Background: Membrane depolarization induced an elevation of membrane PIP2 through increased PI4 kinase activity in Xenopus oocytes.
Results: The depolarization activated PKC βII and enhanced its interaction with PI4 kinase β.
Conclusion: The depolarization-induced elevation of PIP2 is through activation of PKC and the subsequent increased activity of PI4K β.
Significance: This study presents a novel mechanism for phosphoinositide metabolism that could have broad physiological implications.
Keywords: Phosphatidylinositol, Plasma Membrane, Potassium Channels, Protein Kinase C (PKC), Xenopus, Depolarization, PI4-Kinase, PIP2, PKC βII, Membrane Potential
Abstract
In a previous study, we showed that membrane depolarization induced elevation of membrane phosphatidylinositol 4,5-bisphosphates (PtdIns(4,5)P2, also known as PIP2) and subsequently increased the KCNQ2/Q3 currents expressed in Xenopus oocytes through increased PI4 kinase activity. In this study, the underlying mechanism for this depolarization-induced enhancement of PIP2 synthesis was further investigated. Our results indicate that activation of protein kinase C (PKC) isozyme βII was responsible for the enhanced PIP2 synthesis. We found that phorbol-12-myristate, 13-acetate (PMA), an activator of PKC, mimicked the effects of the membrane depolarization by increasing KCNQ2/Q3 activity, elevating membrane PIP2 levels and increasing activity of PI4 kinase β. Furthermore, membrane depolarization enhanced PKC activity. The effects of both depolarization and PMA were blocked by a PKC inhibitor or PI4 kinase β RNA interference. Further results demonstrate that the depolarization selectively activated the PKC βII isoform and enhanced its interaction with PI4 kinase β. These results reveal that the depolarization-induced elevation of membrane PIP2 is through activation of PKC and the subsequent increased activity of PI4 kinase β.
Introduction
PtdIns(4,5)P2 (PIP2)3 is a membrane phospholipid that regulates many important cellular processes, including the attachment of the cytoskeleton to the plasma membrane, exocytosis, endocytosis, membrane trafficking, and the activation of enzymes (1–3). At the same time, PIP2 is the phospholipid precursor of 3 second messengers: inositol trisphosphate (IP3), diacylglycerol (DAG), and phosphatidylinositol 3,4,5-trisphosphate (PIP3) (4). In addition, it is now clear that phosphoinositides are not only precursors, but can also act as second messengers themselves and play a role in different important nuclear signaling events such as cell cycle progression, apoptosis, chromatin remodeling, transcriptional regulation, and mRNA processing (5, 6). Finally, PIP2 modulates the function of ion transporters and ion channels (7–9), including potassium channels (i.e. inwardly rectifying K+ channels, M/KCNQ channels, two-pore channels, and EAG/ERG channels), voltage-gated calcium channels (i.e. P/Q-type, N-type, and L-type channels), and transient receptor potential channels (i.e. RPC, TRPM, TRPV1, and TRPA1). The mechanism underlying receptor-mediated inhibition of M/KCNQ potassium currents involves the hydrolysis of PIP2 by PLC, which is initiated by the activation of Gq-coupled receptors (9, 10).
Apart from PLC-induced, events-related cleavage of membrane PIP2, the steady-state PIP2 level in the cellular membrane is dynamically balanced by the activity of specific phosphoinositide kinases and specific lipid phosphatases. The dominant pathway of PIP2 synthesis is the sequential phosphorylation in the sarcolemma of phosphatidylinositol (PI) at the 4- and then the 5-positions of inositol (4). PI4P, the PI(4,5)P2 precursor, is synthesized from PI through phosphorylation by the PI4 kinase family; PIP is further catalyzed by PIP kinases to phosphatidylinositol 4,5-bisphosphate (PI4,5P2). Type I PIP kinases use PI4P as a substrate to create PI4,5P2, and type II kinases prefer PI5P as a substrate (11). Alteration of the activity of these kinases will inevitably change PIP2 levels and subsequently the PIP2-dependent protein or cell functions. The membrane PIP2 levels can be determined by assessing the function of a membrane ion channel whose activity depends on PIP2. Blockage of PI4 kinase by wortmannin or phenylarsine oxide blocks the resynthesis of PIP2 and thus the reactivation of M/KCNQ currents after inhibition induced by PIP2 hydrolysis (12, 13). When membrane PIP2 abundance was elevated by overexpression of PI(4)P5K, the channel activity of KCNQ2 and KCNQ2/3 dramatically increased (14); a similar maneuver greatly blunts the extent of M/KCNQ current inhibition by Gq/11-coupled receptor stimulation (15).
In our previous study, we described a novel mechanism for membrane PIP2 modulation. In this case, the membrane depolarization elevated membrane PIP2 levels and enhanced PIP2-dependent KCNQ2/Q3 currents expressed in Xenopus oocytes, and the enhanced PIP2 synthesis was possibly due to increased activity of PI4 kinase (16). However, it was not clear how the activity of PI4 kinase was enhanced. In the present study, we found that protein kinase C (PKC) βII was responsible for the activation of PI4 kinase.
EXPERIMENTAL PROCEDURES
cDNAs
The accession numbers for the KCNQ channels used are AF110020 for human KCNQ2 and AF091247 for rat KCNQ3. cDNAs were subcloned into the pGEMHE expression vector for Xenopus oocytes. cRNA was transcribed in vitro using a RiboMAXTM Large Scale RNA Production System, T7 kit (Promega, Madison, WI).
Xenopus Oocyte Isolation and cRNA Injection
Oocytes were surgically isolated from anesthetized adult female frogs (Xenopus laevis) as previously described (17) and treated with 2 mg/ml collagenase (Type II, Sigma) in OR2 solution (in mm: 82.5 NaCl, 2 KCl, 1 MgCl2, 5 HEPES, pH 7.4) for 90 min at room temperature (∼25 °C). After three washes with OR2 solution, the oocytes were incubated at 18 °C in ND96 solution (in mm: 96 NaCl, 1 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES, pH 7.4). cRNA was injected in the range of 0.1–1 ng/oocyte depending on the expression level of the given constructs. Recordings were performed 1–2 days after cRNA injection of the Xenopus oocytes.
Two-electrode Voltage Clamp (TEVC)
Currents from Xenopus oocytes were measured in oocytes 1–2 days after cRNA injection using a two-electrode voltage clamp with 0.5–1.0 MΩ microelectrodes filled with 3 m KCl (pH 7.2) and a Geneclamp 500B amplifier (Axon Instruments). The two external solutions were as follows: 1) ND96 solution (in mm): 96 NaCl, 1 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES; and 2) ND96-K solution (in mm): 96 KCl, 1 NaCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES. All solutions were adjusted with NaOH to pH 7.4. All experiments were carried out at room temperature (20–25 °C). The membrane depolarization was induced either by voltage-clamped depolarization or by high K+ (ND96-K) solution.
Western Blots
The protein lysates were prepared as previously described (16). 100 oocytes from either the control group or the drug-treated group were used for Western blot studies. Oocytes were solubilized in 500 μl of lysis buffer on ice. The lysis buffer contained the following reagents (in mm): 5 Tris-HCl, 1 EDTA, 1 EGTA, 10 Na3VO4, and 10 NaF, and (in μg/ml) 30 PMSF, 10 pepstatin A, 2.5 leupeptin, and 10 aprotinin. Homogenates were centrifuged at 400 rpm for 10 min at 4 °C to remove yolk granules. The supernatant was centrifuged at 12,000 × g for 30 min at 4 °C, yielding a whole cell protein extract that was used for protein interaction experiments. The membrane proteins used for PKC isoform identification were separated by ultracentrifugation (18). In brief, oocytes were homogenized in pre-cooled homogenizer and centrifuged at 5,000 rpm for 5 min. The supernatant was further centrifuged at 100,000 × g for 60 min. The pellet containing the crude membrane fraction was resuspended in lysis buffer with 0.2% Triton X-100. The proteins were mixed with loading buffer (10% glycerol, 50 mm Tris HCl, 2% SDS, 5% β-mercaptoethanol, and 0.02% bromphenol blue), heat denatured at 99 °C for 5 min and subjected to SDS-PAGE. The proteins resolved by 10% SDS-PAGE were transferred to polyvinylidene difluoride (PVDF, Millipore, Billerica, MA) membranes in transfer buffer (20% methanol, 15.6 mm Tris base, and 120 mm glycine) for 3 h at 100 V. The membranes were probed with antibodies against PI4K β (1:500; Upstate, Lake Placid, NY) or PKC isoforms (1:2,000; Epitomics, CA) for 1 h at room temperature or overnight at 4 °C. Nonspecific binding was blocked with 1.5% (w/v) evaporated skim milk (Difco, Becton Drive Franklin Lakes, NJ) in TBS (154 mm NaCl, 10 mm Tris base). Anti-rabbit or anti-mouse secondary antibodies conjugated to IRDye 700DX or IRDye 800CW (1:5,000; Rockland, Gilbertsville, PA) were used to probe primary antibodies. Protein bands were detected and quantified on an Odyssey two-color infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Co-immunoprecipitation Assay
Lysates from Xenopus oocytes were incubated with 0.5 μg of the appropriate antibody and 100 μl oocyte lysate with gentle rocking for 4 h at 4 °C. Protein G beads (30 μl, Santa Cruz, CA) were then added with rocking for 2 h at 4 °C. After washing five times with washing buffer (PBS buffer, protease inhibitors, 0.5% Nonidet P-40, and 0.1% Triton X-100), the co-IP samples were subjected to SDS-PAGE and immunoblot analysis using the appropriate antibodies. Cell lysates incubated with protein G beads alone were routinely used as a negative control. Studies were repeated at least three times.
dsRNAs and Small Interfering RNA (siRNA)
Detailed information for PI4K dsRNA has been previously described (16). The siRNA templates targeting the Xenopus PKC β mRNA sequence were designed and synthesized by Invitrogen (Eugene, OR). The siRNA sequences were as follows (19): siRNA for PKC β: 5′-GCGGUGUCAUGAUGAGUUUGUCACGUUU-3′, 5′-AAACGUGACAAACUCAUGACACCGC-3′, 5′-CGUUUGGUAUCUCGGAGCUACUGAA-3′; Scrambled siRNA for PKC β: 5′-ACT ACATCAGTAGCCTGTCTC-3′, 5′-ATGTAGTCTCACGCCTGTCTC-3′.
The siRNAs were stored at −20 °C in aliquots (final concentration of 100 μm), and a volume of 43 nl was injected into the cytoplasm of the oocytes the day prior to their testing.
Membrane PIP2 Assay by TLC
The method using thin layer chromatography (TLC) to measure PIP2 has been previously described (16). Briefly, oocyte membrane lipids were extracted with chloroform-methanol. The mobile phase for TLC was chloroform/methanol/4N NH4OH (45/35/10, v/v/v). Phospholipids were visualized with iodine vapor.
In Vitro PKC Activity Assay
The PKC activity of Xenopus oocytes was measured using the PepTag assay kit for non-radioactive detection of phosphorylated and non-phosphorylated PKC (Promega). Briefly, 100–200 oocytes were homogenized in 1 ml of cold PKC extraction buffer. The lysate was centrifuged at 14,000 rpm for 5 min at 4 °C, and the supernatant was saved for PKC activity assays.
Chemicals
Chemicals were purchased from Sigma, Invitrogen, or Selleck Chemicals (Houston, TX). Stock solutions were made in DMSO, stored at −20 °C, and diluted in the appropriate solution immediately before use. The final concentration of DMSO was less than 0.1%.
Data Analysis and Statistics
Data were analyzed and fitted using Clampfit 9.2 (Axon Instruments), Origin 7.5 (OriginLab Corp.) software and the Odyssey analysis system. Results are expressed as mean ± S.E. Each experiment was replicated between three to fifteen times. Differences were analyzed with Student's paired/unpaired t test or one-way ANOVA when appropriate, and were considered significant when *, p < 0.05 or **, p < 0.01.
RESULTS
PMA Mimics the Effects of the Membrane Depolarization
In a previous study, we showed that membrane depolarization elevated membrane PIP2 levels and enhanced PIP2-dependent KCNQ2/Q3 currents expressed in Xenopus oocytes; the depolarization-induced elevation of PIP2 levels was a result of increased PI4 kinase activity (16). However, the underlying mechanism for the increased PI4K kinase activity was unknown. In a separate experiment, we found that phorbol 12-myristate 13-acetate (PMA), an activator of PKC, was also able to increase the activity of KCNQ2/Q3 currents expressed in Xenopus oocytes. This prompted us to hypothesize that PKC could be involved in the depolarization-induced effects on the phospholipid metabolism.
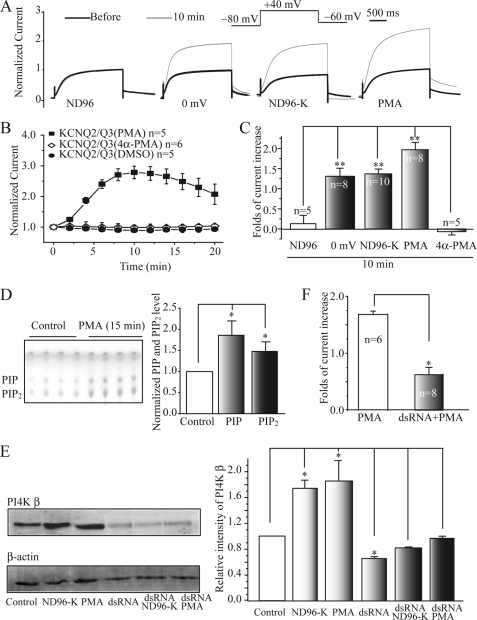
PMA affected the function of KCNQ2/Q3 channels expressed in Xenopus oocytes in a similar way to the depolarization (Fig. 1A). The membrane depolarization was induced by either voltage-clamp at 0 mV or by incubation in a high K+ (ND96-K) solution (16). Treatment with PMA (50 nm) for 10 min increased KCNQ2/Q3 by 1.98 ± 0.21 fold (n = 8). Fig. 1B shows the time course of PMA action on KCNQ currents. The oocytes were held at −80 mV continuously, and multiple brief depolarizations (2 s) to +40 mV were applied to activate KCNQ2/Q3 and to get a measure of the current amplitude at each given time. This protocol minimized the effect of the depolarization (16). 4α-PMA was used as a negative control. PMA, but not 4α-PMA, increased KCNQ2/Q3 currents (Fig. 1B). The results shown in Fig. 1C demonstrate that PMA increased KCNQ2/Q3 currents to a greater extent than the depolarization did.
FIGURE 1.
PMA and membrane depolarization increase KCNQ2/Q3 currents through PI4 kinase β-mediated synthesis of PIP2. A, PMA and membrane depolarization (0 mV and high K+ solution, ND96-K) augment the amplitude of KCNQ2/Q3 currents expressed in Xenopus oocytes. The KCNQ2/Q3 currents were induced by the protocol shown at the top using the TEVC technique. The two current traces (before and 10 min) represent the current traces recorded before and after 10 min treatments of the oocytes with ND96 solution (control), 0 mV (depolarization), ND96-K solution (depolarization), or PMA (50 nm). During treatments, the oocytes were constantly held at −80 mV. B, time course for the effects of PMA (50 nm), 4α-PMA (50 nm), and solvent DMSO on normalized KCNQ2/Q3 currents. The KCNQ currents were measured at +40 mV. The 2 s depolarization at +40 mV was always followed by a longer (1 min) holding potential of −80 mV to avoid the effect of the depolarization. C, summary data for fold increase of KCNQ2/Q3 current after the 10 min treatments described in A (0 mV, 1.31 ± 0.04, n = 8), (ND96-K, 1.38 ± 0.03, n = 10), (PMA, 1.98 ± 0.06, n = 8). **, p < 0.01 compared with ND96 group. D, TLC method was used to measure PIP2 and PIP levels in oocytes from the control group and from the PMA-treated group (50 nm, 15 min). Triplicate samples from a single experiment are shown in the left panel. Summary data from three independent experiments are shown in the right panel. *, p < 0.05 compared with the control group. E, dsRNA for PI4 kinase β blocked PMA- or ND96-K (depolarization)-induced enhancement of PI4 kinase β (PI4K β) expression. The left panel shows the Western blot results for PI4Kβ expression after treatment with control, PMA, ND96K, dsRNA, PMA plus dsRNA, or dsRNA plus ND96K. The right panel shows the relative intensity of the blots from four independent experiments. *, p < 0.05 compared with control. F, in the oocytes injected with dsRNA for PI4K β, PMA increased KCNQ2/Q3 current by 0.65 ± 0.12-fold, which was significantly less than the control (1.68 ± 0.06, *, p < 0.05).
We measured the phosphoinositide levels directly using the TLC method (16). Fig. 1D shows the results of PIP and PIP2 dots visualized with iodine vapor. The identity of PIP and PIP2 was confirmed using mass spectrometry (16). Incubation with PMA for 15 min increased PIP and PIP2 levels in oocyte membranes by 87 ± 33% and 48 ± 23% (Fig. 1D), respectively. We then tested whether the PMA-induced potentiation of KCNQ currents was also due to increased PI4 kinase activity. Previously (16) and in this study (Fig. 1E), we showed that depolarization (ND96-K) increased expression of PI4 kinase β. Similarly, PMA treatment also induced an increase in PI4 kinase β expression by 88 ± 23% (Fig. 1E). To assess the role of PI4 kinase β in PMA-induced enhancement of KCNQ2/Q3 currents, the expression of PI4 kinase β in the oocytes was inhibited by dsRNA for PI4 kinase β (Fig. 1E). Importantly, PMA-induced enhancement of KCNQ2/Q3 currents was abolished by the PI4 kinase β knockdown (Fig. 1F). All of these results suggest that like the membrane depolarization, PMA increased KCNQ current activity and PIP2 levels through increasing the activity of PI4 kinase β.
Depolarization Increases the Activity of PKC
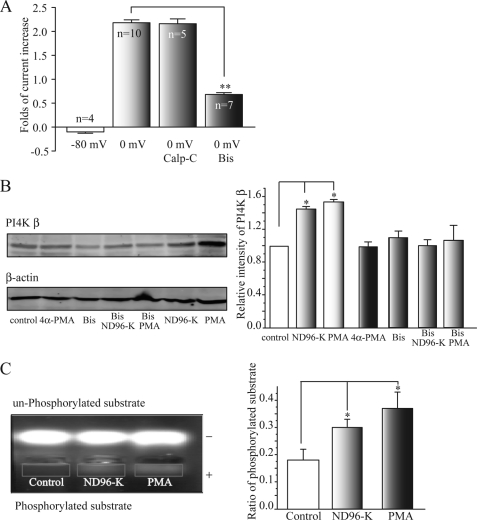
The above results suggest that the depolarization-induced enhancement of PI4 kinase β activity might be caused by PKC activation. To confirm this hypothesis, we first tested the effects of PKC blockers on the effects of the depolarization. We used two PKC blockers, calphostin C (Calp-C) and bisindolylmaleimide (Bis). Bis significantly reduced the depolarization-induced KCNQ2/Q3 current increase (from 2.27 ± 0.10-fold, to 0.68 ± 0.04-fold, n = 7, **, p < 0.01), whereas Calp-C did not. Bis was further tested for its effects on the depolarization- and PMA-induced increase in PI4 kinase β expression. Bis blocked both increases (Fig. 2B).
FIGURE 2.
PKC is involved in the depolarization-induced increased activity of PI4 kinase β. A, PKC blocker Bis significantly inhibited the depolarization (0 mV)-induced potentiation of KCNQ2/Q3 currents. Bis (1 μm) was applied for 2 h before PMA (50 nm) application. Calp-C did not inhibit the potentiation. **, p < 0.01 compared with the 0 mV group. B, bisindolylmaleimide blocked the depolarization- (ND-96K) and PMA-induced enhancement of PI4K β expression. Western blot band intensity was first normalized to the corresponding β-actin intensity and then was normalized again to the control. *, p < 0.05 compared with the control. C, depolarization (ND96-K) or PMA increases PKC activity. The oocytes were exposed to ND96-K for 15 min or PMA (50 nm) for 20 min. PKC activity, as demonstrated by the density of the downward-shifted PKC substrate peptide, was increased by incubation with either ND96-K or PMA. The phosphorylated peptide that served as a PKC substrate migrated toward the cathode (+), while the nonphosphorylated peptide migrated toward the anode (−). The right panel shows summary data for the ratio of phosphorylated substrate (band intensity of phosphorylated substrate (rectangle)/unphosphorylated) from seven independent experiments. *, p < 0.05 compared with the control.
Finally, the effects of the depolarization on the activity of PKC were assessed using a commercial kit (see “Experimental Procedures”). In this assay (20), the PKC substrate (a short 12-amino acid peptide) shifts in the electrophoresis in a distinct direction and quantity that is determined by the charges that the peptide carries. Upon being phosphorylated (by PKC), the peptide is negatively charged and will shift downward; otherwise it will shift upwards. Both the depolarization and PMA increased the amount of the phosphorylated peptide; thus the depolarization and PMA increased the activity of PKC by 31 ± 3% (n = 7) and 37 ± 6% (n = 7), respectively (Fig. 2C).
PKC βII May Be Involved in the Effects of Membrane Depolarization
Previous studies indicate that all 11 known mammalian isozymes of PKC are present in Xenopus oocytes (21, 22). However, after trying antibodies from different sources, we were only able to detect the presence of the PKC α, βI, βII, δ, and ϵ isozymes. Therefore, we studied which of these 5 isozymes could be involved in the effects of the membrane depolarization described above. The molecular weights of the PKC isozyme proteins in the Xenopus oocytes vary from 75 to 90 kDa, similar to their counterparts in mammals (23).
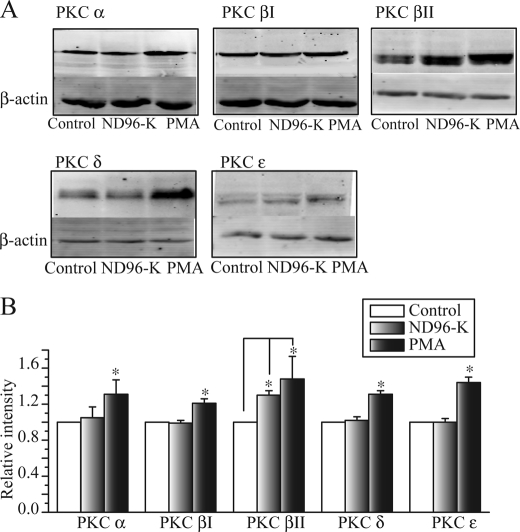
PKC translocation to the plasma membrane has been generally considered to be the hallmark of activation (24) and has been frequently used as a surrogate measure of PKC isoform activation in cells (19, 25). Translocation of the PKC isozymes after exposure to ND96-K (depolarization) for 15 min or PMA (50 nm) for 20 min was studied by Western blot, and the results are shown in Fig. 3. Among the 5 isozymes, only PKC βII was increased in the membrane by 30 ± 5% (n = 5) by the depolarization, whereas all 5 isozymes were increased by PMA treatment (Fig. 3, A and B).
FIGURE 3.
The depolarization and PMA selectively increase membrane PKC βII. The Xenopus oocytes were exposed to PMA (50 nm) for 20 min or depolarization (ND96-K) for 15 min. A, representative Western blots of membrane proteins from oocytes treated with control, ND96-K or PMA were probed with antibodies against PKC α, βI, βII, δ or ϵ. B, average values from densitometry analyses of more than three separate experiments. *, p < 0.05 compared with the control. The Western blot band intensity of each group was first normalized to each corresponding actin band intensity and then was normalized again to the control.
The Membrane Depolarization Selectively Enhances the Interaction between PKC βII and PI4 Kinase β
The above results suggest that there might be a direct interaction between PKC and PI4 kinase, such that the activation and translocation of PKC would bring more PI4 kinase to the plasma membrane. To test this hypothesis, we studied the interactions between PKC βII and PI4 kinase using co-immunoprecipitation assays. As a control, PKC α was also tested for its interaction with PI4 kinase β because PKC α was activated by PMA but not by the depolarization (Fig. 3), and both PKC α and PKC βII are members of the conventional PKC subfamily.
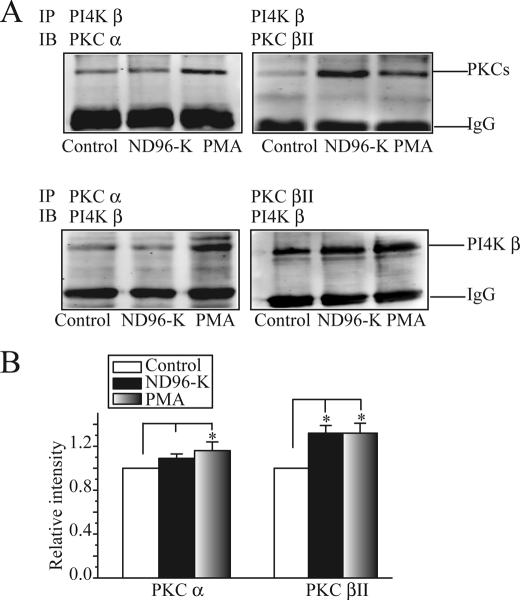
As shown in Fig. 4, when the proteins were immunoprecipitated with antibodies against PKC isozymes or PI4 kinase β, the interaction between PKC and PI4 kinase was clearly visible (Fig. 4A). We saw a low level of basal interactions between both PKC isozymes and PI4 kinase β. PMA (50 nm) enhanced the interaction of PI4 kinase with both PKC α and PKC βII, whereas the membrane depolarization only enhanced the interaction of PI4 kinase with PKC βII (Fig. 4, A and B).
FIGURE 4.
The depolarization enhances the interaction between PKC βII and PI4 kinase β. A, co-immunoprecipitation results for PKC α or PKC βII interaction. Protein lysates from oocytes treated with ND96, ND96-K, or PMA (50 nm) were immunoprecipitated and blotted with anti-PI4 kinase β, anti-PKC α, or anti-PKC βII antibodies. B, summary data for the relative intensity of the precipitates blotted with anti-PI4 kinase β, anti-PKC α, or anti-PKC βII antibodies from five independent experiments. *, p < 0.05 compared with the control.
We then tested the effects of knocking down PKC βII on the depolarization- and PMA-induced potentiation of KCNQ currents. In the Western blot results shown in Fig. 5A, homogenates from the control oocytes or oocytes injected for 16 h with either scrambled siRNA or PKC β siRNA were probed with the PKC βII antibody (see “Experimental Procedures”). Clearly, PKC β siRNA significantly inhibited PKC βII levels in oocytes compared with either control or scrambled siRNA (Fig. 5A). Under these conditions, the depolarization-induced enhancement of KCNQ currents was greatly reduced in siRNA-injected oocytes (1.5 ± 0.04, 0.12 ± 0.04- and 1.16 ± 0.07-fold increase in current for control, siRNA and scrambled siRNA, respectively) (Fig. 5B). In contrast, the PMA-induced potentiation of KCNQ currents was not significantly affected by siRNA for PKC βII (Fig. 5B). Furthermore, the siRNA for PKC α, which significantly inhibited PKC α expression, did not affect the depolarization-induced enhancement of KCNQ currents (data not shown). Thus the depolarization selectively activated PKC βII.
FIGURE 5.
PKC βII contributes to the depolarization-induced enhancement of KCNQ currents. A, PKC βII siRNA sufficiently suppressed PKC βII expression. B, PKC βII siRNA reduced the depolarization, but not PMA-induced increase of KCNQ2/Q3 currents. C, PKC β inhibitor enzastaurin (2 μm) significantly reduced the depolarization (ND96-K)-induced increase in membrane PKC βII levels. D, enzastaurin blocked the depolarization (0 mV)-induced increase of KCNQ2/Q3 current. **, p < 0.01 compared with the control.
Enzastaurin (LY317615) is a selective inhibitor of PKC β (26). Oocytes pretreated with enzastaurin (2 μm) significantly decreased the membrane translocation of PKC βII induced by the depolarization (Fig. 5C). Consistent with this result, enzastaurin significantly inhibited the depolarization-induced potentiation of KCNQ currents (Fig. 5D; the current increase was reduced from 1.57 ± 0.23 to 0.43 ± 0.28 fold).
DISCUSSION
In a previous report, we demonstrated that membrane depolarization increases cellular PIP2 levels through increased PIP2 synthesis mediated by PI4 kinase (16). However, the mechanism by which the depolarization increased the PI4 kinase activity was unknown. In this study, we used functional and biochemical assays to show that the increased activity of PI4 kinase β could be the result of PKC activation. Several pieces of evidence support this conclusion (1). PMA, an activator of PKC, increased KCNQ2/Q3 currents with a similar time course to the depolarization-induced increase (2). Similar to the depolarization, PMA increased cellular PIP2 levels (3). PMA and depolarization increased PI4 kinase β expression, and this effect was abrogated by dsRNA for PI4 kinase β or by the PKC blocker bisindolylmaleimide (4). PKC activity was increased by PMA and by depolarization (high K+ incubation).
Two PI4 kinase isoforms from Xenopus laevis were described: PI4 kinase α (PubMed, BC077604.1) and PI4 kinase β (PubMed, BC073706.1). However, we were unable to detect the 230-kDa form of PI4 kinase α protein. We clearly show that PI4 kinase β was activated by PKC, which led to increased PIP2 synthesis in both the case of depolarization and of PMA action. Similar observations have been reported in rat basophilic leukemia cells (27) and bovine sperm cells (28), where PMA increases the activity of PI4 kinase β and the synthesis of PIP and PIP2 through activation of PKC. However, in these studies, the relationship between PKC and PI4 kinase was established using pharmacological tools. No phosphorylation or any other mechanisms were determined.
PKC isoforms have unique (and in some cases opposing) functions in cells, at least in part as a result of isoform-specific subcellular compartmentalization patterns, protein-protein interactions, and post-translational modifications that influence catalytic function (24, 29–31). Many PKCs are pharmacologically activated by tumor-promoting phorbol esters such as PMA that anchor PKCs in their active conformations to membranes. According to the classical model of PKC activation, cellular PKC responses result from the collective actions of individual PKC isoforms (which traditionally are viewed as having only relatively limited in vitro substrate specificity) that are coexpressed in a particular cell type and localized to their distinctive subcellular compartments (in close proximity to their specific membrane substrate). Studies have revealed that PtdIns(4,5)P2 itself binds to a polybasic region in the C2 domain of PKC (32, 33). Studies have shown that eleven PKC isozymes are present in Xenopus oocytes (21, 22). We detected PKC α, βI, βII, δ, and ϵ isozymes in this present study (Fig. 3). Of the five PKCs (α, βI, βII, δ, and ϵ), PKC βII was translocated to the membrane in response to the depolarization (ND96-K, see Fig. 3A). The actions of the membrane depolarization were blocked by the PKC inhibitor enzastaurin (LY317615) and by PKC βII siRNA (see Fig. 5), further supporting the involvement of PKC βII in the effects of the membrane depolarization. The observation that siRNA for the PKC α isozyme did not affect depolarization actions demonstrates the specificity of PKC βII in the actions of ND96-K. Contrary to the depolarization, PMA caused translocation of all PKC isozymes present to the membrane (see Fig. 3).
Furthermore, the co-immunoprecipitation results presented in Fig. 4 suggest a direct interaction between PKC βII and PI4 kinase β. The membrane depolarization selectively enhanced the interaction between PKC βII and PI4 kinase β but not between PKC α and PI4 kinase β; in contrast, PMA enhanced the interaction between both PKC isozymes and PI4 kinase. These results clearly demonstrate the selective activation of PKC βII by the membrane depolarization. In agreement with this finding, siRNA for PKC βII abolished the depolarization-induced but not the PMA-induced enhancement of KCNQ current (Fig. 5), the latter of which could possibly be due to other PKC isozymes (i.e. PKC α). Our results indicate that PKC may mediate the depolarization-induced effects on phosphoinositide metabolism. However, it is still not clear how the PKC is activated by the depolarization. In skeletal muscle cells, the depolarization increases production of inositol 1, 4,5-trisphosphate (IP3) through activation of PLC and subsequently initiates a slow Ca2+ signal in the nuclei and the adjacent cytoplasm (34) that has been shown to increase the activity of PKC (35) and subsequently alter gene expression (36). l-type Ca2+ channels are believed to be the voltage-sensing mechanism (37). Thus in skeletal muscle cells, and possibly in other muscle cells such as vascular smooth muscle cells (38), the depolarization-initiated localized changes in Ca2+ levels seem to be responsible for the increased activity of PKC. Under these circumstances, extracellular Ca2+ is generally not needed. In neurons, although the membrane depolarization does increase the production of phosphatidylinositols such as IP3, which is also suggested to be the result of PLC activation, this process totally depends on the presence of extracellular Ca2+ (39). Our experiments do not support a similar mechanism in oocytes as described above for muscle cells. We have tried techniques that would greatly alter the intracellular Ca2+ level in Xenopus oocytes, and none of these affected the depolarization (ND96K)-induced enhancement of KCNQ currents (supplemental Fig. S1A). Additionally, no changes in intracellular Ca2+ levels were observed upon depolarization (supplemental Fig. S1B). Furthermore, the absence of extracellular Ca2+ did not affect the depolarization (ND96-K)-induced translocation of PKC βII to the membrane (supplemental Fig. S1C).
Next we questioned whether DAG, the endogenous activator of PKC produced by phospholipase C (PLC) and upstream mechanisms, could be the missing link to membrane potential. The results presented in supplemental Fig. S2 suggest this may not be the case. Treatments of oocytes with either OAG (10 μm, an analog of DAG) or m-3M3FBS (20 μm, a activator of PLC) did not affect the KCNQ2/Q3 currents in Xenopus oocytes (supplemental Fig. S2A). Activation of the EGF receptor has been shown to activate PLC in Xenopus oocytes (40). However, genistein (41), a tyrosine kinase blocker, did not inhibit the depolarization-induced potentiation of KCNQ2/Q3 currents (supplemental Fig. S2B).
Membrane proteins other than ion channel could also be direct voltage sensors. Accumulating evidence has begun to emerge showing that voltage-sensitive GPCR signaling can occur independently of Ca2+ signals. For example, membrane depolarization modulates signaling of muscarinic receptors and bradykinin receptors (42) in neuronal cells, M2 muscarinic receptors in Xenopus oocytes (43), and purinergic Y2Y1 and other Gq-coupled receptors in megakaryocytes (44); it has been suggested that all of these events use mechanisms localized at the GPCR per se, or involve a direct coupling interface between GPCR and immediate downstream effectors. Recently, two muscarinic receptors (M2R and M1R) were shown to have charge-movement-associated currents analogous to the gating currents of voltage-gated channels. The results indicate that GPCRs serve as sensors for both transmembrane potential and external chemical signals (45). It should be noted that while the depolarization-induced modulation of GPCR signaling is graded and has no apparent threshold or upper limit (42), our voltage-dependent potentiation of KCNQ2/Q3 currents by the depolarization resembles the voltage-dependent activation property of ion channels. Further studies are warranted to elucidate the voltage-sensing mechanism underlying activation of PKC.
What is the physiological relevance of the present study? First, the described mechanism may be involved in egg fertilization. During oocyte fertilization, the union of egg and sperm promotes a series of biochemical and cell biological changes within the fertilized egg. This phenomenon is termed egg activation (46). The egg activation is companied by an activation potential induced by an inward current (47), which could contribute to the increased synthesis of PIP2 seen during the fertilization of Xenopus oocytes (48). Secondly, the membrane voltage-dependent cell events have been reported in cells other than oocytes. For example, both voltage-dependent vesicle endo- and exocytosis have been seen in dorsal root ganglion neurons (49). It is well established that PIP2 plays an important role in endo- and exocytosis (1). Finally, the PKC-mediated activation of PI4 kinase could have wide physiological implications. We have observed the interaction of PKC β and PI4 kinase in rat cardiomyocytes,4 given that electrical activity increases PIP2 levels in cardiac myocytes (50), the mechanism described in this study should also be explored in the heart.
Supplementary Material
This work was supported by the National Natural Science Foundation of China (30730031, to H. Z.) and the National Basic Research Program (2007CB512100).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
H. Zhang, unpublished data.
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- PMA
- phorbol-12-myristate, 13-acetate
- TEVC
- two-electrode voltage clamp
- Bis
- bisindolylmaleimide
- Calp-C
- calphostin C.
REFERENCES
- 1. McLaughlin S., Wang J., Gambhir A., Murray D. (2002) Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 [DOI] [PubMed] [Google Scholar]
- 2. Di Paolo D., Lenci I., Trinito M. O., Carbone M., Longhi C., Tisone G., Angelico M. (2006) Dig. Liver Dis. 38, 749–754 [DOI] [PubMed] [Google Scholar]
- 3. Krauss M., Haucke V. (2007) FEBS Lett. 581, 2105–2111 [DOI] [PubMed] [Google Scholar]
- 4. Tolias K. F., Cantley L. C. (1999) Chem. Phys. Lipids 98, 69–77 [DOI] [PubMed] [Google Scholar]
- 5. Mortier E., Wuytens G., Leenaerts I., Hannes F., Heung M. Y., Degeest G., David G., Zimmermann P. (2005) EMBO J. 24, 2556–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonzales M. L., Anderson R. A. (2006) J. Cell. Biochem. 97, 252–260 [DOI] [PubMed] [Google Scholar]
- 7. Hilgemann D. W., Feng S., Nasuhoglu C. (2001) Sci. STKE 2001, re19. [DOI] [PubMed] [Google Scholar]
- 8. Suh B. C., Hille B. (2008) Annu. Rev. Biophys. 37, 175–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gamper N., Shapiro M. S. (2007) J. Physiol. 582, 967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suh B. C., Hille B. (2005) Curr. Opin Neurobiol. 15, 370–378 [DOI] [PubMed] [Google Scholar]
- 11. Rameh L. E., Tolias K. F., Duckworth B. C., Cantley L. C. (1997) Nature 390, 192–196 [DOI] [PubMed] [Google Scholar]
- 12. Suh B. C., Hille B. (2002) Neuron 35, 507–520 [DOI] [PubMed] [Google Scholar]
- 13. Zhang H., Craciun L. C., Mirshahi T., Rohács T., Lopes C. M., Jin T., Logothetis D. E. (2003) Neuron 37, 963–975 [DOI] [PubMed] [Google Scholar]
- 14. Li Y., Gamper N., Hilgemann D. W., Shapiro M. S. (2005) J. Neurosci. 25, 9825–9835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winks J. S., Hughes S., Filippov A. K., Tatulian L., Abogadie F. C., Brown D. A., Marsh S. J. (2005) J. Neurosci. 25, 3400–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X., Chen X., Jia C., Geng X., Du X., Zhang H. (2010) J. Biol. Chem. 285, 9402–9409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Du X., Zhang H., Lopes C., Mirshahi T., Rohacs T., Logothetis D. E. (2004) J. Biol. Chem. 279, 37271–37281 [DOI] [PubMed] [Google Scholar]
- 18. Zhao Z., Liu B., Zhang G., Jia Z., Jia Q., Geng X., Zhang H. (2008) Pflugers Arch. 456, 413–423 [DOI] [PubMed] [Google Scholar]
- 19. Rajagopal S., Fang H., Patanavanich S., Sando J. J., Kamatchi G. L. (2008) Brain Res. 1210, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goueli B. S., Hsiao K., Tereba A., Goueli S. A. (1995) Anal. Biochem. 225, 10–17 [DOI] [PubMed] [Google Scholar]
- 21. Johnson J., Capco D. G. (1997) Mech. Dev. 67, 215–226 [DOI] [PubMed] [Google Scholar]
- 22. Stith B. J., Woronoff K., Espinoza R., Smart T. (1997) Mol. Biol. Cell 8, 755–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stumpo D. J., Haupt D. M., Blackshear P. J. (1994) J. Biol. Chem. 269, 21184–21190 [PubMed] [Google Scholar]
- 24. Steinberg S. F. (2008) Physiol. Rev. 88, 1341–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamplova B., Novak F., Kolar F., Novakova O. (2010) Physiol. Res. 59, 25–33 [DOI] [PubMed] [Google Scholar]
- 26. Lee S. H., Chen T., Zhou J., Hofmann J., Bepler G. (2010) Clin. Lung Cancer 11, 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Apgar J. R. (1995) Mol. Biol. Cell 6, 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Etkovitz N., Rubinstein S., Daniel L., Breitbart H. (2007) Biol. Reprod 77, 263–273 [DOI] [PubMed] [Google Scholar]
- 29. Babwah A. V., Dale L. B., Ferguson S. S. (2003) J. Biol. Chem. 278, 5419–5426 [DOI] [PubMed] [Google Scholar]
- 30. Becker K. P., Hannun Y. A. (2004) J. Biol. Chem. 279, 28251–28256 [DOI] [PubMed] [Google Scholar]
- 31. Cenni V., Döppler H., Sonnenburg E. D., Maraldi N., Newton A. C., Toker A. (2002) Biochem. J. 363, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guerrero-Valero M., Marín-Vicente C., Gómez-Fernández J. C., Corbalán-García S. (2007) J. Mol. Biol. 371, 608–621 [DOI] [PubMed] [Google Scholar]
- 33. Corbalán-García S., Guerrero-Valero M., Marín-Vicente C., Gómez-Fernández J. C. (2007) Biochem. Soc. Trans. 35, 1046–1048 [DOI] [PubMed] [Google Scholar]
- 34. Eltit J. M., Hidalgo J., Liberona J. L., Jaimovich E. (2004) Biophys. J. 86, 3042–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cárdenas C., Müller M., Jaimovich E., Pérez F., Buchuk D., Quest A. F., Carrasco M. A. (2004) J. Biol. Chem. 279, 39122–39131 [DOI] [PubMed] [Google Scholar]
- 36. Powell J. A., Carrasco M. A., Adams D. S., Drouet B., Rios J., Müller M., Estrada M., Jaimovich E. (2001) J. Cell Sci. 114, 3673–3683 [DOI] [PubMed] [Google Scholar]
- 37. Araya R., Liberona J. L., Cárdenas J. C., Riveros N., Estrada M., Powell J. A., Carrasco M. A., Jaimovich E. (2003) J. Gen Physiol. 121, 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maasch C., Wagner S., Lindschau C., Alexander G., Buchner K., Gollasch M., Luft F. C., Haller H. (2000) FASEB J. 14, 1653–1663 [DOI] [PubMed] [Google Scholar]
- 39. Audigier C., Jacob P., Tanière P., Boulez J., Chayvialle J. A., Lombard-Bohas C. (1998) Gastroenterol Clin. Biol. 22, 471–473 [PubMed] [Google Scholar]
- 40. Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. (1989) Cell 57, 1101–1107 [DOI] [PubMed] [Google Scholar]
- 41. Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. (1987) J. Biol. Chem. 262, 5592–5595 [PubMed] [Google Scholar]
- 42. Billups D., Billups B., Challiss R. A., Nahorski S. R. (2006) J. Neurosci. 26, 9983–9995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ben-Chaim Y., Tour O., Dascal N., Parnas I., Parnas H. (2003) J. Biol. Chem. 278, 22482–22491 [DOI] [PubMed] [Google Scholar]
- 44. Martinez-Pinna J., Gurung I. S., Vial C., Leon C., Gachet C., Evans R. J., Mahaut-Smith M. P. (2005) J. Biol. Chem. 280, 1490–1498 [DOI] [PubMed] [Google Scholar]
- 45. Ben-Chaim Y., Chanda B., Dascal N., Bezanilla F., Parnas I., Parnas H. (2006) Nature 444, 106–109 [DOI] [PubMed] [Google Scholar]
- 46. Evans J. P., Florman H. M. (2002) Nat. Cell Biol. 4, suppl., s57–s63 [DOI] [PubMed] [Google Scholar]
- 47. Kline D., Nuccitelli R. (1985) Dev. Biol. 111, 471–487 [DOI] [PubMed] [Google Scholar]
- 48. Snow P., Yim D. L., Leibow J. D., Saini S., Nuccitelli R. (1996) Dev. Biol. 180, 108–118 [DOI] [PubMed] [Google Scholar]
- 49. Zhang C., Xiong W., Zheng H., Wang L., Lu B., Zhou Z. (2004) Neuron 42, 225–236 [DOI] [PubMed] [Google Scholar]
- 50. Nasuhoglu C., Feng S., Mao Y., Shammat I., Yamamato M., Earnest S., Lemmon M., Hilgemann D. W. (2002) Am. J. Physiol. Cell Physiol. 283, C223–C234 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.