Background: Cytochrome c maturation (Ccm) is the covalent ligation of heme b to an apocytochrome c by the Ccm apparatus.
Results: CcmI subunit of CcmFHI heme ligation complex recognizes and binds specifically apo- and not holocytochrome c2.
Conclusion: CcmI and its homologues are apocytochrome c chaperones.
Significance: A first glimpse to how the heme ligation complex recognizes an apocytochrome c before, and releases a holocytochrome c after, cofactor addition.
Keywords: Bioenergetics, Cytochrome b, Cytochrome c, Electron Transport, Heme, Cytochrome c Maturation Ccm, Rhodobacter capsulatus, TPR Motifs, Apocytochrome c Chaperone, Protein-Protein Interactions
Abstract
Cytochrome c maturation (Ccm) is a sophisticated post-translational process. It occurs after translocation of apocytochromes c to the p side of energy transducing membranes and forms stereo-specific thioether bonds between the vinyl groups of heme b (protoporphyrin IX-Fe) and the thiol groups of cysteines at their conserved heme binding sites. In many organisms this process involves up to 10 (CcmABCDEFGHI and CcdA) membrane proteins. One of these proteins is CcmI, which has an N-terminal membrane-embedded domain with two transmembrane helices and a large C-terminal periplasmic domain with protein-protein interaction motifs. Together with CcmF and CcmH, CcmI forms a multisubunit heme ligation complex. How the CcmFHI complex recognizes its apocytochrome c substrates remained unknown. In this study, using Rhodobacter capsulatus apocytochrome c2 as a Ccm substrate, we demonstrate for the first time that CcmI binds apocytochrome c2 but not holocytochrome c2. Mainly the C-terminal portions of both CcmI and apocytochrome c2 mediate this binding. Other physical interactions via the conserved structural elements in apocytochrome c2, like the heme ligating cysteines or heme iron axial ligands, are less crucial. Furthermore, we show that the N-terminal domain of CcmI can also weakly bind apocytochrome c2, but this interaction requires a free thiol group at apocytochrome c2 heme binding site. We conclude that the CcmI subunit of the CcmFHI complex functions as an apocytochrome c chaperone during the Ccm process used by proteobacteria, archaea, mitochondria of plants and red algae.
Introduction
The c-type cytochromes are widespread electron carrier proteins found in all domains of life (1). They have conserved features despite their diverse three-dimensional structures, and their functions extend from electron transport in biological systems (2) to apoptosis in eukaryotic cells (3). All cytochromes c have at least one protoporphyrin IX-Fe (heme b) cofactor that is stereo-specifically attached to the polypeptide chain via two thioether bonds (4). These bonds are formed between the vinyl groups at positions 2 and 4 of the heme tetrapyrrole ring and the two thiol groups of Cys1 and Cys2 of apocytochrome c (C1XXC2H) heme binding site, respectively. In α- and γ-proteobacteria like Rhodobacter capsulatus, cytochrome c maturation (Ccm)4 occurs in the periplasm after translation and translocation of apocytochromes c from the cytoplasm via the Sec secretion pathway (see Fig. 1). It is a complex process that involves up to 10 membrane proteins: CcmABCDEFGHI and CcdA (5, 6). These components are responsible for (i) transport and relay of heme (CcmABCDE), (ii) preparation and chaperoning of ligation-competent apocytochromes c (CcdA and CcmG), and (iii) ligation of heme to apocytochromes c (CcmFHI) to produce mature holocytochromes c (5, 6). The final step of this intricate process is the ligation per se of heme b to apocytochrome c to form mature cytochrome c, and this is attributed to the multisubunit membrane complex CcmFHI (7) (see Fig. 1). CcmF contains heme b and belongs to the heme handling protein family (8, 9). It has a conserved periplasmic WWD motif and two conserved His residues that are proposed to acquire heme from holoCcmE (9). CcmH has a single transmembrane helix and a periplasmic domain with a non-canonical thioredox-like motif (10). Last, CcmI is a bipartite protein with an N-terminal membrane integral part (CcmI-1) composed by two transmembrane helices linked through a cytoplasmic loop with a leucine zipper-like motif (11, 12). Its second part is a large C-terminal periplasmic domain (CcmI-2) with three tetratricopeptide repeats (TPR) (see Fig. 2A). Earlier genetic studies inferred that the two parts of CcmI play different roles during Ccm (11, 13–16). In R. capsulatus, the absence of CcmI differently affects the production of membrane-bound (i.e. cytochrome c1 subunit of the cytochrome bc1 complex, cytochrome cy, and cytochromes co and cp subunits of cbb3-type cytochrome c oxidase) and periplasmic (i.e. cytochromes c2 and c′) electron carrier c-type cytochromes (17–20). As expected, CcmI-null mutants produce no c-type cytochromes (11, 12). However, mutants containing only the CcmI-1 domain still produce cytochrome c1. In contrast, mutants lacking CcmI-1 do not produce any c-type cytochromes (11, 12). These findings suggested that CcmI-1 is required for maturation of all c-type cytochromes, whereas CcmI-2 is unnecessary for that of the C-terminally anchored cytochrome c1. Overall, studies suggested that CcmI-1 might be involved in heme ligation together with CcmF and CcmH, whereas CcmI-2 cooperates with CcmG for delivery of apocytochromes c to the heme ligation complex (12, 21, 22). In fact, recent data established that R. capsulatus CcmFHI forms a membrane-integral complex (7) (Fig. 1) and suggested that CcmG might function as a apocytochrome c2 “holdase” (23). How CcmFHI recognized apocytochromes c remained unknown.
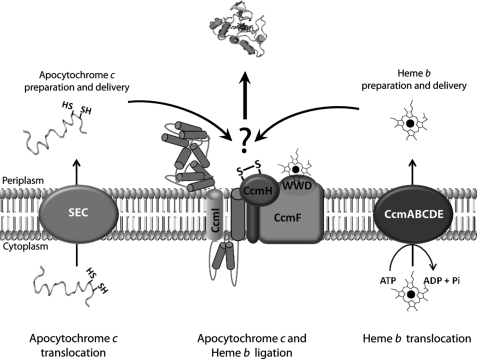
FIGURE 1.
Overall organization of Ccm system I. After translocation to the periplasm via the general secretory pathway (SEC), a pre-apocytochrome is processed to an apocytochrome that undergoes a series of thio-redox reactions. CcdA and CcmG (not shown for the sake of simplicity) keep reduced the heme binding site cysteines of apocytochrome to render it ligation-competent (left, apocytochrome c translocation, preparation, and delivery). CcmABCDE proteins mediate translocation and relay of heme b to the ligation site (right, heme b translocation, preparation, and delivery). The final step of Ccm is attributed to the CcmFHI proteins that form a multisubunit membrane complex responsible for apocytochrome c-heme b ligation reaction per se (middle, apocytochrome c and heme b ligation).
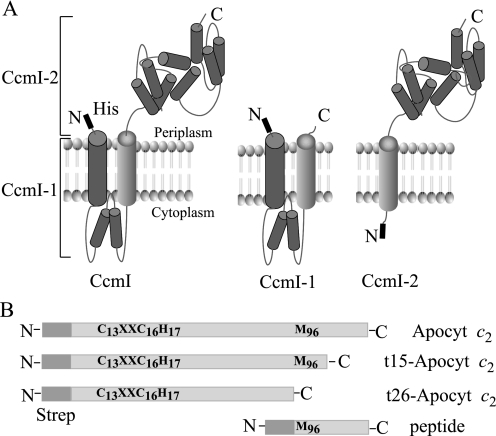
FIGURE 2.
R. capsulatus CcmI and apocytochrome c2 and their derivatives. Panel A, a hypothetical model of native CcmI with its two functionally distinct domains (left), N-terminal membrane-embedded CcmI-1 (middle), and C-terminal periplasmic, TPR containing CcmI-2 (right) domains with their N-terminal His10 epitopes are depicted based on topological predictions (57) and structural data (PDB code 2E2E (2E2E-TPR) domain of E. coli NrfG protein (49)). Note that only the second transmembrane helix of CcmI is present in CcmI-2. Panel B, salient features of apocytochrome c2 are shown. Different N-terminal Strep-tagged apocytochrome c2 constructs, with a full-length apocytochrome c2 containing both the heme binding site Cys residues (Cys-13 and Cys-16) and the heme iron axial ligands (His-17 and Met-96) (Apocyt c2), two derivatives lacking the last C-terminal 26 and 15 amino acid residues (t26-Apocyt c2 and t15-Apocyt c2, respectively), and a synthetic peptide corresponding to the C-terminal 26 amino acid residues with a Strep Tag II-epitope fused to its N terminus, are shown.
In this study we chose the apocytochrome form of R. capsulatus cytochrome c2 as a substrate to test the putative chaperone role of CcmI during Ccm. Cytochrome c2 is a periplasmic electron transfer protein involved in both respiration and photosynthesis and belongs to the Class I c-type cytochromes (24). These c-type cytochromes are characterized by an N-terminal-located heme binding motif (C1XXC2H) and a C-terminal-located methionine residue acting as the sixth axial ligand of heme iron. We heterologously expressed in Escherichia coli and affinity-purified R. capsulatus CcmI and apocytochrome c2. Using a reciprocal in vitro protein-protein interaction assay, we demonstrated for the first time that R. capsulatus CcmI tightly binds apocytochrome c2 but not holocytochrome c2. This binding occurs via strong interactions between the periplasmic CcmI-2 domain of CcmI and the C-terminal helical portion of apocytochrome c2. Other salient structural elements of apocytochrome c2, such as its heme binding site Cys residues or its heme iron axial ligands, and the membrane-integral CcmI-1 domain of CcmI play less critical roles in these interactions. We conclude that the CcmI subunit of the CcmFHI complex functions as an apocytochrome c chaperone during the Ccm process.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids, and Growth Conditions
The bacterial strains and plasmids used in this work are described in Table 1. R. capsulatus strains were grown chemo-heterotrophically (i.e. by respiration) at 35 °C on enriched (mineral-peptone-yeast extract medium) medium supplemented as needed with tetracycline at 2.5 μg per ml final concentration (25). E. coli strains were grown aerobically at 37 °C in Luria-Bertani (LB) broth medium supplemented with ampicillin at a final concentration of 100 μg/ml. The E. coli RP4182, which lacks supE, was used as an expression strain for apocytochrome c2 to avoid any undesired C-terminal extension via translational read-through across its stop codon.
TABLE 1.
Strains and Plasmids used in this work
| Strains/Plasmids | Relevant characteristicsa | References |
|---|---|---|
| Bacteria | ||
| R. capsulatus | ||
| MT1131 | Wild type, ctrD121 Rifr Res+ Nadi+ Ps+ | (58) |
| MT-SRP1 | Δ (ccmI::kan) Res+ Nadi− Ps− | (11) |
| Escherichia coli | ||
| HB101 | F− Δ(gpt-proA)62 araC14 leuB6(Am) glnV44(AS) galK2(Oc) lacY1 Δ(mcrC-mrr) rpsL20(Strr) xylA5 mtl-1 thi-1 | Stratagene |
| XL1-Blue | endA1 gyrA96(NalR) thi-1 recA1 relA1 lac glnV44 F′[::Tn10 proAB+lacIq Δ(lacZ)M15] hsdR17(rK−mK+) | Stratagene |
| RP4182 | trp, gal, rpsL Δ(supE, dcm, fla) | (59) |
| Plasmids | ||
| pBSK | pBluescriptIISK+, Ampr | Stratagene |
| pRK415 | Broad host-range vector, gene expression mediated by E. coli lacZ promoter, Tetr | (60) |
| pCHB500 | Broad host-range vector with R. capsulatus cycA promoter, Tetr | (61) |
| pRK404 | Broad host-range vector, Tetr | (60) |
| pCS1302 | pCS905 derivative, Strep-tag II sequence fused to GFP, rendering GFP replaceable by cloning any gene of interest in-frame into NdeI and BamHI sites, Ampr | (7) |
| pCS905 | pET-3a derivative (Novagen) with T7 promoter region replaced by a DNA fragment encoding lacI and the tac promoter region, Ampr | |
| pCS1726 | R. capsulatus cycA encoding mature cyt c2 with a N-terminal Strep-tag cloned into pCS1302 using NdeI and BamHI sites, Ampr | (27) |
| pSVEN | 1.65-kb StuI-BbsI fragment containing R. capsulatus CcmI cloned in pRK404 | (11) |
| pAV1 | pCS1726 derivative with an additional in-frame stop codon (TAA) 15 bp downstream from the native TAG of cycA, Ampr | This work |
| pAV1C13S | Cys-13 of R. capsulatus cycA in pAV1 mutated to Ser, Ampr | This work |
| pAV1C16S | Cys-16 of R. capsulatus cycA in pAV1 mutated to Ser, Ampr | This work |
| pAV1H17S | His-17 of R. capsulatus cycA in pAV1 mutated to Ser, Ampr | This work |
| pAV1M96S | Met-96 of R. capsulatus cycA in pAV1 mutated to Ser, Ampr | This work |
| pAV1C13SC16S | Cys-13 and Cys-16 of R. capsulatus cycA in pAV1 mutated to Ser, Ampr | This work |
| pAV2 | pAV1 derivative with an in-frame stop codon (TAA) 78-bp upstream of the native TAG codon, deleting the 26 last amino acids of cycA, Ampr | This work |
| pAV2C13SC16S | Cys-13 and Cys-16 of R. capsulatus cycA in pAV2 mutated to Ser, Ampr | This work |
| pAV3 | pAV1 derivative with an in-frame stop codon (TAA) 42 bp upstream of the native TAG codon, deleting the 15 last amino acids of cycA, Ampr | This work |
| pCS1303 | pCS905 derivative, His10-tag sequence fused to GFP, rendering GFP replaceable by cloning any gene of interest in-frame into NdeI and BamHI sites, Ampr | This work |
| pMADO3 | PCR amplified full length ccmI with introduced 5′-NdeI and 3′-BamHI sites, phosphorylated and cloned into EcoRV-restricted pBSK, Ampr | This work |
| pMADO5 | ccmI from pMADO3 cloned into NdeI-BamHI in pCS1303, Ampr | This work |
| pMADO5–1 | pCS1303 derivative with a 360-bp 5′-NdeI and 3′-BamHI PCR fragment of R. capsulatus MT1131 genomic DNA, yielding a truncated ccmI with its first 121 amino acid residues (CcmI-1 domain), Ampr | This work |
| pMADO5–2 | pMADO5 derivative, obtained by in-frame deletion of the first 250 bp, yielding a ccmI derivative lacking the first 83 amino acid residues (CcmI-2 domain), Ampr | This work |
| pCS1587 | 1.36-kb XbaI-BamHI fragment from pMADO5 cloned into pCHB500 | This work |
a Res and Ps refer to respiratory and photosynthetic growth, respectively. Nadi stain, α-naphthol + N,N-dimethyl-p-phenylenediamine, yielding indophenol blue in the presence of O2 to reveal cytochrome c oxidase activities of bacterial colonies. R. capsulatus MT1131 strain is referred to as a wild-type strain with respect to its cytochrome c profile and growth properties.
Molecular Genetic Techniques
Molecular genetic techniques were performed according to Sambrook et al. (26), and all constructs were confirmed by DNA sequencing and analyzed using the Serial Cloner 2.1 and BLAST software. Nucleotide sequences of the primers used are described in supplemental Table S1). Plasmid pAV1 was generated from pCS1726 (7), carrying an in-frame Strep-tag II epitope (WSHPQFEK) fused at the 5′-end of cycA encoding R. capsulatus cytochrome c2 without its signal sequence by creating an additional stop codon (TAA) 15 bp downstream from the native TAG codon of cycA (Fig. 2B). Plasmids pAV1C13S, pAV1C16S, and pAV1C13S/C16S containing the single and double Cys to Ser substitutions at the 13CKTCH17 heme binding motif of apocytochrome c2 were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer's instructions and plasmid pAV1 as template. Similarly, pAV1H17S or pAV1M96S with the apocytochrome c2 heme iron axial ligands His-17 or Met-96 to Ser substitutions, respectively, were obtained. The truncated versions of apocytochrome c2 were generated by inserting a stop codon (TAA) 78 or 42 bp upstream the native TAG stop codon of cycA using pAV1 as template, yielding pAV2 or pAV3, respectively. The C-terminal apocytochrome c2 truncation in pAV2 eliminated the last C-terminal 26 amino acids of holocytochrome c2 including Met-96, whereas that in pAV3 eliminated only the last 15, excluding Met-96 (Fig. 2B). Plasmid pAV2C13S/C16S was constructed using pAV2 as a template and the primers used before for the full-length Cys-less mutant. Apocytochrome c2 derivatives contained the N-terminal in-frame Strep-tag II epitope sequence followed by the Factor Xa cleavage site.
PCR amplification using plasmid pSVEN containing ccmI (previously called cycH) (11) and primers listed in supplemental Table S1, adding a 5′-NdeI and 3′-BamHI restriction sites, yielded a 1.3-kb fragment that was phosphorylated and cloned into the EcoRV site of pBSK to yield pMADO3. This plasmid was digested with NdeI and BamHI sites, and the fragment-containing ccmI was cloned into the same sites of pCS1303 (a derivative of pCS905 (27)) to obtain pMADO5 (Fig. 2A, CcmI). Plasmid pMADO5 contained an in-frame 10-histidine-long (His10) epitope tag and a Factor Xa cleavage site fused at the N-terminal end of CcmI. An appropriate derivative (i.e. pCS1587, Table 1) carrying the His10-CcmI complemented fully in vivo an R. capsulatus mutant strain (MT-SRP1, Table 1) lacking CcmI, establishing that it was functional. The CcmI-1 portion of CcmI, corresponding to the N-terminal 360 bp of ccmI, was cloned from a wild type R. capsulatus (MT1131) genomic DNA by PCR again using primers with NdeI and BamHI restriction sites. The PCR product thus obtained was digested with NdeI and BamHI and cloned into pCS1303 using the same sites to yield pMADO5–1. This plasmid contained the N-terminal 121 amino acids of CcmI with its two transmembrane helices and its cytoplasmic loop (Fig. 2A, CcmI-1). Plasmid pMADO5 was used as a template for site-directed mutagenesis to delete the first in-frame 249 bp of ccmI to yield pMADO5–2, producing CcmI-2 lacking the N-terminal 83 amino acids forming the first transmembrane helix and the cytoplasmic loop of native CcmI (Fig. 2A, CcmI-2). All CcmI derivatives contained the N-terminal His10 epitope tag followed by the Factor Xa cleavage site.
Preparation of R. capsulatus or E. coli Detergent-solubilized Membrane Proteins
R. capsulatus MT1131 cells were resuspended in buffer A (50 mm Tris-HCl, pH 8.0, 50 mm NaCl, 5 mm EDTA, 1 mm PMSF) and intracytoplasmic membrane vesicles (chromotophores) prepared using a French pressure cell (25). After a 30-min low speed centrifugation (4 °C and 14,000 × g) the supernatant was further centrifuged for 2 h at 4 °C and 138,000 × g to pellet chromatophores. Pellets were resuspended in buffer A, and membrane protein concentrations were determined according to the bicinchoninic acid kit (Sigma, Inc.) using bovine serum albumin as a standard. Membranes were dispersed by the addition of n-dodecyl-β-d-maltoside (DDM) (Anatrace, Inc.) at a protein:detergent ratio of 1:1 (w/w) under continuous stirring for 1 h at 4 °C, and non-solubilized membranes were removed by centrifugation at 138,000 × g for 1 h to yield “clear” supernatants that were stored at −20 °C until further use. In the case of E. coli, cells expressing CcmI-1 were resuspended in buffer A without EDTA, and dispersed membrane fractions were obtained as described for R. capsulatus cells.
Heterologous Expression and Purification of His10-CcmI and Strep-apocytochrome c2 and Their Derivatives
E. coli strains were grown at 37 °C (with 200 rpm shaking) in 1 liter of LB medium supplemented with 100 μg/ml ampicillin until an A600 of 0.6–0.8. Expression of the desired proteins in E. coli was controlled by a Ptac-lac promoter-operator system. Cultures were induced by the addition of 1 mm isopropyl β-d-1-thiogalactopyranoside for 4 h. For purification of His10-CcmI, His10-CcmI-2, and Strep-apocytochrome c2 as well as its mutant derivatives, cells were lysed using 5 ml of CelLytic™ B 2× Cell Lysis Reagent (Sigma) per g of wet cells supplemented with lysozyme (0.2 mg/ml), DNase (0.05 mg/ml), MgCl2 (10 mm), and EDTA-free protease inhibitor mixture (Sigma) for 1 h at 4 °C with gentle shaking. Crude lysates were centrifuged for 30 min at 20,000 × g, and cleared supernatants were filtered through a 0.45-μm filter.
For purification of His10-CcmI or His10-CcmI-2, cleared lysates were diluted 5-fold with a buffer containing 25 mm Tris-HCl, pH 7.4, 500 mm NaCl, 40 mm imidazole, and 0.01% DDM and loaded onto a Ni2+-Sepharose high performance column (GE Healthcare) equilibrated with the same buffer. Elution of His10-CcmI or His10-CcmI-2 was done using the same buffer supplemented with 500 mm imidazole. Proteins were concentrated using Amicon Ultra YM-30 filters (Millipore, Inc.) and desalted with a PD-10 column (GE Healthcare) using a buffer containing 50 mm Tris-HCl, pH 8, 50 mm NaCl and 0.01% DDM.
For purification of His10-CcmI-1, solubilized membranes were loaded initially onto a Ni2+-Sepharose high performance column. However, because of poor binding efficiency of His10-CcmI-1, the flow-through was re-loaded onto a Q-Sepharose Fast Flow column (GE Healthcare) previously equilibrated with a buffer containing 50 mm Tris-HCl, pH 8, and 0.01% DDM. In this way His10-CcmI-1 eluted before applying a salt gradient and purified away from the majority of E. coli membrane proteins. After concentration using Amicon YM-10 filters (Millipore Inc.) and desalting as described above, partially purified His10-CcmI-1 was identified with anti-CcmI antibodies (see below).
For purification of Strep-apocytochrome c2 and its mutant variants, cell lysates were diluted 5-fold with “wash buffer” composed of 100 mm Tris-HCl, pH 8, and 150 mm NaCl, loaded onto a 5-ml Strep Tactin-Sepharose (IBA, Inc.) column, washed extensively, and eluted by adding 2.5 mm desthiobiotin to the wash buffer following the manufacturer's instructions. Eluted proteins were concentrated via Amicon YM-3 filters (Millipore Inc.) and desalted using a buffer with 50 mm Tris-HCl, pH 8, 50 mm NaCl. Purification of R. capsulatus cytochrome c2 was done as in Holden et al. (28).
Production of Anti-CcmI Polyclonal Antibodies
Nickel affinity chromatography-purified R. capsulatus His10-CcmI (∼3 mg) was subjected to SDS-PAGE, electro-eluted from the gel matrix, and used as an antigen for rabbit polyclonal antibodies production, which was carried out by Open Biosystems, Inc.
Protein-Protein Interaction Studies Using Reciprocal Co-purification Assays
Protein-protein interactions between CcmI and its derivatives and apocytochrome c2 and its mutant variants were assayed as follows. His10-CcmI (5 or 10 μg) and Strep-apocytochrome c2 (15 or 30 μg) were mixed in binding/wash buffer containing 50 mm Tris-HCl, pH 8, 50 mm NaCl, 50 mm imidazole, and 0.01% DDM (final volume of 400 or 800 μl, respectively) and incubated for 2 h at 25 °C with gentle shaking. After incubation, epitope-tagged components of the assay mixture were re-purified by using as appropriate 200 μl of Strep Tactin or Ni2+-Sepharose resins packed into small columns equilibrated with wash buffer and washed extensively with at least 10 times the column volume. Elution with 2.5 times the column volume was performed by adding 2.5 mm desthiobiotin or 500 mm imidazole to the wash buffer for the Strep-tag or His-tag purifications, respectively. Flow-through and elution fractions were precipitated with methanol:acetone (7:2, v/v) overnight at −20 °C and analyzed by SDS-PAGE. For experiments using solubilized membranes of R. capsulatus MT1131 cells, 10, 20, and 30 μg of Strep-apocytochrome c2 were incubated with 400 μg of solubilized membrane proteins for 16 h at 4 °C with gentle shaking. After incubation, the mixtures were loaded onto Strep Tactin columns as described above. Elution fractions were concentrated using Amicon YM-3 filters (Millipore Inc.) and analyzed by SDS-PAGE. Protein-protein interactions between His10-CcmI and Strep-apocytochrome c2 were also tested by size exclusion chromatography using a Sephacryl S200 column (GE Healthcare). The two proteins were co-incubated under the conditions used for the co-purification assays (see above) and loaded onto the S200 column equilibrated with the binding/wash buffer, and elution profiles were determined using SDS-PAGE.
SDS-PAGE and Immunoblot Analyses
SDS-PAGE was performed using 15% polyacrylamide gels according to Laemmli (29). For CcmI, CcmI-1, and CcmI-2 immunodetection, gel-resolved proteins were electroblotted onto Immobilon-PVDF membranes (Millipore, Inc.) and probed with rabbit polyclonal antibodies that were raised against R. capsulatus CcmI. Horseradish peroxidase conjugated anti-rabbit IgG antibodies (GE Healthcare) were used as secondary antibodies, and detection was performed using the SuperSignal West Pico Chemiluminescent Substrate® from Thermo Scientific, Inc.
Reduction and Alkylation of Apocytochrome c2, Its Mutant Derivatives, and CcmI-1
Approximately 1 mg (70 μm) of purified Strep-apocytochrome c2 or its appropriate mutant derivatives in 50 mm Tris-HCl, pH 8, 50 mm NaCl were incubated with 10 mm dithiothreitol (DTT) for 1 h at 25 °C followed by the addition of freshly prepared 50 mm iodoacetamide (IAM) for another 1 h in the dark. After incubation, excess of DTT and IAM was removed using a PD-10 desalting column previously equilibrated with the same buffer. Reduction/alkylation of CcmI-1 was conducted in a similar fashion using ∼400 μg of partially purified CcmI-1 except that all buffers contained 0.01% DDM.
Circular Dichroism Spectroscopy
The far-UV circular dichroism (CD) spectra (195–250 nm) were recorded with a Model 202 spectropolarimeter (AVIV® Instruments, Inc.) using a 2-mm path length cuvette (Hellma, Inc.). CD spectra of proteins were recorded at 25 °C using a 3-nm bandwidth and a step size of 1 nm. A time constant of 10 s was used to improve the signal to noise ratio and to decrease the contribution of the solvent at lower wavelengths. CD spectra were recorded using protein concentrations of 1–100 μm depending on the number of amino acid residues of proteins examined in 20 mm sodium phosphate buffer, pH 7.5, and corrected by subtracting the spectrum of the buffer alone. Raw CD data were converted into the mean residue ellipticity [θ]λ (deg cm2 dmol−1) at each wavelength using the relation, [θ]λ = θλ/(10CNl), where θλ is the observed ellipticity in millidegrees at wavelength λ, C is the molar protein concentration, N is the number of amino acids of the protein, and l is the path length of the cuvette in cm. To monitor the conformational changes induced by CcmI-apocytochrome c2 interactions, protein concentrations identical to those used for acquisition of individual CD spectra were incubated using the sodium phosphate buffer described above for 2 h at 25 °C. The CD spectrum of the protein mixtures was then compared with the sum of the spectra of the individual proteins. As a negative control, CD spectrum of CcmI incubated with bovine serum albumin was also recorded.
Chemicals
All chemicals and solvents were of high purity and HPLC spectral grades and purchased from commercial sources.
RESULTS
Overproduction and Purification of Epitope-tagged CcmI and Apocytochrome c2
Defining the molecular nature of specific interactions between various apocytochromes c and Ccm components is important for our understanding of how Ccm proceeds. Earlier genetic studies (11, 12) led us to initiate a detailed biochemical analysis of the interactions between CcmI and apocytochrome c2. The in vivo accumulation of apocytochromes c is not readily observed in R. capsulatus wild type or Ccm-defective strains, and the purification of a large amount of native membrane-integral Ccm components is also difficult. Thus, we used in vitro approaches with heterologously expressed, N-terminally epitope-tagged apocytochrome c2 and CcmI (Table 1, Fig. 2). These proteins were overproduced in E. coli and purified in large amounts (∼mg) by affinity chromatography as described under “Experimental Procedures.” The purity of His10-CcmI and Strep-apocytochrome c2 was assessed by SDS-PAGE (Fig. 3A) as well as by nanoLC-MS/MS and immunoblot analyses using anti-CcmI and anti-cytochrome c2 polyclonal antibodies, respectively (not shown). Purified His10-CcmI samples contained a major band (>90%) of 50 kDa (i.e. full-length CcmI) and trace amounts of three minor bands of 33, 27, and 21 kDa molecular masses, whereas Strep-apocytochrome c2 samples had a major band (>90%) of 13.5 kDa and a minor band of 12.9 kDa molecular mass. In all cases nanoLC-tandem mass spectrometry analyses (data not shown) confirmed that the minor bands corresponded to N-terminal-tagged, C-terminal-truncated derivatives of CcmI or apocytochrome c2, produced and co-purified under the conditions used.
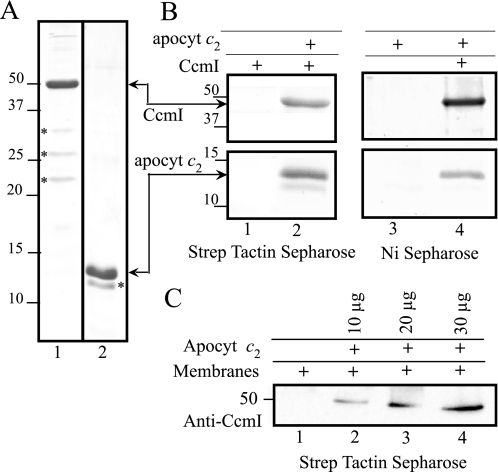
FIGURE 3.
Co-purification of CcmI with apocytochrome c2. Panel A, Coomassie Blue staining of purified His10-CcmI (lane 1) and Strep-apocytochrome c2 (apocyt c2, lane 2) (3 μg of protein/lane). The minor C-terminal-truncated forms of CcmI of 33-, 27-, and 21-kDa and of apocytochrome c2 of 12.9-kDa molecular masses, identified and sequenced by nanoLC-MS/MS, are indicated by a asterisks. Panel B, co-purification of His10-CcmI and Strep-apocytochrome c2 using Strep Tactin-Sepharose (lanes 1 and 2) and Ni2+-Sepharose (lanes 3 and 4) resins, respectively, is shown. One-half of the CcmI-apocytochrome c2 incubation mixture (see “Experimental Procedures”) was loaded on Strep Tactin-Sepharose, and the remaining half was loaded on Ni2+-Sepharose resin columns. Lanes 1 and 3 show that CcmI and apocytochrome c2 do not bind to Strep Tactin or Ni2+-Sepharose resins, respectively, unless their appropriately tagged partners are present (lanes 2 and 4). For CcmI, only the 50-kDa region of the gel is shown, but minor C-terminal-truncated forms of CcmI of 33, 27, and 21 kDa are also present in lanes 2 and 4; and note the absence of the C-terminal-truncated form of apocytochrome c2 in lane 4. Panel C, co-purification of native CcmI from DDM-solubilized membranes from R. capsulatus strain MT1131 using different amounts of purified Strep-apocytochrome c2 (10, 20, and 30 μg, lanes 2, 3, and 4, respectively) using the Strep Tactin resin. Co-eluted CcmI was detected by immunoblot analysis with anti-CcmI polyclonal antibodies. Note that native CcmI does not bind to the Strep Tactin-Sepharose in the absence of apocytochrome c2 (lane 1). Molecular mass markers (in kDa) are shown on the left.
CcmI Interacts Directly with Apocytochrome c2 in Vitro
To probe whether CcmI interacts directly with apocytochrome c2 during Ccm, in vitro reciprocal protein-protein interaction assays were performed. Purified His10-CcmI and Strep-apocytochrome c2 were co-incubated and subjected to affinity chromatography using Strep Tactin or Ni2+-Sepharose resin columns, as described under “Experimental Procedures.” Different column fractions were analyzed by SDS-PAGE and immunoblots as needed. Control experiments revealed neither unspecific binding of His10-CcmI to the Strep Tactin resin nor Step-apocytochrome c2 to Ni2+-Sepharose resin columns (Fig. 3B, lanes 1 and 3). In contrast, when His10-CcmI and Strep-apocytochrome c2 were co-incubated, they were co-eluted from either of the tag-affinity columns (Fig. 3B, lanes 2 and 4), indicating that CcmI and apocytochrome c2 interact with each other to form a stable binary complex. We note the presence of minor, C-terminal-truncated forms of CcmI in lanes 2 and 4 (not shown) and the absence of the C-terminal-truncated form of apocytochrome c2 in lane 4 only.
The co-elution assays were repeated with DDM-dispersed membranes of wild type R. capsulatus strain MT1131 to assess the specificity of CcmI and apocytochrome c2 interactions. Different amounts of purified Strep-apocytochrome c2 were incubated, with DDM-dispersed membrane proteins under our assay conditions. Strep Tactin elution fractions analyzed by SDS-PAGE and immunoblots with anti-CcmI specific antibodies showed that native CcmI and Strep-tagged apocytochrome c2 co-eluted in quantities proportional to the amounts of apocytochrome c2 used (Fig. 3C). Thus, apocytochrome c2 interacts specifically with CcmI. Furthermore, formation of a stable complex between His10-CcmI and Strep-apocytochrome c2 was also confirmed by size exclusion chromatography with Sephacryl S-200 column (data not shown).
CcmI Interacts with Apocytochrome c2 but Not with Holocytochrome c2 in Vitro
The finding that CcmI binds to apocytochrome c2 led us to investigate if it also interacted with holocytochrome c2. We co-incubated purified His10-CcmI and R. capsulatus holocytochrome c2 (28) and subjected the mixture to the co-purification assay using Ni2+-Sepharose resin under the conditions that evidenced CcmI-apocytochrome c2 interactions. The data showed that holocytochrome c2 does not co-purify with His10-CcmI under the conditions used. Thus, CcmI binds only apocytochrome c2, and not holocytochrome c2, in agreement with its role as an apocytochrome c chaperone during Ccm (Fig. 4).
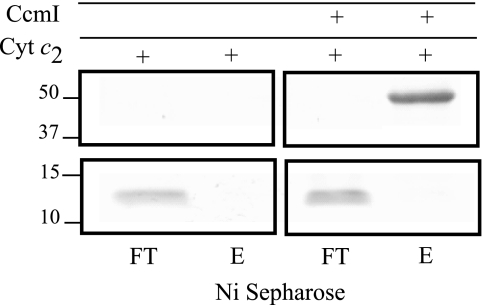
FIGURE 4.
Holocytochrome c2 does not co-purify with His10-CcmI. Co-purification experiments were conducted as in Fig. 3, panel B, except that His10-CcmI (5 μg) was incubated with holocytochrome c2 (15 μg) instead of apocytochrome c2. Flow-through (FT) and elution (E) fractions from the Ni2+-Sepharose resin were analyzed by SDS-PAGE as above. Note that holocytochrome c2 was not retained by Ni2+-Sepharose resin either when alone or when incubated together with CcmI. Molecular markers (in kDa) are shown on the left.
Co-incubation of Apocytochrome c2 and CcmI Induces Conformational Changes in Interacting Partners
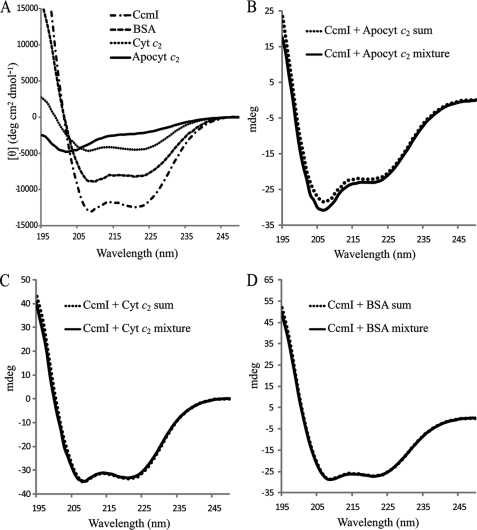
CD spectroscopy in the far UV region (178–260 nm) is a powerful technique to probe conformational changes that occur during protein-protein interactions in solution (30, 31). As expected, the CD spectrum of purified Strep-apocytochrome c2 showed low ellipticity above 215 nm and a negative peak around 202 nm, typical of proteins with “random coil” conformations (Fig. 5A) (32, 33). Similar spectra were also obtained for the heme binding site cysteine and heme iron axial ligands mutants, the C-terminal-truncated derivatives of apocytochrome c2, and the synthetic peptide (see below) (data not shown). On the contrary, the CD spectrum of holocytochrome c2 and purified His10-CcmI showed the typical characteristics of highly α-helical proteins, with negative bands at 222 and 208 nm (Fig. 5A) (32–37).
FIGURE 5.
Circular dichroism spectra to monitor binding apocytochrome c2 to CcmI. Panel A, shown are far-UV (195–250 nm) CD spectra of 1.5 μm CcmI, 15 μm apocytochrome c2 (Apocyt c2), 15 μm cytochrome c2 (Cyt c2), and 1 μm BSA in 20 mm sodium phosphate buffer, pH 7.5, recorded at 25 °C. A CD spectrum of apocytochrome c2 indicates a disordered random coil conformation, and the CD spectra of CcmI, BSA, and cytochrome c2 show typical features of α-helical proteins. Panel B, shown is a comparison of the CD spectra of the (CcmI + Apocyt c2) mixture after 2 h of incubation at 25 °C with the sum of the individual spectrum of each protein. Panel C, shown is a comparison of the CD spectra of the (CcmI + Cyt c2) mixture with the sum of the corresponding individual spectrum of each protein as in B. Panel D, shown is a comparison of the CD spectra of the (CcmI + BSA) mixture with the sum of the corresponding individual spectrum of each protein as in C. Note that only the (CcmI + Apocyt c2) mixture shows a spectrum that is different from the sum of the individual spectra.
Next, purified His10-CcmI and Strep-apocytochrome c2 were coincubated, and their CD spectra between 195 and 250 nm were acquired (“Experimental Procedures”) to assess whether their conformations changed upon their interactions. When the CD spectrum of the protein mixture was compared with the sum of the individual spectrum of each interacting partner, an increase in the helical content of the mixture, as diagnosed by the increase in intensity of the negative bands at 208 and 222 nm, was observed (Fig. 5B). As expected, because CcmI is a highly helical protein, this change is small, but it provides additional evidence for direct association between CcmI and apocytochrome c2 (31). As controls, His10-CcmI incubated either with holocytochrome c2 or bovine serum albumin instead of Strep-apocytochrome c2 exhibited no CD spectral changes (Fig. 5, C and D, respectively). Thus, CcmI interacts directly only with the apo form of cytochrome c2 and not with its holo form.
Structural Determinants Responsible for CcmI and Apocytochrome c2 Interactions
Considering the putative role of CcmI as an apocytochrome c chaperone (11, 12, 15, 16), defining the molecular basis of their interactions is of paramount importance. R. capsulatus cytochrome c2, like its mitochondrial counterpart horse heart cytochrome c, belongs to Class I of c-type cytochromes (24). These proteins have a heme binding (C1XXC2H) motif located at the N-terminal, a methionine residue acting as the sixth axial ligand of heme iron close to the C-terminus (Fig. 2B), and a globular fold with interacting N- and C-terminal helices (38–40).
Heme Binding Site Cys Residues
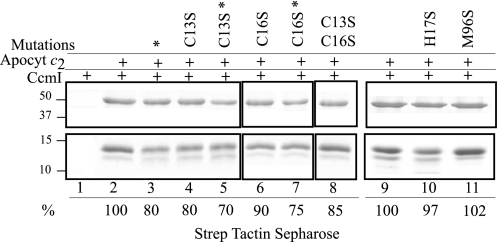
Participation of the heme binding site (13CXXCH17) Cys residues of apocytochrome c2 in CcmI interactions was examined by using single and double Cys to Ser substitutions as well as by alkylating with IAM the free Cys-thiol groups of Strep-apocytochrome c2 and its mutant derivatives. Binding of purified apocytochrome c2 (-C13S, -C16S, and -C13S/C16S) mutants to His10-CcmI were probed via affinity co-purification as for the native Step-apocytochrome c2. The data indicated that apocytochrome c2 mutants lacking either or both of the heme binding site Cys residues still co-purified with CcmI. Image analyses of Coomassie Blue-stained gels indicated that the amount of CcmI that co-purifies with the -C13S, -C16S, and -C13S/C16S apocytochrome c2 mutants decreased by only ∼ 20, 10, and 15%, respectively, relative to that observed with the native apocytochrome c2 (Fig. 6, lanes 4, 6, and 8). Thus, the heme binding site Cys residues have a small contribution to CcmI-apocytochrome c2 interactions.
FIGURE 6.
Co-purification of CcmI with apocytochrome c2 and its derivatives lacking the heme binding site Cys residues Cys-13 and C-16 or the heme iron axial ligands His-17 and Met-96. Co-purification experiments were conducted as in Fig. 3, panel B, except that His10-CcmI (5 μg) was incubated with apocytochrome c2 (15 μg) or its appropriate derivatives. Lane 1 shows that CcmI does not exhibit unspecific binding to Strep Tactin resin. Lanes 2 and 9 show co-purification of CcmI with native apocytochrome c2, and the amount of CcmI present in the elution fraction was taken as 100% for image quantification using Image J program and used for comparison with the amounts seen in lanes 3–8, 10, and 11. Lanes 4, 6, and 8 show slightly lower amounts of CcmI present in the elution fractions when apocytochrome c2 (Apocyt c2)-C13S (80%), -C16S (90%), and -C13S/C16S (85%), respectively, were used. Lanes 3, 5, and 7 also show lower amounts of CcmI present in the elution fractions when wild type apocytochrome c2 (80%) and single Cys mutants (70% for C13S and 75% for C16S) treated with DTT and IAM, respectively, were used. Lanes 10 and 11 show similar amounts of CcmI present in the elution fractions when incubated with apocyt-H17S (97%) and -M96S (102%) mutants, respectively, as compared with native apocytochrome c2 (lane 9). The asterisks indicate that apocytochrome c2 Cys thiols were treated with DTT/IAM, and the molecular markers (in kDa) are indicated on the left.
During Ccm, heme binding site Cys-thiols are thought to undergo oxidation reduction via the periplasmic thio-redox pathways (5, 6, 23). Any requirement for a defined redox state of the heme binding site Cys residues for CcmI-apocytochrome c2 interaction was tested by DTT/IAM treatments of native and mutated apocytochrome c2 proteins. Co-purification assays revealed that chemical modification of the Cys residues decreased by ∼ 20–30% that of the amount of co-purifying CcmI as compared with that seen with untreated, native apocytochrome c2 (Fig. 6, lanes 3, 5, and 7). Thus, the presence of a disulfide bond at the heme binding site of native apocytochrome c2 only slightly favored its interaction with CcmI. We note that similar CcmI co-purification patterns observed with the native or DTT/IAM-treated or Cys to Ser mutant proteins exclude the occurrence of excessive intermolecular disulfide bonds forming apocytochrome c2 polymers under the conditions used.
Axial Ligands His-17 and Met-96 of Heme Iron Atom
The His-17 to Ser and Met-96 to Ser variants of Strep-apocytochrome c2 were constructed and purified, and their binding ability to His10-CcmI was tested. The data indicated that apocytochrome c2 mutants lacking the heme iron axial ligands His-17 or Met-96 co-purified with CcmI at amounts similar to those observed with the native apocytochrome c2. Thus, the heme axial ligands are not important for CcmI-apocytochrome c2 interactions (Fig. 6, lanes 9–11).
The C-terminal α-Helix of Apocytochrome c2
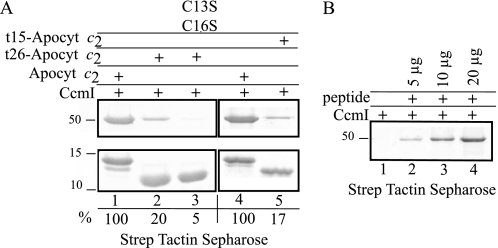
Minor contributions of the residues involved in heme ligation of apocytochrome c2 to its interactions with CcmI suggested that additional structural elements might be involved in apocytochrome c-chaperone interactions. Strong hydrophobic interactions between the N- and C-terminal α-helices of apocytochrome c are important for folding of Class I c-type cytochromes (41). Thus, two apocytochrome c2 derivatives lacking their C-terminal helices (t26- and t15-apocytochrome c2, missing the last 26 and 15 amino acids of apocytochrome c2, respectively) were constructed. Based on R. capsulatus cytochrome c2 three-dimensional structure, the t15-apocytochrome c2 mutant lacked only the C-terminal helix that interacts with the N-terminal region but retained the axial heme iron ligand Met-96, whereas the t26-apocytochrome c2 lacked both of these features (Table 1 and Fig. 2B). A Cys-less derivative of the truncated t26-apocytochrome c2 was also produced to further assess the contribution of the heme binding site cysteine residues to these interactions. Again, purified truncated apocytochrome c2 derivatives were subjected to co-purification assays similar to those carried out with native apocytochrome c2 (Fig. 7A). The amount of CcmI that co-purified with t26-apocytochrome c2 (apparent molecular mass of 11.5 kDa) decreased significantly to ∼20% of that observed with native apocytochrome c2 (Fig. 7A, lanes 1 and 2). A similar situation was also observed with the t15-apocytochrome c2 (apparent molecular mass of 13 kDa) variant (Fig. 7A, lanes 4 and 5). Moreover, the amount of CcmI retained by the truncated t26-apocytochrome c2 became barely detectable (<5%) when its Cys-less derivative was used (Fig. 7A, lane 3). These data indicated that the C-terminal portion of apocytochrome c2 was a major determinant for binding CcmI, whereas its heme binding site Cys residues provided a small but additive contribution. Furthermore, the comparable binding abilities seen with the t15- and t26-apocytochrome c2 derivatives confirmed that the heme iron sixth axial ligand is not involved in this interaction.
FIGURE 7.
CcmI interacts directly with the C-terminal portion of apocytochrome c2. Panel A, co-purification of His10-CcmI with full-length and C-terminal-truncated derivatives t15- and t26- apocytochrome c2 and appropriate double Cys mutants is shown. Co-purification was done as in Fig. 3, panel B, except that His10-CcmI (5 μg) was incubated with apocytochrome c2 (Apocyt c2, 15 μg) or the appropriate derivatives as before. Lanes 1 and 4 show co-purification of CcmI with full-length apocytochrome c2, and the amount of CcmI present in the elution fraction was taken as 100% for image quantification as in Fig. 6. The amount of CcmI present in the elution fraction after incubation with t26-apocytochrome c2 was decreased to 20% as compared with the full-length apocytochrome c2 (lane 2). Lane 3 shows that after mutation of the Cys residues of t26-apocytochrome c2, the amount of CcmI that co-purifies is barely detectable. The amount of CcmI present in the elution fraction after incubation with t15-apocytochrome c2 (lane 5) was also decreased to 17% as compared with the full-length apocytochrome c2 shown in lane 4, similar to that seen with the t26-apocytochrome c2 (lane 2). Panel B, co-purification of His10-CcmI using different amounts of a synthetic peptide corresponding to the C-terminal 26-amino acid residues of apocytochrome c2 is shown. Lane 1 shows that His10-CcmI is not retained by the Strep Tactin resin in the absence of the synthetic peptide, and increasing amounts of His10-CcmI co-purified with 5, 10, and 20 μg (lanes 2, 3, and 4, respectively) of the synthetic peptide.
A Synthetic Peptide Corresponding to the C-terminal Helix of Apocytochrome c2 Is Sufficient to Recognize and Bind CcmI
CcmI binding ability to the C-terminal helix of apocytochrome c2 was assessed using a synthetic peptide (NH2-WSHPQFEKIEGRKAKTGMAFKLAKGGEDVAAYLASVVK-COOH) corresponding to the C-terminal 26 amino acid residues of native apocytochrome c2 (deleted in t26-apocytochrome c2 mutant) (Fig. 2B). Incubation of increasing amounts (5, 10, and 20 μg) of this peptide instead of apocytochrome c2, with purified His10-CcmI led to concentration-dependent co-purification of His10-CcmI-peptide complex (Fig. 7B). Thus, the last 26 C-terminal amino acids of apocytochrome c2 are sufficient for its interaction with CcmI in vitro. Based on overall data we concluded that the C-terminal helix of apocytochrome c2 promotes its binding to CcmI during Ccm.
Periplasmic Domain of CcmI Is Responsible for Binding of Apocytochrome c2 C-terminal Helix
To establish which functional domain of CcmI interacts with apocytochrome c2, two truncated versions of His10-CcmI were produced by site-directed mutagenesis (Fig. 2A). The His10-CcmI-2 derivative of CcmI (apparent molecular mass of 42 kDa) contained only the second transmembrane helix followed by the large periplasmic portion containing the three TPR motifs of CcmI (Fig. 2A, CcmI-2). Co-purification assays with purified His10-CcmI-2 were conducted as done with the intact CcmI using native apocytochrome c2 and its derivatives. The data indicated that CcmI-2 co-purified with apocytochrome c2 and its derivatives in amounts similar to those seen with intact CcmI (supplemental Fig. S1, A and B). Finally, like intact CcmI, CcmI-2 also bound quantitatively to the synthetic peptide mimicking the C-terminal portion of apocytochrome c2 (supplemental Fig. S1C). We therefore concluded that the periplasmic CcmI-2 domain of CcmI is sufficient to bind apocytochrome c2 via its C-terminal helical portion.
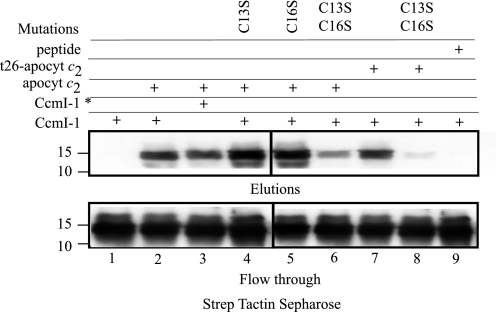
Membrane-embedded CcmI-1 Domain of CcmI Binds Weakly to Apocytochrome c2
A His10-CcmI-1 derivative (apparent molecular mass of 14 kDa) that contains only the N-terminal 121 amino acid residues corresponding to the two transmembrane helices and the cytoplasmic loop of CcmI was also produced (Fig. 2A, CcmI-1). However, this membrane-integral protein was present in low amounts in E. coli membranes, and its purification was very difficult. We succeeded in obtaining His10-CcmI-1-enriched fractions and used them for co-purification assays with Strep-apocytochrome c2 and derivatives as done before. Identification of His10-CcmI-1 in the flow-through and elution fractions was accomplished using anti-CcmI antibodies. Incubation with apocytochrome c2 allowed retention and co-elution of a small amount of CcmI-1 by the Strep Tactin column, as detected in the elution fractions with anti-CcmI antibodies. Most of His10-CcmI-1 was present in the flow-through (Fig. 8, lane 2) and wash fractions (not shown), indicating that binding of CcmI-1 to apocytochrome c2 was poor, sharply contrasting that seen with intact CcmI or CcmI-2 proteins. When in the previous experiments a 10-fold excess of apocytochrome c2 over His10-CcmI or His10-CcmI-2 was used, the amounts of CcmI or CcmI-2 that co-eluted with apocytochrome c2 were virtually close to 100%, and these proteins were undetectable in the flow-through fractions (data not shown). The ratio of apocytochrome c2 to CcmI-1 was much larger in the latter experiments, and only a small fraction of CcmI-1 co-purified with apocytochrome c2 (Fig. 8, top and bottom panels), suggesting that it interacted very weakly with apocytochrome c2.
FIGURE 8.
Co-purification of membrane-embedded CcmI-1 domain with apocytochrome c2 and its derivatives. Co-purification experiments were conducted as in Fig. 3, panel B, except that partially purified His10-CcmI-1 (20 μg) was incubated with apocytochrome c2 (apocyt c2, 15 μg) or single or double Cys apocytochrome c2 mutants. The assay mixtures were then subjected to affinity chromatography using Strep Tactin-Sepharose, and elution and flow through fractions were analyzed by SDS-PAGE/immunoblot using anti-CcmI antibodies. Lane 1 shows that CcmI-1 does not exhibit any unspecific binding to the Strep Tactin resin. Lane 2 shows co-purification of CcmI-1 with full-length apocytochrome c2. Lane 3 shows similar amounts of DTT/AIM-treated CcmI-1 co-purified with apocytochrome c2. Lanes 4, 5, and 7 show CcmI-1 co-purified with the single Cys mutants apocytochrome c2-C13S and -C16S and the truncated t26-apocytochrome c2, respectively. Lanes 6 and 8 show lower amounts of CcmI-I being co-purified with apocytochrome c2 and the truncated variant double Cys mutants apocytochrome c2- C13S/C16S and t26-apocytochrome c2-C13S/C16S, respectively. Lane 9 shows that CcmI-1 does not bind or recognize the synthetic peptide corresponding to the 26 C-terminal amino acids of apocytochrome c2. Molecular mass markers (in kDa) are indicated on the left, and CcmI-1* indicates that it was treated with DTT/IAM as described under “Experimental Procedures.”
Heme Binding Site Cys Residues of Apocytochrome c2 Are More Important Than Its C-terminal Helix for CcmI-1 Interactions
Comparison of the amounts of CcmI-1 that co-purified with the native, the heme binding site single (C13S or C16S) mutants, and the double (C13S/C16S) mutant of apocytochrome c2 indicated that those that retained at least one Cys residue at their heme binding site interacted more strongly with CcmI-1 than those that lacked both of them (Fig. 8, lanes 2, 4, 5,and 6). As R. capsulatus CcmI has a unique Cys residue at its position 7, DTT/IAM-treated CcmI-1 was tested for its interactions with the native apocytochrome c2. The data indicated that DTT/IAM-treated CcmI-1 still bound apocytochrome c2 in amounts similar to its untreated form (Fig. 8, lanes 2 and 3). Similarly, co-purification assays using the truncated t26-apocytochrome c2 and its double Cys mutant derivative confirmed that the C-terminal portion of apocytochrome c2 was not important, but the absence of the Cys residues decreased the CcmI-1-apocytochrome c2 interactions (Fig. 8, lanes 7 and 8). Therefore, of the two domains of CcmI, the membrane integral CcmI-1 domain interacts poorly with the N-terminal heme binding region, whereas the periplasmic CcmI-2 domain binds strongly the C-terminal helical portion of apocytochrome c2.
DISCUSSION
In most Gram-negative bacteria like R. capsulatus that use Ccm (System I) for cytochrome c production, CcmI is an important subunit of the CcmFHI complex thought to perform the final heme-apocytochrome c ligation step (7). Earlier findings suggested that CcmI acts as a chaperone for apocytochromes c to facilitate their delivery to the heme ligation complex (11, 12, 21, 22). In this work, by probing in vitro protein-protein interactions between purified R. capsulatus CcmI and apocytochrome c2, we demonstrated unequivocally that CcmI tightly binds apocytochrome c2 but not holocytochrome c2. This conclusion is supported by reciprocal co-elutions, CD spectroscopy, and size exclusion chromatography. In particular, CD spectra indicated that conformational changes occur in CcmI upon its incubation with only apocytochrome c2, and not holocytochrome c2 or bovine serum albumin, corroborating that CcmI binds the apo- but not the holo-form of cytochrome c2 in solution. Moreover, experiments using DDM-dispersed total membrane proteins indicated that these interactions are specific.
Current knowledge about the protein-protein interactions between the Ccm components and apocytochromes c is limited. Earlier, the ability of E. coli CcmH to reduce in vitro a disulfide bond formed at the heme binding site of an apocytochrome c-like peptide was reported (10, 42). Also, using a yeast two-hybrid system, the interactions in vivo between Arabidopsis thaliana CcmH or CcmFN2 and apocytochrome c were described (43). More recently, in vitro weak binding of apocytochrome c2 to a Cys-less CcmG protein was detected (23). This work provides the first biochemical evidence that the CcmI subunit of the CcmFHI complex acts as an apocytochrome c chaperone for R. capsulatus apocytochrome c2 and possibly for other c-type cytochromes during Ccm.
The observation that CcmI binds specifically apocytochrome c2 and the well known structural changes that apocytochromes c undergo during their folding process (41, 44, 45) allowed identification of the structural elements that mediate interactions between these partners. A major finding is that CcmI recognizes and binds the C-terminal helix of apocytochrome c2 rather than the heme binding (13CXXCH17) site at the N terminus or the 5th (i.e. His-17) or 6th (i.e. Met-96) axial ligands of heme iron. Moreover, under our in vitro assay conditions, CcmI-apocytochrome c2 interactions were unaffected by the redox state of heme binding site Cys residues. Future quantitative measurements or in vivo assays may reveal different CcmI binding affinities for various derivatives of apocytochrome c2.
The weakened binding of CcmI to the C-terminal-truncated apocytochrome c2 derivatives as well as the binding of a synthetic peptide corresponding to the last 26 residues demonstrate clearly that this portion of apocytochrome c2 is necessary and sufficient for recognition and binding of CcmI. In class I-type cytochromes c, like the horse heart cytochrome c or R. capsulatus cytochrome c2, interactions between apocytochrome c and heme are well studied (24, 32, 45, 46). Upon the addition of a tetrapyrrole ring, the N- and C-terminal helices of cytochrome c pair together in a tight perpendicular arrangement. These helix-helix interactions are mediated via Gly-6 and Phe-10/Leu-94 and Tyr-97 in horse heart mitochondrial cytochrome c and via Gly-6 and Phe-10/Val-107 and Tyr-110 in R. capsulatus cytochrome c2. Moreover, a third helix adjacent to the sixth axial Met ligand provides conserved hydrophobic contacts to the heme cofactor (24, 45, 46). Thus, like any class I-type cytochrome c in the absence of heme, the C-terminal helix of R. capsulatus apocytochrome c2 is available to interact with CcmI, whereas this helix is packed against the N-terminal helix together with heme in holocytochrome c2.
The fact that purified CcmI-2 binds apocytochrome c2 as well as the intact CcmI indicates that the periplasmic C-terminal domain with its TPR motifs is responsible for these interactions. The TPR motifs are widely spread modules composed by 34 hydrophobic residues forming two antiparallel α-helices. Tandem arrays of TPR motifs form right-handed super helical structures with concave and convex surfaces for protein-protein interactions (47, 48). Interactions similar to those seen here were also observed between the E. coli TPR-containing NrfG, belonging to the nitrite reductase specific NrfEFG heme ligation complex, and the C-terminal helical portion of NrfA apocytochrome c (49). Remarkably though, when both the C-terminal portion of apocytochrome c2 and its heme binding site Cys residues are eliminated, the CcmI-apocytochrome c2 interactions are completely abolished. Thus, the heme binding site Cys residues of apocytochrome c2 also contribute, although to a lesser extent, to its interaction with CcmI.
Unexpectedly, we observed that apocytochrome c2 also interacts in vitro with the membrane integral CcmI-1 domain of CcmI. Under our experimental conditions, these interactions were much weaker than those seen with CcmI-2 and involved different structural elements of apocytochrome c2, including a free thiol group at its heme binding site. Earlier studies indicated that deletion of CcmI-1 abolished the production of all c-type cytochromes (inclusive of the C-terminal membrane anchored cytochrome c1) (11, 12, 22) and that a CcmI mutant lacking 42 amino acids of the cytoplasmic loop and its leucine zipper motif more strongly affected the production of N-terminal membrane-anchored cytochromes cy, co, or cp than the C-terminal-anchored cytochrome c1 in R. capsulatus (12). Similar phenotypes showing that cytochrome c1 is still produced as long as the CcmI-1 domain is functional was also seen with Paracoccus denitrificans (50) and rhizobial species (13, 14, 16). Leucine zipper motifs, like those in the cytoplasmic loop of CcmI-1, typically promote protein-protein interactions between their amphipathic α-helices and parallel α-helices of interacting partners (51, 52). In the absence of CcmI, lack of these interactions restricts efficient delivery of apocytochrome c to the ligation complex upon translocation across the cytoplasmic membrane. Consequently, overproduction of the Ccm components (e.g. CcmF, CcmH, and CcmG) or apoprotein substrates (e.g. apocytochrome c2) becomes necessary to compensate for the decreased Ccm efficiency to render cells capable of producing physiologically sufficient amounts of c-type cytochromes to support their growth (21, 22). Thus, in addition to being a subunit of the CcmFHI complex, CcmI-1 is also important for the production of all c-type cytochromes via its low affinity interaction with their N-terminal heme binding sites. In this respect we note that enteric bacteria like E. coli lack a separate CcmI component and do not produce a cytochrome c1 homologue. However, they still contain a homologue of CcmI-2 domain that is fused to CcmH as a “CcmH-CcmI-2” variant, most likely acting like the CcmI of other species. Whether one of the two transmembrane helices of CcmI-1, which is eliminated during this fusion event (53), is required to produce the C-terminal anchored cytochromes c like cytochrome c1 remains to be seen.
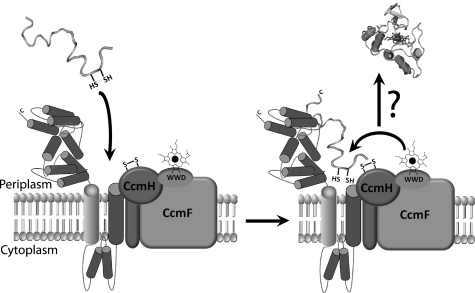
In conclusion, this work established that CcmI binds tightly to the C-terminal portion of apocytochrome c2 via its large periplasmic TPR-containing CcmI-2, whereas it probes the integrity of the heme binding site via its membrane anchored CcmI-1 domains. These findings suggest that apocytochromes c that are not membrane-anchored, like apocytochrome c2, might be recognized upon their translocation by CcmI via their freely accessible C-terminal domains. They are brought in contact with the CcmFHI complex from which they are released upon interaction with heme, which is locally available in the complex, as depicted in Fig. 9.
FIGURE 9.
A hypothetical model depicting the role of CcmI subunit of CcmFHI complex as an apocytochrome c2 chaperone during Ccm. Upon translocation from the cytoplasm to the periplasm via the SEC translocon, apocytochrome c2 is captured via its C-terminal helical and N-terminal heme binding regions by the CcmI-2 and CcmI-1 domains, respectively, of the CcmFHI heme ligation complex. Release of apocytochrome c2 from CcmI-2 is proposed to occur upon folding of its N- and C-terminal helical regions, promoted by binding of heme present in the CcmFHI complex. This would yield first a transient b-type apocytochrome c2 followed by the formation of covalent thioether bonds during the Ccm process.
It remains to be seen whether CcmI-2 chaperones only the soluble apocytochrome c2 or if it can also chaperone other soluble cytochromes c via their C-terminal helical regions. Also, if CcmI-2 is restricted exclusively to the Class 1-type globular cytochromes c, how are the four helical bundle cytochromes c, like R. capsulatus cytochrome c′, chaperoned? Could they be produced independently of Ccm, as recently proposed? (54) Second, do the N-terminal membrane-anchored apocytochromes, like the monoheme cytochromes cy or co as well as the diheme cytochrome cp, interact with CcmI? If so, then which regions of these proteins interact directly? Third, does CcmI play any role in chaperoning multiheme cytochromes c, such as the R. capsulatus pentaheme DorC involved in DMSO reduction? Finally and most intriguing, does the local availability of heme on the CcmFHI complex trigger release of apocytochrome c from CcmI, initially converting it to a b-type apocytochrome folding intermediate? Such intermediates have been seen during in vitro holocytochrome c folding (41, 44, 45, 55, 56) or organic synthesis of P. denitrificans cytochrome c550 and mitochondrial cytochrome c (36) upon incubation of apocytochrome c together with heme under reducing conditions. Ongoing studies will address these and other related questions.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant GM38239 (to F. D.). This work was also supported by Department of Energy Grant 91ER20052 (to F. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Fig. S1.
- Ccm
- cytochrome c maturation
- DDM
- n-dodecyl-β-D-maltoside
- IAM
- iodoacetamide
- TPR
- tetratricopeptide repeat.
REFERENCES
- 1. Keilin D. (1966) The History of Cell Respiration and Cytochrome, Butler & Tanner, London [Google Scholar]
- 2. Bertini I., Cavallaro G., Rosato A. (2006) Chem. Rev. 106, 90–115 [DOI] [PubMed] [Google Scholar]
- 3. Ow Y. P., Green D. R., Hao Z., Mak T. W. (2008) Nat. Rev. Mol. Cell Biol. 9, 532–542 [DOI] [PubMed] [Google Scholar]
- 4. Bowman S. E., Bren K. L. (2008) Nat. Prod. Rep. 25, 1118–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanders C., Turkarslan S., Lee D. W., Daldal F. (2010) Trends Microbiol. 18, 266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kranz R. G., Richard-Fogal C., Taylor J. S., Frawley E. R. (2009) Microbiol. Mol. Biol. Rev. 73, 510–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanders C., Turkarslan S., Lee D. W., Onder O., Kranz R. G., Daldal F. (2008) J. Biol. Chem. 283, 29715–29722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee J. H., Harvat E. M., Stevens J. M., Ferguson S. J., Saier M. H., Jr. (2007) Biochim. Biophys. Acta 1768, 2164–2181 [DOI] [PubMed] [Google Scholar]
- 9. Richard-Fogal C. L., Frawley E. R., Bonner E. R., Zhu H., San Francisco B., Kranz R. G. (2009) EMBO J. 28, 2349–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Matteo A., Gianni S., Schininà M. E., Giorgi A., Altieri F., Calosci N., Brunori M., Travaglini-Allocatelli C. (2007) J. Biol. Chem. 282, 27012–27019 [DOI] [PubMed] [Google Scholar]
- 11. Lang S. E., Jenney F. E., Jr., Daldal F. (1996) J. Bacteriol. 178, 5279–5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanders C., Boulay C., Daldal F. (2007) J. Bacteriol. 189, 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delgado M. J., Yeoman K. H., Wu G., Vargas C., Davies A. E., Poole R. K., Johnston A. W., Downie J. A. (1995) J. Bacteriol. 177, 4927–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kereszt A., Slaska-Kiss K., Putnoky P., Banfalvi Z., Kondorosi A. (1995) Mol. Gen. Genet. 247, 39–47 [DOI] [PubMed] [Google Scholar]
- 15. Ritz D., Bott M., Hennecke H. (1993) Mol. Microbiol. 9, 729–740 [DOI] [PubMed] [Google Scholar]
- 16. Cinege G., Kereszt A., Kertész S., Balogh G., Dusha I. (2004) Mol. Genet. Genomics 271, 171–179 [DOI] [PubMed] [Google Scholar]
- 17. Zannoni D., Daldal F. (1993) Arch. Microbiol. 160, 413–423 [DOI] [PubMed] [Google Scholar]
- 18. Davidson E., Daldal F. (1987) J. Mol. Biol. 195, 13–24 [DOI] [PubMed] [Google Scholar]
- 19. Gray K. A., Grooms M., Myllykallio H., Moomaw C., Slaughter C., Daldal F. (1994) Biochemistry 33, 3120–3127 [DOI] [PubMed] [Google Scholar]
- 20. Koch H. G., Hwang O., Daldal F. (1998) J. Bacteriol. 180, 969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deshmukh M., May M., Zhang Y., Gabbert K. K., Karberg K. A., Kranz R. G., Daldal F. (2002) Mol. Microbiol. 46, 1069–1080 [DOI] [PubMed] [Google Scholar]
- 22. Sanders C., Deshmukh M., Astor D., Kranz R. G., Daldal F. (2005) J. Bacteriol. 187, 4245–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turkarslan S., Sanders C., Ekici S., Daldal F. (2008) Mol. Microbiol. 70, 652–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ambler R. P. (1991) Biochim. Biophys. Acta 1058, 42–47 [DOI] [PubMed] [Google Scholar]
- 25. Daldal F., Cheng S., Applebaum J., Davidson E., Prince R. C. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 2012–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring harbor Laboratory Press, NY [Google Scholar]
- 27. Sanders C., Wethkamp N., Lill H. (2001) Mol. Microbiol. 41, 241–246 [DOI] [PubMed] [Google Scholar]
- 28. Holden H. M., Meyer T. E., Cusanovich M. A., Daldal F., Rayment I. (1987) J. Mol. Biol. 195, 229–231 [DOI] [PubMed] [Google Scholar]
- 29. Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 30. Johnson W. C., Jr. (1990) Proteins 7, 205–214 [DOI] [PubMed] [Google Scholar]
- 31. Greenfield N. J. (2004) Methods. Mol. Biol. 261, 55–78 [DOI] [PubMed] [Google Scholar]
- 32. Fisher W. R., Taniuchi H., Anfinsen C. B. (1973) J. Biol. Chem. 248, 3188–3195 [PubMed] [Google Scholar]
- 33. Hamada D., Hoshino M., Kataoka M., Fink A. L., Goto Y. (1993) Biochemistry 32, 10351–10358 [DOI] [PubMed] [Google Scholar]
- 34. Stellwagen E., Rysavy R., Babul G. (1972) J. Biol. Chem. 247, 8074–8077 [PubMed] [Google Scholar]
- 35. Damaschun G., Damaschun H., Gast K., Gernat C., Zirwer D. (1991) Biochim. Biophys. Acta 1078, 289–295 [DOI] [PubMed] [Google Scholar]
- 36. Daltrop O., Ferguson S. J. (2003) J. Biol. Chem. 278, 4404–4409 [DOI] [PubMed] [Google Scholar]
- 37. Tomlinson E. J., Ferguson S. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5156–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pielak G. J., Auld D. S., Beasley J. R., Betz S. F., Cohen D. S., Doyle D. F., Finger S. A., Fredericks Z. L., Hilgen-Willis S., Saunders A. J. (1995) Biochemistry 34, 3268–3276 [DOI] [PubMed] [Google Scholar]
- 39. Benning M. M., Wesenberg G., Caffrey M. S., Bartsch R. G., Meyer T. E., Cusanovich M. A., Rayment I., Holden H. M. (1991) J. Mol. Biol. 220, 673–685 [DOI] [PubMed] [Google Scholar]
- 40. Bushnell G. W., Louie G. V., Brayer G. D. (1990) J. Mol. Biol. 214, 585–595 [DOI] [PubMed] [Google Scholar]
- 41. Akiyama S., Takahashi S., Ishimori K., Morishima I. (2000) Nat. Struct. Biol. 7, 514–520 [DOI] [PubMed] [Google Scholar]
- 42. Monika E. M., Goldman B. S., Beckman D. L., Kranz R. G. (1997) J. Mol. Biol. 271, 679–692 [DOI] [PubMed] [Google Scholar]
- 43. Meyer E. H., Giegé P., Gelhaye E., Rayapuram N., Ahuja U., Thöny-Meyer L., Grienenberger J. M., Bonnard G. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Travaglini-Allocatelli C., Gianni S., Brunori M. (2004) Trends Biochem. Sci. 29, 535–541 [DOI] [PubMed] [Google Scholar]
- 45. Colón W., Elöve G. A., Wakem L. P., Sherman F., Roder H. (1996) Biochemistry 35, 5538–5549 [DOI] [PubMed] [Google Scholar]
- 46. Sauder J. M., MacKenzie N. E., Roder H. (1996) Biochemistry 35, 16852–16862 [DOI] [PubMed] [Google Scholar]
- 47. D'Andrea L. D., Regan L. (2003) Trends Biochem. Sci. 28, 655–662 [DOI] [PubMed] [Google Scholar]
- 48. Blatch G. L., Lässle M. (1999) Bioessays 21, 932–939 [DOI] [PubMed] [Google Scholar]
- 49. Han D., Kim K., Oh J., Park J., Kim Y. (2008) Proteins 70, 900–914 [DOI] [PubMed] [Google Scholar]
- 50. Page M. D., Ferguson S. J. (1995) Mol. Microbiol. 15, 307–318 [DOI] [PubMed] [Google Scholar]
- 51. Landschulz W. H., Johnson P. F., McKnight S. L. (1988) Science 240, 1759–1764 [DOI] [PubMed] [Google Scholar]
- 52. Alber T. (1992) Curr. Opin Genet. Dev. 2, 205–210 [DOI] [PubMed] [Google Scholar]
- 53. Fabianek R. A., Hofer T., Thöny-Meyer L. (1999) Arch. Microbiol. 171, 92–100 [DOI] [PubMed] [Google Scholar]
- 54. Inoue H., Wakai S., Nishihara H., Sambongi Y. (2011) FEBS J. 278, 2341–2348 [DOI] [PubMed] [Google Scholar]
- 55. Dumont M. E., Corin A. F., Campbell G. A. (1994) Biochemistry 33, 7368–7378 [DOI] [PubMed] [Google Scholar]
- 56. Alam Khan M. K., Das U., Rahaman M. H., Hassan M. I., Srinivasan A., Singh T. P., Ahmad F. (2009) J. Biol. Inorg. Chem. 14, 751–760 [DOI] [PubMed] [Google Scholar]
- 57. Arai M., Mitsuke H., Ikeda M., Xia J. X., Kikuchi T., Satake M., Shimizu T. (2004) Nucleic Acids Res. 32, W390–W393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scolnik P. A., Walker M. A., Marrs B. L. (1980) J. Biol. Chem. 255, 2427–2432 [PubMed] [Google Scholar]
- 59. Bhagwat A. S., Sohail A., Roberts R. J. (1986) J. Bacteriol. 166, 751–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Keen N. T., Tamaki S., Kobayashi D., Trollinger D. (1988) Gene 70, 191–197 [DOI] [PubMed] [Google Scholar]
- 61. Benning C., Somerville C. R. (1992) J. Bacteriol. 174, 2352–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.