Abstract
TNF-α potently stimulates basal lipolysis in adipocytes, which may contribute to hyperlipidemia and peripheral insulin resistance in obesity. Recent studies show that adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) act sequentially in catalyzing the first two steps of adipose lipolysis in response to β-adrenergic stimulation. Here, we sought to determine their functional roles in TNF-α-induced lipolysis. Silencing of ATGL expression in adipocytes almost completely abolished basal and TNF-α-induced glycerol release. In comparison, the glycerol release under the same conditions was only partially decreased upon reduction in expression of either HSL or the ATGL coactivator CGI-58. Interestingly, overexpression of ATGL restored the lipolytic rates in cells with silenced HSL or CGI-58, indicating a predominant role for ATGL. While expression of ATGL, HSL and CGI-58 remains mostly unaffected, TNF-α treatment caused a rapid abrogation of the ATGL inhibitory protein G0S2. TNF-α drastically decreased the level of G0S2 mRNA, and the level of G0S2 protein could be maintained by inhibiting proteasomal protein degradation using MG-132. Furthermore, coexpression of G0S2 was able to significantly decrease TNF-α-stimulated lipolysis mediated by overexpressed ATGL or CGI-58. We propose that the early reduction in G0S2 content is permissive for TNF-α-induced lipolysis.
Keywords: Adipocyte, Lipase, Lipolysis, Triacylglycerol, Tumor Necrosis Factor (TNF)
Introduction
During extended starvation and exercise, catecholamines stimulate the hydrolysis of triacylglycerol (TAG)2 stores within the lipid droplets (LDs) in adipocytes. The so-called lipolysis makes available surplus fuel in the form of free fatty acids (FFAs) and glycerol for use by other organs and tissues. During the instances of nutritional overload such as obesity, elevated basal adipose lipolysis contributes to the increase of systemic FFA levels, thereby exacerbating peripheral lipotoxicity and insulin resistance (1–4). Considering the importance of TAG turnover at both physiological and pathological levels, it is not surprising that enzymes governing lipolysis have attracted immense research interest over the years. Among many intracellular lipases identified thus far that possess in vitro capacity to hydrolyze TAG, hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) are the most characterized functionally (5, 6). A body of evidence has emerged recently, suggesting that ATGL and HSL act sequentially in mediating adipocyte lipolysis in response to β-adrenergic stimulation (7–12). In this newly established model, ATGL initiates lipolysis by specifically removing the first FA from TAGs to produce DAG substrates, which are then hydrolyzed by HSL to generate an additional FA and MAG substrates. MAGs are converted into FA and glycerol by MAG lipase in the final step of lipolysis.
HSL was discovered nearly half a century ago due to its induction during fasting and by catabolic hormones (13, 14). It has broad substrate specificity toward acylglycerols, including TAG, DAG, and MAG, as well as cholesteryl, retinyl, and lipoidal esters. Data from numerous previous studies suggested a key role for HSL in hydrolysis of both TAG and DAG in adipocytes (15, 16). However, animals deficient in HSL showed no obese phenotype though their adipocytes had a markedly blunted glycerol release upon β-adrenergic simulation (8, 9, 17–19). That these mice displayed an accumulation of DAG instead of TAG in several tissues led to the conclusion that HSL mainly functions as a rate-limiting enzyme for DAG hydrolysis (8). Therefore, there might be separate TAG hydrolases in adipocytes. In 2004, three laboratories independently identified ATGL as a lipase with selective affinity for TAG (7, 20, 21). Analysis using cultured cells and mutant mouse lines has demonstrated that ATGL plays a governing role in both basal and adrenergically stimulated TAG breakdown in adipocytes (5). Most notably, ATGL-null mice showed enhanced adiposity along with impaired adipocyte lipolysis under both basal and isoproterenol-stimulated conditions (23). Conversely, mice overexpressing ATGL specifically in adipose tissue were leaner with decreased TAG content in adipocytes and were resistant to diet-induced obesity with improved insulin sensitivity (24).
At the cell biology level, enzyme action of both ATGL and HSL is decided by their subcellular localization. HSL is a well known substrate for PKA downstream of β-adrenergic stimulation. Phosphorylation of HSL together with the lipid droplet coat protein perilipin promotes translocation of HSL from the cytoplasm to the surface of the LDs (25, 26). In comparison, although ATGL undergoes a similar LD translocation (12, 27), the responsible mechanisms are unclear because PKA does not appear to directly phosphorylate ATGL (7). A major advance in understanding ATGL-mediated lipolysis is the discovery that ATGL activity is highly dependent on association with a co-activator protein called CGI-58 (comparative gene identification-58; also known as ABHD5 (α/β hydrolase fold-containing protein 5)) (28). Recent reports have attributed the activation of ATGL in adipocytes to the dissociation of CGI-58 from perilipin (29–31). In the non-stimulated state, CGI-58 is in complex with perilipin at the surface of LDs and therefore is separated from ATGL. Upon stimulation, perilipin is phosphorylated on Ser-492 or Ser-517 by PKA, thereby releasing CGI-58 to act on ATGL. An earlier study has also demonstrated that phosphorylation of Ser-517 in perilipin is an essential step for ATGL activation (32).
Recently, our laboratory identified a protein encoded by G0S2 (G0/G1 switch gene 2) as a selective inhibitor of ATGL (27). G0S2 is a small basic protein with 78% identity between mouse and human orthologs. It binds directly to ATGL and is capable of inhibiting its lipase activity both in the absence and the presence of CGI-58. Ectopic expression of G0S2 prevented LD degradation in HeLa cells mediated by ATGL/CGI-58. In cultured adipocytes and fat explants, overexpression of G0S2 decreased basal and isoproterenol-stimulated lipolysis. Knockdown of endogenous G0S2, on the other hand, enhanced lipolysis in mature adipocytes (27). Moreover, G0S2 is highly expressed in adipose tissue as well as liver and heart. The expression of G0S2 was shown to increase in response to insulin, glucose, and ligands for the PPAR family of transcription factors (27, 33–35). Therefore, G0S2 likely controls TAG turnover in adipocytes as well as in nonadipocyte cells, and alteration of its expression may be a way via which nutritional and hormonal factors regulate the lipid homeostasis.
In obesity, a major factor implicated in the stimulation of adipose lipolysis is TNF-α (36, 37), a cytokine secreted by macrophages and adipocytes with a well established role in immunomodulatory and inflammatory response. In healthy human, TNF-α infusion led to enhanced systemic lipolysis with a concomitant increase in FFA clearance (38). In various types of cultured adipocytes, treatment with TNF-α consistently increased basal lipolytic rate (39–43). Mechanistically, activation of TNF receptor-1-dependent pathways is both necessary and sufficient for such lipolytic induction (44). The downstream signals involve the activation of several kinases such as ERK1/2, JNK, and IκB kinase (41, 42, 45–47) with resultant effects, including down-regulation of several genes involved in preventing basal lipolysis. Decreased expression of perilipin (48) and another LD protein FSP27 (49) is proposed to promote lipolysis through augmentation of action of the lipases. In this respect, prevention of protein depletion using adenoviral-mediated expression of either perilipin or FSP27 was shown to protect against TNF-α-induced lipolysis. Other evidence has also suggested that the extracellular glucose is required for the lipolytic action of TNF-α (43).
Although it becomes increasingly clear that multiple factors are involved in TNF-α-induced lipolysis, very few reports have directly addressed the contribution of relevant lipases and their regulators. In the present study, we examined the functional involvement of ATGL, HSL, CGI-58, and G0S2 in 3T3-L1 adipocytes treated with TNF-α. The differentiated 3T3-L1 adipocytes have been used extensively as the model system for investigation of hormone-stimulated lipolysis. We analyzed the expression pattern of aforementioned proteins, and performed both loss- and gain-of-function experiments via siRNA-mediated gene knockdown and adenovirus-mediated protein overexpression. Our results provide novel information on the molecular mechanisms by which TNF-α enhances lipolysis in adipocytes.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
The rabbit polyclonal antibodies against ATGL (catalog no. 2138) and HSL (catalog no. 4107) were from Cell Signaling. Monoclonal β-actin antibody (catalog no. A1978) was obtained from Sigma-Aldrich. Polyclonal anti-CGI-58 antibody (catalog no. 12201-1-AP) was purchased from Proteintech Group, Inc. Affinity-purified rabbit polyclonal antibody G0S2 was generated against recombinant GST fusion protein containing the C-terminal region of murine G0S2 (residues 43–103) by Proteintech Group, Inc. The goat polyclonal antibody against perilipin A (catalog no. ab616682) was purchased from Abcam, Inc. Horseradish peroxidase-linked secondary antibodies were from Pierce. The protease inhibitor mini tablets (catalog no. 11 836 170 001) were obtained from Roche Diagnostics. A lipolysis assay kit (catalog no. LIP-1-NC-L1) was purchased from Zenbio. 3H-labeled triolein was from PerkinElmer Life Sciences. The RNeasy Mini Kit (catalog no. 74104) and LongRange 2Step RT-PCR kit (catalog no. 205922) were from Qiagen. Reagents for tissue culture were obtained from Invitrogen. Rosiglitazone was obtained from Gateway Chemical Technology, LLC. Recombinant TNF-α (catalog no. T7539), insulin, dexamethasone, and 3-isobutyl-1-methylxanthine were purchased from Sigma-Aldrich.
Cell Culture
Mouse 3T3-L1 preadipocytes were maintained in high glucose DMEM supplemented with 10% newborn calf serum, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate. Differentiation to adipocytes was induced by treatment of postconfluent cells with 10% FBS, 1 μg/ml insulin, 1 μm dexamethasone, and 0.5 mm isobutyl-1-methylzanthine. The differentiation medium was withdrawn 3 days later and replaced with medium supplemented with 10% FBS and 1 μg/ml insulin. After 2 days in insulin-containing medium, the cells were then cultured in DMEM containing 10% FBS.
RNA Extraction, Reverse Transcription, and Real-time PCR
Total RNA was isolated from 3T3-L1 adipocytes using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. Reverse transcription was performed with LongRange reverse transcriptase (Qiagen) and an oligo(dT) primer using 1 μg of total RNA. For quantification of G0S2 expression, real-time PCR was performed using the LightCycler® System (Roche Applied Science) according to the manufacturer's instructions. Briefly, reaction conditions included 1× real-time PCR Master Mix (Roche Applied Science), 100 nm forward and reverse primers, and 50 ng of cDNA. PCR was carried out for 45 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 34 s with an initial cycle of 50 °C for 2 min and 95 °C for 10 min. The Universal ProbeLibrary (UPL) probe 26 and the following G0S2 primers were used: sense, 5′-TCTCTTCCCACTGCACCCTA-3′ and antisense, 5′-TCCTGCACACTTTCCATCTG-3′. As a positive control, the 18 S gene was also amplified in the same cDNA samples using the Universal ProbeLibrary (UPL) probe 81 with the following primers: sense, 5′-CGATTGGATGGTTTAGTGAGG-3′ and antisense, 5′-AGTTCGACCGTCTTCTCAGC-3.
Cell Lysis and Immunoblotting
For immunoblotting, 3T3-L1 cells were washed twice with ice-cold PBS and were lysed at 4 °C with a buffer containing 50 mm Tris-HCl (pH 8.0), 135 mm NaCl, 10 mm NaF, 1% Nonidet P-40, 0.1% SDS, 0.5% sodium deoxycholate, 1.0 mm EDTA, 5% glycerol, and protease inhibitors (1 mini tablet per 7 ml of buffer). The lysates were clarified by centrifugation at 10,000 × g for 10 min and then mixed with equal volume of 2× SDS sample buffer. The solubilized proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Individual proteins were blotted with primary antibodies at appropriate dilutions. Peroxide-conjugated secondary antibodies were incubated with the membrane at a dilution of (1:5000). The signals were then visualized by enhanced chemiluminescence (ECL Reagents, GE Healthcare).
siRNA-mediated Gene Silencing
For ATGL, HSL, and CGI-58 silencing in 3T3-L1 adipocytes, 4 nmol of mixed oligonucleotides were delivered into cells via electroporation using a Bio-Rad Gene Pulser II at 950 μFaraday and 160 V as described previously (50). After 3 days of incubation, cells were processed for designated assays.
The following double-stranded stealth siRNA oligonucleotides (Invitrogen) were used: set 1 for mouse ATGL, 5′-UCAGACGGAGACGAGAACGUCAUCAUAU-3′ (sense) and 5′-AUAUGAUGACGUUCUCUCCGUCUGA-3′ (antisense); set 2 for mouse ATGL, 5′-CCAGGCCAAUGUCUGCAGCACAUUU-3′ (sense) and 5′-AAAUGUGCUGCAGACAUUGGCCUGG-3′ (antisense); set 1 from mouse HSL, 5′-CCCUCUACACGUCACCAUAGUCAA-3′ (sense) and 5′-UUGACUAUGGGUGACGUGUAGAGGG-3′ (antisense); set 2 from mouse HSL, 5′-CAGGAGCUAGGAGUCCCUAUCUUCU-3 (sense) and 5′-AGAAGAUAGGGACUCCUAGCUCCUG-3′ (antisense); set 1 from mouse CGI-58, 5′-CCCAAGUGGUGAGACAGCUUUCAAA-3′ (sense) and 5′-UUUGAAAGCUGUCUCACCACUUGGG-3′ (antisense); set 2 from mouse CGI-58, 5′-GGAUAGGUGGCUUGCAUCCUGACAU-3 (sense) and 5′-AUGUCAGGAUGCAAGCCACCUAUCC-3′ (antisense). Control oligonucleotides with comparable GC content were also from Invitrogen.
Adenoviral Infection of Adipocytes
Recombinant adenoviruses containing murine ATGL, HSL, G0S2, and CGI-58 cDNA under the control of a CMV promoter were custom generated by Vector Biolabs. A CMV-null virus (Ad-null) was also obtained for use in control experiments. Infection of differentiated adipocytes was performed by using a previously published method (51) with minor modifications. For infection of 3T3-L1 adipocytes, Ad-G0S2, Ad-ATGL, Ad-CGI-58, or Ad-null was diluted to 3 × 107 pfu/ml in serum-free Opti-MEM containing 5.0 μg/ml polybrene and preincubated for 45 min at room temperature. Adipocytes plated in six-well dishes were washed once with PBS, and 1.2 ml of preincubated virus mix was added to each well. Cells were centrifuged at 800 × g for 1 h at room temperature followed by incubation at 37 °C. Fresh DMEM containing 10% FBS (1.0 ml/well) was added after 4 h, and cells were incubated for 48–72 h before use.
Assay for TAG Hydrolase Activity and Lipolysis
For assays with 3T3-L1 adipocyte extracts, cells in six-well dishes were washed twice in ice-cold PBS and then lysed on ice by sonication in 0.5 ml of cell extraction buffer (0.25 m sucrose, 1 mm EDTA, 1 mm DTT, and protease inhibitors (1 mini tablet per 7 ml of volume)). The cell extracts were clarified by centrifugation at 1000 × g for 15 min. The TAG hydrolase activity against 3H-labeled triolein was measured as described previously (28) (7) by mixing 0.1 ml of extracts with 0.1 ml of substrate solution. Lipolysis was measured as the rate of glycerol release. In brief, adipocytes were incubated in 2.5 ml of assay buffer in the presence or absence of 50 ng/ml TNF-α. Aliquots of assay buffer were collected over a 30 min to 2 h period. The amounts of glycerol released were determined by using a lipolysis assay kit (Zenbio) according to the manufacturer's instructions. Lysates were then prepared from the remaining cells, and protein concentrations in the lysates were used to normalize the lipolytic signals. Statistical analysis was determined by Student's t test or one-way analysis of variance.
RESULTS
Lipolysis and Lipolytic Protein Expression in Response to TNF-α
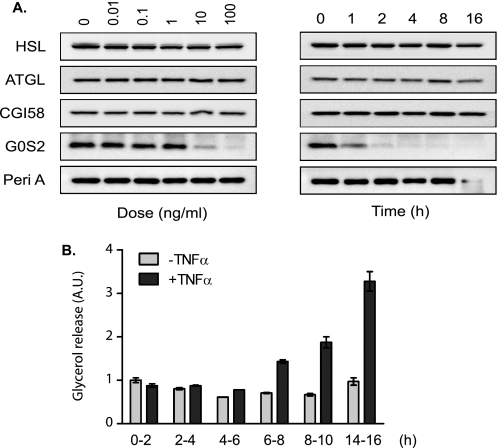
Numerous studies have demonstrated that TNF-α increases the rate of lipolysis in adipocytes (39, 41, 45, 46). To examine the modulation of individual players in the lipolytic proteome, we treated fully differentiated 3T3-L1 adipocytes with TNF-α for various periods of time. Western blot analysis revealed that levels of HSL, ATGL, and CGI-58 remained steady over a 16-h span (Fig. 1A). The expression of perilipin was unchanged during the first 8 h of treatment but decreased significantly after 16 h. The level of G0S2 protein, on the other hand, was progressively reduced over time starting at the 1-h time point and almost completely disappeared after 4 h of TNF-α treatment. A dose-response experiment then showed that 8-h treatment with TNF-α at concentrations greater than 10 ng/ml was sufficient to induce a noticeable decrease in the level of G0S2 protein. These results indicate that although down-regulation of perilipin expression requires prolonged exposure to TNF-α, the effect of TNF-α treatment on G0S2 expression is potent and rapid in adipocytes.
FIGURE 1.
Effects of TNF-α on protein expression and lipolysis in 3T3-L1 adipocytes. A, time course and dose-response analysis of TNF-α treatment were performed by using differentiated 3T3-L1 adipocytes. Left panel, cells were treated with 50 ng/ml of TNF-α for different periods of time. Right panel, cells were treated with varying concentrations of TNF-α in serum-free DMEM/0.5%BSA for 8 h. Afterward, cells were lysed, and immunoblotting was performed using antibodies specific for HSL, ATGL, CGI-58, G0S2, and perilipin A (Peri A). B, differentiated 3T3-L1 adipocytes were pretreated for 30 min in serum-free DMEM/0.5% BSA and then cultured in the same medium without or with 50 ng/ml TNF-α. After 0, 2, 4, 6, 8, or 14 h, cells were switched to fresh medium without or with TNF-α for another 2-h incubation. The lipolytic rates during various time windows were determined by measuring glycerol concentration in the medium and expressed as fold change from untreated cells during the 0–2-h time period. The data are presented as the means ± S.E. of three independent experiments. *, p < 0.05; **, p < 0.01.
For determining the lipolytic rates during different treatment periods, we pretreated adipocytes with TNF-α for varying length of time and thereafter followed the glycerol release as an index of lipolysis over a 2-h period. As shown in Fig. 1B, TNF-α treatment had little effect during the time periods of 0–2 h and 2–4 h. A slight increase in the rate of glycerol release was observed during the 4–6 h period. By the 6–8h period, lipolysis increased drastically (∼2-fold) in cells treated with TNF-α when compared with that in untreated controls. Longer treatments resulted in further stimulation as evidenced by a 3.3-fold increase of glycerol release in TNF-α-treated cells by the 14–16 h period. Therefore, the increase in the lipolytic rate occurs early in the course of the TNF-α treatment and becomes more marked as the treatment is prolonged.
ATGL and HSL Both Contribute to TNF-α-induced Lipolysis
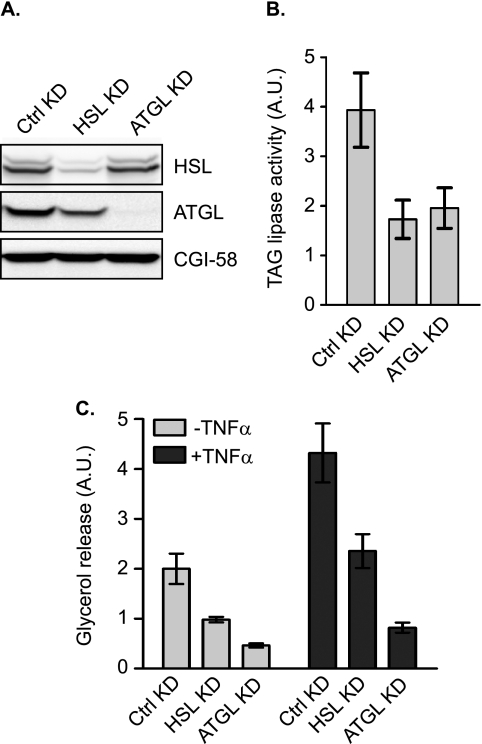
To directly assess the relative contribution of ATGL and HSL, we introduced siRNA via electroporation to knockdown each lipase in 3T3-L1 adipocytes. Compared with the endogenous protein levels in cells transfected with a nonsilencing control siRNA, <10% of HSL and <5% of ATGL were present in cells transfected with a pool of siRNA directed against respective proteins (Fig. 2A). There was no effect of ATGL siRNA on HSL protein expression or vice versa. As measured against triolein as a substrate, silencing of HSL expression reduced the TAG hydrolase activity in total cell extracts by 56% (Fig. 2B). In comparison, a 50% reduction in lipase activity was achieved by ATGL siRNA. The respective contributions of HSL and ATGL to the overall activity against triolein are in line with what was observed previously in cultured adipocytes and adipose tissue explants.
FIGURE 2.
Contribution of HSL and ATGL to TAG hydrolase activity and TNF-α-induced lipolysis in adipocytes. 3T3-L1 adipocytes were electroporated with nonspecific control (Ctrl) siRNA, ATGL-specific siRNA, or HSL-specific siRNA oligonucleotides. A and B, cells were extracted and expression of ATGL, CGI-58, or HSL proteins was analyzed by immunoblotting with specific antibodies . The TAG hydrolase activity in the cell extracts was measured using 3H-labeled triolein as substrate. The activity was normalized with the total protein levels. A.U., arbitrary units. C, cells were pretreated for 6 h in serum-free DMEM/0.5% BSA without or with 50 ng/ml TNF-α and then were switched to fresh medium without or with TNF-α for another 2-h incubation. The glycerol concentration in the medium was measured and expressed as fold change from untreated cells receiving control siRNA. The data are presented as the means ± S.E. of three independent experiments. *, p < 0.05; **, p < 0.01. KD, knockdown.
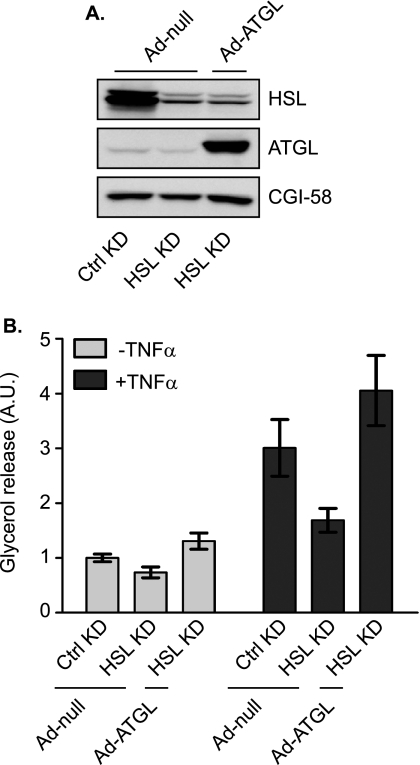
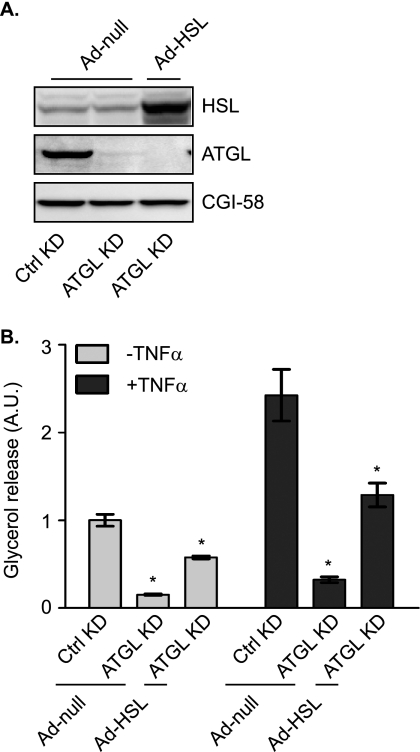
To evaluate the effects on TAG breakdown, we next measured basal and TNF-α-induced glycerol release in siRNA-transfected cells (Fig. 2). A confounding factor in studying lipolysis during chronic TNF-α treatment is that many adipose genes are down-regulated, making it difficult to accurately assess the contribution of individual regulators. Because 6–8 h of treatment with TNF-α was sufficient to induce a significant increase in lipolysis without notable impact on the levels of major lipolytic players (Fig. 1), we chose 6 h as the length for TNF-α pretreatment of 3T3-L1 adipocytes in the following experiments. As shown in Fig. 2C, TNF-α caused a ∼2-fold increase in glycerol release from cells transfected with the control siRNA. In cells transfected with ATGL siRNA, the release of glycerol at basal state and in response to TNF-α treatment was decreased by 86 and 81%, respectively, suggesting a predominant role for ATGL under both conditions. On the other hand, with resultant reductions in glycerol release at 51 and 45%, respectively, the knockdown of HSL was only able to partially inhibit lipolysis under the two conditions (Fig. 2C). To test whether increased expression of ATGL would compensate for the loss of HSL or vice versa, we overexpressed ATGL or HSL in adipocytes where expression of the other enzyme was silenced (Figs. 3 and 4). To this end, we generated ATGL and HSL constructs incorporated into an adenovirus expression system (Ad-ATGL and Ad-HSL). A null adenovirus (Ad-null) was used to infect control cells. When these reagents were used, infection with Ad-ATGL and Ad-HSL led to a profound increase in expression of respective lipases when compared with the level of endogenous protein in Ad-null-infected cells (Figs. 3A and 4A). Importantly, overexpression of ATGL was more than sufficient to increase both basal and TNF-α-induced glycerol release from adipocytes where endogenous HSL was knocked down (Fig. 3B). In the other words, under both conditions the lipolytic rates were greater in cells whose endogenous HSL was replaced by ectopic ATGL than in cells where both HSL and ATGL were at endogenous levels. In comparison, overexpression of HSL only partially restored lipolysis in cells with silenced ATGL expression under the two conditions (Fig. 4B).
FIGURE 3.
Effect of overexpression ATGL on adipocyte lipolysis in the absence of HSL. 3T3-L1 adipocytes were electroporated with either control (Ctrl) or HSL-specific siRNA oligonucleotides. 24 h after electroporation, cells were infected with either a null adenovirus (Ad-null) or a virus containing ATGL cDNA (Ad-ATGL) followed by incubation for another 48 h. A, cells were lysed, and then protein expression was analyzed by immunoblotting with antibodies specific for HSL, ATGL, and CGI-58. B, cells were pretreated for 6 h in serum-free DMEM/0.5% BSA without or with 50 ng/ml TNF-α and then were switched to fresh medium without or with TNF-α for another 2-h incubation. The glycerol concentration in the medium was measured and expressed as fold change from untreated cells receiving control siRNA. The data are presented as the means ± S.E. of three independent experiments. *, p < 0.05. A.U., arbitrary units; KD, knockdown.
FIGURE 4.
Effect of overexpression HSL on adipocyte lipolysis in the absence of ATGL. 3T3-L1 adipocytes were electroporated with either control or ATGL specific siRNA oligonucleotides. 24 h after electroporation, cells were infected with either a null adenovirus (Ad-null) or a virus containing HSL cDNA (Ad-HSL) followed by incubation for another 48 h. A, cells were lysed, and then protein expression was analyzed by immunoblotting with antibodies specific for HSL, ATGL, and CGI-58. B, cells were pretreated for 6 h in serum-free DMEM/0.5% BSA without or with 50 ng/ml TNF-α and were then switched to fresh medium without or with TNF-α for another 2-h incubation. The glycerol concentration in the medium was measured and expressed as fold change from untreated cells receiving control siRNA. The data are presented as the means ± S.E. of three independent experiments. *, p < 0.05. A.U., arbitrary units; KD, knockdown.
Dependence of TNF-α-induced Lipolysis on CGI-58
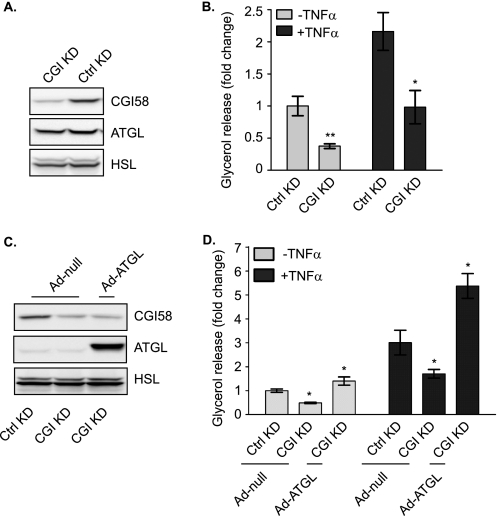
ATGL activity largely depends on the presence of CGI-58 (28). We next performed an siRNA silencing experiment to demonstrate the requirement of CGI-58 for TNF-α-induced lipolysis in adipocytes (Fig. 5). Transfection of adipocytes with CGI-58 siRNA, when compared with the control siRNA, decreased the expression of CGI-58 by ∼70% (Fig. 5A). Following down-regulation of CGI-58, a 63% decrease of basal and a 55% decrease of TNF-α-induced lipolysis were observed (Fig. 5B), indicating that endogenous CGI-58 is critically involved in lipolysis under both conditions. Because the function of CGI-58 is to activate ATGL and itself possesses no intrinsic lipase activity, we tested whether increased expression of ATGL would restore lipolytic rates in the absence of CGI-58. To this end, we overexpressed ATGL in adipocytes where expression of endogenous CGI-58 was silenced. In comparison with infection with the null virus, infection of adipocytes with Ad-ATGL led to a profound increase in ATGL expression (Fig. 5C). Interestingly, under both basal and TNF-α-treated conditions, increased expression of ATGL in cells with silenced expression of CGI-58 was sufficient to boost lipolysis to levels even higher than in null virus-infected control cells (Fig. 5D). These results indicate that while CGI-58 plays a critical role in activating ATGL, the dependence on CGI-58 can be ameliorated by the enzyme in excess amount.
FIGURE 5.
Involvement of CGI-58 in basal and TNF-α-induced lipolysis. 3T3-L1 adipocytes were incubated for 72 h after electroporation with either control or CGI-58-specific (CGI) siRNA oligonucleotides. A, cells were lysed, and protein expression was analyzed by immunoblotting with antibodies specific for HSL, ATGL, and CGI-58. B, cells were pretreated for 6 h in serum-free DMEM/0.5% BSA without or with 50 ng/ml TNF-α and then were switched to fresh medium without or with TNF-α for another 2-h incubation. The glycerol concentration in the medium was measured and expressed as fold change from untreated cells receiving control siRNA. The data are presented as the means ± S.E. of three independent experiments. C and D, 24 h after electroporation, cells were infected with either Ad-null or Ad-ATGL followed by a 48-h incubation. Immunoblotting and glycerol release were measured as described in A and B. *, p < 0.05; **, p < 0.01. A.U., arbitrary units; KD, knockdown; Ctrl, control.
TNF-α Decreases G0S2 Levels in Adipocytes
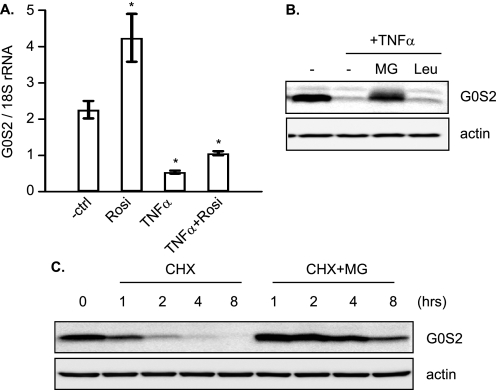
G0S2 is a selective inhibitor of ATGL (27). We have shown previously that TNF-α down-regulates the protein level of G0S2. To study the role of G0S2 in TNF-α-induced lipolysis, we first examined the transcriptional regulation of the G0S2 gene. 3T3-L1 adipocytes were incubated for 4 h in the presence or absence of TNF-α. As a positive control, cells were treated with rosiglitazone, a PPARγ agonist known to promote G0S2 gene expression. The effect on G0S2 mRNA relative to 18 S ribosomal RNA was assessed by real-time PCR. As shown in Fig. 6A, TNF-α treatment resulted in a reduction of G0S2 mRNA to 24% of control, whereas treatment with rosiglitazone increased the level of G0S2 mRNA by 87%. In addition, TNF-α was able to drastically prevent the effect of rosiglitazone when cells were incubated in the presence of both drugs. Next, we determined whether the protein level of G0S2 could be maintained by prevention of protein degradation. As shown in Fig. 6B, addition of a proteasomal inhibitor, MG-132, blocked the degradation of G0S2 in cells treated with TNF-α. By contrast, a general peptidase inhibitor leupeptin was incapable of preventing such turnover. To examine the stability of G0S2 protein, we treated adipocytes with cycloheximide to block new protein synthesis and measured the amount of intact G0S2 over an 8-h period (Fig. 6C). We found that the G0S2 protein is unusually short-lived and has a half-life <1 h. MG-132 was effective in averting the G0S2 degradation in the presence of cycloheximide. Collectively, these results suggest that upon blockade of G0S2 gene transcription in response to TNF-α, the existing G0S2 protein is rapidly destroyed by proteasomal degradation pathway.
FIGURE 6.
Regulation of G0S2 expression in adipocytes. A, 3T3-L1 adipocytes were serum-deprived for 30 min before different combinations of 50 ng/ml TNF-α and 1 μm rosiglitazone (Rosi) were added for 4 h. Total RNA was subjected to quantitative real-time RT-PCR determining G0S2 mRNA levels. The results were normalized to the amount of 18 S rRNA and are the means ± S.E. of at least three independent experiments. *, p < 0.05. B, 3T3-L1 adipocytes were incubated without or with TNF-α in the absence or presence of 10 μm MG-132 (MG) or 10 μg/ml leupeptin (Leu) for 8 h. Afterward, the cells were lysed and analyzed by anti-G0S2 or anti-β-actin immunoblotting. C, 3T3-L1 adipocytes were incubated with 5 μg/ml cycloheximide (CHX) alone or in the presence of 10 μm MG-132 for varying length of time. At each time point, the cells were lysed, and immunoblotting was performed as described in B. ctrl, control.
Adenoviral Expression of G0S2 Protects against TNF-α-induced Lipolysis
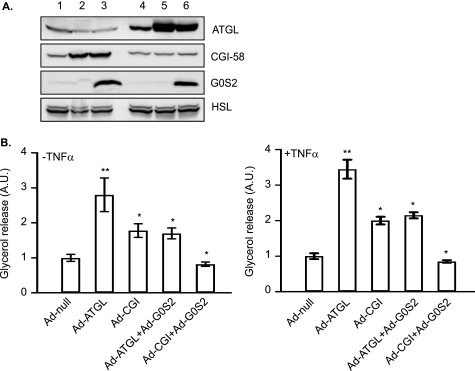
Our previous studies have shown that G0S2 depletion increases lipolysis in adipocytes. To test the hypothesis that the ATGL action in TNF-α-induced lipolysis is promoted by the depletion of G0S2 protein content, we examined the effect of G0S2 coexpression on the lipolytic response of adipocytes overexpression either ATGL or CGI-58. As described previously, we generated a recombinant adenovirus encoding murine G0S2 (Ad-G0S2). Unlike endogenous G0S2, G0S2 expression mediated by Ad-G0S2 is under the control of a CMV promoter and thus is insusceptible to the transcriptional down-modulation by TNF-α. To achieve coexpression, we infected adipocytes with Ad-ATGL with or without Ad-G0S2. As shown in Fig. 7A (lanes 4–6), infection with Ad-ATGL increased ATGL expression to comparable levels in the absence of presence of co-expressed G0S2. Cells infected by Ad-G0S2 contained increased G0S2 content compared with cells infected with the null virus. Overexpression of ATGL resulted in an over 2-fold increase of glycerol release under both basal and TNF-α-induced conditions. However, coexpression of G0S2 significantly diminished the effect of ATGL (Fig. 7B). Similarly, co-expression of G0S2 was also able to abolish the impact of increased levels of CGI-58 on basal and TNF-α-induce lipolysis (Fig. 7, A, lanes 1–3, and B). Therefore, the presence of G0S2 in adipocytes substantially inhibits the lipolytic action mediated by ATGL/CGI-58 in response to TNF-α.
FIGURE 7.
Effects of G0S2 expression on basal and TNF-α-induced lipolysis mediated by ATGL and CGI-58. 3T3-L1 adipocytes were incubated for 72 h following co-infection with adenoviruses in different combinations: lanes 1 and 4, Ad-null alone; lane 2, Ad-CGI-58 and Ad-null; lane 3, Ad-CGI-58+Ad-G0S2; lane 5, Ad-ATGL+Ad-null; and lane 6, Ad-ATGL+Ad-G0S2. A, cells were lysed, and protein expression was analyzed by immunoblotting with antibodies specific for HSL, ATGL, and CGI-58. B, cells were pretreated for 6 h in serum-free DMEM/0.5% BSA without or with 50 ng/ml TNF-α and then were switched to fresh medium without or with TNF-α for another 2-h incubation. The glycerol concentration in the medium was measured and expressed as fold change from Ad-null-infected cells. The data are presented as the means ± S.E. of three independent experiments. *, p < 0.05; **, p < 0.01. A.U., arbitrary units.
DISCUSSION
TNF-α has long been recognized for its major roles in obesity by modulating fat deposition, energy homeostasis, and especially insulin resistance (36, 37, 52). Of interest, TNF-α acts to increase the basal lipolytic rate in adipocytes, which contributes to the elevation of plasma FFA levels associated with lipotoxicity in peripheral tissues. The prevalence of lipotoxicity as a pathogenic mechanism in insulin resistance and type 2 diabetes has sharpened the research focus on lipolysis for the past decade. Regardless of the type of external stimuli and intracellular signaling pathways involved, lipolysis ultimately is dependent on the action of acylglycerol lipases and their regulators. ATGL and HSL are the two major lipases in adipocytes responsible for mediating adrenergically stimulated FFA and glycerol release (5, 6). In this study, we sought to gain new insights into their respective roles in adipocyte lipolysis induced by TNF-α. Although a previous report indicates that TNF-α down-regulates ATGL and HSL mRNA (53), we find that TNF-α does not alter their protein levels in 3T3-L1 adipocytes. Parallel observations were obtained from primary rat adipocytes treated with TNF-α (39, 54). Most importantly, we demonstrate that HSL and ATGL are both required for the lipolytic response to TNF-α. Our data show that the maximum stimulatory effect of TNF-α also depends on the ATGL coactivator CGI-58. Although CGI-58 expression remains mostly unaffected, TNF-α treatment causes a rapid abrogation of ATGL inhibitory protein G0S2. Furthermore, we provide evidence that early reduction in G0S2 content serves as a facilitative mechanism for TNF-α-induced lipolysis.
The elevation of adipocyte lipolysis in response to TNF-α occurs in a progressive manner. Prolonged TNF-α exposure (>24 h) is known to suppress the expression of numerous proteins that prevent basal lipolysis. For example, treatment of 3T3-L1 adipocytes with TNF-α for 24–48 h have been shown previously to down-regulate expression of phosphordiesterase 3B (55) and the inhibitory Gi protein (22). The decrease in phosphordiesterase 3B and Gi expression correlates with activation of PKA and stimulation of lipolysis, presumably reflecting an increase in intracellular cAMP concentration. In addition, it has been proposed that lipolysis mediated by the chronic TNF-α treatment is also dependent on the down-regulation of perilipin (48). Prevention of perilipin depletion via adenovirus-mediated overexpression was shown to be sufficient to protect TNF-α-induced lipolysis (48). However, by comparing the chronology of protein expression with glycerol release, we and others (54) find that during the early stage of treatment, TNF-α is able to induce a significant increase of lipolysis without affecting the protein level of perilipin in adipocytes. We observe that the basal lipolytic rate starts to go up as early as 4–6 h after TNF-α treatment and the increase precedes the decrease in perilipin content. For the purpose of avoiding the confounding effects resulted from decreased expression of perilipin and other proteins, we choose in this study to assess the function of ATGL and HSL during the early stage of TNF-α treatment.
ATGL and HSL are both known to possess the in vitro capacity to cleave the first FA bond of a TAG molecule. Our gene silencing experiments demonstrate that ATGL and HSL together account for the majority of the hydrolytic activity toward TAG in adipocyte extracts when assayed in vitro against a triolein substrate. This is in accordance with a previous measurement of TAG hydrolase activity in white adipose tissue extracts of ATGL-null and HSL-null mice (11). More importantly, we find that both enzymes contribute to the TAG breakdown induced by TNF-α because adipocytes with reduced levels of either ATGL or HSL exhibit decreased lipolytic rates in response to the drug. Cells with silenced expression of ATGL show a more profound reduction in glycerol release than those with silenced expression of HSL, indicating that ATGL plays a more prominent role in initiating TAG hydrolysis. Consistently, overexpression of HSL only partially restores lipolysis in the absence of ATGL, whereas overexpression of AGTL is more than sufficient to compensate for the loss of HSL during basal and TNF-α-induced lipolysis.
Studies on the regulation of adrenergically stimulated lipolysis suggest that ATGL and HSL act sequentially in the first two steps of TAG breakdown (7–12). Specifically, ATGL is believed to be rate-limiting in converting TAG to DAG with the release of one FA during the first step of lipolysis. HSL, on the other hand, functions mainly in the hydrolysis of DAG to MAG and another FA. However, we find that overexpression of ATGL was fully capable of restoring glycerol release from cells with silenced expression of HSL. Considering that the glycerol release is strictly dependent on the completion of the hydrolytic process (i.e. TAG → DAG → MAG → glycerol), our results strongly suggest that ATGL may function in vivo to hydrolyze both TAG and DAG in the absence of HSL. With both enzymes expressed at endogenous levels, however, it remains highly likely that HSL is the major DAG lipase, whereas ATGL favors TAG substrates. Aside from being a TAG hydrolase, ATGL is known to possess activity as an acyl-CoA-independent transacylase, utilizing MAGs or DAGs as acyl donors/acceptors in the production of TAGs (21). Therefore, it is also possible that ATGL at high levels promotes glycerol production as a result of acyl donation from MAGs to DAGs.
The discovery of ATGL is relatively more recent. How the enzyme action of ATGL is regulated was largely unclear until the identification of CGI-58 as a coactivator (28) and G0S2 as an inhibitor (27). Our data show that both CGI-58 and G0S2 are critically involved in TNF-α-induced lipolysis in adipocytes. Silencing of CGI-58 expression profoundly reduced the rates of glycerol release under basal and TNF-α-induced conditions. ATGL overexpression was able to compensate for the loss of CGI-58, which is consistent with the coactivating nature of the role of CGI-58 in ATGL-mediated lipolysis. In contrast to the steady expression of CGI-58, a rapid and drastic depletion of G0S2 occurs in adipocytes treated with TNF-α. Remarkably, restoration of G0S2 protein levels by adenovirus-mediated ectopic expression was sufficient to prevent TNF-α-induced increase of glycerol release. We further demonstrate that G0S2 has the capacity to specifically target ATGL/CGI-58 system because coexpression of G0S2 was able to inhibit lipolytic enhancement resulted from overexpression of either ATGL or CGI-58. Because there is a significant temporal disconnect between the reduction of G0S2 levels and subsequent activation of lipolysis by TNF-α, we conclude that down-regulation of G0S2 is a permissive but not by itself a sufficient step in the sequence of TNF-α-triggered early events that lead to increased TAG turnover in adipocytes.
Data presented here show a pronounced decrease in G0S2 mRNA along with a nearly complete disappearance of G0S2 protein upon short TNF-α treatment. The mechanisms by which TNF-α down-regulates G0S2 transcription are currently unknown. It has been shown that transcription of G0S2 is PPARγ-dependent in adipocytes (33). Thus, we speculate that the effect of TNF-α on G0S2 may be due to the previously observed decrease in PPAR protein levels in TNF-α-treated adipocytes. The evidence that TNF-α is able to suppress the activating effect of the PPARγ agonist rosiglitazone on G0S2 expression supports such possibility. Moreover, previous experiments using selective kinase inhibitors demonstrate that TNF-α regulates lipolysis in adipocytes through rapid and transient activation of downstream kinases such as ERK1/2, JNK, and IκB kinase (41, 42, 45–47). Whether signaling pathways dependent on these kinases are involved in the switch-off of G0S2 transcription is an important topic for future investigation. Furthermore, the direct measurement upon cycloheximide-induced translational inhibition has revealed that G0S2 is a highly unstable protein with a protein half-life (t½) shorter than 1 h. Under both TNF-α and cycloheximide-treated conditions, G0S2 protein content in adipocytes can be restored by the treatment with the proteasome inhibitor MG-132. Therefore, we conclude that the combination of decreased transcription of G0S2 gene and fast turnover of the existing protein accounts for the reduced level of G0S2 in response to TNF-α treatment. It remains to be determined whether gene transcription and proteasomal degradation of G0S2 protein may be altered by other hormonal or nutritional cues as ways to regulate the basal rate of adipocyte lipolysis.
Based on our and others' findings, we propose that TNF-α-induced lipolysis in adipocytes is a two-stage process controlled by multiple factors. During the short term treatment, our data implicate a mechanism that involves the down-regulation of G0S2, which could act in concert with additional mechanisms. For example, TNF-α was recently shown to stimulate rapid turnover of FSP27 (49), a lipid droplet protein whose expression is required for the maintenance of unilocular LDs and low lipolytic rates. We speculate that although loss of G0S2 could liberate the activity of ATGL, decreased expression of FSP27 may result in multilocularization of LDs, which, in turn, enhances the action of ATGL and HSL due to expansion of the LD surface area. During prolonged exposure to TNF-α, decreased expression of proteins such as phosphordiesterase 3B, Gi, and perilipin allows further and sustained activation of lipolysis. Although decreased expression of phosphordiesterase 3B and Gαi elevates intracellular cAMP levels followed by activation of PKA and HSL, loss of the lipolytic barrier protein perilipin enhances the substrate access of the lipases as well as the enzyme action of ATGL via releasing its coactivator CGI-58.
This work was supported by National Institutes of Health Grant DK089178. This work was also supported by a Junior Faculty Award from the American Diabetes Association (to J. L.).
- TAG
- triacylglycerol
- MAG
- monoacylglycerol
- ATGL
- adipose triglyceride lipase
- HSL
- hormone-sensitive lipase
- LD
- lipid droplet
- FFA
- free fatty acid
- DAG
- diacylglycerol
- PPAR
- peroxisome proliferator-activated receptor
- Ad
- adenovirus.
REFERENCES
- 1. Unger R. H., Clark G. O., Scherer P. E., Orci L. (2010) Biochim. Biophys. Acta 1801, 209–214 [DOI] [PubMed] [Google Scholar]
- 2. Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. (2007) Annu. Rev. Nutr. 27, 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeFronzo R. A. (2004) Int. J. Clin. Pract. Suppl. 143, 9–21 [DOI] [PubMed] [Google Scholar]
- 4. Lafontan M., Langin D. (2009) Prog. Lipid Res. 48, 275–297 [DOI] [PubMed] [Google Scholar]
- 5. Lass A., Zimmermann R., Oberer M., Zechner R. (2011) Prog. Lipid Res. 50, 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kolditz C. I., Langin D. (2010) Curr. Opin. Clin. Nutr. Metab. Care 13, 377–381 [DOI] [PubMed] [Google Scholar]
- 7. Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. (2004) Science 306, 1383–1386 [DOI] [PubMed] [Google Scholar]
- 8. Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T. M., Wagner E. F., Zechner R. (2002) J. Biol. Chem. 277, 4806–4815 [DOI] [PubMed] [Google Scholar]
- 9. Osuga J., Ishibashi S., Oka T., Yagyu H., Tozawa R., Fujimoto A., Shionoiri F., Yahagi N., Kraemer F. B., Tsutsumi O., Yamada N. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 787–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karlsson M., Contreras J. A., Hellman U., Tornqvist H., Holm C. (1997) J. Biol. Chem. 272, 27218–27223 [DOI] [PubMed] [Google Scholar]
- 11. Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. (2006) J. Biol. Chem. 281, 40236–40241 [DOI] [PubMed] [Google Scholar]
- 12. Bezaire V., Mairal A., Ribet C., Lefort C., Girousse A., Jocken J., Laurencikiene J., Anesia R., Rodriguez A. M., Ryden M., Stenson B. M., Dani C., Ailhaud G., Arner P., Langin D. (2009) J. Biol. Chem. 284, 18282–18291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaughan M., Berger J. E., Steinberg D. (1964) J. Biol. Chem. 239, 401–409 [PubMed] [Google Scholar]
- 14. Rizack M. A. (1964) J. Biol. Chem. 239, 392–395 [PubMed] [Google Scholar]
- 15. Yeaman S. J., Smith G. M., Jepson C. A., Wood S. L., Emmison N. (1994) Adv. Enzyme Regul. 34, 355–370 [DOI] [PubMed] [Google Scholar]
- 16. Kraemer F. B., Shen W. J. (2002) J. Lipid Res. 43, 1585–1594 [DOI] [PubMed] [Google Scholar]
- 17. Okazaki H., Osuga J., Tamura Y., Yahagi N., Tomita S., Shionoiri F., Iizuka Y., Ohashi K., Harada K., Kimura S., Gotoda T., Shimano H., Yamada N., Ishibashi S. (2002) Diabetes 51, 3368–3375 [DOI] [PubMed] [Google Scholar]
- 18. Fortier M., Wang S. P., Mauriège P., Semache M., Mfuma L., Li H., Levy E., Richard D., Mitchell G. A. (2004) Am. J. Physiol. Endocrinol. Metab. 287, E282–288 [DOI] [PubMed] [Google Scholar]
- 19. Wang S. P., Laurin N., Himms-Hagen J., Rudnicki M. A., Levy E., Robert M. F., Pan L., Oligny L., Mitchell G. A. (2001) Obes. Res. 9, 119–128 [DOI] [PubMed] [Google Scholar]
- 20. Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. (2004) J. Biol. Chem. 279, 47066–47075 [DOI] [PubMed] [Google Scholar]
- 21. Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. (2004) J. Biol. Chem. 279, 48968–48975 [DOI] [PubMed] [Google Scholar]
- 22. Gasic S., Tian B., Green A. (1999) J. Biol. Chem. 274, 6770–6775 [DOI] [PubMed] [Google Scholar]
- 23. Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E. F., Klingenspor M., Hoefler G., Zechner R. (2006) Science 312, 734–737 [DOI] [PubMed] [Google Scholar]
- 24. Ahmadian M., Duncan R. E., Varady K. A., Frasson D., Hellerstein M. K., Birkenfeld A. L., Samuel V. T., Shulman G. I., Wang Y., Kang C., Sul H. S. (2009) Diabetes 58, 855–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egan J. J., Greenberg A. S., Chang M. K., Wek S. A., Moos M. C., Jr., Londos C. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 8537–8541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sztalryd C., Xu G., Dorward H., Tansey J. T., Contreras J. A., Kimmel A. R., Londos C. (2003) J. Cell Biol. 161, 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang X., Lu X., Lombès M., Rha G. B., Chi Y. I., Guerin T. M., Smart E. J., Liu J. (2010) Cell Metab. 11, 194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., Zechner R. (2006) Cell Metab. 3, 309–319 [DOI] [PubMed] [Google Scholar]
- 29. Subramanian V., Rothenberg A., Gomez C., Cohen A. W., Garcia A., Bhattacharyya S., Shapiro L., Dolios G., Wang R., Lisanti M. P., Brasaemle D. L. (2004) J. Biol. Chem. 279, 42062–42071 [DOI] [PubMed] [Google Scholar]
- 30. Granneman J. G., Moore H. P., Krishnamoorthy R., Rathod M. (2009) J. Biol. Chem. 284, 34538–34544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Granneman J. G., Moore H. P., Granneman R. L., Greenberg A. S., Obin M. S., Zhu Z. (2007) J. Biol. Chem. 282, 5726–5735 [DOI] [PubMed] [Google Scholar]
- 32. Miyoshi H., Perfield J. W., 2nd, Souza S. C., Shen W. J., Zhang H. H., Stancheva Z. S., Kraemer F. B., Obin M. S., Greenberg A. S. (2007) J. Biol. Chem. 282, 996–1002 [DOI] [PubMed] [Google Scholar]
- 33. Zandbergen F., Mandard S., Escher P., Tan N. S., Patsouris D., Jatkoe T., Rojas-Caro S., Madore S., Wahli W., Tafuri S., Müller M., Kersten S. (2005) Biochem. J. 392, 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parikh H., Carlsson E., Chutkow W. A., Johansson L. E., Storgaard H., Poulsen P., Saxena R., Ladd C., Schulze P. C., Mazzini M. J., Jensen C. B., Krook A., Björnholm M., Tornqvist H., Zierath J. R., Ridderstråle M., Altshuler D., Lee R. T., Vaag A., Groop L. C., Mootha V. K. (2007) PLoS Med. 4, e158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ma L., Robinson L. N., Towle H. C. (2006) J. Biol. Chem. 281, 28721–28730 [DOI] [PubMed] [Google Scholar]
- 36. Cawthorn W. P., Sethi J. K. (2008) FEBS Lett. 582, 117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen X., Xun K., Chen L., Wang Y. (2009) Cell Biochem. Funct. 27, 407–416 [DOI] [PubMed] [Google Scholar]
- 38. Plomgaard P., Fischer C. P., Ibfelt T., Pedersen B. K., van Hall G. (2008) J. Clin. Endocrinol. Metab. 93, 543–549 [DOI] [PubMed] [Google Scholar]
- 39. Green A., Dobias S. B., Walters D. J., Brasier A. R. (1994) Endocrinology 134, 2581–2588 [DOI] [PubMed] [Google Scholar]
- 40. Ren T., He J., Jiang H., Zu L., Pu S., Guo X., Xu G. (2006) J. Mol. Endocrinol. 37, 175–183 [DOI] [PubMed] [Google Scholar]
- 41. Souza S. C., Palmer H. J., Kang Y. H., Yamamoto M. T., Muliro K. V., Paulson K. E., Greenberg A. S. (2003) J. Cell. Biochem. 89, 1077–1086 [DOI] [PubMed] [Google Scholar]
- 42. Ryden M., Dicker A., van Harmelen V., Hauner H., Brunnberg M., Perbeck L., Lonnqvist F., Arner P. (2002) J. Biol. Chem. 277, 1085–1091 [DOI] [PubMed] [Google Scholar]
- 43. Green A., Rumberger J. M., Stuart C. A., Ruhoff M. S. (2004) Diabetes 53, 74–81 [DOI] [PubMed] [Google Scholar]
- 44. Sethi J. K., Xu H., Uysal K. T., Wiesbrock S. M., Scheja L., Hotamisligil G. S. (2000) FEBS Lett. 469, 77–82 [DOI] [PubMed] [Google Scholar]
- 45. Zhang H. H., Halbleib M., Ahmad F., Manganiello V. C., Greenberg A. S. (2002) Diabetes 51, 2929–2935 [DOI] [PubMed] [Google Scholar]
- 46. Rydén M., Arvidsson E., Blomqvist L., Perbeck L., Dicker A., Arner P. (2004) Biochem. Biophys. Res. Commun. 318, 168–175 [DOI] [PubMed] [Google Scholar]
- 47. Lien C. C., Au L. C., Tsai Y. L., Ho L. T., Juan C. C. (2009) Endocrinology 150, 4892–4900 [DOI] [PubMed] [Google Scholar]
- 48. Souza S. C., de Vargas L. M., Yamamoto M. T., Lien P., Franciosa M. D., Moss L. G., Greenberg A. S. (1998) J. Biol. Chem. 273, 24665–24669 [DOI] [PubMed] [Google Scholar]
- 49. Ranjit S., Boutet E., Gandhi P., Prot M., Tamori Y., Chawla A., Greenberg A. S., Puri V., Czech M. P. (2011) J. Lipid Res. 52, 221–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen X. W., Leto D., Chiang S. H., Wang Q., Saltiel A. R. (2007) Dev. Cell 13, 391–404 [DOI] [PubMed] [Google Scholar]
- 51. Greenberg C. C., Meredith K. N., Yan L., Brady M. J. (2003) J. Biol. Chem. 278, 30835–30842 [DOI] [PubMed] [Google Scholar]
- 52. Uysal K. T., Wiesbrock S. M., Marino M. W., Hotamisligil G. S. (1997) Nature 389, 610–614 [DOI] [PubMed] [Google Scholar]
- 53. Kralisch S., Klein J., Lossner U., Bluher M., Paschke R., Stumvoll M., Fasshauer M. (2005) Mol. Cell. Endocrinol. 240, 43–49 [DOI] [PubMed] [Google Scholar]
- 54. Zu L., Jiang H., He J., Xu C., Pu S., Liu M., Xu G. (2008) Mol. Pharmacol. 73, 215–223 [DOI] [PubMed] [Google Scholar]
- 55. Rahn Landström T., Mei J., Karlsson M., Manganiello V., Degerman E. (2000) Biochem. J. 346, 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]