoform (TrkB-t) (e.g. see Ref. 28). Only the TrkB-fl possesses the catalytic kinase domain, and tyrosine kinase inhibitors, such as K252a (29), can thus be used to evaluate if a given effect of BDNF occurs through TrkB-fl or through another receptor isoform. We first evaluated whether tyrosine phosphorylation of GAT molecules could play a role in the functional regulation of GABA transport in astrocytes. For this purpose, astrocytic cultures were treated with K252a, and changes in Km and Vmax values were calculated from Michaelis-Menten fitting of saturation curves. K252a induced a significant (p < 0.05, n = 5) decrease in Vmax of GAT-1-mediated (Fig. 2A) and GAT-3-mediated (Fig. 2B) transport, without significant (p > 0.05) changes in Km values. It is worthwhile to note that data obtained with K252a somehow contrast with data obtained with BDNF because whereas the neurotrophin affects GAT-1-mediated but not GAT-3-mediated transport, the inhibitor of tyrosine phosphorylation affects both transporters.
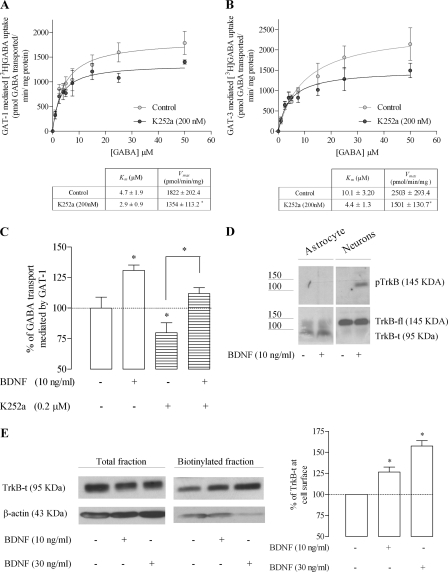
FIGURE 2.
BDNF modulates GAT-1 through activation of the TrkB-t receptor. Saturation analysis of GAT-1 (A) and GAT-3 (B) transport is shown. Cells were incubated in the absence (open circles) or presence of K252a (filled circles) before the addition of [3H]GABA at the concentrations indicated on the abscissa; data shown in the upper panel are the mean values from five experiments performed in quadruplicate (four wells per GABA concentration), the averaged Km and Vmax values being shown in the lower panel; *, p < 0.05 (Student's t test, as compared with control). C, effect of BDNF upon [3H]GABA uptake in the presence or absence of K252a; *, p < 0.05 (one-way ANOVA followed by Bonferroni's post-test, as compared with control conditions (first bar on the left) except where otherwise indicated by the connecting lines above the bars. D, analysis of TrkB and pTrkB staining in total lysates of astrocytes or neurons treated with/without BDNF, as indicated. E, Western blot analysis of TrkB-t immunoreactivity in cell lysates and biotinylated fractions of astrocytes. In the left panel is shown a representative immunoblot, and the right panel depicts the quantitative densitometric analysis of the immunoreactivity in the biotinylated fraction. Blots were probed with anti-TrkB (1:1000), and β-actin immunolabeling (1:10,000) was used as a loading control. In C, 100% on the ordinate corresponds to the amount of [3H]GABA taken up by astrocytes in the same experiments in the absence of any drug. In E, 100% on the ordinate corresponds to TrkB-t staining in the absence of BDNF, after normalization for β-actin immunoreactivity. The results are expressed as mean ± S.E. (error bars) from five (A and B), six (C), or four (E) independent experiments.
To evaluate if the effect of BDNF requires a tyrosine phosphorylation signaling cascade, we then tested if K252a could prevent the effect of BDNF. Given the marked inhibitory effect of K252a per se, the strategy was to compare, within the same astrocytic culture, GABA transport under the following four different conditions (no drug added, only BDNF added, only K252a added, and BDNF added in the presence of K252a), therefore allowing the comparison of the effect of BDNF under similar conditions in the absence and in the presence of K252a. From data shown in Fig. 2C, it is evident that despite the presence of K252, BDNF was able to enhance GABA transport. Indeed, upon calculation of the effect of BDNF as percentage increase (i.e. taking as control the transport of GABA in the same drug conditions but absence of BDNF), it became clear that BDNF increased GABA transport by 31 ± 4.4% (n = 6, p < 0.05) in the absence of K252a (value calculated by considering the control condition as 100%) and by 32 ± 4.9% (n = 6, p < 0.05) in the presence of K252a (value calculated by considering the K252a-treated astrocyte condition as 100%) with no statistically significant differences (n = 6, p > 0.05) between both BDNF effects. These results indicate that tyrosine kinase activity is not required for the effect of BDNF and therefore are highly suggestive of a non-TrkB-fl-mediated effect.
In accordance with the results obtained with K252a, data from Western blot analysis showed that cultured astrocytes express TrkB-t receptors but not TrkB-fl receptors. Indeed, a band corresponding to the molecular mass of TrkB-fl isoform (145 kDa) was never detected in the Western blots of homogenates of astrocytic cultures, whereas the TrkB antibody clearly recognized a 95-kDa protein in the samples, a molecular mass compatible with that of the TrkB-t isoform (Fig. 2D). As a control for the antibody used, homogenates from cultured hippocampal neurons, non-treated or treated with 10 ng/ml BDNF, were also assayed, and, as illustrated in Fig. 2D, a 145-kDa protein corresponding to the molecular mass of the TrkB-fl was clearly detected in the neurons. Because activation of TrkB-fl by BDNF leads to receptor autophosphorylation, we assessed the levels of pTrkB in astrocytes and neurons treated with BDNF (10 ng/ml) by using an antibody against pTrkB. It became clear that pTrkB staining could be detected in neurons but not astrocytes (Fig. 2D). Altogether, these data strongly suggest that astrocytes express the truncated form but not the full-length form of the TrkB receptor. Interestingly, exposure of astrocytes to different concentrations of BDNF (10–30 ng/ml) for 10 min, leads to a concentration-dependent increase (p < 0.05, n = 4) in the expression of TrkB-t receptor on the surface membrane, as assessed by Western blot analysis of the biotinylated astrocyte membrane fractions (Fig. 2E).
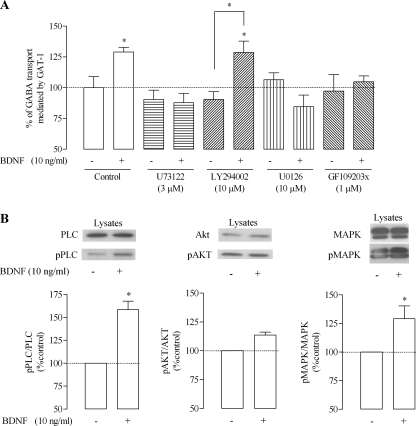
Experiments were then designed to assess signaling pathways involved in the modulatory action of BDNF upon GAT-1. The effects of BDNF in the absence and in the presence of inhibitors of known TrkB-fl signaling cascades were compared in the same astrocytic cultures. As shown in Fig. 3A, the PI3K inhibitor LY294002 (10 μm) (30) had no significant inhibitory effect on GAT-1-mediated GABA uptake (p > 0.05, n = 4); also, it did not prevent the facilitatory action of BDNF, which remained virtually unchanged in the presence of this inhibitor. On the other hand, inhibition of the ERK/MAPK signaling pathway with the selective MEK1 and -2 inhibitor U0126 (10 μm) (31) had no effect upon GABA transport in rat astrocytes when tested alone but fully prevented the facilitatory effect of BDNF on GAT-1-mediated GABA transport. Indeed, GAT-1-mediated GABA uptake in astrocytes in the presence of U0126 (10 μm) was not significantly (n = 4, p > 0.05) altered by BDNF (10 ng/ml; Fig. 3A). Likewise, the PLC-γ inhibitor U73122 (3 μm) (32) also prevented the effect of BDNF while being by itself devoid of effect (p > 0.05, n = 4; Fig. 3A) upon GABA transport. These results suggest that the BDNF-induced increase in GAT-1-mediated GABA uptake involves the activity of at least two transduction pathways, namely the PLC-γ and the ERK/MAPK pathways. Because TrkB receptor activation coupled to PLC-γ phosphorylation leads to a subsequent activation of PKC-δ (33), and in order to assess if PKC-δ is involved in the BDNF-induced GAT-1 modulation, we tested the influence of the PKC-δ inhibitor, GF109203x (1 μm) (34). This inhibitor per se did not affect GAT-1-mediated GABA transport but abolished the effect of BDNF, also implicating PKC-δ in the signaling cascade. BDNF (10 ng/ml) induced a significant increase of the phosphorylation state of PLC and MAPK (p < 0.05, n = 3) but no alteration for the phosphorylation state of Akt (Fig. 3B), which fits to the results obtained in uptake experiments with PI3K, ERK/MAPK, or PLC-δ blockers. In summary, the results so far suggest that BDNF-induced modulation of GAT-1 is not mediated by TrkB-fl, because it does not involve phosphorylation at tyrosine residues, and that it is most likely mediated through a TrkB-t isoform coupled to a non-classic PLC and ERK/MAPK mechanism, involving PKC-δ.
FIGURE 3.
Transduction pathways involved in GAT-1 modulation by BDNF. A, effect of BDNF upon [3H]GABA uptake in the presence of inhibitors of the different signal-transducing pathways of TrkB receptor, namely U73122, LY294002, U0126, and GF109203x (for details, see “Results”), as indicated below the bars. B, Western blot analysis of PLC/pPLC, Akt/pAkt, and MAPK/pMAPK staining in total lysates of cells treated with BDNF, as indicated. In the upper panels are shown representative immunoblots, and the lower panels depict the quantitative densitometric analysis of the immunoreactivity of phosphorylated proteins; blots were probed with anti-PLC (1:750), pPLC (1:250), Akt (1:1500), pAkt (1:500), MAPK (1:6000), and pMAPK (1:500). In A, 100% on the ordinate corresponds to the amount of [3H]GABA taken up by astrocytes in the same experiments in the absence of any drug; in B, 100% on the ordinate corresponds to PLC/pPLC, Akt/pAkt, or MAPK/pMAPK staining in the absence of BDNF. The results are expressed as mean ± S.E. (error bars) from four (A) or three (B) independent experiments. *, p < 0.05 (one-way ANOVA followed by Bonferroni's post-test), as compared with no drug (first bar on the left) or, whenever indicated, with the closest bar on the left (absence of BDNF under same drug conditions).
Incorporation of an HA Epitope into EL2 of GAT-1 Affects neither GAT-1 Affinity for GABA nor Sensitivity to BDNF
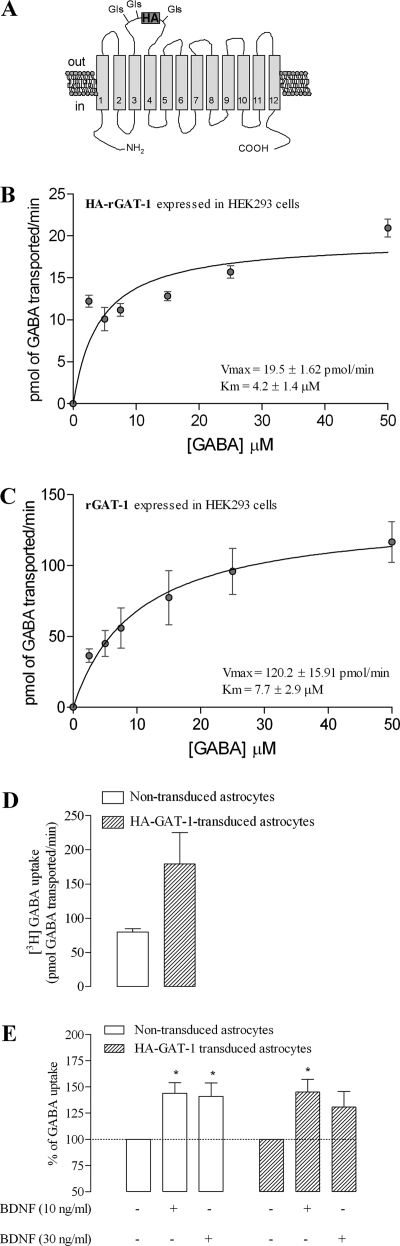
In order to test the hypothesis that BDNF increases GABA uptake through GAT-1 by increasing the density of transporters at the cell surface, we generated a functional rat GAT-1 transporter that has an antibody-accessible extracellular epitope, enabling the measurement of changes in rGAT-1 surface expression by ELISA. For the closely related dopamine transporter, an HA epitope has previously been inserted into EL2 without altering the function of the transporter (19). Accordingly, an HA tag of nine amino acid residues (YPYDVPDYA) was introduced in the EL2 loop of rGAT-1, between residues 698 and 699 (Fig. 4A), to generate HA-rGAT-1. Kinetic measurements of [3H]GABA uptake into HEK293 cells expressing HA-rGAT-1 (Fig. 4B) yielded a Km (4.2 ± 1.4 μm, n = 3) value that did not differ from the Km value obtained in HEK293 cells expressing wild type rGAT-1 (7.7 ± 2.9 μm, n = 2) (Fig. 3C). Notably, the Km values obtained in HEK293 cells expressing rGAT-1 or HA-rGAT-1 were similar to the Km value for rGAT-1 endogenously expressed in astrocyte primary cultures (Figs. 1A and 4, B and C). The Vmax value for HA-rGAT-1 expressed in HEK293 (19.5 ± 1.62 pmol/min) was significantly different from the value obtained in a parallel experiments on cells expressing wild type rGAT-1 (120 ± 15.9 pmol/min). Nevertheless, these uptake experiments demonstrate that introduction of the HA epitope in EL2 yielded a functional transporter that was expressed at the cell surface (although to a lower extent than the wild type transporter) and had an unaltered apparent affinity for GABA.
FIGURE 4.
Characterization of HA-GAT-1-mediated GABA transport. A, schematic representation of the localization of the HA tag, which was placed in EL2 of rat GAT-1; predicted N-glycosylation sites in the EL2 are indicated. B and C, saturation kinetics of GAT-1-mediated GABA uptake in HEK293 cells stably expressing HA-GAT-1 (B) or rGAT-1 (C). The Km and Vmax values are shown as an inset in the corresponding graph. D, [3H]GABA uptake through GAT-1 in HA-GAT-1-transduced (filled bars) and non-transduced (open bars) astrocytes. E, BDNF effects on GABA uptake in HA-rGAT-1-transduced (filled bars) and non-transduced (open bars) astrocytes from the same cell batch. The ordinates represent the [3H]GABA uptake as a percentage of the control value (no BDNF added) in the same experiment and in the same cell batch in similar conditions. In all panels, the results are expressed as mean ± S.E. (error bars) from three (B), two (C and D), or five (E) independent experiments. *, p < 0.05 (one-way ANOVA followed by Bonferroni's post-test) as compared with control (no drug added, first column on the left) in the same group of cells.
We next evaluated the expression of HA-rGAT-1 in rat astrocytes. Following transduction of astrocytes with lentivirus encoding HA-rGAT-1, GABA uptake was increased more than 2-fold compared with non-transduced astrocytes (Fig. 4D). Importantly, treatment of HA-rGAT-1-transduced astrocytes with BDNF (10 and 30 ng/ml) led to an increase in GABA uptake that was similar to the increase caused by BDNF in non-transduced astrocytes from the same batch (Fig. 4E), indicating that HA-rGAT-1 and endogenous rGAT-1 have similar sensitivity to BDNF.
BDNF Enhances Expression of rGAT-1 at the Plasma Membrane of Astrocytes
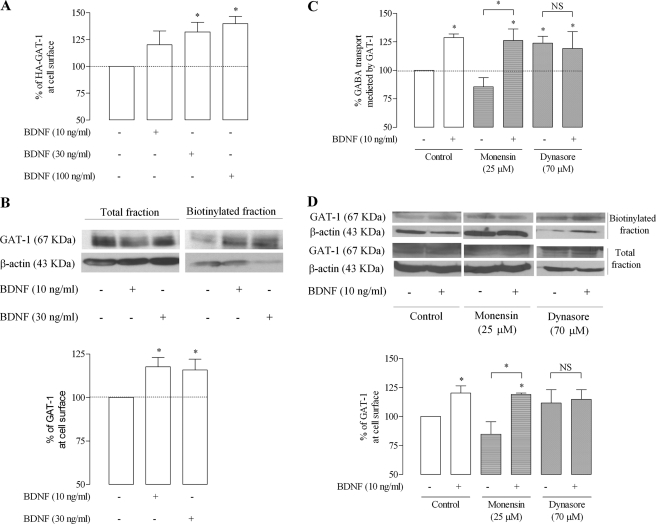
The influence of BDNF upon GAT-1 trafficking in rat astrocytes was first assessed by cell surface ELISA using an antibody against the HA tag. HA-rGAT-1-transduced astrocytes were incubated with BDNF (10–100 ng/ml) for 10 min before the assay, inducing a significant increase (20.1 ± 12.90%, 32.2 ± 8.97%, and 39.9 ± 6.68% for 10, 30, and 100 ng/ml BNDF, respectively, n = 4, p < 0.05) in immunodetected HA-tagged surface transporter as compared with astrocytes that were not incubated with the neurotrophin (Fig. 5A).
FIGURE 5.
BDNF enhances surface expression of GAT-1 in astrocytes. A, HA-rGAT-1-transduced astrocytes were incubated for 10 min with (+) or without (−) BDNF, as indicated below each bar and then assayed by ELISA. 100% on the ordinate represents normalized HA-GAT-1 expression in plasma membrane of astrocytes in the control situation (absence of BDNF). B, rGAT-1-transduced astrocytes were incubated as in A, but changes in surface GAT-1 immunoreactivity were assessed by surface biotinylation. In the upper panel is shown a representative immunoblot from total lysate and biotinylated astrocyte fractions. Blots were probed with anti-GAT-1 (1:500), and β-actin (1:10000) immunoreactivity was used as loading control. In the lower panel is shown the average densitometric analysis, where 100% on the ordinate represents normalized rGAT-1 expression in the biotinylated fraction in the absence of BDNF. C and D, influence of monensin and dynasore (for details, see “Results”) upon the effect of BDNF on GABA uptake (C) or surface expression of rGAT-1 (D). In the upper panel in D is shown a representative immunoblot from total lysate and biotinylated astrocyte fractions, and in the lower panel is shown the average densitometric analysis, where 100% on the ordinates represents normalized rGAT-1 expression in the biotinylated fraction in the absence of BDNF. The results are expressed as mean ± S.E. (error bars) from four (A), five (B and C), or three (D) individual experiments. *, p < 0.05 (one-way ANOVA followed by the Bonferroni's post-test), as compared with control conditions (first column on the left) except where otherwise indicated by the connecting lines above the bars. NS, not statistically significant (p > 0.05).
To rule out the possibility that the effect of BDNF upon trafficking was related to the HA tag, biotinylation experiments using astrocytes not transfected with the HA-tagged GAT-1 were performed. Because GAT-1 levels in biotinylated astrocytic membranes are below the detection limit by Western blot (data not shown), rGAT-1 was overexpressed in the astrocytes by transduction with lentivirus encoding wild type rGAT-1. Cells were then incubated in the absence or presence of BDNF before biotinylation of surface proteins. As shown in the representative immunoblot (Fig. 5B, top) and in summary plot (Fig. 5B, bottom), incubation of cells with BDNF for 10 min leads to an increase (17.7 ± 5.38 and 20.9 ± 4.76%, for 10 or 30 ng/ml BDNF, respectively, n = 5, p < 0.05) in GAT-1 immunoreactivity in the biotinylated fraction, without appreciable change in total GAT-1 immunoreactivity.
Altogether, the data above clearly show that BDNF enhances the expression of GAT-1 transporters at the plasma membrane. To assess whether BDNF affects the rate of internalization of GAT-1 or its recycling back to the plasma membrane, we tested the influence of dynasore, a dynamin inhibitor therefore blocking dynamin/clathrin-dependent endocytosis (35), as well as of the cation ionophore monensin, which blocks protein recycling back to the membrane without influencing protein internalization (36). Monensin, used in conditions (25 μm, 1 h) previously shown to inhibit recycling of dopamine transporters in neuronal cell lines (37), slightly decreased GABA uptake by around 10% (n = 5; Fig. 5C), suggestive of constitutive recycling of GAT-1 in astrocytes. Similarly, biotinylation assays showed that monensin slightly decreases GAT-1 expression at surface membranes (n = 3; Fig. 5D). In the presence of monensin, however, BDNF still increased GAT-1-mediated GABA uptake by 26 ± 10.2% (n = 5, p < 0.05; Fig. 5C) as well as GAT-1 expression at surface membranes (n = 3, p < 0.05; Fig. 5D). This suggests that BDNF does not operate by enhancing GAT-1 recycling back to the plasma membrane. The dynamin inhibitor, dynasore (70 μm), increased GAT-1-mediated GABA uptake by 24 ± 6.1% (n = 5, p < 0.05; Fig. 5C) as well as increasing GAT-1 expression at surface membranes (Fig. 5D), indicating that in astrocytes, as it occurs in neurons (5), GAT-1 internalization involves the dynamin/clathin-dependent mechanism rather than the Ca2+-regulated dynamin-independent endocytotic pathway, which is also present in cortical astrocytes (37). Dynasore (70 μm) fully abrogates the facilitatory effect of BDNF upon GAT-1-mediated GABA transport (n = 5; Fig. 5C) as well as upon GAT-1 expression at surface membranes (n = 3; Fig. 5D), indicating that BDNF inhibits constitutive internalization of GAT-1 rather than enhancing the insertion/recycling pathway.
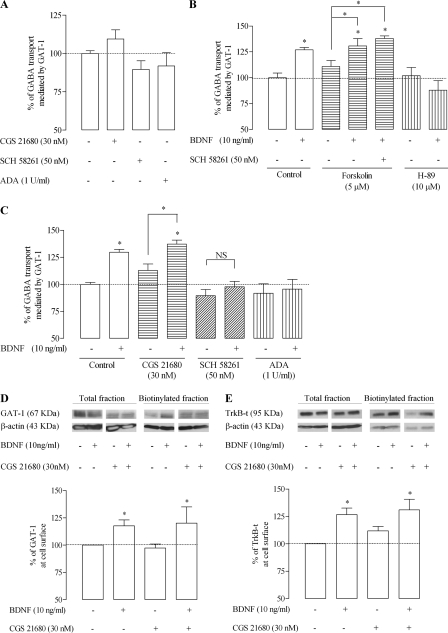
Tonic Levels of Extracellular Adenosine Are Enough to Trigger the Effect of BDNF
It has been repeatedly observed that actions of BDNF at synapses require co-activation of adenosine A2A receptors, a mechanism that involves activation of PKA and TrkB translocation to lipid rafts (38, 39). An exception is BDNF-induced inhibition of GABA transport into nerve terminals because the effect of BDNF on GAT-1 in nerve terminals is not prevented by the removal of extracellular adenosine or by blockade of adenosine A2A receptors (11). In order to evaluate if adenosine A2A receptors could influence the facilitatory action of BDNF on GAT-1-mediated GABA transport into astrocytes, we tested whether A2A receptor blockade with a selective antagonist, SCH 58261 (50 nm) (40), or A2A receptor activation with a selective agonist, CGS 21680 (30 nm) (41), affected the action of BDNF. Per se, these drugs did not significantly (n = 4, p > 0.05) affect GABA transport, although there was a tendency for a facilitation by the A2A receptor agonist and an inhibition by the A2A receptor antagonist (Fig. 6A). Similarly, adenosine deaminase (1 unit/ml), an enzyme that catabolizes adenosine into inosine, therefore removing extracellular adenosine, caused a slight but non-significant decrease in GAT-1-mediated GABA transport (n = 4, p > 0.05). To evaluate the effect of BDNF in the presence or absence of the A2A receptor ligands, experiments were designed so that GABA transport in the presence of each A2A receptor ligand was taken as control for the effect of BDNF, which was tested in the same astrocytic culture in the absence or in the presence of the ligand. A summary of the results is shown in Fig. 6C. The facilitatory effect of BDNF was fully lost upon incubation of astrocytes with the A2A receptor antagonist, SCH 58261 (50 nm, n = 8). The same occurred when extracellular endogenous adenosine was removed by incubating the cells with adenosine deaminase (1 unit/ml, n = 4; Fig. 6C, ADA). Activation of A2A receptors with CGS 21680 (30 nm) caused a slight but non-significant (n = 4, p > 0.05; Fig. 6B) enhancement of the facilitatory action of BDNF, suggesting that tonic activation of A2A receptors by endogenous adenosine is enough to fully trigger the facilitatory effect of BDNF on GAT-1 mediated GABA transport into astrocytes. A similar conclusion can be drawn from evaluating the facilitatory action of BDNF upon surface expression of GAT-1 (Fig. 6D) or BDNF-induced enhancement of TrkB-t surface expression (Fig. 6E) because in no case did a further activation of A2A receptors with GCS 21680 induce a further effect of BDNF.
FIGURE 6.
Modulation of the effect of BDNF by adenosine A2A receptors. A, influence of A2A receptor activation with a selective agonist, CGS 21680, or blockade with a selective antagonist, SCH 58261, or removal of extracellular adenosine with adenosine deaminase (ADA) on [3H]GABA uptake mediated by GAT-1. B, influence of drugs that affect cAMP signaling upon the effect of BDNF on [3H]GABA uptake. Forskolin was used as an activator of adenylate cyclase, and H-89 was used as an inhibitor of PKA (for details, see “Results”). C, influence of CGS 21680, SCH 58261, and adenosine deaminase on the effect of BDNF upon [3H]GABA uptake. D and E, A2A receptor agonist, CGS 21680, effects on the BDNF-induced increase in surface expression of GAT-1 (D) or TrkB-t (E). In the upper panels are shown representative immunoblots for GAT-1 (D) and TrkB-t (E) immunoreactivity in cell lysate and biotinylated fractions of astrocytes. Blots were probed with anti-GAT-1 or anti-TrkB antibodies, and β-actin immunoreactivity was used as loading control. In the lower panel is shown the average densitometric analysis, where 100% on the ordinate represents rGAT-1 expression or TrkB-t levels (both normalized for β-actin in the same lane) in the biotinylated fraction in the absence of BDNF. For A, B, and C, 100% on the ordinates correspond to the amount of GABA taken up in the absence of any drug. In all panels, data are expressed as mean ± S.E. (error bars) from 4–8 (A–C) or six (D and E) individual experiments. Drug presence (+) or absence (−) is indicated below each bar. *, p < 0.05 (one-way ANOVA followed by Bonferroni's post-test), as compared with control conditions (no BDNF added) except where otherwise indicated by the connecting lines above the bars; NS, not statistically significant (p > 0.05).
Activation or inhibition of the canonical signal-transducing pathway of adenosine A2A receptors should influence the effect of BDNF in a way similar to what is observed when A2A receptor activity is manipulated by receptor ligands. Accordingly, the adenylate cyclase activator, forskolin (5 μm) (42), did not cause a further enhancement of the effect of BDNF (n = 6; Fig. 6B), whereas the protein kinase A inhibitor, H-89 (10 μm) (43), fully prevented the effect of BDNF on GAT-1-mediated GABA transport (n = 6; Fig. 6B). Upon activation of adenylate cyclase with forskolin, the A2A receptor antagonist was no longer able to block the effect of BDNF (n = 4; Fig. 6B), strongly indicating that blockade of the action of BDNF by A2A receptor antagonism is upstream of adenylate cyclase, therefore reinforcing the conclusion that A2A receptors operate through this transducing pathway to allow BDNF actions upon GABA transport into astrocytes.
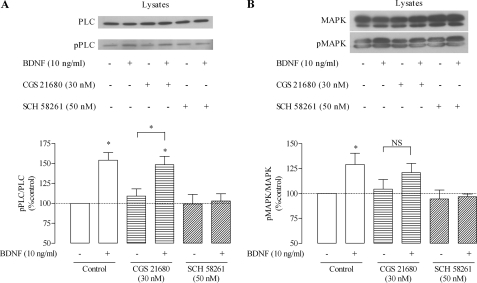
Finally, we evaluated whether the BDNF-induced enhancement of the phosphorylation state of PLC and MAPK (Fig. 3B) was also under the control of adenosine A2A receptors. As shown in Fig. 7, BDNF-induced phosphorylation of PLC and MAPK was also fully blocked by the A2A receptor antagonist, SCH 58261 (50 nm), but not significantly affected by a further activation of A2A receptors with the agonist, CGS 21680 (30 nm), therefore mimicking what we observed while measuring GABA transport. Per se, neither the A2A receptor agonist (CGS 21680, 30 nm) nor the antagonist (SCH 58261, 50 nm) significantly affected the phosphorylation state of PLC and MAPK in astrocytes.
FIGURE 7.
A2A receptor-mediated modulation of the signaling pathways activated by BDNF. Shown is Western blot analysis of PLC/pPLC (A) and MAPK/pMAPK (B) staining in total lysates of cells treated with BDNF, CGS 21680, and SCH 58261, as indicated. In the upper panels are shown representative immunoblots for PLC/pPLC (A) and MAPK/pMAPK (B) in cell lysate; in the lower panels are shown the average densitometric analysis, where the ordinates represent quantitative densitometric analysis of immunoreactivity of phosphorylated proteins. Data are expressed as mean ± S.E. (error bars) from six (A) or four (B) individual experiments. *, p < 0.05 (one-way ANOVA followed by the Bonferroni's post-test), as compared with no drug conditions (first column on the left) except where otherwise indicated by the connecting lines above the bars; NS, not statistically significant (p > 0.05).
DISCUSSION
The main finding of the present work is that BDNF increases GAT-1-mediated GABA transport into astrocytes through a mechanism that involves the TrkB-t isoform of TrkB receptors and a non-classic PLC-γ/PKC-δ and ERK/MAPK pathway, leading to enhanced expression of GAT-1 at the cell surface due to a reduced internalization.
BDNF facilitates the maximum velocity of GAT-1-mediated transport (Vmax) without a significant change in Km value, suggestive of an increase in the number of transporters at the membrane. This was directly confirmed by cell surface biotinylation and surface ELISA in astrocytes overexpressing wild type GAT-1 or HA-tagged GAT-1, respectively. Constitutive recycling of neurotransmitter transporters is known to occur in neurons (6, 37). Therefore, if this would apply to astrocytes, enhanced expression of GAT-1 on astrocytic surface membranes could result either from inhibition of endocytosis or from enhancement of its recycling back to the membrane. The finding that monensin, which inhibits protein recycling back to the membrane without affecting endocytosis (36), does not prevent the effect of BDNF upon surface expression of GAT-1 molecules rules out an influence of BDNF upon membrane reinsertion of the transporters. Endocytosis frequently occurs through clathrin-dependent coated vesicle formation, a process that also depends on dynamin and, in neurons, controls synaptic vesicle turnover. Astrocytes, however, also possess a Ca2+-dependent/dynamin independent endocytic pathway (37), but because the dynamin inhibitor, dynasore, mimics the effect of BDNF and abrogates its influence upon surface expression of GAT-1, it seems likely that the effect of BDNF results from inhibition of GAT-1 internalization through a dynamin-dependent process.
BDNF has a complex pattern of modulation of GABAergic transmission, which is time-dependent and cell- and synapse-specific. In a time frame from minutes to a few h, BDNF inhibits GABAA receptor-mediated responses (44) by down-regulation of GABAA receptor surface expression (45). These fast BDNF actions are synapse-specific, but the trend is toward a postsynaptic inhibition of GABAergic transmission, in particular toward an inhibition of inhibitory inputs to interneurons (46). The inhibitory action of BDNF disappears after prolonged exposure to BDNF (45), turning into a long lasting facilitatory action of GABAA responses, associated with an increase in GABAA receptor clusters (47). These long lasting effects of BDNF are more related to trophic than to fast signaling actions because they were shown to involve establishment of functional inhibitory synapses between interneurons (47). We now report another fast and easily reversible mechanism operated by BDNF that influences GABAergic transmission. Thus, the enhanced GABA transport into astrocytes most probably contributes to transiently fasten the shutdown of GABAergic responses, a process that, if added to the fast decrease in postsynaptic sensitivity to GABA (44–46) and to a facilitation of GABA transport into neurons (10), will lead to a marked inhibition of inhibitory signaling. At the synaptic level, this action of BDNF may, however, be slightly compensated for by an inhibition of GAT-1-mediated GABA uptake by the nerve endings (11) as well as by an enhanced presynaptic release of GABA (46). Therefore, the facilitatory action of BDNF upon GAT-1 might predominantly contribute to decrease tonic inhibition (i.e. to a decreased exposure of extrasynaptic distant GABAA receptors (48) to a persistently low concentration of “ambient” GABA). It is also worthwhile to note that tonic inhibition is predominantly influenced by GAT-1 rather than GAT-3 (17) and that BDNF affected GAT-1-mediated but not GAT-3-mediated transport in astrocytes.
In accordance with previous reports (49, 50), the now described action of BDNF in astrocytes might not involve TrkB-fl receptors because it was still evident when tyrosine kinase activity was blocked with K252a. Accordingly, no TrkB-fl or pTrkB immunoreactivity was detected in astrocytes, which were clearly immunopositive for the TrkB-t isoform. The TrkB-t receptor is characterized by lacking the tyrosine kinase domain, which is present in the full-length TrkB receptor. Because it lacks the tyrosine kinase domain, the TrkB-t receptor does not directly activate the classical transduction pathways that characterize TrkB-fl receptor, namely the activation of ERK, PI3K, and PLC-γ pathways (28, 51). TrkB-t isoforms can, however, operate an intracellular signaling pathway(s), leading to phosphorylation and changes in kinase activity (52, 53). When testing which pathways activated by BDNF are related to its ability to modulate GAT-1, we found that the inhibition of PLC-γ, as well as the inhibition of MAPK pathways, prevented the BDNF action upon the GAT-1 transporter, implicating these pathways in the action of the neurotrophin in the astrocytes. BDNF-induced activation of PLC-γ is often associated with PKC-δ activation (33). Accordingly, blockade of PKC prevented the BDNF effect upon GAT-1 in astrocytes. An inhibitor of PI3K did not prevent the effect of BDNF, suggesting the absence of involvement of the PI3K/Akt pathway on the action of BDNF upon GABA transport into astrocytes. Likewise, after a brief incubation with BDNF, there was an enhanced phosphorylation of PLC and of MAPK but not of Akt. The signaling pathway operated by BDNF to quickly modulate GAT-1 in astrocytes is consistent with its time frame of action because PLC-γ/PKC-δ is associated with fast signaling, whereas the PI3K/Akt pathway is mostly related to long lasting survival-related influences of BDNF (54). Nevertheless, the blockade of tyrosine kinase by K252a diminished the Vmax of both GAT-1 and GAT-3, modulating GABA transporters in a way similar to what was already described in neurons for GAT-1 (10, 55).
Interestingly, astrocytes treated for a few min with BDNF had increased TrkB-t receptor levels in the cell membrane, suggesting a BDNF-induced translocation of the TrkB-t receptor to the plasma membrane. At least in neurons, where membrane translocation of TrkB receptors has been mostly studied, acute exposure to BDNF rapidly (within seconds) increases TrkB surface expression, whereas long lasting (within hours) treatment with BDNF leads to decreased surface TrkB levels (55). A similar process might therefore occur in relation to surface expression of TrkB-t receptors in astrocytes. This process may result in a localized positive feedback loop between neurons and astrocytes, where fast neuronal spiking leads to release of the neurotrophin, which can be taken up by astrocytes, endowing these with the ability to resecrete it upon stimulation (48), a mechanism that, when coupled to a quick and BDNF-dependent overexpression of TrkB receptors at neuronal (56) and astrocytic (present work) membranes, further restricts neurotrophin actions to neuron-astrocyte contacts.
Several studies demonstrated that the excitatory action of BDNF on synaptic transmission is fully dependent on adenosine A2A receptors activation because it is absent when A2A receptors are blocked (38, 57–59) or knocked down (60) or upon removal of extracellular endogenous adenosine (39, 59). Activation of adenosine A2A receptors transactivates TrkB (60) and induces TrkB translocation to lipid rafts (39), which probably underlies the mechanisms behind the A2A/TrkB receptor facilitatory interaction. In the case of the inhibitory action of BDNF on presynaptic GAT-1 activity, it is modulated by A2A receptor activation but remains present either in the presence of A2A receptor antagonists or upon extracellular adenosine removal (11). The now reported facilitatory action of BDNF upon GAT-1 in astrocytes was lost upon blockade of adenosine A2A receptors or extracellular adenosine removal. It therefore appears that fast excitatory actions of BDNF are those that require co-activation of adenosine A2A receptors by endogenously released adenosine. Interestingly, the present results allow us to extend the adenosine/BDNF cross-talk to astrocytes and to TrkB-t. It is noteworthy that the reported results show for the first time a functional consequence of the cross-talk between TrkB-t and adenosine A2A receptors, indicating that the catalytic domain of the TrkB receptor is not involved in the cross-talk with A2A receptors.
GABA has the ability to increase intracellular calcium levels in astrocytes, and this action triggers the release of ATP from the astrocytes (61). On the other hand, ATP is released with several neurotransmitters and triggers astrocytic calcium waves, leading to further release of ATP as well as of gliotransmitters (62, 63). Released ATP can be extracellularly catabolized by a cascade of ectoenzymes, leading to adenosine formation. Therefore, it is expected that the extracellular adenosine levels at the tripartite synapse are enough to activate high affinity adenosine receptors, namely A2A receptors, which will gate BDNF actions in astrocytes. BDNF itself is able to trigger calcium responses in astrocytes (50), therefore most probably further reinforcing the cycle of astrocyte-to-neuron communication involving purines. In our experimental conditions, extracellular levels of adenosine were probably already high enough to maximally activate A2A receptors because further activation of these receptors with a selective A2A receptor agonist did not cause an enhancement of the facilitatory action of BDNF either upon GAT-1-mediated GABA transport or surface expression of GAT-1 transporters or even upon surface expression of TrkB-t receptors.
The present results allow us to conclude that gating of TrkB-t receptors by A2A receptor activation is upstream of adenylate cyclase activation because the effect of BDNF could be observed when A2A receptors were blocked but adenylate cyclase was activated. Receptor tyrosine kinases and G-protein-coupled receptors, by sharing protein signaling components specific for each receptor that are in close proximity, can form platforms, producing an integrated response upon engagement of ligands (64). Examples of such platforms are lipid rafts, and A2A receptors are known to promote translocation of TrkB receptors to lipid rafts in a cyclic AMP-dependent manner (39). Such proximity interaction may also occur with A2A receptors, adenylate cyclase, and TrkB-t receptors in astrocytes, having an impact upon GAT-1-mediated GABA transport.
In conclusion, the data now reported highlight a new role for the TrkB-t receptor in astrocytes, namely the modulation of activity and trafficking of GAT-1. This action of BDNF may impact upon synaptic transmission, namely decreasing tonic inhibition, and in this way add to the plethora of mechanisms operated by the neurotrophin to reinforce synaptic activity.
Acknowledgments
We thank Regeneron Pharmaceuticals for the gift of brain-derived neurotrophic factor and the Institute of Physiology of the Faculty of Medicine of Lisbon for the animal housing facility.
This work was supported by Fundação para a Ciência e a Tecnologia Grants SFRH/BD/227989/2006 (to S. H. V.) and SFRH/BD/38099/2007 (to S. C. F.).
- GAT
- GABA transporter
- rGAT-1
- rat GAT-1
- [3H]GABA
- 4-amino-n-[2,3-3H]butyric acid
- PLC
- phospholipase C
- ANOVA
- analysis of variance
- pPLC
- pAkt, pMAPK, and pTrkB, phosphorylated PLC, Akt, MAPK, and TrkB, respectively
- HEK293
- human embryonic kidney 293 cells
- HEK293T
- variant of the human embryonic kidney 293 cells
- CGS 21680
- 4-[2-[[6-amino-9-(N-ethyl-β-d-ribofuranuro-namidosyl)-9H-purinyl]amino]ethyl]benzene-propanoic acid hydrochloride
- SCH 58261
- 2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine
- U73122
- 1-[6-[[(17b)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione
- SKF 89976a
- 1-(4,4-diphenyl-3-butenyl)-3-piperidine-carboxyliacidhydrochloride)
- SNAP 5114
- 1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-(S)-3-piperid inecarboxylic acid
- LY294002
- 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio) butadiene
- H-89
- N-[2-(p-bromocinnamylamino)ethyl]-5 isoquinolinesulfonamide dihydrochloride.
REFERENCES
- 1. Gether U., Andersen P. H., Larsson O. M., Schousboe A. (2006) Trends Pharmacol. Sci. 27, 375–383 [DOI] [PubMed] [Google Scholar]
- 2. Lee T. S., Bjørnsen L. P., Paz C., Kim J. H., Spencer S. S., Spencer D. D., Eid T., de Lanerolle N. C. (2006) Acta Neuropathol. 111, 351–363 [DOI] [PubMed] [Google Scholar]
- 3. Iversen L. (2006) Br. J. Pharmacol. 147, Suppl. 1, S82–S88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schousboe A., Larsson O. M., Sarup A., White H. S. (2004) Eur. J. Pharmacol. 500, 281–287 [DOI] [PubMed] [Google Scholar]
- 5. Deken S. L., Wang D., Quick M. W. (2003) J. Neurosci. 23, 1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D., Quick M. W. (2005) J. Biol. Chem. 280, 18703–18709 [DOI] [PubMed] [Google Scholar]
- 7. Beckman M. L., Bernstein E. M., Quick M. W. (1999) J. Neurosci. 19, RC9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cristóvão-Ferreira S., Vaz S. H., Ribeiro J. A., Sebastião A. M. (2009) J. Neurochem. 109, 336–347 [DOI] [PubMed] [Google Scholar]
- 9. Whitworth T. L., Quick M. W. (2001) J. Biol. Chem. 276, 42932–42937 [DOI] [PubMed] [Google Scholar]
- 10. Law R. M., Stafford A., Quick M. W. (2000) J. Biol. Chem. 275, 23986–23991 [DOI] [PubMed] [Google Scholar]
- 11. Vaz S. H., Cristóvão-Ferreira S., Ribeiro J. A., Sebastião A. M. (2008) Brain Res. 1219, 19–25 [DOI] [PubMed] [Google Scholar]
- 12. Sebastião A. M., Assaife-Lopes N., Diógenes M. J., Vaz S. H., Ribeiro J. A. (2010) Biochim. Biophys. Acta 1808, 1340–1349 [DOI] [PubMed] [Google Scholar]
- 13. Halassa M. M., Haydon P. G. (2010) Annu. Rev. Physiol. 72, 335–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haydon P. G., Carmignoto G. (2006) Physiol. Rev. 86, 1009–1031 [DOI] [PubMed] [Google Scholar]
- 15. Halassa M. M., Fellin T., Haydon P. G. (2007) Trends Mol. Med. 13, 54–63 [DOI] [PubMed] [Google Scholar]
- 16. Perea G., Navarrete M., Araque A. (2009) Trends Neurosci. 32, 421–431 [DOI] [PubMed] [Google Scholar]
- 17. Kirmse K., Kirischuk S., Grantyn R. (2009) Synapse 63, 921–929 [DOI] [PubMed] [Google Scholar]
- 18. Biber K., Klotz K. N., Berger M., Gebicke-Härter P. J., van Calker D. (1997) J. Neurosci. 17, 4956–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sorkina T., Miranda M., Dionne K. R., Hoover B. R., Zahniser N. R., Sorkin A. (2006) J. Neurosci. 26, 8195–8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leander Johansen J., Dagø L., Tornøe J., Rosenblad C., Kusk P. (2005) Mol. Biotechnol. 29, 47–56 [DOI] [PubMed] [Google Scholar]
- 21. Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F. H., Verma I. M., Trono D. (1996) Science 272, 263–267 [DOI] [PubMed] [Google Scholar]
- 22. Zufferey R., Nagy D., Mandel R. J., Naldini L., Trono D. (1997) Nat. Biotechnol. 15, 871–875 [DOI] [PubMed] [Google Scholar]
- 23. Naldini L., Blömer U., Gage F. H., Trono D., Verma I. M. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 11382–11388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schousboe A., Sarup A., Bak L. K., Waagepetersen H. S., Larsson O. M. (2004) Neurochem. Int. 45, 521–527 [DOI] [PubMed] [Google Scholar]
- 25. Wood J. D., Sidhu H. S. (1986) J. Neurochem. 46, 739–744 [DOI] [PubMed] [Google Scholar]
- 26. Borden L. A., Murali Dhar T. G., Smith K. E., Weinshank R. L., Branchek T. A., Gluchowski C. (1994) Eur. J. Pharmacol. 269, 219–224 [DOI] [PubMed] [Google Scholar]
- 27. Borden L. A., Dhar T. G., Smith K. E., Branchek T. A., Gluchowski C., Weinshank R. L. (1994) Receptors Channels 2, 207–213 [PubMed] [Google Scholar]
- 28. Chao M. V. (2003) Nat. Rev. Neurosci. 4, 299–309 [DOI] [PubMed] [Google Scholar]
- 29. Tapley P., Lamballe F., Barbacid M. (1992) Oncogene 7, 371–381 [PubMed] [Google Scholar]
- 30. Ding J., Vlahos C. J., Liu R., Brown R. F., Badwey J. A. (1995) J. Biol. Chem. 270, 11684–11691 [DOI] [PubMed] [Google Scholar]
- 31. Favata M. F., Horiuchi K. Y., Manos E. J., Daulerio A. J., Stradley D. A., Feeser W. S., Van Dyk D. E., Pitts W. J., Earl R. A., Hobbs F., Copeland R. A., Magolda R. L., Scherle P. A., Trzaskos J. M. (1998) J. Biol. Chem. 273, 18623–18632 [DOI] [PubMed] [Google Scholar]
- 32. Smith R. J., Sam L. M., Justen J. M., Bundy G. L., Bala G. A., Bleasdale J. E. (1990) J. Pharmacol. Exp. Ther. 253, 688–697 [PubMed] [Google Scholar]
- 33. Patapoutian A., Reichardt L. F. (2001) Curr. Opin Neurobiol. 11, 272–280 [DOI] [PubMed] [Google Scholar]
- 34. Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. (1991) J. Biol. Chem. 266, 15771–15781 [PubMed] [Google Scholar]
- 35. Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. (2006) Dev. Cell 10, 839–850 [DOI] [PubMed] [Google Scholar]
- 36. Mollenhauer H. H., Morré D. J., Rowe L. D. (1990) Biochim. Biophys. Acta 1031, 225–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang M., Chen G. (2009) J. Neurosci. 29, 8063–8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diógenes M. J., Assaife-Lopes N., Pinto-Duarte A., Ribeiro J. A., Sebastião A. M. (2007) Hippocampus 17, 577–585 [DOI] [PubMed] [Google Scholar]
- 39. Assaife-Lopes N., Sousa V. C., Pereira D. B., Ribeiro J. A., Chao M. V., Sebastião A. M. (2010) J. Neurosci. 30, 8468–8480 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Zocchi C., Ongini E., Conti A., Monopoli A., Negretti A., Baraldi P. G., Dionisotti S. (1996) J. Pharmacol. Exp. Ther. 276, 398–404 [PubMed] [Google Scholar]
- 41. Jarvis M. F., Schulz R., Hutchison A. J., Do U. H., Sills M. A., Williams M. (1989) J. Pharmacol. Exp. Ther. 251, 888–893 [PubMed] [Google Scholar]
- 42. Awad J. A., Johnson R. A., Jakobs K. H., Schultz G. (1983) J. Biol. Chem. 258, 2960–2965 [PubMed] [Google Scholar]
- 43. Murray A. J. (2008) Sci. Signal. 1, re4 [DOI] [PubMed] [Google Scholar]
- 44. Tanaka T., Saito H., Matsuki N. (1997) J. Neurosci. 17, 2959–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brünig I., Penschuck S., Berninger B., Benson J., Fritschy J. M. (2001) Eur. J. Neurosci. 13, 1320–1328 [DOI] [PubMed] [Google Scholar]
- 46. Wardle R. A., Poo M. M. (2003) J. Neurosci. 23, 8722–8732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elmariah S. B., Oh E. J., Hughes E. G., Balice-Gordon R. J. (2005) J. Neurosci. 25, 3638–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lindquist C. E., Birnir B. (2006) J. Neurochem. 97, 1349–1356 [DOI] [PubMed] [Google Scholar]
- 49. Bergami M., Santi S., Formaggio E., Cagnoli C., Verderio C., Blum R., Berninger B., Matteoli M., Canossa M. (2008) J. Cell Biol. 183, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rose C. R., Blum R., Pichler B., Lepier A., Kafitz K. W., Konnerth A. (2003) Nature 426, 74–78 [DOI] [PubMed] [Google Scholar]
- 51. Huang E. J., Reichardt L. F. (2003) Annu. Rev. Biochem. 72, 609–642 [DOI] [PubMed] [Google Scholar]
- 52. Baxter G. T., Radeke M. J., Kuo R. C., Makrides V., Hinkle B., Hoang R., Medina-Selby A., Coit D., Valenzuela P., Feinstein S. C. (1997) J. Neurosci. 17, 2683–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ohira K., Funatsu N., Homma K. J., Sahara Y., Hayashi M., Kaneko T., Nakamura S. (2007) Eur. J. Neurosci. 25, 406–416 [DOI] [PubMed] [Google Scholar]
- 54. Blum R., Konnerth A. (2005) Physiology 20, 70–78 [DOI] [PubMed] [Google Scholar]
- 55. Quick M. W., Hu J., Wang D., Zhang H. Y. (2004) J. Biol. Chem. 279, 15961–15967 [DOI] [PubMed] [Google Scholar]
- 56. Haapasalo A., Sipola I., Larsson K., Akerman K. E., Stoilov P., Stamm S., Wong G., Castren E. (2002) J. Biol. Chem. 277, 43160–43167 [DOI] [PubMed] [Google Scholar]
- 57. Diógenes M. J., Fernandes C. C., Sebastião A. M., Ribeiro J. A. (2004) J. Neurosci. 24, 2905–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pousinha P. A., Diogenes M. J., Ribeiro J. A., Sebastião A. M. (2006) Neurosci. Lett. 404, 143–147 [DOI] [PubMed] [Google Scholar]
- 59. Fontinha B. M., Diógenes M. J., Ribeiro J. A., Sebastião A. M. (2008) Neuropharmacology 54, 924–933 [DOI] [PubMed] [Google Scholar]
- 60. Tebano M. T., Martire A., Potenza R. L., Grò C., Pepponi R., Armida M., Domenici M. R., Schwarzschild M. A., Chen J. F., Popoli P. (2008) J. Neurochem. 104, 279–286 [DOI] [PubMed] [Google Scholar]
- 61. Serrano A., Haddjeri N., Lacaille J. C., Robitaille R. (2006) J. Neurosci. 26, 5370–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fields R. D., Burnstock G. (2006) Nat. Rev. Neurosci. 7, 423–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hamilton N. B., Attwell D. (2010) Nat. Rev. Neurosci. 11, 227–238 [DOI] [PubMed] [Google Scholar]
- 64. Pyne N. J., Pyne S. (2011) Trends Pharmacol. Sci. 32, 443–450 [DOI] [PubMed] [Google Scholar]