Abstract
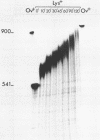
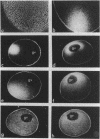
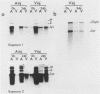
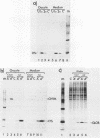
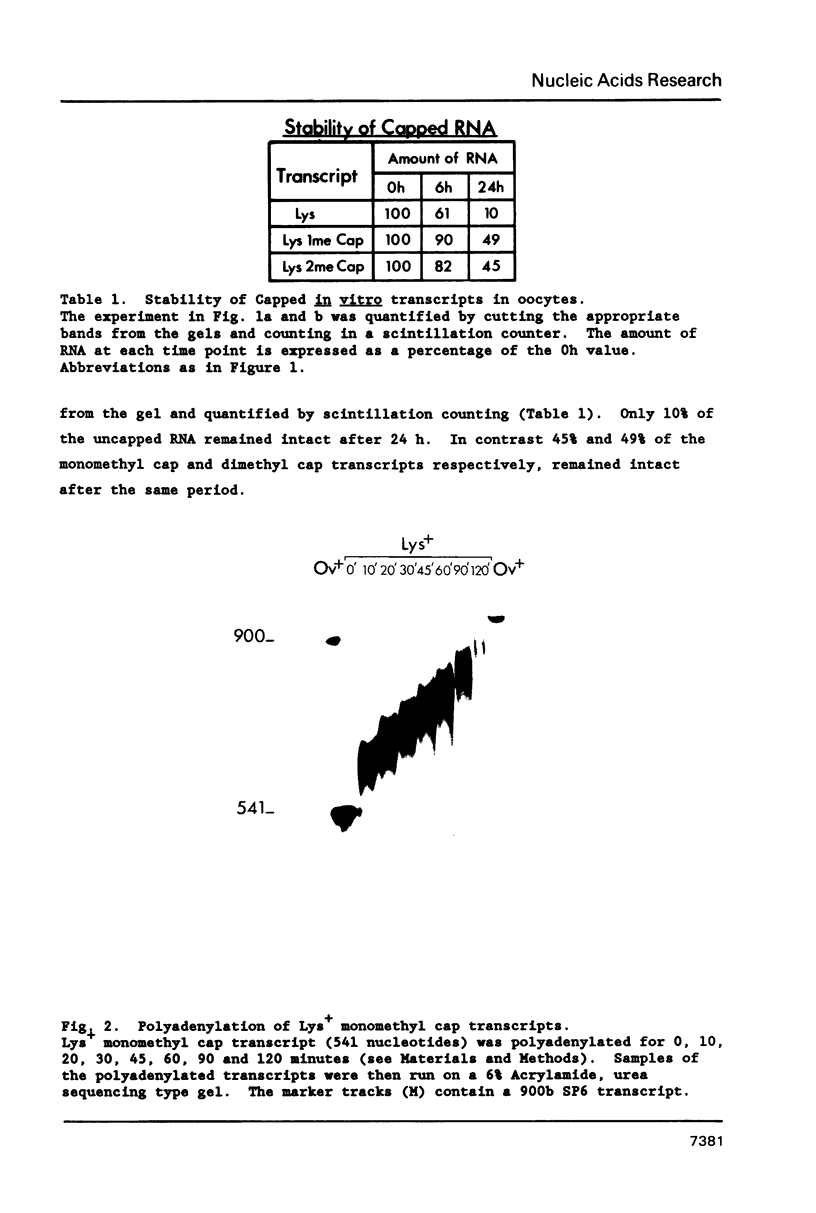
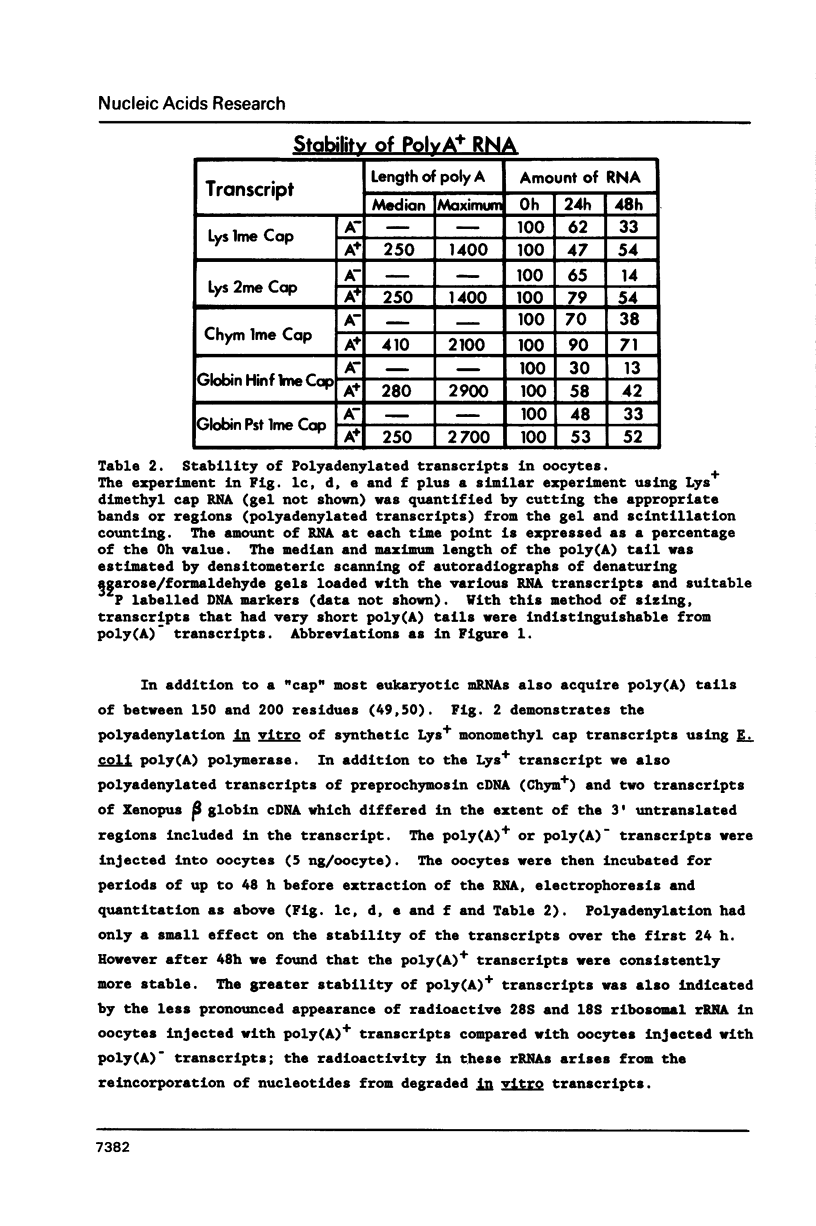
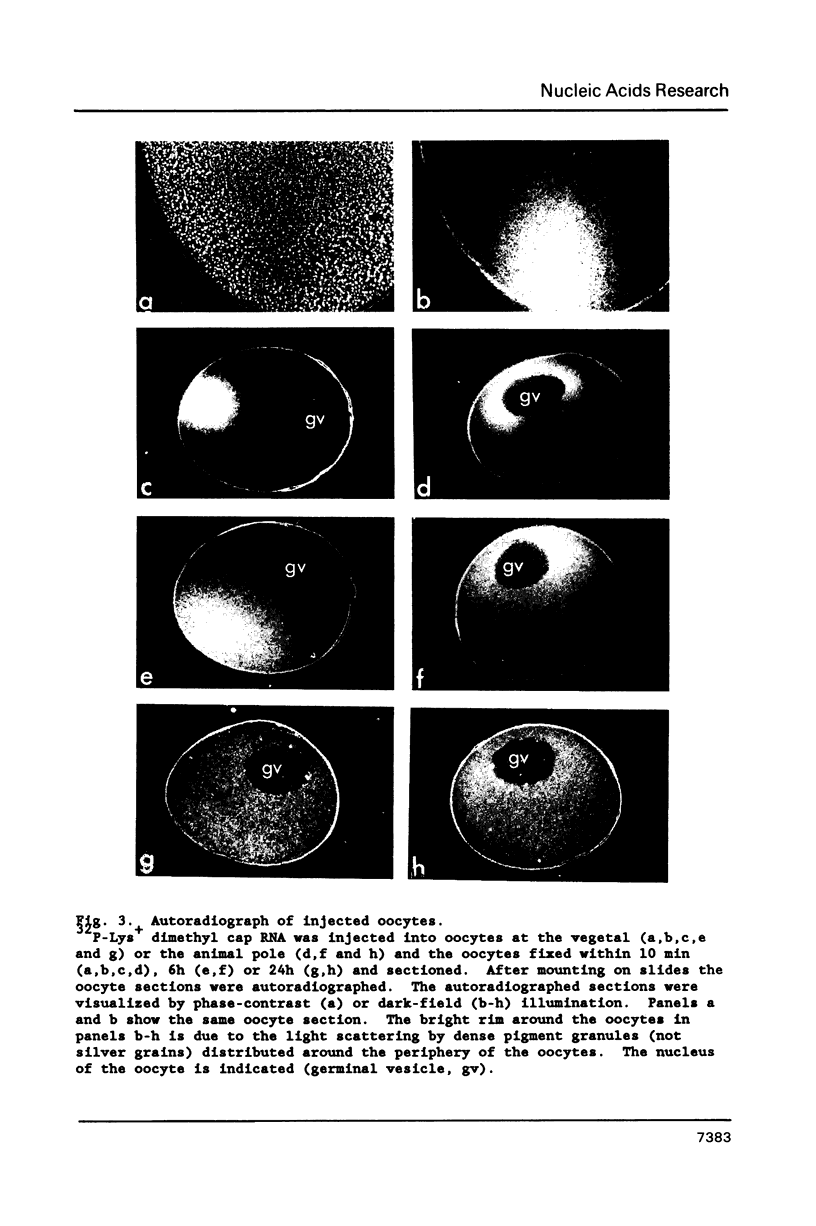
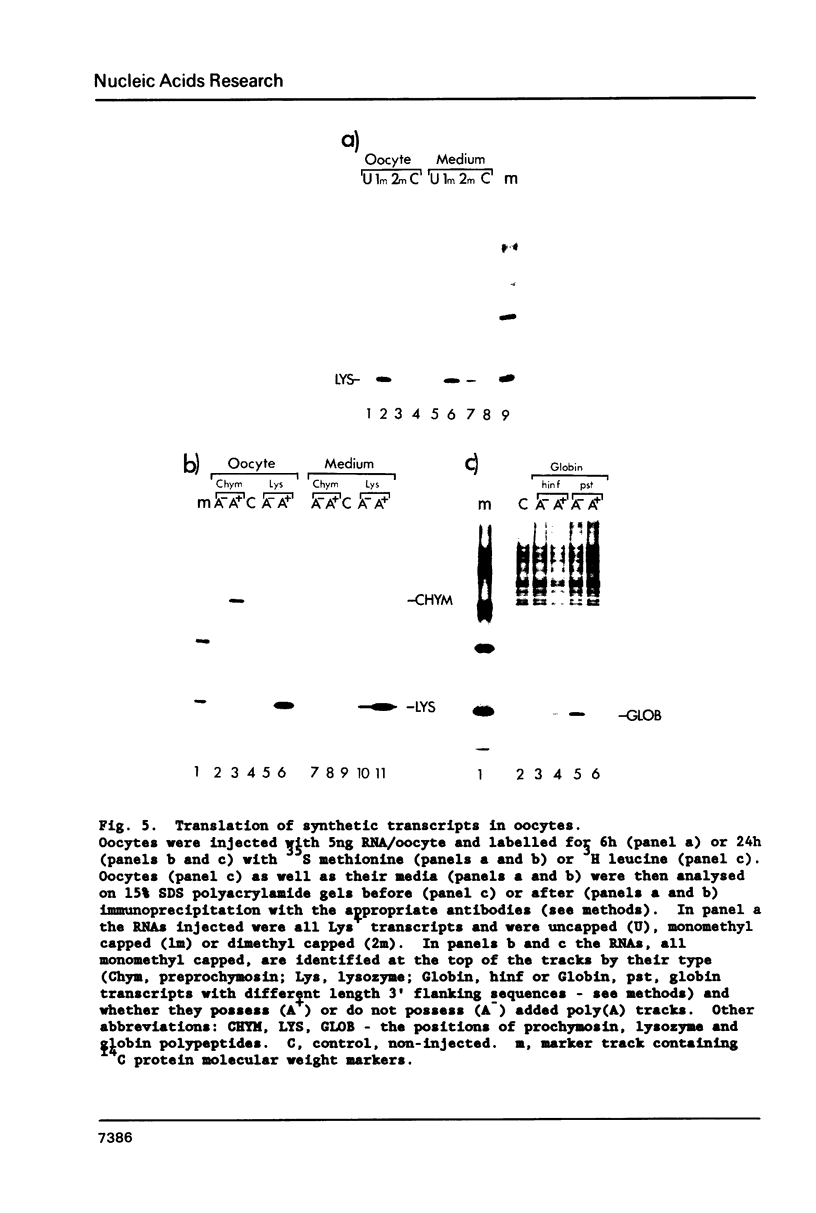
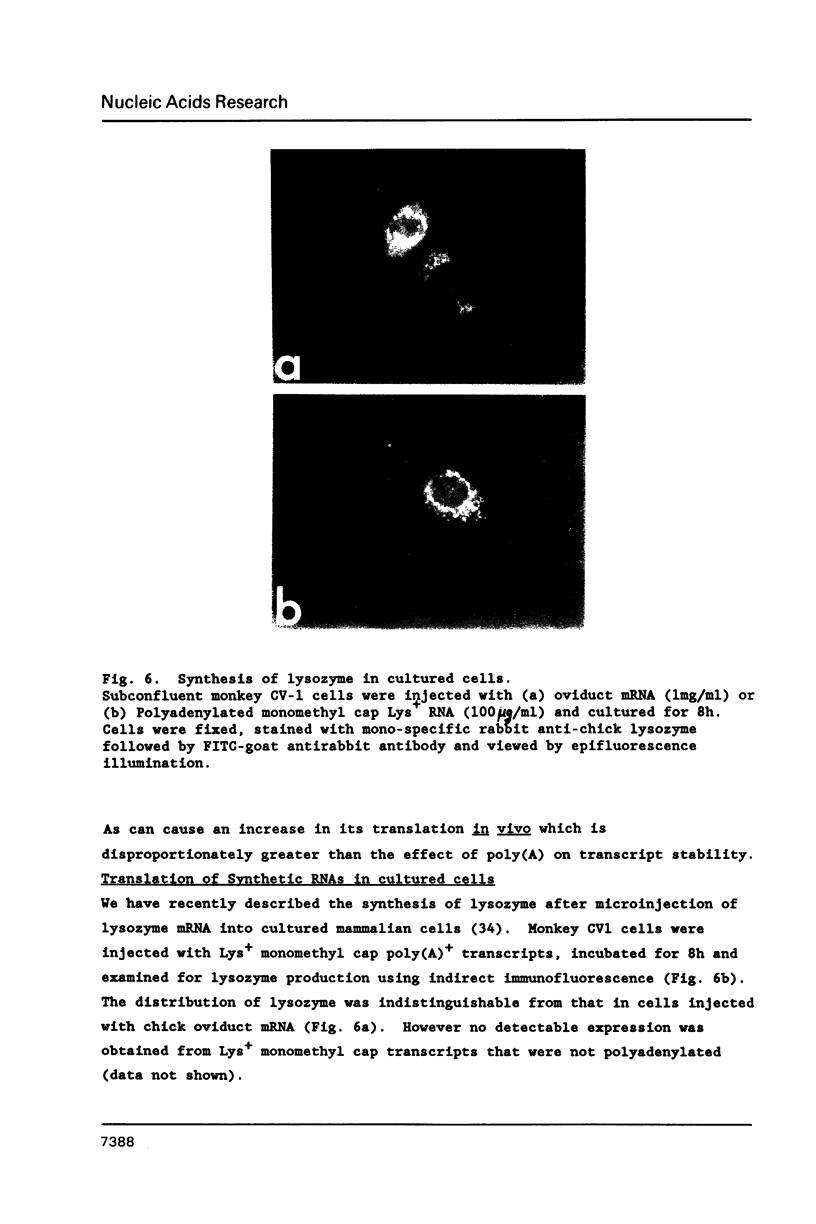
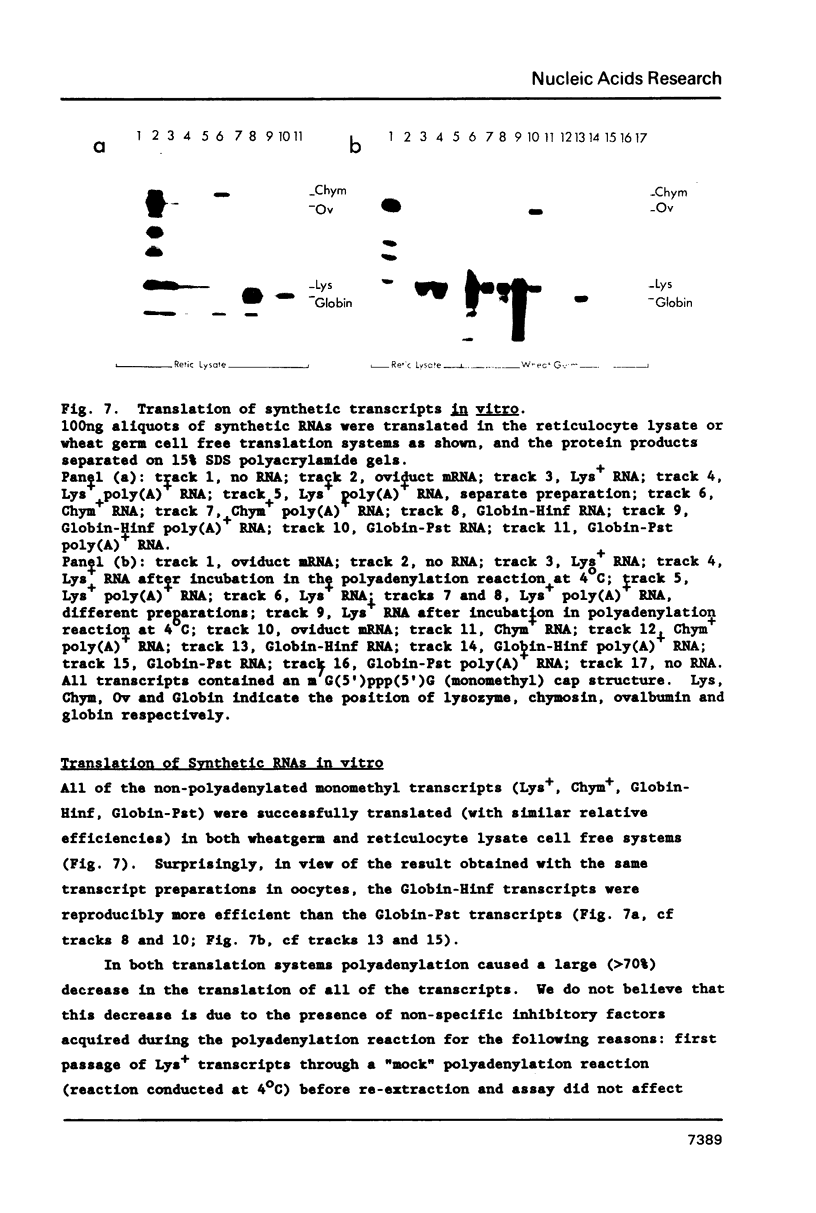
Synthetic RNAs coding for chicken lysozyme, calf preprochymosin and Xenopus globin were transcribed in vitro using Sp6 RNA polymerase. The effects of capping and adding a poly(A) tail on the stability, movement and translation of these RNAs in Xenopus oocytes was examined. Capping and polyadenylation increased stability of the transcripts, with at least 40% remaining intact 48 h after injection into oocytes. Capped poly(A)- transcripts moved more rapidly in oocytes than either capped poly(A)+ transcripts or naturally occurring mRNAs. The translational efficiency of most of the synthetic RNAs in oocytes increased with both capping and polyadenylation. The exception was one Xenopus globin transcript which had an unusual 3' end of 20As and 30Cs, where further polyadenylation decreased translational efficiency. Polyadenylation was essential for detectable expression of the synthetic RNAs in cultured cells, but decreased translation of the synthetic RNAs in vitro.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende C. C., Allende J. E., Firtel R. A. The degradation of ribonucleic acids injected into Xenopus laevis oocytes. Cell. 1974 Jul;2(3):189–196. doi: 10.1016/0092-8674(74)90093-2. [DOI] [PubMed] [Google Scholar]
- Ash J. F., Louvard D., Singer S. J. Antibody-induced linkages of plasma membrane proteins to intracellular actomyosin-containing filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5584–5588. doi: 10.1073/pnas.74.12.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. V., Lane C. D. Translation of Xenopus liver messenger RNA in Xenopus oocytes: vitellogenin synthesis and conversion to yolk platelet proteins. Cell. 1976 Jun;8(2):283–297. doi: 10.1016/0092-8674(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Biomembranes. Part J: Membrane biogenesis: assembly and targeting (general methods, eukaryotes). Methods Enzymol. 1983;96:1–901. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Ribosome binding to reovirus mRNA in protein synthesis requires 5' terminal 7-methylguanosine. Cell. 1975 Oct;6(2):185–195. doi: 10.1016/0092-8674(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Jeffrey W. R. Transient localizations of messenger RNA in Xenopus laevis oocytes. Dev Biol. 1982 Jan;89(1):1–12. doi: 10.1016/0012-1606(82)90288-3. [DOI] [PubMed] [Google Scholar]
- Cashdollar L. W., Chmelo R., Esparza J., Hudson G. R., Joklik W. K. Molecular cloning of the complete genome of reovirus serotype 3. Virology. 1984 Feb;133(1):191–196. doi: 10.1016/0042-6822(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Cashdollar L. W., Esparza J., Hudson G. R., Chmelo R., Lee P. W., Joklik W. K. Cloning the double-stranded RNA genes of reovirus: sequence of the cloned S2 gene. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7644–7648. doi: 10.1073/pnas.79.24.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A. K., Chatterjee B., Roy A. K. Translation and stability of rat liver messenger RNA for alpha 2 mu-globulin in Xenopus oocyte. The role of terminal poly(A). J Biol Chem. 1979 Sep 25;254(18):8937–8942. [PubMed] [Google Scholar]
- Doel M. T., Carey N. H. The translational capacity of deadenylated ovalbumin messenger RNA. Cell. 1976 May;8(1):51–58. doi: 10.1016/0092-8674(76)90184-7. [DOI] [PubMed] [Google Scholar]
- Drummond D. R., McCrae M. A., Colman A. Stability and movement of mRNAs and their encoded proteins in Xenopus oocytes. J Cell Biol. 1985 Apr;100(4):1148–1156. doi: 10.1083/jcb.100.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Microinjection of early SV40 DNA fragments and T antigen. Methods Enzymol. 1980;65(1):816–825. doi: 10.1016/s0076-6879(80)65076-9. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Harris T. J., Lowe P. A., Lyons A., Thomas P. G., Eaton M. A., Millican T. A., Patel T. P., Bose C. C., Carey N. H., Doel M. T. Molecular cloning and nucleotide sequence of cDNA coding for calf preprochymosin. Nucleic Acids Res. 1982 Apr 10;10(7):2177–2187. doi: 10.1093/nar/10.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Bruck C., Cleuter Y. Translational stability of native and deadenylylated rabbit globin mRNA injected into HeLa cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):908–911. doi: 10.1073/pnas.78.2.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Burny A., Hubert E., Leclercq M., Cleuter Y., Chantrenne H., Soreq H., Littauer U. Z. Degradation of deadenylated rabbit alpha-globin mRNA in Xenopus oocytes is associated with its translation. Nature. 1977 Mar 31;266(5601):473–474. doi: 10.1038/266473a0. [DOI] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Gallwitz D., Weinberg E., Devos R., Hubert E., Cleuter Y. Functional stabilisation of HeLa cell histone messenger RNAs injected into Xenopus oocytes by 3'-OH polyadenylation. Nature. 1978 Feb 9;271(5645):572–573. doi: 10.1038/271572a0. [DOI] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Hubert E., Leclercq M., Nudel U., Soreq H., Salomon R., Lebleu B., Revel M., Littauer U. Z. Role of the polyadenylate segment in the translation of globin messenger RNA in Xenopus oocytes. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3143–3146. doi: 10.1073/pnas.71.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A., Favreau M. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 1983 Sep 24;11(18):6353–6368. doi: 10.1093/nar/11.18.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A., Sippel A. E., Grez M., Schütz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984 Oct;38(3):731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Formation of the 3' end of histone mRNA by post-transcriptional processing. Nature. 1984 Mar 8;308(5955):203–206. doi: 10.1038/308203a0. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P., Strachan R., Wallis E., Tabe L., Colman A. Efficient expression of cloned complementary DNAs for secretory proteins after injection into Xenopus oocytes. J Mol Biol. 1984 Dec 15;180(3):615–643. doi: 10.1016/0022-2836(84)90030-5. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littauer U. Z., Soreq H. The regulatory function of poly(A) and adjacent 3' sequences in translated RNA. Prog Nucleic Acid Res Mol Biol. 1982;27:53–83. doi: 10.1016/s0079-6603(08)60597-8. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Lane C. Requirement for 7-methylguanosine in translation of globin mRNA in vivo. Nucleic Acids Res. 1978 Sep;5(9):3237–3247. doi: 10.1093/nar/5.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbaix G., Huez G., Burny A., Cleuter Y., Hubert E., Leclercq M., Chantrenne H., Soreq H., Nudel U., Littauer U. Z. Absence of polyadenylate segment in globin messenger RNA accelerates its degradation in Xenopus oocytes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3065–3067. doi: 10.1073/pnas.72.8.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell I. H., Maxwell F., Hahn W. E. Removal of RNase activity from DNase by affinity chromatography on agarose coupled aminophenylphosphoryl-uridine-2' (3')-phosphate. Nucleic Acids Res. 1977 Jan;4(1):241–246. doi: 10.1093/nar/4.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., Woodland H. R. Stability of non-polyadenylated viral mRNAs injected into frog oocytes. Eur J Biochem. 1981 Jun 1;116(3):467–470. doi: 10.1111/j.1432-1033.1981.tb05359.x. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Skorupa E. S., Kemper B. Single stranded DNA SP6 promoter plasmids for engineering mutant RNAs and proteins: synthesis of a 'stretched' preproparathyroid hormone. Nucleic Acids Res. 1985 Feb 25;13(4):1103–1118. doi: 10.1093/nar/13.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Tobimatsu T., Imoto K., Tanaka K., Fujita Y., Fukuda K., Kurasaki M., Takahashi H., Morimoto Y., Hirose T. Location of functional regions of acetylcholine receptor alpha-subunit by site-directed mutagenesis. 1985 Jan 31-Feb 6Nature. 313(6001):364–369. doi: 10.1038/313364a0. [DOI] [PubMed] [Google Scholar]
- Nakazato H., Kopp D. W., Edmonds M. Localization of the polyadenylate sequences in messenger ribonucleic acid and in the heterogeneous nuclear ribonucleic acid of HeLa cells. J Biol Chem. 1973 Feb 25;248(4):1472–1476. [PubMed] [Google Scholar]
- Palatnik C. M., Wilkins C., Jacobson A. Translational control during early Dictyostelium development: possible involvement of poly(A) sequences. Cell. 1984 Apr;36(4):1017–1025. doi: 10.1016/0092-8674(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Richardson M. A., Furuichi Y. Nucleotide sequence of reovirus genome segment S3, encoding non-structural protein sigma NS. Nucleic Acids Res. 1983 Sep 24;11(18):6399–6408. doi: 10.1093/nar/11.18.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J. D., Smith L. D. Differential capacity for translation and lack of competition between mRNAs that segregate to free and membrane-bound polysomes. Cell. 1981 Nov;27(1 Pt 2):183–191. doi: 10.1016/0092-8674(81)90372-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg U. B., Preiss A., Seifert E., Jäckle H., Knipple D. C. Production of phenocopies by Krüppel antisense RNA injection into Drosophila embryos. Nature. 1985 Feb 21;313(6004):703–706. doi: 10.1038/313703a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Tansey T. R., Ruderman J. V. Sequence-specific adenylations and deadenylations accompany changes in the translation of maternal messenger RNA after fertilization of Spisula oocytes. J Mol Biol. 1983 May 25;166(3):309–327. doi: 10.1016/s0022-2836(83)80087-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Soreq H., Tamm I. Does 3'-terminal poly(A) stabilize human fibroblast interferon mRNA in oocytes of Xenopus laevis? Proc Natl Acad Sci U S A. 1978 Oct;75(10):5030–5033. doi: 10.1073/pnas.75.10.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985 Feb;40(2):223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Sippel A. E. Purification and characterization of adenosine triphosphate: ribonucleic acid adenyltransferase from Escherichia coli. Eur J Biochem. 1973 Aug 1;37(1):31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Soreq H., Sagar A. D., Sehgal P. B. Translational activity and functional stability of human fibroblast beta 1 and beta 2 interferon mRNAs lacking 3'-terminal RNA sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1741–1745. doi: 10.1073/pnas.78.3.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle G., Besley J., Williamson A. R., Mosmann T. R., Colman A. Post-translational fate of variant MOPC 315 lambda chains in Xenopus oocytes and mouse myeloma cells. Eur J Biochem. 1983 Apr 15;132(1):131–138. doi: 10.1111/j.1432-1033.1983.tb07337.x. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Capon D. J., Simonsen C. C., Eaton D. L., Gitschier J., Keyt B., Seeburg P. H., Smith D. H., Hollingshead P., Wion K. L. Expression of active human factor VIII from recombinant DNA clones. Nature. 1984 Nov 22;312(5992):330–337. doi: 10.1038/312330a0. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]