Abstract
The acute-phase response is an inflammatory process triggered mainly by the cytokine IL-6. Signaling of IL-6 is transduced by activation of STAT3 (signal transducer and activator of transcription 3), which rapidly induces the production of acute-phase proteins such as haptoglobin and fibrinogen. Another target of the IL-6/STAT3 signal transduction pathway is the microRNA cluster miR-17/92. Here, we investigated the interplay of miR-17/92 and STAT3 signaling and its impact on the acute-phase response in primary human hepatocytes and hepatoma (HepG2) cells. Employing a reporter gene system consisting of STAT3-sensitive promoter sequences, we show that the miR-17/92 cluster member miR-18a enhanced the transcriptional activity of STAT3. IL-6 stimulation experiments in miR-18a-overexpressing hepatocytes and HepG2 cells revealed an augmented acute-phase response indicated by increased expression and secretion of haptoglobin and fibrinogen. This effect was due, at least in part, to repression of PIAS3 (protein inhibitor of activated STAT, 3), a repressor of STAT3 activity, which we identified as a novel direct target of miR-18a. Finally, we demonstrate that the expression of miR-17/92 in primary hepatocytes and HepG2 cells is modulated by IL-6. Our data reveal, for the first time, a microRNA-mediated positive feedback loop of IL-6 signal transduction leading to an enhanced acute-phase response in human hepatocytes.
Keywords: Fibrinogen, Interleukin, MicroRNA, Signal Transduction, STAT Transcription Factor, Transcription Target Genes, Acute-phase Response
Introduction
Severe inflammation is observed in several conditions, including overwhelming infection, systemic inflammatory response syndrome, sepsis, and septic shock, and results in the activation of a signaling cascade that is commonly referred to as the acute-phase response. Acute-phase proteins are involved in a great variety of physiological and biochemical processes, including growth inhibition of microbes (e.g. complement factors), blood coagulation (e.g. fibrinogen), and binding of proteins (e.g. haptoglobin-mediated binding of hemoglobin) (1). During inflammation, the production of plasma proteins by hepatocytes is altered either by increasing the levels of plasma proteins (positive acute-phase reaction) or by decreasing their levels (negative acute-phase reaction).
IL-6 is one of the most important cytokines that orchestrates the hepatic production of acute-phase proteins (2). For signal transduction, IL-6 binds to a membrane-associated receptor complex, which subsequently leads to the phosphorylation of Tyr-705 of STAT3 (signal transducer and activator of transcription 3) (3). Phosphorylated STAT3 is actively transported to the nucleus, where it activates the transcription of IL-6 target genes such as the acute-phase genes haptoglobin (Hp)2 (4) and fibrinogen γ-chain (FGG) (5). Several negative feedback loops antagonize the activation of STAT3 to avoid persistent downstream signaling and subsequent dysregulation in the production of acute-phase proteins. A well characterized feedback pathway includes the blocking of the phosphorylation of STAT proteins by the action of the cytokine-inducible family of SOCS (suppressors of cytokine signaling) (6).
MicroRNAs (miRNAs) comprise a novel class of short non-protein-coding RNAs that regulate the expression of their target genes in a post-transcriptional manner either by mRNA degradation or by translational repression (7). The importance of miRNAs in the signal transduction of IL-6 has been highlighted by a recent study showing that miRNAs derived from the miRNA cluster miR-17/92 modulate STAT3 phosphorylation in multiple myeloma cells (8); moreover, Fort et al. (9) identified several miRNAs that are directly involved in the production of the acute-phase protein fibrinogen in human hepatoma (HuH-7) cells. The polycistronic miRNA cluster miR-17/92 codes for six mature miRNAs (miR-17-5p, miR-18a, miR-19a, miR-19b, miR-20a, and miR-92) and has primarily attracted attention for being frequently linked to cancer by exhibiting oncogenic activities mainly due to targeting tumor suppressor genes (10). The expression of miR-17/92 is regulated by a number of known transcription factors, including the oncogenic transcription factor c-Myc (11). In a previous work, we provided evidence that, upon stimulation with IL-6, the expression of miR-17/92 is directly regulated by STAT3, thus emphasizing a potential link between miR-17/92 and inflammatory processes (12). On the basis of these findings, we hypothesized that miR-17/92 might represent a key component in IL-6 signal transduction contributing to the regulation of the acute-phase response in hepatocytes.
Because hepatocellular carcinoma (HepG2) cells have been extensively used to investigate the IL-6-induced expression of acute-phase proteins and thus provide an established model to study the acute-phase response (13), we used these cells in this study to address the role of miR-17/92 in the expression of acute-phase genes and to identify novel miR-17/92 targets in the IL-6 signaling cascade. In addition, the interaction between miR-17/92 and the acute-phase response was investigated in primary human hepatocytes to underscore the role of these miRNAs in a physiological setting. Our data reveal, for the first time, a miRNA-mediated positive feedback loop of IL-6 signal transduction leading to an enhanced acute-phase response in human hepatocytes.
EXPERIMENTAL PROCEDURES
Methods
These are described in detail under supplemental “Materials and Methods.”
Cell Culture
HepG2 human hepatocellular carcinoma cells and HEK293 human embryonic kidney cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Primary human hepatocytes were purchased from Lonza (Verviers, Belgium) and cultured according to the manufacturer's instructions. For stimulation experiments, HepG2 cells and primary hepatocytes were serum-starved for 24 h and treated with IL-6 as indicated.
Quantitative Real-time PCR Analysis
Quantification of specific mRNA transcripts was performed by SYBR Green quantitative real-time PCR using the ABI Prism 7700 sequence detection system (Applied Biosystems, Rotkreuz, Switzerland) with normalization to the expression of GAPDH. Mature miRNAs were detected by specific stem-loop primers according to Chen et al. (14). Primer sequences are shown in supplemental Table S1.
Western Blotting
The following primary antibodies were used: anti-PIAS3 (protein inhibitor of activated STAT, 3) and anti-Hp (both from Abcam, Cambridge, United Kingdom), anti-STAT3 (R&D Systems), anti-phospho-STAT3 (Tyr-705; Cell Signaling Technology, Danvers, MA), and anti-α-tubulin (Sigma). Bands were detected with species-specific secondary antibodies coupled to horseradish peroxidase and quantified with AlphaImager software (Alpha Innotech, San Leandro, CA).
Plasmid Construction
An 878-bp fragment of the Hp promoter containing two STAT3-binding sites and a 578-bp fragment of the FGG promoter containing one STAT3-binding site were each cloned into the firefly luciferase-based pGL3-Basic vector (Promega, Dübendorf, Switzerland). The SV40 promoter of pRL-SV40 (Promega) was replaced with the promoter of GAPDH (1063 bp). The 3′-UTR of PIAS3 (924 bp) was cloned into the pGL3-Control vector (Promega). As a negative control, an antisense construct was used (15), as well as a construct with the specifically mutated seed match for miR-18a.
Reporter Gene Assay
For promoter activity studies, HepG2 cells were transfected with pGL3-Basic-Hp or pGL3-Basic-FGG and pRL-GAPDH for normalization. After 24 h, the cells were stimulated with IL-6 for 4 h. Luciferase activity was measured using the Dual-Luciferase reporter assay system (Promega) and normalized to the activity of Renilla luciferase.
For miRNA target validation, HEK293 cells were transfected with pGL3-Control-PIAS3–3′-UTR, pRL-SV40 for normalization, and precursor molecules (pre-miRNAs). After 24 h, the cells were lysed, and luciferase activity was measured using the Dual-Luciferase reporter assay system.
Transfection of siRNAs and miRNAs
HepG2 cells were transfected with 200 nm siRNAs (Qiagen, Hombrechtikon, Switzerland) or 100 nm pre-miRNAs or anti-miRNA (Ambion/Applied Biosystems; pre-miR-18a, pre-miR-19a, pre-miR-20a, pre-miR-92a, or anti-miR-18a) using the cell line Nucleofector kit V (Amaxa, Cologne, Germany) or Lipofectamine 2000. Following an incubation period of 48 or 72 h, cells were stimulated with IL-6 (20 ng/ml).
ELISA
To determine levels of Hp and fibrinogen in cell culture supernatants, we used commercially available ELISA kits (GenWay Biotech Inc., San Diego, CA) according to the manufacturer's instructions.
Statistics
For statistical analysis, GraphPad Prism software was used. To compare samples, Student's paired or unpaired t test was applied, and a p value <0.05 was considered to be statistically significant. All data are shown as means ± S.D. or as single raw data (primary hepatocytes).
RESULTS
miR-17/92 Enhances the Acute-phase Response in HepG2 Cells
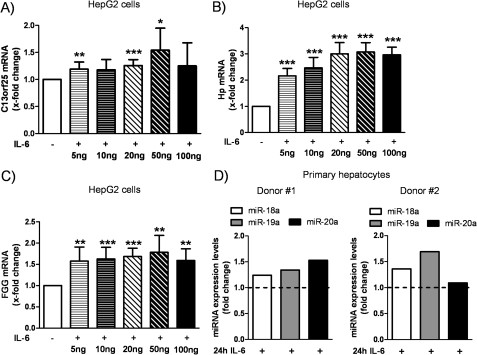
This study was dedicated to investigating the role of miR-17/92 in IL-6 signaling and acute-phase response. Recently, we demonstrated that STAT3 directly activates the transcription of miR-17/92 upon IL-6 stimulation in human pulmonary arterial endothelial cells (12). To address the question of whether IL-6 enhances the expression of miR-17/92 in HepG2 cells and primary hepatocytes similarly, IL-6 stimulation experiments were performed. As shown in Fig. 1A, HepG2 cells were stimulated for 1 h with different amounts of IL-6 ranging from 5 to 100 ng/ml, and expression of the primary transcript of miR-17/92 (C13orf25) was assessed as described by O'Donnell et al. (11). Treatment with the lowest dose of IL-6 led to a small but significant up-regulation of C13orf25 (1.19 ± 0.13-fold, p = 0.008) (Fig. 1A); the strongest induction of C13orf25 mRNA levels was found when stimulated with 50 ng/ml IL-6 (1.54 ± 0.41-fold, p = 0.012).
FIGURE 1.
Dose-dependent induction of C13orf25 and acute-phase genes by IL-6. HepG2 cells and primary hepatocytes were stimulated with IL-6 for 1 or 24 h, respectively. A, the mRNA levels of C13orf25 were dose-dependently induced by IL-6, with 50 ng/ml IL-6 yielding the strongest increase in C13orf25 (n = 7). B and C, IL-6 induced the mRNA expression of the acute-phase genes Hp and FGG (n = 7), respectively. D, IL-6 stimulation (20 ng/ml) for 24 h led to increased expression levels of miR-18a, miR-19a, and miR-20a in primary hepatocytes (n = 2). The dashed lines indicate unstimulated control cells. The respective unstimulated samples were set to 1. Statistical analysis was by Student's paired t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Dose-dependent induction of two acute-phase genes (i.e. Hp and FGG) used as positive controls is shown in Fig. 1 (B and C, respectively). These data reflect the potential of IL-6 to efficiently stimulate the production of acute-phase genes in HepG2 cells.
Next, we used primary hepatocytes to address the role of IL-6 and miR-17/92 in another experimental model of the acute-phase response. We thus measured the expression levels of three miRNAs derived from miR-17/92 (miR-18a, miR-19a, and miR-20a) 24 h after IL-6 treatment and found that the expression levels of these miRNAs were increased in both donors used in this study (Fig. 1D). Together with our previous findings (12), these data support a cell type-independent mechanism for the induction of miR-17/92 by IL-6.
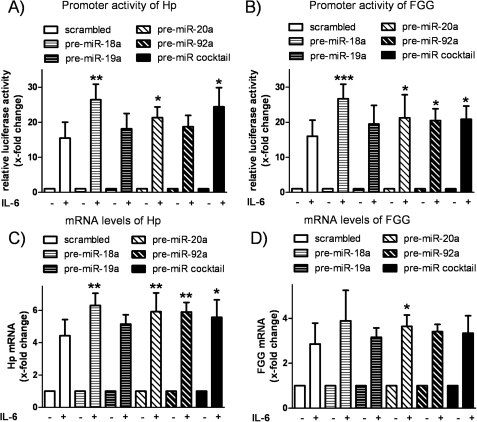
In further study, we investigated the functional consequence of enhanced expression of miR-17/92 in HepG2 cells, in particular the significance for IL-6 signaling and acute-phase response. We performed gain-of-function experiments by transfecting HepG2 cells with precursor molecules of miR-18a, miR-19a, miR-20a, and miR-92a (representing the four functional miRNA families encoded by miR-17/92) and a mixture of all four. Successful overexpression of miRNAs was confirmed by quantitative real-time PCR (supplemental Fig. S1). The impact of each miRNA on the activity of STAT3 was measured employing a luciferase-based reporter gene system consisting of STAT3-sensitive promoter sequences of two selected acute-phase genes, i.e. Hp and FGG. In scrambled transfected cells, IL-6 increased the relative Hp promoter activity by 15.48 ± 4.51-fold compared with unstimulated scrambled transfected cells (Fig. 2A). Interestingly, the increase in promoter activity upon stimulation with IL-6 was significantly more enhanced when HepG2 cells were transfected with miR-18a (26.40 ± 4.47-fold, p = 0.002), miR-20a (21.32 ± 3.04-fold, p = 0.032), or a mixture of all four miRNAs (24.4 ± 5.46-fold, p = 0.011). Similar results were obtained when the promoter activity of FGG in HepG2 cells was analyzed, showing a significantly enhanced IL-6 response after transfection of miR-18a, miR-20a, miR-92a, and the miR-18a/miR-19a/miR-20a/miR-92a mixture compared with scrambled transfection (Fig. 2B).
FIGURE 2.
Effect of miR-17/92 on acute-phase response in HepG2 cells. HepG2 cells were transfected with pre-miRNA (pre-miR-18a, pre-miR-19a, pre-miR-20a, pre-miR-92a, and a mixture of all four) or a scrambled negative control. A, the promoter activity of Hp was assessed by cotransfection of a luciferase-based vector system comprising 878 bp of the Hp promoter sequence. 4 h of IL-6 stimulation (20 ng/ml) induced relative luciferase activity in all samples, whereas the IL-6 response was more pronounced in miR-18a-, miR-20a-, and miR-18a/miR-19a/miR-20a/miR-92a-transfected cells (n = 5). B, a luciferase-based reporter gene system consisting of the promoter sequence of FGG (578 bp) was cotransfected in HepG2 cells. The response to 4 h of IL-6 stimulation was enhanced by cotransfection of miR-18a, miR-20a, miR-92a, and the mixture of all four miRNAs compared with the scrambled control (n = 7). C, quantification of the mRNA levels of Hp after 4 h of IL-6 stimulation (20 ng/ml) showed a similar result as the respective reporter gene studies. The IL-6-inducible effect on Hp transcription was significantly enhanced by transfection of miR-18a, miR-20a, miR-92a, and the mixture of all four miRNAs (n = 6). D, identical samples show significant alteration of FGG mRNA expression by IL-6 in miR-20a-transfected cells (n = 6). The respective unstimulated samples were set to 1. Statistical analysis was by Student's unpaired t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To confirm the results of the reporter gene assay, the mRNA levels of both acute-phase genes in HepG2 cells were quantified. IL-6 stimulation for 4 h thus induced the expression of Hp by 4.43 ± 1-fold in scrambled transfected cells. Overexpression of miR-18a (6.31 ± 0.75-fold, p = 0.001), miR-20a (5.92 ± 1.17-fold, p = 0.005), miR-92a (5.9 ± 0.6-fold, p = 0.005), or the miRNA mixture (5.57 ± 1.09-fold, p = 0.046) significantly enhanced the induction of Hp transcripts (Fig. 2C). Quantification of FGG mRNA levels yielded a similar expression pattern (Fig. 2D). After 4 h of stimulation with IL-6, the expression of FGG was induced in miR-18a-transfected cells (3.89 ± 1.35-fold, p = 0.082) and miR-20a-transfected cells (3.64 ± 0.51-fold, p = 0.03) compared with scrambled transfection (2.86 ± 0.93-fold).
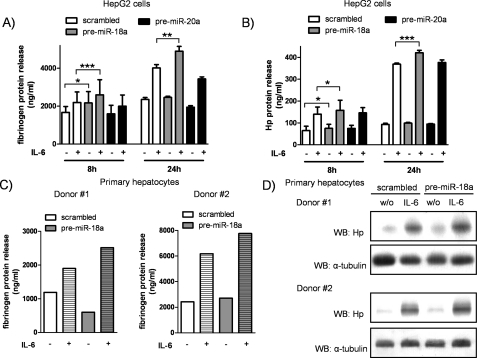
Overexpression of miR-18a Promotes the Expression of Acute-phase Proteins
Because miR-18a and miR-20a showed the most consistent and prominent effects in modulating the acute-phase response at the mRNA level, we assessed the secretion of fibrinogen and Hp into the supernatants of HepG2 cells stimulated with IL-6 for 8 or 24 h. Fibrinogen release from miR-18a-transfected cells collected 8 h after stimulation was significantly increased both in unstimulated cells (2170 ± 602 ng/ml, p = 0.018) and in IL-6-stimulated cells (2760 ± 702.6 ng/ml, p < 0.001) compared with scrambled transfection (unstimulated, 1669 ± 312.1 ng/ml; IL-6-stimulated, 2194 ± 556.3 ng/ml) (Fig. 3A). Enforced expression of miR-20a did not influence the release of fibrinogen either in IL-6 naive cells or under stimulated conditions. Similarly, after 24 h of IL-6 stimulation, miR-18a-overexpressing cells produced significantly more fibrinogen (4899 ± 253.2 versus 4017 ± 173.5 ng/ml in the scrambled control, p = 0.002) (Fig. 3A), whereas transfection of miR-20a failed to enhance the release of fibrinogen.
FIGURE 3.
Overexpression of miR-18a promotes expression and release of acute-phase proteins. The protein levels of fibrinogen and Hp in pre-miRNA-transfected HepG2 cells and hepatocytes were quantified by ELISA or Western blotting. A, release of fibrinogen into the supernatants after 8 and 24 h of IL-6 stimulation (20 ng/ml) was significantly increased in cells overexpressing miR-18a as shown by ELISA. miR-20a transfection failed to increase the release of fibrinogen (n = 5). B, the same supernatants were assessed for quantification of Hp protein levels, showing enhanced secretion of Hp after 8 and 24 h of IL-6 stimulation when miR-18a was overexpressed. Transfection of miR-20a did not change the release of Hp (n = 5). C, primary hepatocytes were transfected with pre-miR-18a and stimulated with IL-6 (20 ng/ml) for 24 h. The total amount of fibrinogen in supernatants was quantified by ELISA, showing that the IL-6-induced release of fibrinogen was enhanced in hepatocytes transfected with pre-miR-18a (n = 2). D, the same cells were analyzed for protein levels of Hp by Western blotting (WB). The expression of Hp was increased by miR-18a transfection compared with scrambled transfection (n = 2). Statistical analysis was by Student's paired t test. w/o, without. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The same supernatants were assessed for Hp protein release, and, again, we found that miR-18a overexpression increased the secretion of the acute-phase protein (Fig. 3B). In detail, miR-18a significantly enhanced Hp release after both 8 h (166.6 ± 41.46 ng/ml, p = 0.02) and 24 h (420.8 ± 11.43 ng/ml, p < 0.001) of IL-6 stimulation compared with scrambled transfected and IL-6-stimulated cells (139.9 ± 32.6 and 368.7 ± 5.25 ng/ml, respectively). Hp release in miR-20a-overexpressing cells remained unaffected. To confirm these findings, identical experiments were performed in primary hepatocytes. As shown in Fig. 3C, the IL-6-induced release of fibrinogen after 24 h was more pronounced in hepatocytes transfected with precursor molecules of miR-18a compared with the scrambled control. Similarly, we found increased expression of Hp in miR-18a-overexpressing hepatocytes as indicated by Western blotting (Fig. 3D).
The ELISA and Western blot experiments thus confirmed the potential of miR-18a (but not of miR-20a) to promote the expression and release of acute-phase proteins. Conversely, the loss-of-function experiments by antagonizing the expression of miR-18a led to a decreased acute-phase response in HepG2 cells (supplemental Fig. S2).
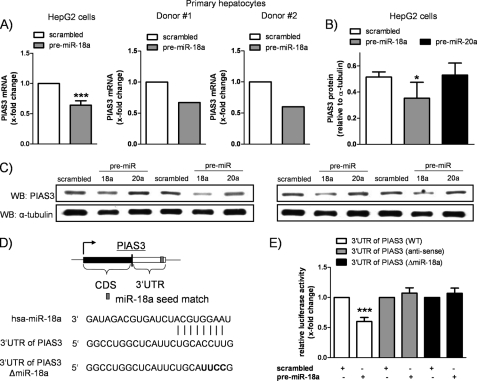
miR-18a Directly Targets PIAS3
The previous experiments revealed an enhanced hepatic acute-phase response upon overexpression of miR-18a. Because miRNAs have been implicated in the repression of gene expression, we speculated that miR-18a might target a negative regulator of IL-6 signaling. Utilizing a computational screening (TargetScan, Whitehead Institute for Biomedical Research) (16), the 3′-UTR of PIAS3 was identified to contain a potential binding site for miR-18a. PIAS3 is endogenously expressed in HepG2 cells and specifically inhibits the DNA-binding activity of STAT3 (17). To investigate whether miR-18a influences PIAS3 expression, we measured the mRNA levels of PIAS3 in miR-18a-transfected HepG2 cells and primary hepatocytes. As shown in Fig. 4A, miR-18a transfection significantly reduced the expression of PIAS3 mRNA in HepG2 cells (by 36 ± 7%, p < 0.001) and in hepatocytes from both donors. Consistent with these data, Western blot experiments showed reduced protein levels of PIAS3 in HepG2 cells transfected with pre-miR-18a (PIAS3/α-tubulin ratio, 0.52 ± 0.04 (scrambled transfection) and 0.35 ± 0.12 (pre-miR-18a transfection), p = 0.044) (Fig. 4B). To prove the specificity of the observed reduction of PIAS3, we additionally quantified PIAS3 protein expression in miR-20a-transfected cells. miR-20a, which was not predicted to target PIAS3, did not change the protein expression of PIAS3 (PIAS3/α-tubulin ratio, 0.53 ± 0.09, p = 0.773) (Fig. 4B), implying an efficient and specific down-regulation of PIAS3 by miR-18a.
FIGURE 4.
PIAS3 is a direct target of miR-18a. A, miR-18a-transfected HepG2 cells revealed a significant down-regulation of PIAS3 mRNA levels compared with the scrambled control (n = 7). The mRNA levels of PIAS3 were also found to be decreased by miR-18a transfection in primary hepatocytes (n = 2). B, Western blotting confirmed the reduced expression of PIAS3 at the protein level in HepG2 cells as assessed by densitometric analysis (n = 4). Specific miR-18a-mediated down-regulation of PIAS3 was proven by transfection of a non-predicted miRNA, miR-20a, showing no alterations in the protein expression of PIAS3. C, Western blots (WB) used for densitometric analysis are shown (n = 4). D, TargetScan prediction software identified one seed match of miR-18a in the 3′-UTR of PIAS3. The predicted base pairing of PIAS3 mRNA and miR-18a is shown. The entire 3′-UTR of PIAS3 was used for reporter gene studies, and the predicted miR-18a seed sequence was destroyed by introducing four point mutations. CDS, coding sequence; hsa, Homo sapiens. E, reporter gene studies in HEK293 cells showed that cotransfection of miR-18a significantly decreased the relative luciferase activity compared with scrambled transfection. The antisense construct and the mutant construct (ΔmiR-18a) were not affected by overexpression of miR-18a (n = 7). Statistical analysis was by Student's paired t test (A and E; scrambled transfection was set to 1) or by Student's unpaired t test (B). *, p < 0.05; ***, p < 0.001.
To address the question of whether the observed reduction in PIAS3 expression is directly miR-18a-driven, we performed reporter gene studies in HEK293 cells. The entire 3′-UTR of PIAS3 was cloned into the pGL3-Control vector, creating a luciferase reporter system with the respective seed sequence for miR-18a (Fig. 4D). As a negative control, the “antisense” construct was generated (15). In addition, we mutated the miR-18a seed match sequence in the “sense” construct by introducing four point mutations (3′-UTR of PIAS3ΔmiR-18a) (Fig. 4D). Cotransfection of the PIAS3 3′-UTR WT construct and pre-miR-18a yielded a significantly reduced relative luciferase activity (0.6 ± 0.07-fold, p < 0.001) (Fig. 4E). The antisense construct, as well as the mutant construct, was not affected by overexpression of miR-18a. These findings imply a direct interaction between the 3′-UTR of PIAS3 and miR-18a.
PIAS3 Is an Important Modulator of the Acute-phase Response
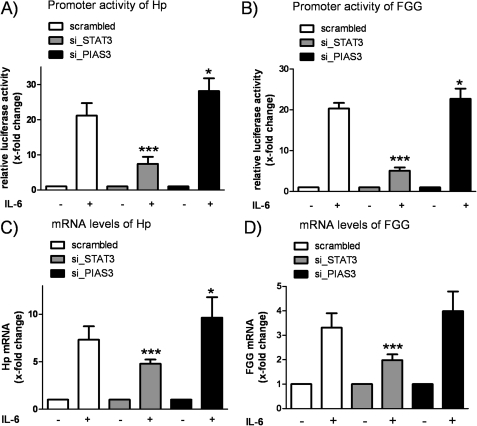
So far, our data demonstrated that miR-18a promotes the acute-phase response and directly inhibits PIAS3, a known repressor of STAT3 activity. Due to the fact that the role of PIAS3 in the acute-phase response was still unknown and to mimic the effects of miR-18a, we silenced PIAS3 and, as an additional control, STAT3 in HepG2 cells. (mRNA levels were reduced by 32 ± 12 and 69 ± 6%, respectively (p < 0.001).) Next, we performed reporter gene studies to monitor the promoter activity of Hp and FGG in siRNA-transfected and IL-6-stimulated HepG2 cells. The IL-6-induced promoter activity of Hp was significantly reduced in STAT3-silenced cells (7.43 ± 2.01-fold, p < 0.001) compared with scrambled transfection (21.17 ± 3.55-fold) (Fig. 5A). Conversely, the response to IL-6 was increased when the expression of PIAS3 was silenced (28.15 ± 3.63-fold, p = 0.015). Promoter activity studies of FGG revealed a similar result showing a significant reduction (silencing of STAT3) and increase (silencing of PIAS3) of the IL-6 response in HepG2 cells (Fig. 5B). To support these findings further, the mRNA levels of Hp and FGG were measured after 4 h of IL-6 stimulation. Fig. 5C summarizes the quantification of Hp mRNA levels, showing an IL-6-induced up-regulation of Hp by 7.32 ± 1.41-fold in scrambled transfected cells. Transfection of STAT3 siRNA reduced the IL-6-induced stimulation of Hp mRNA expression (4.78 ± 0.44-fold, p < 0.001); in contrast, the expression of Hp was further increased when PIAS3 was silenced concomitantly (9.65 ± 2.15-fold, p = 0.015). A similar expression pattern was detected when the same samples were analyzed for the expression of FGG mRNA (Fig. 5D).
FIGURE 5.
PIAS3 is an important mediator of acute-phase response. HepG2 cells were transfected with siRNAs targeting PIAS3 (si_PIAS3) and STAT3 (si_STAT3). A, IL-6 stimulation (20 ng/ml) for 4 h activated the promoter of Hp in all samples, with silencing of STAT3 reducing the activity of luciferase compared with scrambled transfection. Conversely, silencing of PIAS3 increased the relative luciferase activity (n = 5). B, promoter studies of FGG revealed a similar pattern of activation showing a significant reduction (STAT3 siRNA) and enhancement (PIAS3 siRNA) of the IL-6 response in HepG2 cells (n = 7). C, the mRNA levels of Hp after 4 h of IL-6 stimulation were significantly decreased (STAT3 siRNA) or increased (PIAS3 siRNA) compared with scrambled transfection (n = 9). D, the same samples were analyzed for expression of FGG mRNA, showing a similar expression pattern (n = 9). The respective unstimulated samples were set to 1. Statistical analysis was by Student's unpaired t test. *, p < 0.05; ***, p < 0.001.
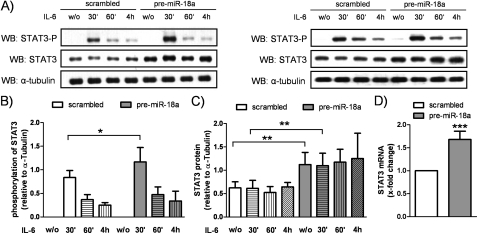
miR-18a Promotes Phosphorylation and Expression of STAT3
The effect of miR-18a on the phosphorylation of STAT3 was tested by performing Western blot experiments. As shown in Fig. 6A, the IL-6-induced phosphorylation of STAT3 was found to be more pronounced in pre-miR-18a-transfected HepG2 cells (STAT3-P/α-tubulin ratio, 0.84 ± 0.15 (scrambled transfection) and 1.17 ± 0.31 (pre-miR-18a transfection), p = 0.039) (Fig. 6B). Induction peaked 30 min after IL-6 stimulation, indicating enhanced activity of STAT3. In addition, the expression of STAT3 was increased at the protein level (STAT3/α-tubulin ratio, 0.62 ± 0.13 (scrambled transfection unstimulated) and 1.12 ± 0.26 (pre-miR-18a transfection unstimulated), p = 0.002) (Fig. 6C) and at the mRNA level (1.68 ± 0.18-fold, p < 0.001) (Fig. 6D) by pre-miR-18a transfection. Taken together, our data present a novel IL-6/miR-17/92 pathway that enhances the expression and release of the acute-phase proteins fibrinogen and Hp by promoting STAT3 phosphorylation and PIAS3 inhibition, thereby augmenting STAT3 activation.
FIGURE 6.
miR-18a promotes phosphorylation and expression of STAT3. HepG2 cells were transfected with pre-miR-18a or the scrambled negative control, serum-starved, and stimulated with IL-6 (20 ng/ml) for 30 min, 1 h, or 4 h. A, Western blot (WB) experiments were performed to analyze the phosphorylation and expression of STAT3. Two representative Western blots are shown. w/o, without. B, phosphorylation of STAT3 (Tyr-705) was markedly enhanced in pre-miR-18a-transfected cells compared with scrambled transfection. Densitometric analysis revealed a significant increase after 30 min of IL-6 stimulation (n = 6). C, transfection of miR-18a led to a significant up-regulation of the protein levels of STAT3 in IL-6 naïve and IL-6-stimulated HepG2 cells. The results from densitometric analysis are shown (n = 6). D, the mRNA levels of STAT3 were found to be up-regulated in miR-18a-overexpressing cells (n = 7). Statistical analysis was by Student's unpaired t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
DISCUSSION
In this study, we have demonstrated a novel regulatory pathway of the acute-phase response. Briefly, we found that IL-6 stimulated the expression of the miRNA cluster miR-17/92 in HepG2 cells and primary hepatocytes, representing a novel positive feedback loop of IL-6 signaling through the repression of the STAT3 inhibitor PIAS3. Thus, overexpression of the miR-17/92-derived miR-18a enhanced the production of the acute-phase proteins fibrinogen and Hp, providing evidence for an important role of miRNAs in the regulation of the inflammatory response.
To a substantial extent, the acute-phase response is controlled by the cytokine IL-6, which is released from monocytes and other immune cells during acute and chronic inflammatory disorders (18). In the liver, the acute-phase response is initiated by the IL-6-induced phosphorylation and dimerization of the transcription factor STAT3, which subsequently shuttles to the nucleus (3). STAT3 homodimers bind to specific DNA sequences in promoter regions and stimulate the transcription of IL-6 target genes (19). We have shown recently that, in human pulmonary arterial endothelial cells, STAT3 directly activates the transcription of miR-17/92 upon stimulation with IL-6 (12). Here, analogous stimulation experiments with IL-6 in HepG2 cells revealed a dose-dependent up-regulation of the primary transcript of miR-17/92 (i.e. C13orf25). Together with our previous results (12), the induction of miR-17/92 by IL-6 thus appears to be a STAT3-mediated and cell type-independent mechanism. In this regard, we identified an evolutionarily conserved core palindromic TT-AA motif with a 4-bp spacing in the promoter of miR-17/92, which is responsible for STAT3 binding (12).
To maintain a physiological steady state under normal conditions, the function and expression of STAT3 need to be finely balanced, which is achieved mainly by the concerted action of intracellular feedback loops. We therefore hypothesized that the STAT3-regulated miR-17/92 cluster might be involved in the feedback signaling of STAT3 as well. We thus measured the promoter activity of the STAT3 target genes Hp and FGG in HepG2 cells transfected with miRNA mimics. Overexpression of miR-18a and miR-20a resulted in enhanced activation of STAT3 upon IL-6 stimulation as shown by promoter studies. These results were further confirmed by showing increased mRNA expression levels of Hp and FGG upon transfection of miR-18a and miR-20a, respectively. With respect to these findings we conclude that miR-17/92 acts as an enhancer of STAT3 activity and represents a positive feedback loop in the IL-6 signaling cascade. Interestingly, Pichiorri et al. (8) demonstrated that another miRNA encoded by miR-17/92, miR-19a, augments STAT3 activity by targeting SOCS1, a known suppressor of STAT3 phosphorylation. In contrast to these findings, we did not observe increased IL-6 signaling in miR-19a-transfected HepG2 cells. These differences are probably due to the respective experimental settings: because SOCS1 is part of a negative feedback loop of STAT3 activity (6) and thus exerts its function with temporal delay, the 4 h of IL-6 stimulation employed in our study might be too short to observe a miR-19a-mediated effect on STAT3 activity.
Because miRNAs have been associated mainly with the repression of gene expression (7), the observed up-regulation of acute-phase genes upon overexpression of miRNAs was most likely due to an indirect effect (i.e. the repression of a yet undefined inhibitor). A computational approach identified PIAS3, which interferes with the DNA-binding activity of STAT3 (17), to contain a potential binding site for miR-18a. Overexpression of miR-18a decreased the expression of PIAS3 at both the mRNA (probably due to mRNA cleavage) and protein levels. Furthermore, by performing reporter gene studies with the 3′-UTR of PIAS3, we confirmed a direct miR-18a-driven repression of PIAS3. We demonstrated the importance of PIAS3 for acute-phase response by showing that knockdown of PIAS3 led to enhanced activation of the promoters of FGG and Hp and thus increased expression of Hp and FGG mRNAs. The correlation between stimulation with IL-6 and expression of C13orf25 and PIAS3 was further investigated by kinetic experiments and revealed a down-regulation of PIAS3 after long-term stimulation (supplemental Fig. S3).
To determine whether miR-18a also enhances the secretion of acute-phase proteins and to put our findings into a physiological context, we quantified the levels of Hp and fibrinogen in the supernatants of pre-miRNA-transfected HepG2 cells. We found the release of Hp and fibrinogen to be enhanced by miR-18a under both IL-6 naïve and IL-6-stimulated conditions. These findings emphasize the potency of miR-18a as a regulator of the acute-phase response via STAT3 signaling.
Loss-of-function experiments by antagonizing miR-18a to inhibit STAT3 signaling were performed in HepG2 cells transfected with small antisense molecules directed against miR-18a (anti-miR-18a). These experiments showed reduced promoter activity and mRNA expression levels of FGG and Hp and decreased secretion of fibrinogen and Hp in anti-miR-18a-treated cells. On the other hand, the protein levels of PIAS3 were found to be up-regulated under these conditions. These data confirm the physiological role of miR-18a in the IL-6 signaling cascade and further demonstrate that silencing of miR-18a may provide a useful tool to interfere with the hepatic acute-phase response.
The phosphorylation of Tyr-705 of STAT3 is an essential trigger of the acute-phase reaction and thus of special importance (3). Here, we found that Tyr-705 was more phosphorylated in pre-miR-18a-transfected cells, indicating enhanced STAT3 activity. These findings support the conclusion that miR-18a acts as enhancer of the IL-6 pathway. Interestingly, the expression of STAT3 by pre-miR-18a transfection was found to be increased at both the mRNA and protein levels. Because the inhibitory action of PIAS3 affects the DNA-binding activity of STAT3, one might propose an additional, PIAS3-independent effect of miR-18a on Tyr-705 phosphorylation. Whether this effect is due to increased expression of the unphosphorylated STAT3 substrate remains unclear and needs to be addressed by further studies.
In addition to miR-18a, the STAT3-dependent activation of the Hp and FGG promoters was significantly enhanced when HepG2 cells were transfected with precursor molecules of miR-20a. ELISAs for Hp and fibrinogen did not reveal, however, an elevated release of these proteins when miR-20a was overexpressed. Because two independent methods (reporter gene assay and quantitative real-time PCR) showed an increase in STAT3 activity by miR-20a, we suggest that the failure of increased acute-phase protein secretion by miR-20a might be due to an impaired protein release, or, alternatively, that miR-20a targets not only repressors but also activators of the IL-6 transduction pathway, e.g. JAK1 (Janus kinase 1). JAK1 is the kinase responsible for STAT3 phosphorylation and is also targeted by miR-20a (20).
The most important findings of this study were confirmed by performing experiments in an additional cell model using primary human hepatocytes. By overexpressing miR-18a in these cells, we could show that miR-18a augments the IL-6-induced acute-phase response by targeting the repressor of IL-6 signaling, PIAS3. Similarly, IL-6 treatment increased the expression of miRNAs derived from the miR-17/92 cluster, suggesting the presence of a positive feedback loop in both primary hepatocytes and HepG2 cells.
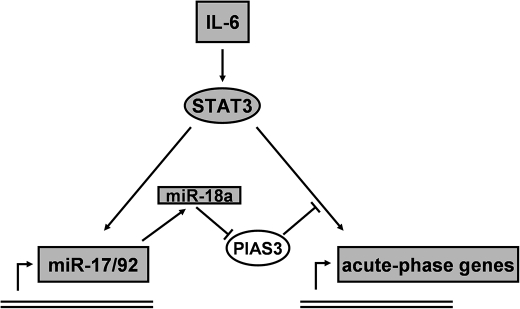
In summary, we propose the following model for the regulation of the acute-phase response by miR-17/92 (Fig. 7). During states of acute or chronic inflammation, immune cells secrete IL-6, which causes the activation of STAT3 in hepatocytes. This triggers the production of acute-phase proteins and their systemic release. At the same time, the expression of miRNAs derived from the cluster miR-17/92 is up-regulated. This leads to a further augmentation of STAT3 activity through the repression of PIAS3 by miR-18a and finally contributes to the overwhelming release of acute-phase proteins.
FIGURE 7.
Regulation of acute-phase gene expression by miR-17/92 through a positive feedback loop involving PIAS3. We propose the following model for the regulation of the acute-phase response by the miR-17/92 cluster. IL-6, released from immune cells, activates STAT3 in hepatocytes, thus triggering the expression of acute-phase genes and the miRNA cluster miR-17/92. In turn, miR-18a targets the STAT3 inhibitor PIAS3, which leads to the enhanced activation of STAT3 and thus results in an increased release of acute-phase proteins.
In conclusion, we identified here a novel IL-6/miR-17/92 pathway that, through the inhibition of PIAS3, enhances the production and release of the acute-phase proteins fibrinogen and Hp. To our knowledge, this is the first report on a miRNA-mediated regulation of PIAS3, thus contributing to the understanding of the complex regulatory mechanisms within the signal transduction of IL-6.
Supplementary Material
This work was supported by Swiss National Foundation Grant SNF-32000-116842, the Schwyzer Foundation, the Zurich Lung League Foundation, and the Masterswitch FP7 and Institute for Arthritis Research Epalinges Programs.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Materials and Methods,” Figs. S1–S3, Table S1, and additional references.
- Hp
- haptoglobin
- FGG
- fibrinogen γ-chain
- miRNA
- microRNA.
REFERENCES
- 1. Gabay C., Kushner I. (1999) N. Engl. J. Med. 340, 448–454 [DOI] [PubMed] [Google Scholar]
- 2. Heinrich P. C., Castell J. V., Andus T. (1990) Biochem. J. 265, 621–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wegenka U. M., Buschmann J., Lütticken C., Heinrich P. C., Horn F. (1993) Mol. Cell. Biol. 13, 276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y., Kinzie E., Berger F. G., Lim S. K., Baumann H. (2001) Redox Rep. 6, 379–385 [DOI] [PubMed] [Google Scholar]
- 5. Zhang Z., Fuentes N. L., Fuller G. M. (1995) J. Biol. Chem. 270, 24287–24291 [DOI] [PubMed] [Google Scholar]
- 6. Krebs D. L., Hilton D. J. (2001) Stem Cells 19, 378–387 [DOI] [PubMed] [Google Scholar]
- 7. Bartel D. P. (2004) Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 8. Pichiorri F., Suh S. S., Ladetto M., Kuehl M., Palumbo T., Drandi D., Taccioli C., Zanesi N., Alder H., Hagan J. P., Munker R., Volinia S., Boccadoro M., Garzon R., Palumbo A., Aqeilan R. I., Croce C. M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12885–12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fort A., Borel C., Migliavacca E., Antonarakis S. E., Fish R. J., Neerman-Arbez M. (2010) Blood 116, 2608–2615 [DOI] [PubMed] [Google Scholar]
- 10. Xiao C., Srinivasan L., Calado D. P., Patterson H. C., Zhang B., Wang J., Henderson J. M., Kutok J. L., Rajewsky K. (2008) Nat. Immunol. 9, 405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Donnell K. A., Wentzel E. A., Zeller K. I., Dang C. V., Mendell J. T. (2005) Nature 435, 839–843 [DOI] [PubMed] [Google Scholar]
- 12. Brock M., Trenkmann M., Gay R. E., Michel B. A., Gay S., Fischler M., Ulrich S., Speich R., Huber L. C. (2009) Circ. Res. 104, 1184–1191 [DOI] [PubMed] [Google Scholar]
- 13. Hou T., Ray S., Brasier A. R. (2007) J. Biol. Chem. 282, 37091–37102 [DOI] [PubMed] [Google Scholar]
- 14. Chen C., Ridzon D. A., Broomer A. J., Zhou Z., Lee D. H., Nguyen J. T., Barbisin M., Xu N. L., Mahuvakar V. R., Andersen M. R., Lao K. Q., Livak K. J., Guegler K. J. (2005) Nucleic Acids Res. 33, e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuhn D. E., Martin M. M., Feldman D. S., Terry A. V., Jr., Nuovo G. J., Elton T. S. (2008) Methods 44, 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis B. P., Burge C. B., Bartel D. P. (2005) Cell 120, 15–20 [DOI] [PubMed] [Google Scholar]
- 17. Chung C. D., Liao J., Liu B., Rao X., Jay P., Berta P., Shuai K. (1997) Science 278, 1803–1805 [DOI] [PubMed] [Google Scholar]
- 18. Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 7251–7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seidel H. M., Milocco L. H., Lamb P., Darnell J. E., Jr., Stein R. B., Rosen J. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 3041–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doebele C., Bonauer A., Fischer A., Scholz A., Reiss Y., Urbich C., Hofmann W. K., Zeiher A. M., Dimmeler S. (2010) Blood 115, 4944–4950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.