Background: The three microRNAs from miRNA cluster MC-let-7a-1∼let-7d are often down-regulated in human cancers.
Results: We functionally characterized the promoter and investigated the transcriptional regulation of MC-let-7a-1∼let-7d.
Conclusion: MYC oncoprotein could inhibit the transcription of MC-let-7a-1∼let-7d via binding to a noncanonical E-box.
Significance: Understanding how MC-let-7a-1∼let-7d is regulated could shed light on identifying new molecular targets and designing novel treatment for cancers.
Keywords: Hepatocellular Carcinoma, MicroRNA, Myc, Proliferation, Transcription Regulation, MC-let-7a-1∼let-7d, MYC, E-box, Transcriptionally Regulate
Abstract
The human microRNA cluster MC-let-7a-1∼let-7d, with three members let-7a-1, let-7f-1, and let-7d, is an important cluster of the let-7 family. These microRNAs play critical roles in regulating development and carcinogenesis. Therefore, precise control of MC-let-7a-1∼let-7d level is critical for cellular functions. In this study, we first showed that the expression of these three members was significantly reduced in human hepatocellular carcinoma HepG2 cells as compared with the immortalized human liver L02 cells. We demonstrated that the MC-let-7a-1∼let-7d cluster was encoded by a single polycistronic transcript driven by a 10-kb upstream promoter, with two MYC-binding sites. Importantly, MYC inhibited MC-let-7a-1∼let-7d promoter activity via binding to the noncanonical E-box 3 downstream of the transcription start sites, whereas it enhanced promoter activity by binding to the canonical E-box 2 upstream of the transcription start sites. We found that although the binding affinity of MYC to E-box 2 was stronger than E-box 3, the binding quantum of MYC to E-box 3 was significantly higher in cancerous HepG2 cells as compared with the noncancerous L02 cells. In addition, forced expression of let-7 could reverse the MYC-mediated cell proliferation. These findings suggested that in L02 cells with a low level of MYC, MYC binds mainly to E-box 2 to enhance MC-let-7a-1∼let-7d expression. However, in HepG2 cells with an elevated MYC, the extra MYC could bind to E-box 3 to suppress the transcription of MC-let-7a-1∼let-7d and thus enable HepG2 cells to maintain a high level of MYC and a low level of let-7 microRNAs simultaneously.
Introduction
MicroRNAs (miRNAs)3 are short noncoding RNAs (∼22 nucleotides) that regulate protein expression and control diverse aspects of biology, including carcinogenesis, development, and numerous cellular processes (1). In principle, miRNAs expression can be regulated at any step during the maturation process. This process initiates from the transcription of primary transcripts, followed by post-transcription regulations, including several processes that generate mature miRNA from primary miRNA (pri-miRNA) and then precursor miRNA (pre-miRNA) (2, 3). Because miRNA genes are frequently located at the chromosomal fragile sites of cancer genomes (4), miRNAs have been considered as novel classes of oncogenes and tumor suppressors (5–7). The altered expressions of several miRNAs (including let-7, miR-9, -21, -122, -151, -221, etc.) have been reported to play pivotal roles in carcinogenesis (8–12).
Among the various families of miRNAs, the let-7 family of miRNAs has become a prototype for miRNAs that function as a tumor suppressor; this is because they inhibit the expressions of multiple oncogenes, including RAS and MYC (13–15). They are often down-regulated in cancer cells and are potential biomarkers as well as prognostic markers that predict disease progression and response to treatment (16, 17).
We and others have demonstrated that let-7 miRNAs act as tumor suppressors and inhibit HCC cell proliferation and invasion by down-regulating MYC and up-regulating p16INK (18, 19). To date, 13 members (let-7a-1, let-7a-2, let-7a-3, let-7b, let-7c, let-7d, let-7e, let-7f-1, let-7f-2, let-7g, let-7i, miR-98, and miR-202) have been found in Homo sapiens (20). They are located in nine different loci on chromosomes 3, 9–12, 19, 21, 22, and X.
The MC-let-7a-1∼let-7d cluster includes let-7a-1, let-7f-1, and let-7d. These three members together accounted for ∼24% of the total let-7 precursors (supplemental Table S1) (21). let-7a-1 is reported to be down-regulated in liver, breast, lung, gastric, pancreatic, melanoma and prostate cancers (16, 22–27). Similarly, let 7f-1 is down-regulated in lung, ovarian, prostate, sarcoma, and papillary thyroid cancers (16, 22, 28, 29) and let-7d in ovarian, head, and neck squamous cell carcinoma and prostate cancers (22, 28). Because the levels of the let-7 family members are important in cancer development, it is therefore extremely important to understand how the expressions of MC-let-7a-1∼let-7d are regulated.
A recent report suggested that MYC could induce LIN28B and LIN28 expressions and subsequently repress let-7 microRNAs expressions post-transcriptionally (30–40). In addition, it is also suggested that MYC may bind to a conserved site upstream of the let-7a-1/let-7f-1/let-7d cluster. However, the precise binding sites, the functions, and the mechanisms involved in the transcription regulation have not been characterized (41).
In this study, we investigated the transcriptional regulation of MC-let-7a-1∼let-7d. We predicted the promoter region and transcription start sites (TSSs) of MC-let-7a-1∼let-7d by bioinformatics and then verified in human liver L02 and hepatocellular carcinoma (HCC) HepG2 cells. We further showed that MYC could directly down-regulate the expression of MC-let-7a-1∼let-7d at the transcription level in HCC. Two MYC-binding sites were identified. One E-box element located downstream of the TSS is critical for the let-7 transcription repression in HepG2 cells and one upstream for stimulation. Taken together, these findings suggested that the MYC oncoprotein could either inhibit or stimulate the transcription of miRNA let-7 tumor suppressors depending on its intracellular levels.
EXPERIMENTAL PROCEDURES
Genomic Features of let-7 Family
We obtained genomic coordinates of the let-7 family from the miRBase microRNA sequence data base (version 16) (42). Because the size of miRNA primary transcripts is unknown, we clustered same strand miRNAs at 50-kb thresholds according to the miRBase. Flanking sequence data, expressed sequence tags data (ESTs) and CpG islands were obtained from the University of California, Santa Cruz, Genome Browser by the ENCODE (ENCyclopedia Of DNA Elements) Project (43). Putative TSSs were predicted from the FANTOM (the Functional Annotation of the Mammalian Genome) web resource (44). Putative binding sites for transcription factors of conserved genomic regions were explored by conducting the UCSC Genome Browser JASPAR and TESS.
Cell Culture
Human HCC cell line HepG2, immortalized human liver cell line L02, and human embryonic kidney cell line HEK293T were obtained from ATCC and maintained in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen), in a humidified atmosphere with 5% CO2 at 37 °C.
Genomic DNA Extraction, RNA Extraction, cDNA Synthesis, and Real Time PCR
For genomic DNA extraction, total cell genomic DNA was extracted by using DNeasy Blood and Tissue (Qiagen), following the manufacturer's instructions. For RNA extraction, the total RNA was extracted by using TRIzol (Invitrogen), in accordance with the manufacturer's instructions. For cDNA synthesis, 1 μg of total RNA was reverse-transcribed by the SuperScriptTM III First-Strand synthesis system (Invitrogen) after DNase I treatment (Invitrogen). Quantitative real time-PCR (qRT-PCR) was subsequently performed in triplicate with a 1:5 dilution of the resultant cDNA by using the Applied Biosystems 7500 real time PCR system (Applied Biosystems) with the Brilliant® II SYBR® Green qPCR master mix (Stratagene). All mRNA quantification data were normalized to GAPDH. The Ct value for each sample was calculated by the ΔΔCt method, and the results were expressed as 2−ΔΔCt (45).
5′-Rapid Amplification of cDNA Ends (5′-RACE)
The 5′-RACE, version 2.0 (Invitrogen), was used in accordance with the manufacturer's instructions. Briefly, cDNA was synthesized by using the MC-let-7a-1∼let-7d specific primers GSP1-1 or GSP1-2 (supplemental Table S2). Primary amplification was carried out with an abridged anchor primer (provided in the kit) and a GSP2-1 primer (supplemental Table S2). Nested PCR was performed with an abridged universal amplification primer (provided in the kit) and a GSP2-2 (supplemental Table S2) by using TaKaRa LA Taq® with GC Buffer I (TaKaRa). The 5′-RACE PCR products were resolved on a 1% agarose gel and stained by EtBr. The bands were excised, cloned into pGL3 basic vector (Promega) via restriction endonuclease MluI sites, and sequenced.
Constructions of Promoter/Reporter and shRNA Expression Plasmids
A 1.9-kb MC-let-7a-1∼let-7d promoter was amplified by PCR by using PrimeSTARTM HS DNA polymerase (TaKaRa) and cloned into pGL3 basic vector (Promega) between Xho I and Hind III sites by using primers PPR-1, as listed in supplemental Table S2. This plasmid was named PPR-1. Subsequent promoter truncations were generated from this template and cloned in a similar manner by using the primers in supplemental Table S2. To construct a MYC expression vector, a human MYC open reading frame (ORF) was amplified by using MYC primers, as indicated in supplemental Table S2, and inserted into the pcDNA3.1(+) vector (Invitrogen) between Hind III and BamH I sites. To construct shRNA vectors for knocking down endogenous MYC, hairpin encoding oligonucleotides (sh-Myc, supplemental Table S2) were annealed and ligated into the pGE-1 shRNA expression vector (Stratagene) between BamH I and Xba I sites. The following targeting sequence was used, GATGAGGAAGAAATCGATG, in accordance with the literature published previously (46). The sequence and orientation of all inserts were confirmed by sequencing.
Mutagenesis
Mutations in the MYC-binding sites were made in the PPR-3 or PPR-10 construct by using two-step PCR (47). Briefly, two pairs of primers, including PPR-3 or PPR-10 sense primers and E-box antisense primers PPR-3 or PPR-10 antisense primers and E-box sense primers, were used in the first PCR step to generate intermediate PCR products. Then these two mutated products were denatured as a template for the second PCR step by using flanking primers PPR-3 or PPR-10. The final PCR products were digested with Xho I and Hind III and then inserted into pGL3 basic vector (Promega). The mutations were confirmed by sequencing.
Transient siRNA Transfection
The si-Lin28 and si-Lin28B were synthesized by GenePharma in accordance with the sequences reported previously (supplemental Table S2) (35). The transient transfection of L02 cells with control siRNA or siRNAs targeting LIN28 and LIN28B mRNAs was performed, as described previously (48).
Luciferase Reporter Assays
HepG2 cells, L02 cells, or HEK293T cells were plated at a density of 50,000 cells per well in a 24-well plate at 24 h before transfection. The cells were co-transfected with 250 ng of MC-let-7a-1∼let-7d promoter/firefly luciferase reporter plasmids and 5 ng of pRL-TK Renilla plasmids (Promega) by using FuGENE® HD transfection reagent (Roche Applied Science). For gain- and loss-of-function experiments, 750 ng of MYC expression plasmids (c-Myc), or knockdown MYC expression plasmids (sh-Myc), or their control vectors (pcDNA3.1 or pGE-1 negative control vector (pGE-NC)) were transfected. After 48 h post-transfection, the cells were either lysed in TRIzol (Invitrogen) for RNA extraction or in passive lysis buffer (Promega) for luciferase assay measured with the Dual-Luciferase reporter assay system (Promega) by using the TD-20/20 luminometer (Turner Designs). The relative luciferase activities were determined by calculating the ratio of firefly luciferase activities over Renilla luciferase activities.
Protein Extraction and Western Blot Analysis
Cells were seeded onto a 6-well plate at a density of 106 cells/well. The cells were lysed for 30 min on ice in RIPA lysis buffer (Thermo) with protease inhibitor mixture (Sigma) and phosphatase inhibitor (Pierce). Protein concentration was determined by a Bio-Rad protein assay kit (Bio-Rad). 50 μg of proteins were separated by 12% SDS-polyacrylamide gels and transferred onto PVDF membranes. Membranes were probed with specific antibodies, including MYC (1:4000; Abcam) and β-actin (1:3000; Abcam), respectively. After washing, membranes were incubated with an HRP-conjugated secondary antibody (1:5000; Abcam). Membranes were exposed by using the enhanced chemiluminescence (ECL) substrate kit (Thermo) and then were photo-documented. The expression level of β-actin was also determined, and it served as an internal control.
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts from HepG2 cells were prepared in accordance with the manufacturer's protocol (NE-PER® Nuclear and Cytoplasmic Extraction Reagents, Pierce). Complementary oligonucleotide pairs corresponding to the three E-boxes embedded in the promoter region of MC-let-7a-1∼let-7d and the mutated E-boxes were synthesized and 5′ end-labeled with biotin by Invitrogen. The oligonucleotide sequences are shown in supplemental Table S2. EMSA was performed by using a LightShift® chemiluminescent EMSA kit (Pierce). Binding reaction with 10 μg of HepG2 nuclear extracts and 100 fmol of 5′ biotin-labeled oligonucleotide was carried out in accordance with the manufacturer's instructions. For the competition assay, a 100-fold molar excess of unlabeled oligonucleotide was added to the binding reaction mixture as a specific competitor. For antibody-supershift assay, a nuclear extract was preincubated with a 3 μl MYC antibody (Abcam) before adding it to the binding reaction. DNA-protein complexes were separated on a pre-electrophoresed 6% polyacrylamide gel in 0.5× TBE, transferred to a nylon membrane, and cross-linked at 120 mJ/cm2 for 1 min and detected by chemiluminescence, in accordance with the manufacturer's directions. Membranes were exposed by using the enhanced chemiluminescence (ECL) substrate kit (Thermo) and then were photo-documented.
Chromatin Immunoprecipitation (ChIP)
ChIP assays were carried out by using an EZ-ChIP assay kit (Upstate Biotechnology, Inc.), in accordance with the manufacturer's instructions. In brief, cells were grown to 90% confluence and added 1% formaldehyde at room temperature for 10 min. The cross-link reaction was quenched with 0.125 m glycine for 5 min at room temperature. Cells were then washed, scraped, and resuspended in 1 ml of lysis buffer. DNA was sonicated into around 400-bp pieces at 4 °C by using Sonics Uibra CellsTM (XINCHEN). Supernatants were recovered by centrifugation and precleared for 1 h at 4 °C with 60 μl of protein G-agarose. Then 10 μl (1%) of supernatant was removed as input. Immunoprecipitations were performed overnight with MYC antibody (Abcam, 9E11, ChIP Grade, 6 μg) or IgG antibody (provided in kit, 1 μg). The immune complexes were captured by incubation with 60 μl of protein G-agarose for 2 h at 4 °C. The immunoprecipitates were washed sequentially with wash buffers. After that, the immunoprecipitates were eluted from the protein G-agarose by incubating with elution buffer (1% SDS, 100 mm NaHCO3). DNA-protein complexes were reversely cross-linked by a high salt solution at 65 °C for 5 h. RNA and protein were eliminated by treating with 10 μg of RNase A at 37 °C for 30 min and then with protease K for 2 h at 45 °C. Finally, DNA was purified by using the spin column provided in the ChIP kit and eluted with 50 μl of elution buffer. qRT-PCR was performed by using the Brilliant® II SYBR® Green qPCR master mix (Stratagene) on the Applied Biosystems 7500 real time PCR system instrument. The primer pairs used for PCR analysis are shown in supplemental Table S2. All data were normalized to input (49).
MTT Analysis of Cell Proliferation
L02 cells were seeded at a density of 2500 cells per well onto a 96-well plate with the complete medium (100 μl/well). After 24 h of incubation, cells were transfected with MYC, let-7a-1 expression plasmids (pll-let-7), or their control vectors (pll3.7 or pcDNA3.1) and their respective negative control by using FuGENE® HD. The cells were then incubated at 37 °C in a humidified environment with 5% CO2. At days 1–6, supernatants were removed, and the cells were incubated with 20 μl of 5 mg/ml MTT reagent (Sigma) at 37 °C for 4 h. At the end of incubation, MTT reagent was removed from each well and replaced by 100 μl of DMSO (Invitrogen). The plate was gently agitated for 10 min, and the absorbance (A) at 490 nm was determined by an ELISA reader (Bio-Rad).
Flow Cytometry
The collected cells were washed twice with PBS, fixed in 70% ethanol overnight, digested with RNase A (10 mg/liter) in PBS for 30 min, then stained with propidium iodide, and analyzed on a FACScalibur (BD Biosciences).
Statistical Analysis
Data were shown as means ± S.D. Statistical analyses for detection of significant differences between the control and the experimental groups were carried out by using an unpaired two-tailed Student's t test (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001).
RESULTS
Bioinformatic Analysis Predicted That Hsa-let-7a-1, let-7f-1, and let-7d Are Co-evolutionary Intergenic miRNAs
We analyzed the phylogenetic relationships of these three let-7 members. As shown in supplemental Table S3, they first emerged as a cluster at a very early stage during evolution and have never separated since then. Hence, we postulated that let-7a-1, let-7f-1, and let-7d were co-transcribed as a cluster MC-let-7a-1∼let-7d. Consistently, the UCSC Genome Browser also indicated that these three members are clustered within a 3-kb region on chromosome 9 (Fig. 1A). The distances between the encoding region of pre-let-7a-1, pre-let-7f-1 and pre-let-7f-1, pre-let-7d are 311 and 2401 bp, respectively. They are transcribed to the same orientation, and no gene is found to overlap with them in GenBankTM.
FIGURE 1.
has-let-7a-1, let-7f-1, and let-7d are encoded by a single polycistronic transcript MC-let-7a-1∼let-7d. A, schematic representation of the has-let-7a-1, let-7f-1, and let-7d gene location, putative promoter, and TSS. B, putative MC-let-7a-1∼let-7d primary transcript was confirmed by qRT-PCR in L02 cells. cDNA was synthesized by HH antisense primer. qRT-PCR products were obtained with primers located within the predicted transcript (BB, CC, DD, EE, FF, GG, and HH), but no product was obtained with primers located upstream of the predicted TSS (AA). As a control, genomic DNA was successfully amplified by all primers. M indicates DNA markers. C, qRT-PCR was performed. All the segments within the primary transcript produced less qRT-PCR product in HepG2 cells than that in L02 cells. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.
To predict the TSS and the promoter region of MC-let-7a-1∼let-7d, we first searched ESTs around their encoding region. As shown in Fig. 1A, EST BG326593 is overlapped with these three let-7 members. In addition, 39 scattered ESTs are found between the 5′ boundary of EST BG326593 and MC-let-7a-1∼let-7 encoding region (data not shown). In addition, in the FANTOM data base (44), a subset of TSSs within 15 kb upstream of the MC-let-7a-1∼let-7d encoding region are found. All of the predicted TSSs are located in the region around the 5′ end of EST BG326593 (chromosome 9: 95,967,500–95,982,000). We also found that a CpG island overlaps with 5′ boundary of EST BG326593 (Fig. 1A). In addition, analysis of the Pol2 (RNA polymerase II) ChIP-seq Signal/Histone Modifications ChIP-seq Signal and Transcription Factors ChIP-seq Signal within 15 kb upstream of MC-let-7a-1∼let-7d encoding region by the UCSC Genome Browser suggested that the TSSs of these three miRNAs were located about 10 kb upstream of their encoding region. Hence, these analyses suggest that the three members were encoded by a single polycistronic transcript MC-let-7a-1∼let-7d with a promoter located about 10 kb upstream of their encoding region (Fig. 1A).
Experimental Confirmation That Hsa-let-7a-1, let-7f-1, and let-7d Are Encoded by a Single Polycistronic Transcript
We performed strand-specific reverse transcription-PCR (RT-PCR) by using cDNA produced by HH antisense primer from HepG2 cells and L02 cells with primers matching segments along the length of the predicted human transcripts (Fig. 1A and supplemental Table S2), as described previously (50). Consistent with the prediction, qRT-PCR products were obtained with primers located within the predicted transcript (BB, CC, DD, EE, FF, GG, and HH), but no product was obtained with primers located upstream of the predicted TSSs (AA). As a control, genomic DNA was successfully amplified by all primer sets (Fig. 1B). Furthermore, we quantified the expression level of the seven segments on the predicted primary transcript by qRT-PCR by using cDNA produced by random hexamers (Invitrogen) from HepG2 cells and L02 cells. As shown in Fig. 1C, all the segments within the primary transcript produced less qRT-PCR product in HepG2 cells than that in L02 cells. Taken together, these data suggested that let-7a-1, let-7f-1, and let-7d were encoded within a single independent non-protein-coding polycistronic transcript, which is transcribed 10 kb upstream from initiation sites.
Alternative Transcription Initiation Sites by HepG2 Cells and L02 Cells
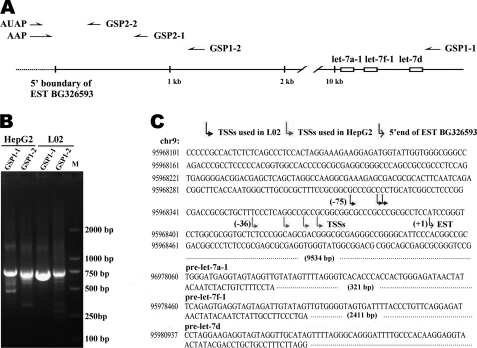
To identify the initiation site of MC-let-7a-1∼let-7, we next performed 5′-RACE by using total RNA prepared from HepG2 cells and L02 cells. Two types of gene-specific primers, GSP1-1 and GSP1-2, were used for the synthesis of the first strand cDNA (Fig. 2A). After nested PCR by GSP2–1 and GSP2–2, the ∼700-bp products were amplified in HepG2 and L02 (Fig. 2B).
FIGURE 2.
Mapping the TSSs of MC-let-7a-1∼let-7d by 5′-RACE. A, primers used for 5′-RACE. Two types of gene-specific primers (GSPs) GSP1-1 or GSP1-2 were, respectively, used to synthesize the first strand cDNA. Primary amplification was carried out with abridged anchor primer (AAP) and GSP2-1 primer. Nested PCR was performed with abridged universal amplification primer (AUAP) and GSP2-2. B, 5′-RACE results. The ∼700-bp products were amplified in HepG2 cells and L02 cells by using both of the first cDNA strands derived from GSP1-1 and GSP1-2. C, sequence analysis of 20 products from each cell lines revealed that variable TSSs were used. The major TSSs in L02 cells were located about 30 bp upstream of TSSs in HepG2 cells.
We cloned the major band of 5′-RACE products and sequenced 10 clones for each sample. Sequence analysis of these 40 5′-RACE products (20 products for each cells) revealed that the products obtained from the HepG2 cells were about 30 bp smaller than those obtained from the L02 cells (Fig. 2C). Specifically, the major TSSs used in the L02 cells were located about 30 bp upstream of the TSSs used in the HepG2 cells.
Characterization of the MC-let-7a-1∼let-7d Promoter
According to the preceding promoter analysis and the 5′-RACE results, the promoter of MC-let-7a-1∼let-7d should be located around the TSSs (Fig. 1A). Because the encoding region of MC-let-7a-1∼let-7d is highly conserved, we hypothesized that its promoters and regulating machinery would also be conserved. As shown in Fig. 3A, the genomic region proximal to the TSSs (from −540 to +1317 with respect to the 5′ end of EST BG326593 as +1) shows high evolutionary conservation among different species. Therefore, we selected a 1.9-kb conserved region as the primary putative promoter to construct a promoter/luciferase reporter construct (PPR-1) and showed that PPR-1 could drive the luciferase expression in the L02 cells (Fig. 3B). The truncations of the promoter further revealed that the promoter (−540 to +1 bp) exhibited the maximal promoter activity. Our results also showed that the truncation of the downstream region from +1317 to +1 dramatically increased the promoter activity (Fig. 3B), suggesting that the 3′ end of PPR-1 contains suppression elements. In addition, truncation of the upstream region from −540 to −227 resulted in a loss of activity (Fig. 3B), thereby indicating that the elements between −540 and −227 contain an activator.
FIGURE 3.
Analysis of MC-let-7a-1∼let-7d promoter. A, plot generated from the UCSC Genome Browser depicts the evolutionary conservation (cons) of MC-let-7a-1∼let-7d encoding region and putative regulation region. Annotated 5′ end of MC-let-7a-1∼let-7d is high evolutionary conservation among different species. B, schematic diagram of the reporter construct (left panel). Truncation of the downstream region to +1 dramatically improved the promoter activity, whereas truncation of the upstream region to −227 resulted in progressive loss of activity.
MYC Down-regulated the Expression of the Primary MC-let-7a-1∼let-7d Transcript
According to the whole genome ChIP-seq data on the UCSC Genome Browser, MYC has a very strong binding signal surrounding the MC-let-7a-1∼let-7d promoter in all of the cells (supplemental Fig. S1). Thus, we sought to determine whether MYC could regulate the MC-let-7a-1∼let-7d expression at the transcription level. We showed that overexpression of MYC in L02 cells caused a decrease in the MC-let-7a-1∼let-7d primary transcript expression (Fig. 4A). Consistently, the knockdown of endogenous MYC by sh-Myc caused an increase (Fig. 4B). According to the published reports, LIN28 and LIN28B, which are induced by MYC, could mediate let-7 post-transcription repression in multiple human and mouse tumor models (30–40). To exclude the possibility that repression of primary MC-let-7a-1∼let-7d is also mediated by LIN28 and LIN28B, we blocked the MYC/LIN28/let-7 cascade by siRNAs targeting LIN28 and LIN28B. As shown in Fig. 4C, the decrease of LIN28 and LIN28B could not eliminate the repression of primary MC-let-7a-1∼let-7d by MYC. These results suggested that the repression of primary MC-let-7a-1∼let-7d by MYC is LIN28-independent.
FIGURE 4.
MYC suppresses the human primary MC-let-7a-1∼let-7d expression level. A, ectopic overexpression of MYC was confirmed by Western blot analysis. Overexpression of MYC significantly reduced the MC-let-7a-1∼let-7d primary transcript expression in L02 cells. B, knockdown of endogenous MYC was confirmed by Western blot analysis, and it increased the MC-let-7a-1∼let-7d primary transcript expression in L02 cells. C, blocking the MYC/LIN28/let-7 pathway by siRNAs targeting LIN28 and LIN28B could not eliminate the repression of primary MC-let-7a-1∼let-7d by MYC. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.
Endogenous MYC Regulated the MC-let-7a-1∼let-7d Expression by Two E-boxes Upstream and Downstream of TSSs
MYC is known to bind to the canonical E-box sequence CACGTG, as well as to the noncanonical sequences, including CGCGTG (51, 52). We noted two presumptive MYC-binding sites CACGTG located upstream and one noncanonical binding site CGCGTG located downstream of the MC-let-7a-1∼let-7d TSSs (Fig. 5A). According to the UCSC Genome Browser, these sites were conserved in vertebrates. Thus, we hypothesized that MYC might bind to these E-boxes to down-regulate MC-let-7a-1∼let-7d promoter activity.
FIGURE 5.
MYC binds to the MC-let-7a-1∼let-7d promoter region through E-boxes both upstream and downstream of the TSSs. A, schematic illustration of the three MYC-binding E-box sites and sequence details. B, consensus sequence of MYC-binding site. Two types of point mutations were introduced into canonical MYC-binding E-box CACGTG, and deletion mutation was performed for noncanonical E-box CGCGTG. C, EMSA and supershift assay analysis of MYC binding to the three E-boxes. Competition assays were performed adding to 100-fold molar unlabeled E-boxes. E-box 1, E-box 2, and E-box 3 mutations could not obtain shift band. Shift bands and supershift bands are observed for E-box 2 and E-box 3 by adding MYC antibody.
To determine the ability of MYC binding to these three E-boxes, EMSAs and supershift analyses were performed with the promoter regions embedding the three E-boxes and the mutated E-boxes. As shown in Fig. 5C, E-box 2 and E-box 3 showed evident shift bands, whereas the E-box 1 could not bind to a nuclear extract. The formation of the complex E-box 2/protein or E-box 3/protein was inhibited by competition (1:100-fold molar excess) with the unlabeled E-box 2 or E-box 3. Furthermore, mutation of the E-box 2- or E-box 3-binding site eliminated the shift bands. In addition, the specificity of MYC binding to the E-box 2 and E-box 3 was confirmed by the supershift of the E-box/protein bands. These results suggest that E-boxes 2 and 3 were necessary for the binding of the nuclear factors.
To assess the effects of these E-boxes in transcription regulation of MC-let-7a-1∼let-7d, co-expression studies were performed using MYC expression or knockdown vector and a PPR-10 promoter/reporter plasmid containing canonical MYC-binding motifs E-box 2 and a PPR-3 promoter/reporter plasmid containing both E-box 2 and the noncanonical E-box 3 (Fig. 6A). To our surprise, we found that the overexpression of MYC significantly activated luciferase activity of PPR-10 both in HepG2 and L02 cells (Fig. 6C), whereas knockdown of MYC resulted in a significant reduction of the PPR-10 activity (Fig. 6C). The positive regulation of PPR-10 (E-box 2) by MYC was further confirmed by E-box mutation assays. As shown in Fig. 6C (right panel), mutations of the E-box 2 reduced the promoter activity and abrogated MYC-mediated regulation of PPR-10 promoter activity. We then examined the regulation of PPR-3. Importantly, we found that overexpression of MYC significantly repressed the PPR-3 activity by 25–50%, whereas knockdown of MYC significantly increased PPR-3 activity (Fig. 6D). Furthermore, the mutation of E-box 2 in PPR-3 reduced promoter activity, but it could not eliminate the negative regulation by MYC. In contrast, the deletion of E-box 3 increased the promoter activity and also eliminated the negative regulation by MYC on PPR-3 activity (Fig. 6D). Taken together, our results showed that the E-box 3 located downstream of TSSs was the essential element required for MYC-mediated repression and E-box 2 located upstream of TSSs for stimulation of the promoter activity of MC-let-7a-1∼let-7d.
FIGURE 6.
MYC regulates the expression of MC-let-7a-1∼let-7d by binding to the E-box 2 and E-box 3. A, schematic illustration of the location of MC-let-7a-1∼let-7d promoter PPR-3 and PPR-10 and the E-boxes. B, overexpression or knockdown of MYC in HepG2 cells and L02 cells was confirmed by Western blot analysis. C, overexpression of MYC increased PPR-10 activity both in HepG2 cells and L02 cells (left panel). Point mutation of E-box 2 suppressed the activity of PPR-10 and MYC-mediated repression (right panel). D, overexpression of MYC suppressed PPR-3 activity, whereas knockdown of MYC significantly increased PPR-3 activity (left panel). The mutation of E-box 2 in PPR-3 cannot eliminate the transcription suppression by MYC, whereas the deletion of E-box 3 significantly reversed the MYC effect on the PPR-3 activity (right panel). *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.
Consistently, upstream of the TSS, the ChIP-seq signal of the MYC binding partner MAX (supplemental Fig. S1) is as strong as MYC, whereas downstream of the TSS, it is much lower. These data further support the hypothesis that MYC regulates the transcription of MC-let-7a-1∼let-7d through these two E-boxes.
Binding of MYC with the Two MC-let-7a-1∼let-7d E-boxes in HepG2 and L02 Cells
To confirm whether MC-let-7a-1∼let-7d promoter is a direct MYC target, we further performed the ChIP assay by using a MYC-specific antibody in HepG2 cells and L02 cells. Because these two E-boxes are located within 650 bp, we sheared the fixed chromatin to an average length of 400 bp by sonication to enhance resolution (Fig. 7A). Real time PCR amplicons were designed to detect two putative E-box-binding sites in ChIP samples. Strong MYC binding with this locus was observed in both E-box 2 and E-box 3 but not in the control upstream AA site, which lacks the MYC binding E-box, or in the IgG negative control (Fig. 7, B and C). Importantly, we found that in the HepG2 cells, the binding quantum of MYC to E-box 3 is similar or slightly higher than that of the E-box 2 (Fig. 7C), and in L02 cells, the binding quantum of MYC to E-box 3 was significantly lower (50% lower) than that of the E-box 2.
FIGURE 7.
Characterization of MC-let-7a-1∼let-7d as a direct MYC target gene and binding depends upon cell type. A, agarose gel demonstrates sonication of chromatin from HepG2 and L02 cells to a length between 300 and 500 bp. B, chromatin from HepG2 and L02 cells was immunoprecipitated by using antibodies against MYC protein. PCR detection of the E-box sites showed binding of MYC to E-box 2. Antibody against IgG was used as a negative control. C, qRT-PCR assays were performed to quantify the MYC binding quantum. Schematic representation of the location of AA, E-box 2, and E-box 3 (upper panel) is shown. The results are summarized as relative binding (MYC ChIP relative to input ChIP). The enrichment of the E-box 3 was significantly less in comparison with the enrichment of the E-box 2 in L02 cells, whereas no significant difference was found in HepG2 cells. D, binding activity of MYC to E-box 2 is stronger than that to E-box 3. 200-Fold unlabeled E-box 3 could not compete 5′ biotin-labeled E-box 2, whereas 200-fold unlabeled E-box 2 could compete 5′ biotin-labeled E-box 3. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.
We further analyzed the relative binding activity of E-box 2 and E-box 3 by EMSA. As shown in Fig. 7D, unlabeled E-box 3 could not compete with E-box 2 (1:200-fold molar excess), and 200-fold unlabeled E-box 2 could abrogate the shift band of the E-box 3-protein complex, suggesting that the binding activity of E-box 2 is stronger than that of E-box 3. These results suggest that the relative binding quantum of a MYC to E-box 3 and E-box 2 is at least partially responsible for the MYC-mediated decrease of the transcription of the MC-let-7a-1∼let-7d in the cancerous HepG2 cells.
let-7 Could Partially Decrease MYC-induced Cell Proliferation
We overexpressed MYC in L02 cells and observed enhanced cell proliferation (Fig. 8A) and reduced expression of let-7 family members (Fig. 8B), and cell cycle distribution analysis by flow cytometry showed increased cell entry into S phase (Fig. 8C). Consistently, when we overexpressed let-7a-1 in L02 cells, we observed decreased cell proliferation rate (Fig. 8A), and a reversed MYC-mediated shift in cell distribution, with an accumulation of G0/G1- and G2/M-phase cells and a corresponding reduction of S phase cells (Fig. 8C).
FIGURE 8.
let-7 could partially slow down cell proliferation induced by MYC. A, forced expression of MYC in L02 cells induced the cell proliferation by MTT assays. Forced expression of let-7a-1 simultaneously could reverse the MYC-mediated cell proliferation induction. B, qRT-PCR analysis demonstrated that overexpression of MYC down-regulated all the let-7 family members. C, forced expression of MYC in L02 cells promoted entry into S phase. let-7a-1 reversed the MYC-mediated shift of the cell cycle distribution, with an accumulation of G0/G1 and G2/M phase cells and a corresponding reduction of S phase cells.
DISCUSSION
Emerging evidence has shown that let-7 family members are tumor-suppressor miRNAs that are down-regulated in many types of cancers (13, 18). Elegant works have been performed and showed that let-7 is regulated post-transcriptionally (30–40). However, the regulation at the transcription level is not fully understood. In this study, we characterized the transcription machinery of MC-let-7a-1∼let-7d and revealed molecular mechanisms underlying the differential MYC-mediated transcription repression in human hepatocarcinoma HepG2 cells as compared with the noncancerous liver L02 cells. We showed that the binding quantum of MYC to the E-box 3 (suppress) relative to E-box 2 (activate) is significantly lower in noncancerous L02 cells than in those of HepG2 liver cancer cells, which may contribute to the differential transcriptional activity in these two cell lines.
The let-7 family accounts for a very high percentage of the miRNAs (21, 53). The evolutionarily conserved E-box binding MYC is one of the most commonly activated oncoproteins associated with the pathogenesis of liver cancer (54, 55). Overexpression of MYC can induce HCC with a high frequency in mice, whereas inhibition of MYC expression results in a loss of the neoplastic properties of carcinoma (56, 57). This is consistent with the recent report suggesting that widespread miRNA repression by MYC contributes to tumorigenesis (41). MYC has been demonstrated to repress the expression of let-7 post-transcriptionally through LIN28B and LIN28 in multiple human and mouse tumor models (30–40). The possibility that MYC could also regulate the transcription was first suggested by a report that MYC binds to a conserved site upstream of the microRNA let-7a-1/let-7f-1/let-7d cluster (41). However, to the best of our knowledge, the transcription machinery and the exact role of MYC in let-7 transcription regulation have not been reported.
In this study, we identified the TSSs of MC-let-7a-1∼let-7d, and demonstrated that multiple TSSs were used in HepG2 cells and L02 cells. An SNP site (rs11792471) was found in the 30-bp region. According to the UCSC Genome Browser, in this region the CTCF ChIP-seq signal was different in different cells. This raised the possibility that alternative transcription initiation sites might influence the transcription of MC-let-7a-1∼let-7.
The combination of computational prediction and strand-specific RT-PCR data suggested that the three let-7 family members in this cluster are encoded by a single polycistronic transcript with a promoter located about 10 kb upstream of their encoding region. The transcription activity of the core promoter (−540 to +1 bp), with the maximal promoter activity, is almost equivalent to the SV40 promoter. The truncation of the downstream region from +1317 to +1 dramatically enhanced the promoter activity, suggesting the presence of suppression elements downstream of the transcription initiation site.
In fact, we found two presumptive canonical MYC-binding sites (CACGTG) located upstream and a noncanonical binding site (CGCGTG) located downstream of the MC-let-7a-1∼let-7d TSS. Our study revealed that MYC acts as an activator in binding to the canonical MYC-binding site upstream of the TSSs (E-box 2), although it acts as suppressor in binding to the noncanonical binding site downstream of the TSSs (E-box 3).
When MYC stimulates transcription, it dimerizes with its binding partner MAX and binds to genomic DNA directly (58, 59). When it represses transcription, MYC does not directly bind to DNA, but it is recruited through protein-protein interactions to the promoters (60). As shown in supplemental Fig. S1, the MAX ChIP-seq signal upstream of the TSSs, is strong, suggesting that MYC activates MC-let-7a-1∼let-7d promoter by directly binding and dimerizes with MAX to enhance MC-let-7a-1∼let-7d promoter activity through binding with E-box 2. In contrast, the MAX ChIP-seq signal downstream of the TSSs is much lower than MYC, suggesting that MYC does not dimerize with MAX, and this is consistent with its role in repressing MC-let-7a-1∼let-7d transcription through binding E-box 3.
Taken together, these results suggest that the ratio of MYC and MAX in the nucleus is the key factor controlling the transcription of MC-let-7a-1∼let-7d. As we have demonstrated, the relative binding activity of MYC and E-box 2 is higher than that of MYC and E-box 3. Therefore, in normal cells, when MYC/MAX ratio is normal, MYC may dimerize with MAX and bind E-box 2 to enhance the transcription of MC-let-7a-1∼let-7d. The let-7 produced in turn binds to c-MYC mRNA and inhibits MYC translation. However, in cancer cells, when the MYC/MAX ratio is high, the extra MYC will bind to E-box 3 and repress the transcription of MC-let-7a-1∼let-7d. This will in turn decrease the let-7 level and abolish its inhibitory effect to MYC and ensure that the MYC protein will be translated. This mechanism may be partly responsible for the differential rate of MC-let-7a-1∼let-7d transcription in different cancer and noncancer cells. Further studies are also required to determine whether this transcription regulation is HCC specific or exists in other types of cancers.
Supplementary Material
Acknowledgments
We thank Dr. David Wilmshurst for editing and Dr. William K. C. Cheung for constructive comments.
This work was supported by National Basic Research Program of China (973 Program) Grants 2010CB529400 and 2010CB912800, National Natural Science Foundation of China Grant PC:81001023, and Research Grants Council of Hong Kong Grant 467109.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1 and Tables S1–S3.
- miRNA
- microRNA
- HCC
- hepatocellular carcinoma
- TSS
- transcription start site
- RT-PCR
- reverse transcription-PCR
- qRT-PCR
- quantitative real time-PCR
- 5′-RACE
- 5′-rapid amplification Of cDNA ends
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
REFERENCES
- 1. Croce C. M., Calin G. A. (2005) Cell 122, 6–7 [DOI] [PubMed] [Google Scholar]
- 2. Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Rådmark O., Kim S., Kim V. N. (2003) Nature 425, 415–419 [DOI] [PubMed] [Google Scholar]
- 3. Gregory R. I., Yan K. P., Amuthan G., Chendrimada T., Doratotaj B., Cooch N., Shiekhattar R. (2004) Nature 432, 235–240 [DOI] [PubMed] [Google Scholar]
- 4. Calin G. A., Sevignani C., Dumitru C. D., Hyslop T., Noch E., Yendamuri S., Shimizu M., Rattan S., Bullrich F., Negrini M., Croce C. M. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2999–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen C. Z. (2005) N. Engl. J. Med. 353, 1768–1771 [DOI] [PubMed] [Google Scholar]
- 6. Kent O. A., Mendell J. T. (2006) Oncogene 25, 6188–6196 [DOI] [PubMed] [Google Scholar]
- 7. Zhang B., Pan X., Cobb G. P., Anderson T. A. (2007) Dev. Biol. 302, 1–12 [DOI] [PubMed] [Google Scholar]
- 8. Bai S., Nasser M. W., Wang B., Hsu S. H., Datta J., Kutay H., Yadav A., Nuovo G., Kumar P., Ghoshal K. (2009) J. Biol. Chem. 284, 32015–32027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyerinas B., Park S. M., Hau A., Murmann A. E., Peter M. E. (2010) Endocr-Relat. Cancer 17, F19–F36 [DOI] [PubMed] [Google Scholar]
- 10. Diao S., Zhang J. F., Wang H., He M. L., Lin M. C., Chen Y., Kung H. F. (2010) Proteomics 10, 3723–3731 [DOI] [PubMed] [Google Scholar]
- 11. Hatley M. E., Patrick D. M., Garcia M. R., Richardson J. A., Bassel-Duby R., van Rooij E., Olson E. N. (2010) Cancer Cell 18, 282–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pineau P., Volinia S., McJunkin K., Marchio A., Battiston C., Terris B., Mazzaferro V., Lowe S. W., Croce C. M., Dejean A. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson S. M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K. L., Brown D., Slack F. J. (2005) Cell 120, 635–647 [DOI] [PubMed] [Google Scholar]
- 14. Sampson V. B., Rong N. H., Han J., Yang Q., Aris V., Soteropoulos P., Petrelli N. J., Dunn S. P., Krueger L. J. (2007) Cancer Res. 67, 9762–9770 [DOI] [PubMed] [Google Scholar]
- 15. Shimizu S., Takehara T., Hikita H., Kodama T., Miyagi T., Hosui A., Tatsumi T., Ishida H., Noda T., Nagano H., Doki Y., Mori M., Hayashi N. (2010) J. Hepatol. 52, 698–704 [DOI] [PubMed] [Google Scholar]
- 16. Takamizawa J., Konishi H., Yanagisawa K., Tomida S., Osada H., Endoh H., Harano T., Yatabe Y., Nagino M., Nimura Y., Mitsudomi T., Takahashi T. (2004) Cancer Res. 64, 3753–3756 [DOI] [PubMed] [Google Scholar]
- 17. Peter M. E. (2009) Cell Cycle 8, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lan F. F., Wang H., Chen Y. C., Chan C. Y., Ng S. S., Li K., Xie D., He M. L., Lin M. C., Kung H. F. (2011) Int. J. Cancer 128, 319–331 [DOI] [PubMed] [Google Scholar]
- 19. Wang Y. C., Chen Y. L., Yuan R. H., Pan H. W., Yang W. C., Hsu H. C., Jeng Y. M. (2010) Carcinogenesis 31, 1516–1522 [DOI] [PubMed] [Google Scholar]
- 20. Roush S., Slack F. J. (2008) Trends Cell Biol. 18, 505–516 [DOI] [PubMed] [Google Scholar]
- 21. Landgraf P., Rusu M., Sheridan R., Sewer A., Iovino N., Aravin A., Pfeffer S., Rice A., Kamphorst A. O., Landthaler M., Lin C., Socci N. D., Hermida L., Fulci V., Chiaretti S., Foà R., Schliwka J., Fuchs U., Novosel A., Müller R. U., Schermer B., Bissels U., Inman J., Phan Q., Chien M., Weir D. B., Choksi R., De Vita G., Frezzetti D., Trompeter H. I., Hornung V., Teng G., Hartmann G., Palkovits M., Di Lauro R., Wernet P., Macino G., Rogler C. E., Nagle J. W., Ju J., Papavasiliou F. N., Benzing T., Lichter P., Tam W., Brownstein M. J., Bosio A., Borkhardt A., Russo J. J., Sander C., Zavolan M., Tuschl T. (2007) Cell 129, 1401–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang J., Lee E. J., Gusev Y., Schmittgen T. D. (2005) Nucleic Acids Res. 33, 5394–5403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yanaihara N., Caplen N., Bowman E., Seike M., Kumamoto K., Yi M., Stephens R. M., Okamoto A., Yokota J., Tanaka T., Calin G. A., Liu C. G., Croce C. M., Harris C. C. (2006) Cancer Cell 9, 189–198 [DOI] [PubMed] [Google Scholar]
- 24. Gramantieri L., Ferracin M., Fornari F., Veronese A., Sabbioni S., Liu C. G., Calin G. A., Giovannini C., Ferrazzi E., Grazi G. L., Croce C. M., Bolondi L., Negrini M. (2007) Cancer Res. 67, 6092–6099 [DOI] [PubMed] [Google Scholar]
- 25. Zhang H. H., Wang X. J., Li G. X., Yang E., Yang N. M. (2007) World J. Gastroenterol. 13, 2883–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Müller D. W., Bosserhoff A. K. (2008) Oncogene 27, 6698–6706 [DOI] [PubMed] [Google Scholar]
- 27. Zhu Y. M., Zhong Z. X., Liu Z. M. (2010) World J. Gastroenterol. 16, 3325–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shell S., Park S. M., Radjabi A. R., Schickel R., Kistner E. O., Jewell D. A., Feig C., Lengyel E., Peter M. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11400–11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ricarte-Filho J. C., Fuziwara C. S., Yamashita A. S., Rezende E., da-Silva M. J., Kimura E. T. (2009) Transl. Oncol. 2, 236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heo I., Joo C., Cho J., Ha M., Han J., Kim V. N. (2008) Mol. Cell 32, 276–284 [DOI] [PubMed] [Google Scholar]
- 31. Newman M. A., Thomson J. M., Hammond S. M. (2008) RNA 14, 1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piskounova E., Viswanathan S. R., Janas M., LaPierre R. J., Daley G. Q., Sliz P., Gregory R. I. (2008) J. Biol. Chem. 283, 21310–21314 [DOI] [PubMed] [Google Scholar]
- 33. Rybak A., Fuchs H., Smirnova L., Brandt C., Pohl E. E., Nitsch R., Wulczyn F. G. (2008) Nat. Cell Biol. 10, 987–993 [DOI] [PubMed] [Google Scholar]
- 34. Viswanathan S. R., Daley G. Q., Gregory R. I. (2008) Science 320, 97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang T. C., Zeitels L. R., Hwang H. W., Chivukula R. R., Wentzel E. A., Dews M., Jung J., Gao P., Dang C. V., Beer M. A., Thomas-Tikhonenko A., Mendell J. T. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3384–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dangi-Garimella S., Yun J., Eves E. M., Newman M., Erkeland S. J., Hammond S. M., Minn A. J., Rosner M. R. (2009) EMBO J. 28, 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu L., Katsaros D., Shaverdashvili K., Qian B., Wu Y., de la Longrais I. A., Preti M., Menato G., Yu H. (2009) Eur. J. Cancer 45, 2212–2218 [DOI] [PubMed] [Google Scholar]
- 38. Sakamoto S., Aoki K., Higuchi T., Todaka H., Morisawa K., Tamaki N., Hatano E., Fukushima A., Taniguchi T., Agata Y. (2009) Mol. Cell. Biol. 29, 3754–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Viswanathan S. R., Powers J. T., Einhorn W., Hoshida Y., Ng T. L., Toffanin S., O'Sullivan M., Lu J., Phillips L. A., Lockhart V. L., Shah S. P., Tanwar P. S., Mermel C. H., Beroukhim R., Azam M., Teixeira J., Meyerson M., Hughes T. P., Llovet J. M., Radich J., Mullighan C. G., Golub T. R., Sorensen P. H., Daley G. Q. (2009) Nat. Genet. 41, 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. West J. A., Viswanathan S. R., Yabuuchi A., Cunniff K., Takeuchi A., Park I. H., Sero J. E., Zhu H., Perez-Atayde A., Frazier A. L., Surani M. A., Daley G. Q. (2009) Nature 460, 909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chang T. C., Yu D., Lee Y. S., Wentzel E. A., Arking D. E., West K. M., Dang C. V., Thomas-Tikhonenko A., Mendell J. T. (2008) Nat. Genet. 40, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Griffiths-Jones S., Saini H. K., van Dongen S., Enright A. J. (2008) Nucleic Acids Res. 36, D154–D158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. ENCODE Project Consortium (2004) Science 306, 636–640 [DOI] [PubMed] [Google Scholar]
- 44. Kawaji H., Severin J., Lizio M., Waterhouse A., Katayama S., Irvine K. M., Hume D. A., Forrest A. R., Suzuki H., Carninci P., Hayashizaki Y., Daub C. O. (2009) Genome Biol. 10, R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Livak K. J., Schmittgen T. D. (2001) Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 46. Popov N., Wanzel M., Madiredjo M., Zhang D., Beijersbergen R., Bernards R., Moll R., Elledge S. J., Eilers M. (2007) Nat. Cell Biol. 9, 765–774 [DOI] [PubMed] [Google Scholar]
- 47. Heckman K. L., Pease L. R. (2007) Nat. Protoc. 2, 924–932 [DOI] [PubMed] [Google Scholar]
- 48. Xia H., Ng S. S., Jiang S., Cheung W. K., Sze J., Bian X. W., Kung H. F., Lin M. C. (2010) Biochem. Biophys. Res. Commun. 391, 535–541 [DOI] [PubMed] [Google Scholar]
- 49. Chakrabarti S. K., James J. C., Mirmira R. G. (2002) J. Biol. Chem. 277, 13286–13293 [DOI] [PubMed] [Google Scholar]
- 50. Bortolin-Cavaillé M. L., Dance M., Weber M., Cavaillé J. (2009) Nucleic Acids Res. 37, 3464–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arabi A., Wu S., Ridderstråle K., Bierhoff H., Shiue C., Fatyol K., Fahlén S., Hydbring P., Söderberg O., Grummt I., Larsson L. G., Wright A. P. (2005) Nat. Cell Biol. 7, 303–310 [DOI] [PubMed] [Google Scholar]
- 52. Kim J. W., Zeller K. I., Wang Y., Jegga A. G., Aronow B. J., O'Donnell K. A., Dang C. V. (2004) Mol. Cell. Biol. 24, 5923–5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marson A., Levine S. S., Cole M. F., Frampton G. M., Brambrink T., Johnstone S., Guenther M. G., Johnston W. K., Wernig M., Newman J., Calabrese J. M., Dennis L. M., Volkert T. L., Gupta S., Love J., Hannett N., Sharp P. A., Bartel D. P., Jaenisch R., Young R. A. (2008) Cell 134, 521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shachaf C. M., Kopelman A. M., Arvanitis C., Karlsson A., Beer S., Mandl S., Bachmann M. H., Borowsky A. D., Ruebner B., Cardiff R. D., Yang Q., Bishop J. M., Contag C. H., Felsher D. W. (2004) Nature 431, 1112–1117 [DOI] [PubMed] [Google Scholar]
- 55. Lüscher B., Eisenman R. N. (1990) Genes Dev. 4, 2025–2035 [DOI] [PubMed] [Google Scholar]
- 56. Simile M. M., De Miglio M. R., Muroni M. R., Frau M., Asara G., Serra S., Muntoni M. D., Seddaiu M. A., Daino L., Feo F., Pascale R. M. (2004) Carcinogenesis 25, 333–341 [DOI] [PubMed] [Google Scholar]
- 57. Wu Y., Renard C. A., Apiou F., Huerre M., Tiollais P., Dutrillaux B., Buendia M. A. (2002) Oncogene 21, 1518–1526 [DOI] [PubMed] [Google Scholar]
- 58. Blackwood E. M., Eisenman R. N. (1991) Science 251, 1211–1217 [DOI] [PubMed] [Google Scholar]
- 59. Zeller K. I., Zhao X., Lee C. W., Chiu K. P., Yao F., Yustein J. T., Ooi H. S., Orlov Y. L., Shahab A., Yong H. C., Fu Y., Weng Z., Kuznetsov V. A., Sung W. K., Ruan Y., Dang C. V., Wei C. L. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 17834–17839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kleine-Kohlbrecher D., Adhikary S., Eilers M. (2006) Curr. Top. Microbiol. Immunol. 302, 51–62 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.