Abstract
Fibroblast growth factor 2 (FGF2) positively modulates osteoblast differentiation and bone formation. However, the mechanism(s) is not fully understood. Because the Wnt canonical pathway is important for bone homeostasis, this study focuses on modulation of Wnt/β-catenin signaling using Fgf2−/− mice (FGF2 all isoforms ablated), both in the absence of endogenous FGF2 and in the presence of exogenous FGF2. This study demonstrates a role of endogenous FGF2 in bone formation through Wnt signaling. Specifically, mRNA expression for the canonical Wnt genes Wnt10b, Lrp6, and β-catenin was decreased significantly in Fgf2−/− bone marrow stromal cells during osteoblast differentiation. In addition, a marked reduction of Wnt10b and β-catenin protein expression was observed in Fgf2−/− mice. Furthermore, Fgf2−/− osteoblasts displayed marked reduction of inactive phosphorylated glycogen synthase kinase-3β, a negative regulator of Wnt/β-catenin pathway as well as a significant decrease of Dkk2 mRNA, which plays a role in terminal osteoblast differentiation. Addition of exogenous FGF2 promoted β-catenin nuclear accumulation and further partially rescued decreased mineralization in Fgf2−/− bone marrow stromal cell cultures. Collectively, our findings suggest that FGF2 stimulation of osteoblast differentiation and bone formation is mediated in part by modulating the Wnt pathway.
Keywords: β-Catenin, Bone, Fibroblast Growth Factor (FGF), Osteoblasts, Wnt Signaling
Introduction
Both embryonic and postnatal skeletogenesis involve mesenchymal precursor cells progressively differentiating into bone-forming cells, osteoblasts (1). Osteoblast differentiation is controlled by extracellular signals, including both the FGF (2) and Wnt pathways (3).
FGF2 is one member of the FGF family (2, 4). It is expressed by osteoblasts (5–7) and stored in the extracellular matrix (7). Our previous studies show that FGF2 positively regulates bone formation and osteoblast differentiation. Disruption of the Fgf2 gene resulted in significantly decreased bone mass and bone formation as revealed by histomorphometry and micro-CT (8). At the cellular level, the effects of FGF2 ablation on osteoblast phenotypes included decreased osteoblast proliferation, reduced colony-forming efficiency in Fgf2−/− bone marrow stromal cell (BMSC)2 cultures, and decreased alkaline phosphatase and von Kossa staining (8–10). The osteoblast function was also confirmed in vivo: bone formation rates were significantly reduced in Fgf2−/− mice compared with wild-type littermates (8) The decreased osteoblast differentiation in Fgf2−/− BMSC cultures can be partially rescued by adding exogenous FGF2 in vitro (8, 9). FGF2 also stimulates in vivo bone formation (11–14). Differentiation of BMSCs into the osteoblast lineage is governed by transcription factors, including runt-related transcription factor 2 (Runx2), Osterix, and activating transcription factor 4 (ATF4) (1). Decreased osteoblast differentiation might be due to reduced expression of transcription factors Runx2 (9) and ATF4 (15), which were associated with reduced bone formation in Fgf2−/− mice. Furthermore, exogenous FGF2 was able to induce Runx2 (16) and ATF4 (15) expression. These studies suggest that FGF2 is a positive regulator of bone formation. In addition, FGF2 is required for osteoclast formation and bone resorption because FGF2 deletion results in reduced osteoclast formation and resorption both in vitro (17) and in vivo (8). The present study focused on potential mechanisms by which FGF2 regulates osteoblast differentiation and bone formation.
Wnts are secreted glycoproteins with a family of 19 proteins in mammals, including Wnt3a and Wnt10b (18). Wnt10b stimulates osteogenic transcription factor Runx2, thereby promoting osteoblastogenesis and bone formation (19, 20). Wnt ligands can signal through either the β-catenin-dependent or -independent pathways (21). The β-catenin-dependent Wnt pathway is well studied in osteoblasts (3). In the absence of Wnt ligands, β-catenin is degraded by a destruction complex which includes glycogen synthase kinase 3β (GSK3β) (3, 21, 22). Thus, GSK3β is a negative regulator of the β-catenin/Wnt pathway. Binding of Wnt ligands to the Frizzled receptor and coreceptors lipoprotein receptor-related protein 5/6 (LRP5/6) inhibits the GSK3β destruction complex (21). Stabilized β-catenin then accumulates in the nucleus, where it interacts with the transcription factor lymphoid enhancer-binding factor 1/T cell-specific transcription factor (LEF/TCF) to activate target gene transcription (3, 21). Therefore, β-catenin is a key signaling factor of the Wnt pathway. Wnt/β-catenin signaling positively regulates osteoblast differentiation and bone formation. β-Catenin expression is increased during osteoblast differentiation (23), and targeted deletion of β-catenin in osteoblast precursors results in a reduction of osteogenesis markers and an absence of bone formation at E18.5 in the developing embryo (24, 25). Moreover, deletion of β-catenin in the late phase of osteoblast differentiation leads to impaired bone maturation and mineralization (26).
The function of the Wnt pathway on bone formation is also regulated by Wnt antagonists including Dickkopfs (Dkks) (27), Sclerostin (28) and secreted Frizzled receptors (sFRP) (29, 30). Dkk1 has been demonstrated to antagonize canonical Wnt signaling and inversely correlates with bone mass (31–34). Besides antagonizing canonical Wnt signaling (31), Dkk2 has been demonstrated to have a role in terminal osteoblast differentiation and mineralized matrix formation (35). Sclerostin (36–38), sFRP1 (29, 30), and sFRP4 (39–41) also negatively regulate bone mass. sFRPs inactivate all of the Wnt pathway (27).
Wnt signaling is particularly important for bone homeostasis as revealed in human disease (3). Patients with loss-of-function mutations in the receptors Lrp5 (43) or Lrp6 (44) are characterized with low bone mineral density (BMD) and skeletal fragility. In contrast, patients with gain-of-function mutations in Lrp5 (reduced affinity of LRP5 for Dkk1) have high bone mass (45–47). Animal studies (48–50) further support the importance of Wnt signaling in osteoblast differentiation and bone formation.
The main question in the present study is whether or not the anabolic action of FGF2 on bone formation mediated by modulation of Wnt signaling. To answer this question, the current studies utilize Fgf2+/+ and Fgf2−/− mice (FGF2 all isoform-null mice) to investigate modulation of the Wnt/β-catenin pathway in osteoblasts both in the absence of endogenous FGF2 and in the presence of exogenous FGF2.
EXPERIMENTAL PROCEDURES
Animals
Fgf2-null mice were previously developed on a Black Swiss 129Sv background (51). Both wild-type and knock-out mice used in this study were on a new mixed genetic background of Black Swiss 129Sv/FVB/N. Littermates of male or female mice were utilized as indicated in the figure legends. Genotyping of mice was performed using primers as described previously (8, 51). Mice were bred and housed in the transgenic facility in the center for laboratory animal care at the University of Connecticut Health Center. Mice were killed by CO2 narcosis and cervical dislocation. The Animal Care and Use Committee of the University of Connecticut Health Center approved all animal protocols.
Bone Mineral Density
Using dual energy x-ray absorptiometry (PIXimus mouse 11 densitometer; GE Medical System, Madison WI), femoral BMD was determined in 6-month-old female Fgf2+/+ and Fgf2−/− mice.
BMSC Cultures
BMSC isolation was modified from previous methods (8). Briefly, femurs and tibiae from Fgf2+/+ and Fgf2−/− mice were dissected. After removing both ends, bones were centrifuged briefly in Eppendorf tubes to release bone marrow cells. The marrow cells were plated at 10 × 106 cells/well in 6-well plates in α-MEM (Invitrogen) containing 10% FBS, 100 units/ml penicillin, and 50 μg/ml streptomycin. To study exogenous FGF2-promoting β-catenin protein accumulation in nucleus, the cells had half-medium changed on day 3 and the entire medium changed on day 6 with FBS-free α-MEM, with serum deprivation 14 h before adding exogenous FGF2. To study β-catenin expression during osteoblast differentiation, the cells were fed α-MEM containing 10% FBS every 3 days in the first 8 days; and then from day 9 the cells were fed with osteogenesis medium (α-MEM, 10% FBS, 8 mm β-glycerophosphate, and 50 μg/ml ascorbic acid) every other day until day 17. The cells were stained for alkaline phosphatase-positive colonies using a kit (Sigma). The alkaline phosphatase-stained dishes were then restained with silver nitrate using the von Kossa method to determine mineralization (52). Colony area was quantified with ImageJ (version 1.61; National Institutes of Health).
RNA Isolation and Quantitative Real-time PCR (qPCR) Analysis
Total RNA was extracted from cells with TRIzol reagent (Invitrogen) according to the manufacturer's protocol. RNA (3 μg) was reverse transcribed using a commercial kit (Clontech). qPCR was carried out with the QuantiTect™ SYBR Green PCR kit using a MyiQ™ instrument (Bio-Rad). GAPDH was used as an internal reference for each sample. Relative mRNA expression was calculated using a formula reported previously (53). The mouse-specific primers are shown in supplemental Table 1.
Western Blot Analysis
Whole cell extracts were harvested in radioimmuneprecipitation assay buffer (Cell Signaling). Cytoplasmic and nuclear extractions were harvested in buffer A and buffer B separately. Components of buffer A and B were described previously (15). Protein concentrations were assayed with BCA protein assay reagent (Pierce). Equal amounts of protein were fractioned on SDS-polyacrylamide gel and transferred onto a PVDF membrane (Bio-Rad). Membranes were blocked for 1 h with 5% nonfat dry milk and then incubated overnight at 4 °C with anti-Wnt10b (Abcam) or anti-β-catenin (BD Transduction Laboratories) or anti-total GSK3β (Cell Signaling). Phosphorylation of GSK3β was detected by Western blotting using rabbit anti-phospho-GSK-3β (Ser-9) (Cell Signaling) that detects GSK3β with phosphorylation at serine 9, the inactive form of phosphorylated GSK3β. Membranes were incubated with appropriate secondary antibody (Amersham Biosciences) at room temperature for 45 min. Blots were developed with ECL Plus reagents (Amersham Biosciences). Finally, blots were reprobed with actin antibody (Santa Cruz Biotechnology) or with anti-tubulin antibody (Sigma). Western blot bands were analyzed by ImageJ.
Statistical Analysis
Results were expressed as the means ± S.E. Differences between wild-type versus knock-out or vehicle versus treatment were analyzed using Student's t test, and differences were considered significant at p < 0.05.
RESULTS
Decreased BMD in Fgf2−/− Mice and Reduced Osteoblast Differentiation in Fgf2−/− BMSCs
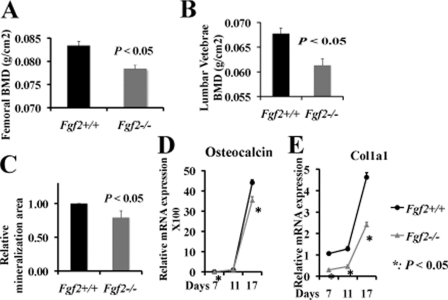
Dual energy x-ray absorptiometry analysis on Fgf2−/− mice with a Black Swiss/129Sv/FVB/N mixed background displayed significant lower femur BMD (Fig. 1A) and lumbar vertebrae BMD (Fig. 1B) compared with wild-type littermates, with no significant change in body weight (supplemental Fig. 1A).
FIGURE 1.
Reduced bone formation and decreased osteoblast differentiation in Fgf2−/− female mice compared with wild-type littermates. Femoral BMD (A) and lumber vertebrae BMD (B) were decreased significantly in Fgf2−/− mice. Reduced osteoblast mineralization (C), reduced osteocalcin (D), and Col1a1 (E) mRNA expression in Fgf2−/− compared with Fgf2+/+ BMSCs are shown. (Black line represents Fgf2+/+, and gray line represents Fgf2−/−). A and B, BMD was determined in 6-month-old female Fgf2+/+ (n = 7) and Fgf2−/− (n = 8) mice. C, freshly isolated BMSCs were cultured in α-MEM/10% FBS for 9 days, then in α-MEM/10% FBS with 50 μg/μl ascorbic acid and 8 mm β-glycerophosphate for another 8 days, followed by alkaline phosphatase, von Kossa staining, and quantification of total mineralization area. Data are mean ± S.E. of four independent experiments using 8-month-old male mice. D and E, freshly isolated BMSCs from 12-month-old female mice were cultured in α-MEM/10% FBS for 3 days, then in α-MEM/10% FBS with 50 μg/μl ascorbic acid and 8 mm β-gly-cerophosphate until day 17, followed by total RNA extraction for gene analysis. Similar results were also obtained in 8- and 10-month-old female mice.
Osteoblast differentiation was examined by von Kossa staining (Fig. 1C) for mineralization in Fgf2+/+ and Fgf2−/− BMSCs that were cultured in osteogenesis medium from day 9 until day 17. As shown in Fig. 1C, and supplemental Fig. 1B, Fgf2−/− BMSCs display significantly less mineralization area versus the Fgf2+/+ control. Consistent with the decreased mineralization, we observed significant reduced mRNA expression for the important osteoblast differentiation genes osteocalcin (Fig. 1D) and Col1a1 (Fig. 1E) in Fgf2−/− cultures during osteoblast differentiation. These results show that loss of endogenous FGF2 resulted in reduced osteoblast differentiation and bone formation.
Modulation of Wnt Pathway Components in the Absence of FGF2
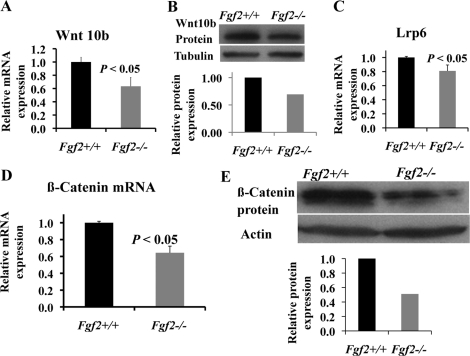
Modulation of Wnt pathway components in the absence of FGF2 was examined in BMSCs cultured in osteogenesis medium at day 17. Wnt10b is known to promote osteoblast differentiation (24). Consistent with decreased osteoblast differentiation in Fgf2−/− BMSCs (Fig. 1), Wnt10b mRNA (Fig. 2A) and protein expression (Fig. 2B) is significantly reduced in Fgf2−/− cultures compared with Fgf2+/+ cultures. We also examined other Wnt ligands and observed no significant change in Wnt3a (supplemental Fig. 2A). To activate the downstream pathway, ligand Wnt10b needs to bind to receptor Lrp5/Lrp6. Lrp5 mRNA expression is decreased (supplemental Fig. 2B) in Fgf2−/− cultures, although not significantly, whereas Lrp6 mRNA expression (Fig. 2C) is decreased significantly in Fgf2−/− cultures. Wnt10b is a known major ligand that functions to activate the Wnt/β-catenin pathway. We observed a significant reduction of β-catenin mRNA expression (Fig. 2D) as well as β-catenin protein expression (Fig. 2E) in Fgf2−/− BMSCs.
FIGURE 2.
Modulation of Wnt pathway in the absence of endogenous FGF2. A–C, compared with Fgf2+/+ BMSCs, Fgf2−/− BMSCs displayed reduced Wnt10b mRNA (A) and protein expression (B) and Lrp6 mRNA expression (C). D and E, β-catenin mRNA (D) and β-catenin protein expression (E) were also markedly reduced in Fgf2−/− BMSCs. A–E, freshly isolated BMSCs were cultured in α-MEM/10% FBS for 9 days, then in α-MEM/10% FBS with 50 μg/μl ascorbic acid and 8 mm β-glycerophosphate for another 8 days, followed by total RNA extraction for gene analysis by qPCR, normalized to GAPDH or whole cell extracts for β-catenin protein analysis by Western blotting. A, C, and D, data are mean ± S.E. of four independent experiments using 8-month-old male mice. B and E, representative images of three experiments using 8-month-old male mice are shown.
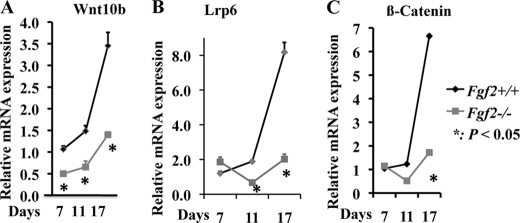
Time Course Expression of Wnt10b, Lrp6, and β-Catenin during Osteoblast Differentiation
Because we observed profound changes in Wnt10b, Lrp6, and β-catenin expression in Fgf2−/− osteoblasts at day 17, we further examined their expression profile in the process of osteoblast differentiation at earlier time points. We found markedly decreased Wnt10b mRNA (Fig. 3A), Lrp6 mRNA (Fig. 3B), and β-catenin (Fig. 3C) expression during osteoblast differentiation in Fgf2−/− BMSCs. Reduced Wnt10b, Lrp6, and β-catenin expression may result in reduced Wnt/β-catenin signaling and contribute to decreased osteoblast differentiation in Fgf2−/− cultures.
FIGURE 3.
Decreased Wnt10b (A), Lrp6 (B), and β-catenin (C) mRNA expression in Fgf2−/− BMSCs during osteoblast differentiation compared with Fgf2+/+ BMSCs. Freshly isolated BMSCs from 12-month-old female mice were cultured in α-MEM/10% FBS for 3 days, then in α-MEM/10% FBS with 50 μg/μl ascorbic acid and 8 mm β-glycerophosphate until day 17, followed by total RNA extraction for gene analysis by qPCR, normalized to GAPDH. *, p < 0.05.
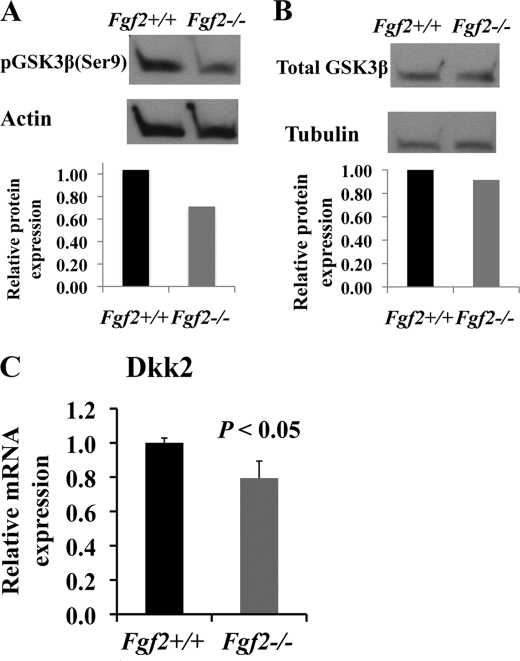
Loss of Endogenous FGF2 Results in Marked Reduction of Inactive Phosphorylated GSK3β
We next investigated the potential mechanisms by which FGF2 modulates the Wnt/β-catenin pathway. GSK3β is a component of the β-catenin destruction complex and a negative regulator of the Wnt pathway. Phosphorylation at Ser-9 inactivates GSK3β, which results in β-catenin stabilization and nuclear accumulation and leads to enhanced Wnt signaling. As shown in Fig. 4A, loss of FGF2 resulted in marked reduction of inactive phosphorylated GSK3β with no alternation in total GSK3β protein expression (Fig. 4B) in Fgf2−/− compared with Fgf2+/+ mice. Reduction of inactive phosphorylated GSK3β could lead to less stabilized β-catenin and decreased β-catenin nuclear signaling, which contributes to reduced osteoblast differentiation in Fgf2−/− osteoblasts.
FIGURE 4.
Potential mechanisms by which FGF2 modulates the Wnt pathway. A and B, loss of endogenous FGF2 results in reduction of inactive phosphorylated GSK3β (A) with no alternation of total GSK3β (B). Images are representative four independent experiments. Two of three independent experiments using 8-month-old male mice showed marked reduction of inactive phosphorylated GSK3β. Marked reduction of inactive GSK3β was also seen in experiments using 2-month-old male mice. C, Fgf2−/− osteoblasts displayed significant reduction of Dkk2 mRNA expression. Freshly isolated BMSCs were cultured in α-MEM/10% FBS for 9 days, then in α-MEM/10% FBS with 50 μg/μl ascorbic acid and 8 mm β-glycerophosphate for another 8 days, followed by total protein extraction for analysis by Western blotting and total RNA extraction for gene analysis by qPCR, normalized to GAPDH. Data are mean ± S.E. (error bars) of four independent experiments using 8-month-old male mice.
Loss of Endogenous FGF2 Results in Marked Reduction of Dkk2 mRNA Expression
The Wnt pathway is also regulated by Wnt antagonists. We examined gene expression of Wnt antagonists and observed that FGF2 ablation did not result in significant changes in mRNA expression of Dkk1, Dkk4, Sclerostin, FrzB/sFRP3, sFRP4, or sFRP1 (supplemental Table 2). Therefore, the Wnt antagonists examined do not seem to contribute to reduce osteoblast differentiation in Fgf2−/− cultures. Interestingly, Fgf2−/− osteoblasts displayed significant reduction of Dkk2 mRNA expression (Fig. 4C). Besides functioning as a Wnt antagonist, Dkk2 has been demonstrated to have a role in terminal osteoblast differentiation and mineralized matrix formation (35). Therefore, reduced Dkk2 expression may contribute to decreased osteoblast differentiation in Fgf2−/− mice.
FGF2 Promotes β-Catenin Nucleus Accumulation and Rescues Osteoblast Differentiation in Fgf2−/− BMSC Cultures
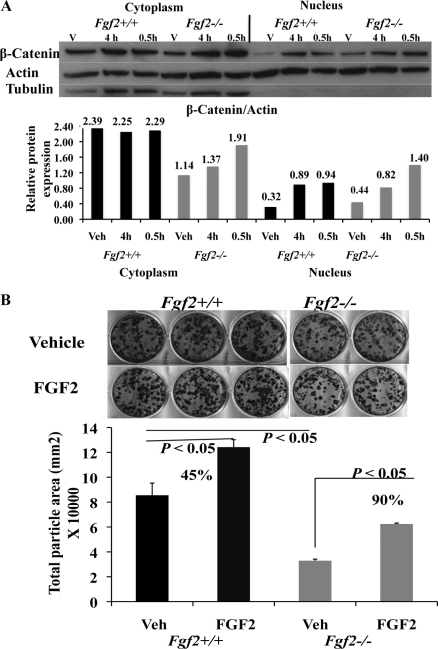
The above results showed modulation of Wnt pathway in the absence of endogenous FGF2, and β-catenin protein was decreased in Fgf2−/− BMSC cultures. Therefore, we next examined modulation of Wnt/β-catenin pathway in the presence of exogenous FGF2. To activate transcription of target genes, β-catenin needs to accumulate in the nucleus. Therefore, we used Western blotting to determine whether exogenous FGF2 is able to promote β-catenin accumulation in the nucleus. After serum deprivation for 14 h, BMSCs were treated with 1 nm FGF2 for 0.5 h or 4 h. Besides increasing β-catenin protein expression in cytoplasm, FGF2 increased β-catenin accumulation in nucleus not only in Fgf2+/+ BMSCs but also in Fgf2−/− BMSCs (Fig. 5A).
FIGURE 5.
Exogenous FGF2 promotes β-catenin nuclear accumulation and further partially rescues the decreased osteoblast differentiation in Fgf2−/− BMSCs. A, exogenous FGF2 promotes β-catenin protein accumulation in nucleus both in Fgf2+/+ BMSCs and in Fgf2−/− BMSCs. Freshly isolated BMSCs from 2-month-old male mice were cultured in α-MEM 10% FBS for 6 days, serum-deprived for 14 h, then treated with 1 nm FGF2 or vehicle at the indicated times, followed by cytoplasm and nuclear extraction and analysis for β-catenin by Western blotting. Similar results were also seen in two other independent experiments using 4-month-old female mice. B, exogenous FGF2 partially rescues decreased osteoblast mineralization in Fgf2−/− BMSCs (90% increase compared with Fgf2−/− vehicle group). Freshly isolated BMSCs from 12-month-old female mice were cultured in α-MEM/10% FBS with 0.1 nm FGF2 or vehicle for 3 days, then in α-MEM/10% FBS with 50 μg/μl ascorbic acid and 8 mm β-glycerophosphate until day 17, followed by alkaline phosphatase, von Kossa staining, and quantification of total mineralization area. Similar results were also obtained in 8-month-old and 10-month-old female mice.
To determine the possible downstream effects of increased β-catenin nuclear accumulation in Fgf2−/− BMSCs, mineralization was assessed at day 17 in osteogenesis medium. FGF2 (0.1 nm) was administered for the first 3 days of culture. We observed that FGF2 was able to partially rescue decreased osteoblast mineralization in Fgf2−/− BMSCs (90% increase compared with Fgf2−/− vehicle group, Fig. 5B). We believe this rescue effect may be due to the increased β-catenin signaling by FGF2.
DISCUSSION
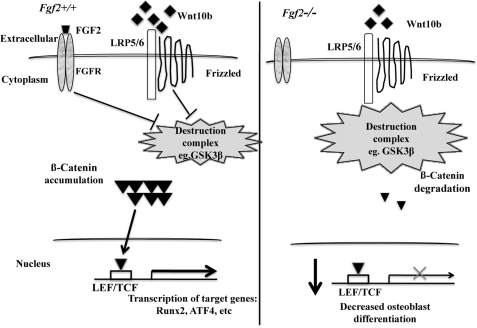
This study examined modulation of the Wnt pathway by FGF2 in osteoblasts. We found the following: (i) Loss of endogenous FGF2 (all isoforms) resulted in significantly reduced mRNA expression of the Wnt ligand Wnt10b, Lrp6, and β-catenin during osteoblast differentiation. (ii) Loss of endogenous FGF2 resulted in markedly reduced protein expression of Wnt10b and β-catenin. (iii) Loss of endogenous FGF2 resulted in reduced protein expression of inactive phosphorylated GSK3β, with no alternation in total GSK3β protein expression. (iv) FGF2 ablation results in decreased mRNA expression of Dkk2, which plays a role in terminal osteoblast differentiation. (v) Addition of exogenous FGF2 promoted β-catenin protein nuclear accumulation not only in Fgf2+/+ BMSCs but also in Fgf2−/− BMSCs in addition to the increase in cytoplasm. (vi) Addition of exogenous FGF2 partially rescued the reduced osteoblast mineralization in Fgf2−/− BMSCs. Collectively, the data reported here support the hypothesis that the anabolic actions of FGF2 on bone formation are partially manifest through modulation of the Wnt pathway (Fig. 6).
FIGURE 6.
Schematic model in which FGF2 stimulation of osteoblast differentiation is partially through modulation of the Wnt/β-catenin pathway. Loss of endogenous FGF2 results in reduced Wnt10b mRNA expression, reduced Lrp5/Lrp6 mRNA expression, reduced β-catenin mRNA and protein expression, and reduced inactive phosphorylated GSK3β protein expression, all of which lead to reduced Wnt/β-catenin signaling. Wnt/β-catenin signaling directly regulates osteoblast transcription factors, including Runx2 and probably ATF4. The reduced Wnt/β-catenin signaling and reduction of Dkk2 mRNA expression (important for terminal osteoblast differentiation) probably contribute to decreased osteoblast differentiation and bone formation in Fgf2−/− mice. However, exogenous FGF2 promoted β-catenin nuclear accumulation and induced inhibited kinase GSK3β, which could increase Wnt signaling in Fgf2−/− BMSCs and probably contributes to rescue the decreased osteoblast differentiation in Fgf2−/− BMSCs. LEF/TCF, lymphoid enhancer-binding factor 1/T cell-specific transcription factor.
The low BMD phenotype in Fgf2−/− mice on a new genetic background of Black Swiss/129Sv/FVB/N is similar to the bone phenotype that we reported previously in mice on a Black Swiss/129Sv genetic background (8, 10). These data further demonstrate that FGF2 is an endogenous, positive regulator of bone mass.
Both FGF2 (8, 10, 54) and Wnt/β-catenin pathways (3) are essential for osteoblast differentiation and bone formation. Thus, it is highly likely that these two pathways cross-talk and function together in osteoblasts. We observed that loss of endogenous FGF2 resulted in decreased expression of Wnt10b, Lrp6, and β-catenin, leading to reduced osteoblast differentiation in Fgf2−/− mice. By contrast, addition of exogenous FGF2 increased β-catenin protein expression in the cytoplasm as well as in the nucleus, and exogenous FGF2 further promoted β-catenin protein nuclear accumulation both in Fgf2+/+ and in Fgf2−/− BMSCs. FGF2 enhancement of β-catenin nuclear accumulation has also been reported in calvarial osteoblasts (55), human endothelial cells (56), neural stem cells (57), as well as in human embryonic stem cells (58). One study (59) reported that FGF2 did not induce β-catenin nuclear accumulation, which is not consistent with our results and other reports (55–58). However, those experiments used different FGF2 treatment durations, and the cell source was different whereby that study (59) used normal human neonatal calvarial osteoblastic cells immortalized by transfection with truncated SV40. Our studies using BMSCs showed that amplified Wnt/β-catenin signaling strongly correlates with higher osteogenic differentiation, consistent with other reports (3, 55). Finally, addition of exogenous FGF2 partially rescued the reduced osteoblast mineralization in Fgf2−/− BMSCs, and that is consistent with the enhanced β-catenin accumulation in the nucleus, suggesting that FGF2-mediated osteoblast mineralization is dependent on β-catenin function.
Our data suggest complementary mechanisms involving both direct and indirect regulatory effects of FGF2 on β-catenin. The direct mechanism is that FGF2 may regulate β-catenin mRNA and protein expression. We provided data (Figs. 2, D and E, and 5A) clearly demonstrating that FGF2 deletion results in decreased β-catenin mRNA and protein expression and that treatment with FGF2 increases β-catenin protein expression both in cytoplasm and nucleus. The basal reduction in β-catenin in Fgf2−/− cells reflects decreased production and FGF2 treatment increases β-catenin production and accumulation in the nucleus, suggesting that FGF2 may regulate β-catenin directly.
The indirect mechanism is that FGF2 may regulate β-catenin through kinase GSK3β. GSK3β is a negative regulator of Wnt/β-catenin pathway (3, 60). β-Catenin is stabilized instead of degraded, if GSK3β is phosphorylated and inhibited. We observed that loss of endogenous FGF2 resulted in decreased expression of inactive phosphorylated GSK3β with no alternation in total GSK3β protein expression in Fgf2−/− compared with Fgf2+/+ mice. In contrast, addition of exogenous FGF2 induced higher levels of inactive GSK3β (55–58), probably through PI3K/Akt signaling (55, 58). We also observed that treatment with FGF2 increased expression of the inactive phosphorylated GSK3β as early as 5 min in BMSCs (supplemental Fig. 3). β-Catenin expression was markedly decreased in Fgf2−/− mice, and that was coincident with the FGF2-mediated reduction of inactive GSK3β. These results suggest that modulation of Wnt/β-catenin signaling, in concert with GSK3β, causes reduced osteoblast differentiation in Fgf2−/− mice.
Besides controlling β-catenin signaling, GSK3β itself has been demonstrated as a key attenuator of osteogenesis. GSK3β heterozygous mice displayed increased bone formation due to enhanced Runx2 activity (61). Interestingly, we also published that Runx2 expression was decreased in Fgf2−/− mice (9). Therefore, reduction of inactive phosphorylated GSK3β alone could also contribute to the reduced osteoblast differentiation in Fgf2−/− mice.
FGF2 modulation of the Wnt pathway might also occur through Wnt antagonists. A single Fgf2 gene codes several protein isoforms in humans, including high molecular mass (22, 23, and 24 kDa) and low molecular mass 18-kDa FGF2 (2). High molecular mass FGF2 isoforms localize preferentially in the nucleus, whereas low molecular mass 18-kDa FGF2 localizes mainly in the cytoplasm and in the extracellular matrix, indicating unique biological functions of different FGF2 isoforms (2). The present study using the FGF2 all isoforms-null mouse model showed that sFRP1 gene expression was not significantly increased whereas the selective 18-kDa FGF2 knock-out mice showed a marked increase of sFRP1 gene and protein expression and decreased activity of Wnt/β-catenin signaling shown by TOP-FLASH reporter assay (62). However, both FGF2 all isoform-knock-out mice (8, 10) and 18-kDa FGF2-deficient mice (62) show a similar decreased osteoblast differentiation and bone formation phenotype. Overall, the results from these two mouse models suggest a differential function between the low molecular mass (18 kDa) and the high molecular mass (22-, 23-, 24-kDa) FGF2 isoforms for regulation of osteoblast differentiation. The continued characterization of the different signaling pathways between the FGF2 isoforms is the focus of ongoing studies in our laboratory.
Interestingly, Wnt antagonist Dkk2 mRNA expression was decreased significantly in Fgf2−/− compared with Fgf2+/+ osteoblasts. Besides binding to receptors Lrp5/6 thus inhibiting Wnt/β-catenin signaling, Dkk2 was demonstrated to play a role in terminal osteoblast differentiation and mineralized matrix formation (35). Osteoblasts from Dkk2-null mice were poorly mineralized (35). Therefore, decreased Dkk2 expression may contribute to reduced osteoblast differentiation and mineralization in Fgf2−/− mice. We speculate that endogenous FGF2 is necessary for terminal osteoblast differentiation and mineralization.
Another interesting result from the present study shows that FGF2 ablation resulted in significant decrease of Lrp6 mRNA expression, suggesting that FGF2 might regulate LRP6 protein expression. Consistent with our observation, a recent report indicated that FGF2 increased phosphorylation of LRP6, thereby enhancing Wnt signaling (63). The observed decreased Wnt10b mRNA and protein expression in Fgf2-null BMSCs is interesting, because other investigators reported that Wnt10b is important in maintaining bone mass as knock-out of Wnt10b is associated with increased bone marrow adipogenesis and decreased bone mass (19). Interestingly, we recently reported increased adipogenesis in bone marrow of Fgf2-null mice and that exogenous FGF2 treatment decreased bone marrow adipogenesis (10). Future studies will determine how FGF2 signaling regulates Wnt10b expression. Wnt10b has been demonstrated to enhance osteoblast differentiation and bone formation (19, 20). Reduction of Wnt10b expression may lead to decreased osteoblast differentiation and bone formation in Fgf2−/− mice.
Fgf2−/− mice also displayed reduced bone cell proliferation. As shown in our previous studies (8), thymidine incorporation into DNA was reduced significantly in calvarial osteoblast cultures from Fgf2−/− mice compared with wild-type littermates. Moreover, the addition of exogenous FGF2 increased cell proliferation in osteoblasts from both genotypes (8). Osteoblast proliferation is regulated by Wnt/β-catenin pathway. For example, bone formation was markedly decreased in patients with loss-of-function mutations in Lrp5 or Lrp6 (43, 44), Wnt coreceptors that are required for optimal Wnt/β-catenin signaling. In contrast, gain-of-function mutants of Lrp5 results in high bone mass (45–47). In addition, osteoblast proliferation was reduced in osteoblasts in the absence of LRP5 (43, 48), and LRP6 was previously reported to regulate proliferation of vascular smooth muscle cells (65). Therefore, reduced Wnt/β-catenin signaling may contribute to the decreased osteoblast proliferation in Fgf2−/− mice.
Reduction of osteoblast differentiation in Fgf2−/− mice was coincident with decreased expression of osteoblast transcription factors Runx2, shown in our previous report (9). Runx2 has been characterized as a direct target of Wnt/β-catenin signaling for stimulation of bone formation (66) and ATF4 is another critical transcription factor controlling osteoblast differentiation (1). We also reported that ATF4 expression was markedly reduced in Fgf2−/− mice (15). Our future experiments will examine whether ATF4 is also a target of Wnt/β-catenin signaling and whether FGF2 is required for Wnt/β-catenin signaling to activate ATF4. Overall, our results suggest that FGF2 signaling modulates a Wnt/β-catenin-GSK3β pathway, that consequently activates Runx2 and ATF4 the transcription factors, thereby regulating osteoblast differentiation (Fig. 6).
Our findings support the hypothesis that FGF2 stimulation of osteoblast differentiation and bone formation occurs through modulation of the Wnt/β-catenin pathway. This study further demonstrates a role of endogenous FGF2 in bone formation through Wnt signaling. These results will advance our understanding of cross-talk between FGF2 and Wnt/β-catenin signaling in bone biology. Both FGF2 (13, 14, 42) and Wnt signaling (3, 64) are being actively investigated in clinics to improve therapeutics for fracture healing and/or bone regeneration. Our studies on FGF2 and Wnt/β-catenin signaling might offer insights and suggest new approaches for treatment of bone disease with low bone formation
Supplementary Material
Acknowledgment
We thank Dr. Gloria Gronowicz for a critical reading of the paper.
This work was supported, in whole or in part, National Institutes of Health Grant AG021189 (to M. M. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. 1–3.
- BMSC
- bone marrow stromal cell
- ATF4
- activating transcription factor 4
- BMD
- bone mineral density
- Col1a1
- type I collagen α1
- Dkk
- Dickkopf
- GSK3β
- glycogen synthase kinase-3β
- LRP
- lipoprotein receptor-related protein
- MEM
- minimum Eagle's medium
- qPCR
- quantitative real-time PCR
- Runx2
- runt-related transcription factor 2
- sFRP
- secreted Frizzled-related protein.
REFERENCES
- 1. Karsenty G. (2008) Annu. Rev. Genomics Hum. Genet. 9, 183–196 [DOI] [PubMed] [Google Scholar]
- 2. Hurley M. M., Marie P. J., Florkiewics R. Z. (2002) Principles of Bone Biology (Bilezikian J. P., Raisz L. G., Rodan G. eds) pp. 627–645, Academic Press, San Diego [Google Scholar]
- 3. Krishnan V., Bryant H. U., Macdougald O. A. (2006) J. Clin. Invest. 116, 1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powers C. J., McLeskey S. W., Wellstein A. (2000) Endocr. Relat. Cancer 7, 165–197 [DOI] [PubMed] [Google Scholar]
- 5. Hurley M. M., Abreu C., Gronowicz G., Kawaguchi H., Lorenzo J. (1994) J. Biol. Chem. 269, 9392–9396 [PubMed] [Google Scholar]
- 6. Hurley M. M., Tetradis S., Huang Y. F., Hock J., Kream B. E., Raisz L. G., Sabbieti M. G. (1999) J. Bone Miner. Res. 14, 776–783 [DOI] [PubMed] [Google Scholar]
- 7. Globus R. K., Plouet J., Gospodarowicz D. (1989) Endocrinology 124, 1539–1547 [DOI] [PubMed] [Google Scholar]
- 8. Montero A., Okada Y., Tomita M., Ito M., Tsurukami H., Nakamura T., Doetschman T., Coffin J. D., Hurley M. M. (2000) J. Clin. Invest. 105, 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naganawa T., Xiao L., Abogunde E., Sobue T., Kalajzic I., Sabbieti M., Agas D., Hurley M. M. (2006) Biochem. Biophys. Res. Commun. 339, 490–498 [DOI] [PubMed] [Google Scholar]
- 10. Xiao L., Sobue T., Esliger A., Kronenberg M. S., Coffin J. D., Doetschman T., Hurley M. M. (2010) Bone 47, 360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mayahara H., Ito T., Nagai H., Miyajima H., Tsukuda R., Taketomi S., Mizoguchi J., Kato K. (1993) Growth Factors 9, 73–80 [DOI] [PubMed] [Google Scholar]
- 12. Nakamura K., Kurokawa T., Aoyama I., Hanada K., Tamura M., Kawaguchi H. (1998) Int. Orthop. 22, 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawaguchi H., Oka H., Jingushi S., Izumi T., Fukunaga M., Sato K., Matsushita T., Nakamura K.; TESK Group (2010) J. Bone Miner. Res. 25, 2735–2743 [DOI] [PubMed] [Google Scholar]
- 14. Kitamura M., Nakashima K., Kowashi Y., Fujii T., Shimauchi H., Sasano T., Furuuchi T., Fukuda M., Noguchi T., Shibutani T., Iwayama Y., Takashiba S., Kurihara H., Ninomiya M., Kido J., Nagata T., Hamachi T., Maeda K., Hara Y., Izumi Y., Hirofuji T., Imai E., Omae M., Watanuki M., Murakami S. (2008) PLoS One 3, e2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fei Y., Xiao L., Hurley M. M. (2010) Biochem. Biophys. Res. Commun. 391, 335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim H. J., Kim J. H., Bae S. C., Choi J. Y., Kim H. J., Ryoo H. M. (2003) J. Biol. Chem. 278, 319–326 [DOI] [PubMed] [Google Scholar]
- 17. Okada Y., Montero A., Zhang X., Sobue T., Lorenzo J., Doetschman T., Coffin J. D., Hurley M. M. (2003) J. Biol. Chem. 278, 21258–21266 [DOI] [PubMed] [Google Scholar]
- 18. Miller J. R. (2002) Genome Biol. 3, REVIEWS3001–REVIEWS3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bennett C. N., Longo K. A., Wright W. S., Suva L. J., Lane T. F., Hankenson K. D., MacDougald O. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3324–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bennett C. N., Ouyang H., Ma Y. L., Zeng Q., Gerin I., Sousa K. M., Lane T. F., Krishnan V., Hankenson K. D., MacDougald O. A. (2007) J. Bone Miner. Res. 22, 1924–1932 [DOI] [PubMed] [Google Scholar]
- 21. Piters E., Boudin E., Van Hul W. (2008) Arch. Biochem. Biophys. 473, 112–116 [DOI] [PubMed] [Google Scholar]
- 22. Moon R. T., Kohn A. D., Ferrari G. V., Kaykas A. (2004) Nat. Rev. Genet. 5, 691–701 [DOI] [PubMed] [Google Scholar]
- 23. Day T. F., Guo X., Garrett-Beal L., Yang Y. (2005) Dev. Cell 8, 739–750 [DOI] [PubMed] [Google Scholar]
- 24. Hu H., Hilton M. J., Tu X., Yu K., Ornitz D. M., Long F. (2005) Development 132, 49–60 [DOI] [PubMed] [Google Scholar]
- 25. Hill T. P., Später D., Taketo M. M., Birchmeier W., Hartmann C. (2005) Dev. Cell 8, 727–738 [DOI] [PubMed] [Google Scholar]
- 26. Holmen S. L., Zylstra C. R., Mukherjee A., Sigler R. E., Faugere M. C., Bouxsein M. L., Deng L., Clemens T. L., Williams B. O. (2005) J. Biol. Chem. 280, 21162–21168 [DOI] [PubMed] [Google Scholar]
- 27. Kawano Y., Kypta R. (2003) J. Cell Sci. 116, 2627–2634 [DOI] [PubMed] [Google Scholar]
- 28. Kamiya N., Ye L., Kobayashi T., Mochida Y., Yamauchi M., Kronenberg H. M., Feng J. Q., Mishina Y. (2008) Development 135, 3801–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bodine P. V., Zhao W., Kharode Y. P., Bex F. J., Lambert A. J., Goad M. B., Gaur T., Stein G. S., Lian J. B., Komm B. S. (2004) Mol. Endocrinol. 18, 1222–1237 [DOI] [PubMed] [Google Scholar]
- 30. Yao W., Cheng Z., Shahnazari M., Dai W., Johnson M. L., Lane N. E. (2010) J. Bone Miner. Res. 25, 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mao B., Wu W., Li Y., Hoppe D., Stannek P., Glinka A., Niehrs C. (2001) Nature 411, 321–325 [DOI] [PubMed] [Google Scholar]
- 32. MacDonald B. T., Joiner D. M., Oyserman S. M., Sharma P., Goldstein S. A., He X., Hauschka P. V. (2007) Bone 41, 331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morvan F., Boulukos K., Clément-Lacroix P., Roman Roman S., Suc-Royer I., Vayssière B., Ammann P., Martin P., Pinho S., Pognonec P., Mollat P., Niehrs C., Baron R., Rawadi G. (2006) J. Bone Miner. Res. 21, 934–945 [DOI] [PubMed] [Google Scholar]
- 34. Li J., Sarosi I., Cattley R. C., Pretorius J., Asuncion F., Grisanti M., Morony S., Adamu S., Geng Z., Qiu W., Kostenuik P., Lacey D. L., Simonet W. S., Bolon B., Qian X., Shalhoub V., Ominsky M. S., Zhu Ke H., Li X., Richards W. G. (2006) Bone 39, 754–766 [DOI] [PubMed] [Google Scholar]
- 35. Li X., Liu P., Liu W., Maye P., Zhang J., Zhang Y., Hurley M., Guo C., Boskey A., Sun L., Harris S. E., Rowe D. W., Ke H. Z., Wu D. (2005) Nat. Genet. 37, 945–952 [DOI] [PubMed] [Google Scholar]
- 36. Brunkow M. E., Gardner J. C., Van Ness J., Paeper B. W., Kovacevich B. R., Proll S., Skonier J. E., Zhao L., Sabo P. J., Fu Y., Alisch R. S., Gillett L., Colbert T., Tacconi P., Galas D., Hamersma H., Beighton P., Mulligan J. T. (2001) Am. J. Hum. Genet. 68, 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li X., Ominsky M. S., Warmington K. S., Morony S., Gong J., Cao J., Gao Y., Shalhoub V., Tipton B., Haldankar R., Chen Q., Winters A., Boone T., Geng Z., Niu Q. T., Ke H. Z., Kostenuik P. J., Simonet W. S., Lacey D. L., Paszty C. (2009) J. Bone Miner. Res. 24, 578–588 [DOI] [PubMed] [Google Scholar]
- 38. Li X., Ominsky M. S., Niu Q. T., Sun N., Daugherty B., D'Agostin D., Kurahara C., Gao Y., Cao J., Gong J., Asuncion F., Barrero M., Warmington K., Dwyer D., Stolina M., Morony S., Sarosi I., Kostenuik P. J., Lacey D. L., Simonet W. S., Ke H. Z., Paszty C. (2008) J. Bone Miner. Res. 23, 860–869 [DOI] [PubMed] [Google Scholar]
- 39. Cho H. Y., Choi H. J., Sun H. J., Yang J. Y., An J. H., Cho S. W., Kim S. W., Kim S. Y., Kim J. E., Shin C. S. (2010) Bone 47, 263–271 [DOI] [PubMed] [Google Scholar]
- 40. Nakanishi R., Akiyama H., Kimura H., Otsuki B., Shimizu M., Tsuboyama T., Nakamura T. (2008) J. Bone Miner. Res. 23, 271–277 [DOI] [PubMed] [Google Scholar]
- 41. Nakanishi R., Shimizu M., Mori M., Akiyama H., Okudaira S., Otsuki B., Hashimoto M., Higuchi K., Hosokawa M., Tsuboyama T., Nakamura T. (2006) J. Bone Miner. Res. 21, 1713–1721 [DOI] [PubMed] [Google Scholar]
- 42. Kitamura M., Akamatsu M., Machigashira M., Hara Y., Sakagami R., Hirofuji T., Hamachi T., Maeda K., Yokota M., Kido J., Nagata T., Kurihara H., Takashiba S., Sibutani T., Fukuda M., Noguchi T., Yamazaki K., Yoshie H., Ioroi K., Arai T., Nakagawa T., Ito K., Oda S., Izumi Y., Ogata Y., Yamada S., Shimauchi H., Kunimatsu K., Kawanami M., Fujii T., Furuichi Y., Furuuchi T., Sasano T., Imai E., Omae M., Yamada S., Watanuki M., Murakami S. (2011) J. Dent. Res. 90, 35–40 [DOI] [PubMed] [Google Scholar]
- 43. Gong Y., Slee R. B., Fukai N., Rawadi G., Roman-Roman S., Reginato A. M., Wang H., Cundy T., Glorieux F. H., Lev D., Zacharin M., Oexle K., Marcelino J., Suwairi W., Heeger S., Sabatakos G., Apte S., Adkins W. N., Allgrove J., Arslan-Kirchner M., Batch J. A., Beighton P., Black G. C., Boles R. G., Boon L. M., Borrone C., Brunner H. G., Carle G. F., Dallapiccola B., De Paepe A., Floege B., Halfhide M. L., Hall B., Hennekam R. C., Hirose T., Jans A., Jüppner H., Kim C. A., Keppler-Noreuil K., Kohlschuetter A., LaCombe D., Lambert M., Lemyre E., Letteboer T., Peltonen L., Ramesar R. S., Romanengo M., Somer H., Steichen-Gersdorf E., Steinmann B., Sullivan B., Superti-Furga A., Swoboda W., van den Boogaard M. J., Van Hul W., Vikkula M., Votruba M., Zabel B., Garcia T., Baron R., Olsen B. R., Warman M. L.. (2001) Cell 107, 513–523 [DOI] [PubMed] [Google Scholar]
- 44. Mani A., Radhakrishnan J., Wang H., Mani A., Mani M. A., Nelson-Williams C., Carew K. S., Mane S., Najmabadi H., Wu D., Lifton R. P. (2007) Science 315, 1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boyden L. M., Mao J., Belsky J., Mitzner L., Farhi A., Mitnick M. A., Wu D., Insogna K., Lifton R. P. (2002) N. Engl. J. Med. 346, 1513–1521 [DOI] [PubMed] [Google Scholar]
- 46. Van Wesenbeeck L., Cleiren E., Gram J., Beals R. K., Bénichou O., Scopelliti D., Key L., Renton T., Bartels C., Gong Y., Warman M. L., De Vernejoul M. C., Bollerslev J., Van Hul W. (2003) Am. J. Hum. Genet. 72, 763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ai M., Holmen S. L., Van Hul W., Williams B. O., Warman M. L. (2005) Mol. Cell. Biol. 25, 4946–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kato M., Patel M. S., Levasseur R., Lobov I., Chang B. H., Glass D. A., 2nd, Hartmann C., Li L., Hwang T. H., Brayton C. F., Lang R. A., Karsenty G., Chan L. (2002) J. Cell Biol. 157, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holmen S. L., Giambernardi T. A., Zylstra C. R., Buckner-Berghuis B. D., Resau J. H., Hess J. F., Glatt V., Bouxsein M. L., Ai M., Warman M. L., Williams B. O. (2004) J. Bone Miner. Res. 19, 2033–2040 [DOI] [PubMed] [Google Scholar]
- 50. Babij P., Zhao W., Small C., Kharode Y., Yaworsky P. J., Bouxsein M. L., Reddy PS., Bodine P. V., Robinson J. A., Bhat B., Marzolf J., Moran RA., Bex F. (2003) J. Bone Miner. Res. 18, 960–974 [DOI] [PubMed] [Google Scholar]
- 51. Zhou M., Sutliff R. L., Paul R. J., Lorenz J. N., Hoying J. B., Haudenschild C. C., Yin M., Coffin J. D., Kong L., Kranias E. G., Luo W., Boivin G. P., Duffy J. J., Pawlowski S. A., Doetschman T. (1998) Nat. Med. 4, 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sher L. B., Harrison J. R., Adams D. J., Kream B. E. (2006) Calcif. Tissue Int. 79, 118–125 [DOI] [PubMed] [Google Scholar]
- 53. Pfaffl M. W. (2001) Nucleic Acids Res. 29, e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marie P. J. (2003) Gene 316, 23–32 [DOI] [PubMed] [Google Scholar]
- 55. Quarto N., Wan D. C., Kwan M. D., Panetta N. J., Li S., Longaker M. T. (2010) J. Bone Miner. Res. 25, 1680–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Holnthoner W., Pillinger M., Groger M., Wolff K., Ashton A. W., Albanese C., Neumeister P., Pestell R. G., Petzelbauer P. (2002) J. Biol. Chem. 277, 45847–45853 [DOI] [PubMed] [Google Scholar]
- 57. Israsena N., Hu M., Fu W., Kan L., Kessler J. A. (2004) Dev. Biol. 268, 220–231 [DOI] [PubMed] [Google Scholar]
- 58. Ding V. M., Ling L., Natarajan S., Yap M. G., Cool S. M., Choo A. B. (2010) J. Cell. Physiol. 225, 417–428 [DOI] [PubMed] [Google Scholar]
- 59. Debiais F., Lefèvre G., Lemonnier J., Le Mée S., Lasmoles F., Mascarelli F., Marie P. J. (2004) Exp. Cell Res. 297, 235–246 [DOI] [PubMed] [Google Scholar]
- 60. Logan C. Y., Nusse R. (2004) Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 61. Kugimiya F., Kawaguchi H., Ohba S., Kawamura N., Hirata M., Chikuda H., Azuma Y., Woodgett J. R., Nakamura K., Chung U. I. (2007) PLoS One 2, e837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xiao L., Liu P., Li X., Doetschman T., Coffin J. D., Drissi H., Hurley M. M. (2009) J. Biol. Chem. 284, 3170–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. ervenka I., Wolf J., Mašek J., Krejci P., Wilcox W. R., Kozubík A., Schulte G., Gutkind J. S., Bryja V. (2011) Mol. Cell. Biol. 31, 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Minear S., Leucht P., Jiang J., Liu B., Zeng A., Fuerer C., Nusse R., Helms J. A. (2010) Sci. Transl. Med. 2, 29ra30 [DOI] [PubMed] [Google Scholar]
- 65. Wang X., Adhikari N., Li Q., Hall J. L. (2004) Am. J. Physiol. Heart Circ. Physiol. 287, H2376–2383 [DOI] [PubMed] [Google Scholar]
- 66. Gaur T., Lengner C. J., Hovhannisyan H., Bhat R. A., Bodine P. V., Komm B. S., Javed A., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2005) J. Biol. Chem. 280, 33132–33140 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.