Background: Amyloid β (Aβ) induces neuronal and synaptic loss in Alzheimer disease.
Results: Nanomolar Aβ induced microglia-dependent neuronal death and synaptic loss that was prevented by four inhibitors of phagocytosis.
Conclusion: Microglial phagocytosis was the primary cause of neuronal death and synaptic loss induced by nanomolar Aβ.
Significance: This is a new mechanism of cell death, suggesting a new treatment strategy for Alzheimer disease.
Keywords: Alzheimer Disease, Inflammation, Microglia, Phagocytosis, Phosphatidylserine
Abstract
Alzheimer disease is characterized by neuronal loss and brain plaques of extracellular amyloid β (Aβ), but the means by which Aβ may induce neuronal loss is not entirely clear. Although high concentrations of Aβ (μm) can induce direct toxicity to neurons, we find that low concentration (nm) induce neuronal loss through a microglia-mediated mechanism. In mixed neuronal-glial cultures from rat cerebellum, 250 nm Aβ1–42 (added as monomers, oligomers or fibers) induced about 30% loss of neurons between 2 and 3 days. This neuronal loss occurred without any increase in neuronal apoptosis or necrosis, and no neuronal loss occurred with Aβ42–1. Aβ greatly increased the phagocytic capacity of microglia and induced phosphatidylserine exposure (an “eat-me” signal) on neuronal processes. Blocking exposed phosphatidylserine by adding annexin V or an antibody to phosphatidylserine or inhibiting microglial phagocytosis by adding either cytochalasin D (to block actin polymerization) or cyclo(RGDfV) (to block vitronectin receptors) significantly prevented neuronal loss. Loss of neuronal synapses occurred in parallel with loss of cell bodies and was also prevented by blocking phagocytosis. Inhibition of phagocytosis prevented neuronal loss with no increase in neuronal death, even after 7 days, suggesting that microglial phagocytosis was the primary cause of neuronal death induced by nanomolar Aβ.
Introduction
Alzheimer disease (AD)2 is characterized by extracellular plaques of which the main constituent is amyloid β (Aβ), as well as intracellular tangles of which the main constituent is the tau protein. Alzheimer disease is also characterized by extensive loss of neurons and synapses. However, there is little or no evidence of excess neuronal death in AD patient brains compared with age-matched controls (1–3). One possible explanation for this is that neuronal death rates are low, and dead neurons are removed almost immediately after they are generated (1). An alternative explanation is that the neurons are removed by phagocytosis before they die (4). This type of cell death we shall refer to as “primary phagocytosis” with the defining characteristic that inhibition of phagocytosis prevents cell death.
The main phagocytes in the brain are microglia, which are essentially resident brain macrophages. In AD, neuritic plaques of fibrillar amyloid contain inflammatory-activated microglia (5), and in culture, Aβ potently activates microglia and their phagocytic capacity (6). Fibrillar Aβ (at high concentrations) activates microglia via a variety of receptors including TLR2, TLR4, CD36, CD47, and class A scavenger receptors, which activate Src, Vav, and Rac, which in turn activate both the phagocyte NADPH oxidase and phagocytosis (7). Phagocytosis of cells, however, requires recognition by the microglia of signals present on the target cell. Exposure of phosphatidylserine (PS) on the outer leaflet of the plasma membrane of cells is a signal recognized by phagocytes to eat that cell (i.e. it is an “eat-me” signal). Receptors/adaptors thought to be involved in PS recognition include the vitronectin receptor, an integrin αvβ3/5, binding PS via adaptor proteins such as MFG-E8 (8, 9). The most well known cause of PS exposure on the surface of a cell is as a result of apoptotic signaling (10, 11). However, PS can be exposed reversibly or irreversibly for a variety of other reasons, including the following: calcium- or oxidant-induced activation of the phospholipid scramblase (which transports PS between the inner and outer leaflet of the plasma membrane) and oxidant- or ATP-depletion-induced inactivation of the aminophospholipid translocase (which pumps PS from the outer to inner leaflet) (12–14). Aβ itself can induce neurons to expose PS (15), and PS exposure may be elevated on neurons in Alzheimer disease and mild cognitive deficit (16, 17). Thus, Aβ may both activate phagocytosis by microglia and cause neurons to expose the “eat-me” signal PS. This suggests the possibility that Aβ may cause microglial phagocytosis of viable PS-exposing neurons.
At high concentrations (μm), Aβ can directly kill neurons in culture, but at lower concentrations (nm), Aβ kills neurons at least partly via inflammatory activation of glia (18). The mechanisms of the direct Aβ neurotoxicity are unclear but may involve activation of receptors or formation of amyloid pores (19). However, because the concentrations of Aβ1–42 required to induce direct neurotoxicity are so high (10–100 μm) (20), relative to levels present in AD patient brains (∼1–20 μg/g (200–4500 nm) of insoluble Aβ1–42 and 10–300 ng/mg (2–65 nm) of soluble Aβ1–42 (21–25)), it is unclear whether this direct neurotoxicity is ever relevant in vivo. The mechanism of the indirect neurotoxicity of Aβ at low concentrations is also unclear, but in general, inflammatory-activated microglia kill neurons via oxidants from phagocyte NADPH oxidase, nitric oxide from inducible NO synthase, glutamate, or proteases (26). However, recently, we have characterized a novel mechanism by which microglia activated by LPS or lipoteichoic acid (agonists for TLR4 and TLR2, respectively) induce neuronal death. This involved activated microglia inducing the reversible exposure of PS on neurons and then phagocytosing those neurons via a PS/MFG-E8/vitronectin receptor-mediated pathway (27). Aβ can also activate microglia and their phagocytic capacity via TLR2/4 (7, 28, 29), and the potential involvement of phagocytosis in neuronal loss was indicated in preliminary experiments using Aβ25–35 (27). Therefore, we tested here whether Aβ-induced neuronal loss in culture is mediated by microglial phagocytosis. We find here that nanomolar Aβ1–42 induces microglia-dependent neuronal loss. Blocking phagocytosis prevents this neuronal loss and leaves live neurons, suggesting that Aβ is inducing the phagocytosis of live neurons and that blocking phagocytosis might be of therapeutic benefit.
EXPERIMENTAL PROCEDURES
Materials
Amyloid β 1–42 peptide was from EZBiolab, reverse amyloid β 42–1 peptide was from AnaSpec, Inc., cyclo(RGDfV/RADfV) was from Bachem, and authentic peroxynitrite was from Cayman Chemical. Unlabeled annexin V reagent and annexin V-EGFP were from BioVision, and SYTOX Red and Alexa Fluor 488-labeled Griffonia simplicifolia isolectin B4 were from Invitrogen. NeuN antibody was from Chemicon, glial fibrillary acidic protein (GFAP) antibody was from Dako, β-tubulin III antibody was from Sigma, synapsin I antibody was from Millipore, synaptosomal-associated protein 25 (SNAP-25) (SMI 81) antibody was from Covance, phosphatidylserine antibody was from Abcam, and mouse control IgG was from eBioscience. Secondary antibody goat anti-rabbit Alexa Fluor 488 was from Invitrogen, goat anti-rabbit-Cy3, goat anti-mouse-Cy3, and Fc region-specific anti-mouse F(ab)2 fragment were purchased from Jackson ImmunoResearch Laboratories. Carboxylate-modified fluorescent microspheres were from Invitrogen. All other materials were purchased from Sigma.
Preparation of Amyloid β Monomers, Oligomers, and Fibrils
Different conformations of amyloid β 1–42 were prepared as described previously (30, 31). 1.0 mg of peptide was dissolved in 400 μl of 1,1,1,3,3,3-hexafluorisopropanol for 30–60 min at room temperature. 100 μl of the resulting seedless solution was added to 900 μl of double-distilled water. After 10–20 min of incubation at room temperature, the solution was centrifuged for 15 min at 12,000 rpm, supernatant was transferred to a new tube, and HFIP was evaporated. For soluble oligomers, the solution was incubated for 24 h at room temperature with shaking. Fibrils were prepared by incubating the solution for 7 days at room temperature. Monomers were prepared by dissolving Aβ1–42 in HFIP and, after removal of HFIP by evaporation, resuspending in dimethyl sulfoxide at a concentration of 0.5 mm.
Primary Cell Culture
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act (1986) and approved by the Cambridge University local ethical committee. Primary mixed neuronal/glial cultures from postnatal day 5–7 rat cerebella were prepared as described previously (32). Cells were plated at a density of 5 × 105 cells/well on poly-l-lysine coated 24-well plates and stimulated after 7–9 days in vitro. Glial cultures and pure microglial cultures were prepared as described previously (33).
Microglia Depletion
To remove microglial cells, mixed neuronal/glial cultures were treated with 50 mm l-leucine-methyl-ester for 4 h, and then medium was replaced with conditioned medium from sister cultures.
Culture Treatment
Cells were treated with 10 nm–10 μm of amyloid β 1–42. Cytochalasin D (1 μm) was added 48 h after stimulation. Annexin V (100 nm) and phosphatidylserine antibody (5 μg/ml) were added together with Aβ1–42 or 48 h after stimulation as specified. Cyclo(RGDfV)/cyclo(RADfV) peptides (50 μm) were added together with Aβ1–42. Phosphatidylserine antibody and mouse control IgG were washed with PBS using Amicon Ultra centrifugal filters (10-kDa molecular mass cut-off, Millipore). Antibodies were blocked with 5-fold molar excess of a Fc region-specific F(ab′) fragment to prevent recognition of the antibodies through their Fc domains by microglial Fcγ receptors. To test for LPS contamination, Aβ1–42 was preincubated with polymyxin B (1000 units/ml, 30 min at 37 °C) and then diluted into the culture medium (final polymyxin B concentration was 10 units/ml). LPS was used at 100 ng/ml. To induce neuronal apoptosis or necrosis, cultures were treated for 2 h with glutamate (200 μm) or peroxynitrite (100 μm), respectively.
Quantification of Cell Densities
Cell densities were assessed 1, 2, 3, or 7 days after treatment. Cultures were labeled with nuclear stains Hoechst 33342 (10 μg/ml) and propidium iodide (1 μg/ml) as well as microglia-specific marker Alexa Fluor 488-tagged isolectin B4 (2 μg/ml). Four images per well were taken using a Zeiss Axiovert S100 microscope. Healthy neurons were recognized by their morphology in phase contrast images, neurons with condensed chromatin were scored as apoptotic, whereas propidium iodide-positive neurons were scored as necrotic.
Phagocytosis Assay
Microglia cells separated from glial cultures were plated at a density of 5 × 104 cells/well on poly-l-lysine coated 24-well plates and left to adhere overnight. The culture was then treated with 250 nm Aβ1–42 for 1 h or 24 h before 3 μl of 1:10 dilution of 1 μm fluorescently labeled carboxylate-modified microspheres were added, and cells were incubated for 2 h at 37 °C, 5% CO2. The medium was removed, and the culture was washed several times to remove excess beads. Cells were labeled with Hoechst 33342 (10 μg/ml), propidium iodide (1 μg/ml), and Alexa Fluor 488-tagged isolectin B4 (2 μg/ml). Four images per well were taken using a Leica DMI6000 CS microscope. Bead number per cell was evaluated in >50 cells per condition.
Annexin V Staining
Three days after treatment, live cells were incubated with EGFP-tagged annexin V reagent (1:250) for 30 min at 37 °C, 5% CO2. Cells were washed once with PBS and fixed with paraformaldehyde as described below.
Immunostaining
Cultures were grown on poly-l-lysine-coated glass coverslips. Cells were fixed with 4% paraformaldehyde for 20 min at room temperature and quenched with 30 mm glycine solution in PBS. Cell membranes were permeabilized with 0.1% Triton X-100 for 5 min. Annexin V staining was detergent-sensitive; therefore, for annexin V-labeling of cells, the permeabilization step was omitted. Unspecific epitopes were blocked with 5% normal serum of secondary antibody host species for 1 h. Cells were incubated with primary antibody for 1 h at room temperature and then with secondary antibody in 2.5% normal serum of secondary antibody host for 1 h at room temperature. Coverslips were mounted with FluorSave reagent (Calbiochem) and imaged under a Leica DMI6000 CS microscope or a confocal Olympus Fluoview 300 microscope.
Statistical Analysis
For all experiments, all conditions were repeated in duplicate. Experiments were replicated in at least three independent cultures. All data presented are expressed as means ± S.E. Statistical analysis was performed using PASW Statistics software. Normality of data was verified by a Shapiro-Wilk test. Means were compared by one-way analysis of variance and post hoc Bonferroni test. p values < 0.05 were considered as significant.
RESULTS
Nanomolar Aβ-induced Neuronal Loss in Primary Neuronal/Glial Cultures
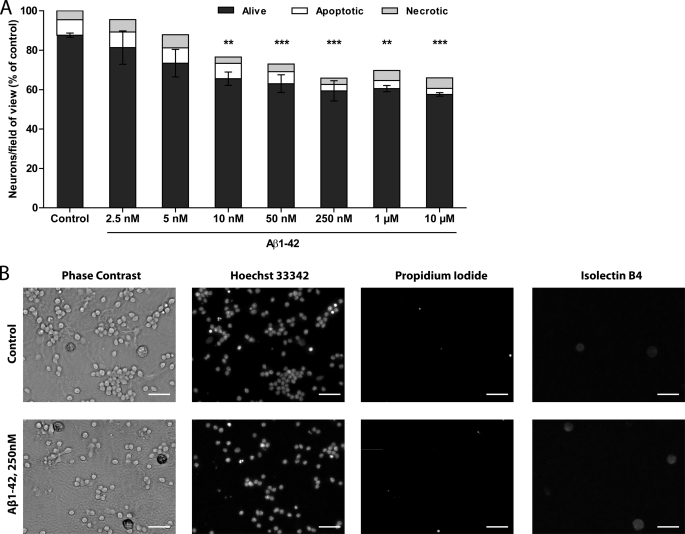
We investigated the neurotoxicity of amyloid β 1–42 peptide (Aβ1–42) in mixed neuronal/glial cultures from rat cerebellum. These cultures consisted of 72 ± 7% of NeuN-positive neurons (almost all cerebellar granule neurons), 6 ± 1% of glial fibrillary acidic protein (GFAP) positive astrocytes and 3 ± 1% of isolectin B4-positive microglia. Cultures were treated with different concentrations of Aβ1–42 (2.5 nm–10 μm) for 3 days. There was significant loss of neurons in the cultures treated with 10 nm to 10 μm of Aβ1–42 without visible neuronal death by apoptosis or necrosis (Fig. 1, A and B). Because these neurons are post-mitotic, and the number of neurons in untreated cultures changes little with time (see Fig. 3A), loss of neurons corresponds to either phagocytosis or neuronal disintegration. Half-maximal neuronal loss was at ∼5 nm Aβ1–42, but neuronal loss was not reliably reproducible at this concentration (Fig. 1A). Further experiments were performed using Aβ1–42 concentration of 250 nm, as this concentration caused maximal and reproducible neuronal loss of 28 ± 5%.
FIGURE 1.
Monomeric Aβ1–42 induces neuronal loss at sub-micromolar concentrations without increasing neuronal death. A, mixed neuronal/glial cultures were treated with different concentrations of Aβ1–42 for 3 days. Data are presented as means ± S.E. for ≥3 independent experiments; ** and ***, p < 0.01/0.001 versus control. B, 3 day treatment with 250 nm Aβ1–42 causes loss of healthy neurons (phase contrast, Hoechst 33342); numbers of apoptotic (condensed Hoechst 33342 positive nuclei) or necrotic (propidium iodide positive nuclei) neurons do not increase. Scale bars, 25 μm.
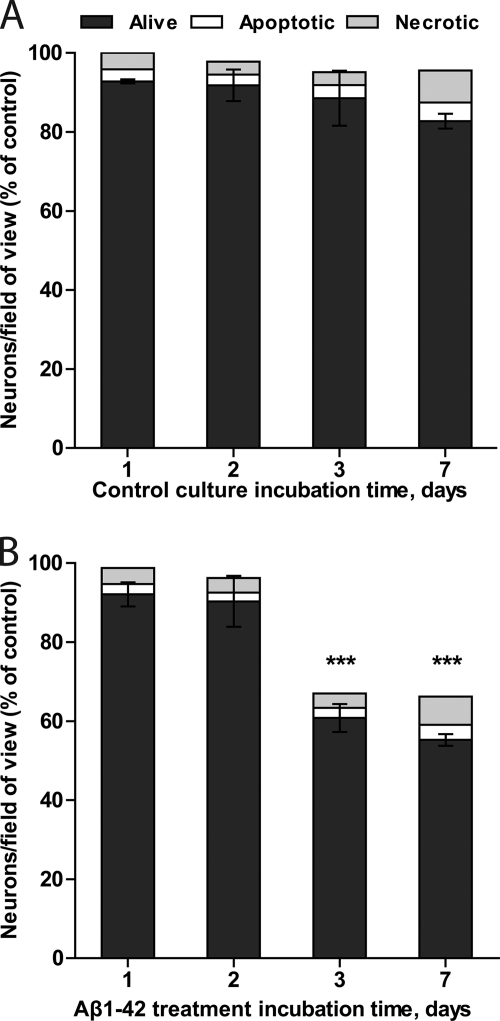
FIGURE 3.
Neuronal loss occurs between 48 and 72 h of treatment. Mixed neuronal/glial cultures were treated with 250 nm Aβ1–42. Little or no loss is observed in non-treated control cultures (A) or during the first 2 days as well as from 3 to 7 days of Aβ1–42 treatment (B). Data are presented as means ± S.E. for ≥3 independent experiments. ***, p < 0.001 versus control.
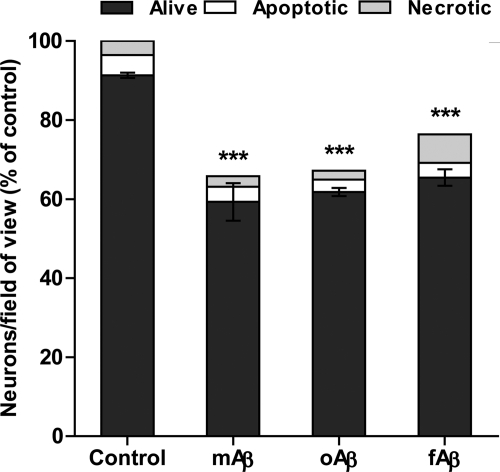
Different Aβ Conformations Had Similar Effect on Neuronal Loss
Different aggregation states of Aβ1–42 have been reported to induce different levels and types of neurotoxicity (31, 34). To investigate whether various conformations of Aβ1–42 peptide had different effects in mixed neuronal/glial culture, cells were treated with monomeric, oligomeric, and fibrillar forms of Aβ1–42 at the same concentration. All conformations caused significant neuronal loss; however, there was no difference between monomeric and preaggregated Aβ1–42 forms (Fig. 2). The concentration dependence of neuronal loss induced by various Aβ1–42 conformations was also similar in the range of 2.5 nm to 1 μm (data not shown). This is perhaps unsurprising as over the 3 days of the experiment the different preparations of Aβ1–42 are likely to adopt the same conformation. The monomeric form of Aβ1–42 was used for all further experiments.
FIGURE 2.
Different conformations of Aβ1–42 at nanomolar concentrations have similar neurotoxicity in mixed neuronal/glial cultures. Cells were treated with 250 nm of monomeric (mAβ), oligomeric (oAβ) and fibrillar (fAβ) Aβ1–42 for 3 days. Data are presented as means ± S.E. for ≥3 independent experiments; ***, p < 0.001 versus control.
Neuronal Loss Occurred between 48 and 72 h of Treatment
To investigate the time course of Aβ1–42-induced neuronal loss, cultures were assessed at 24 h, 48 h, 72 h, and 7 days after Aβ1–42 was added (Fig. 3). Little or no neuronal loss was observed during the first 2 days of treatment; however, between 48 and 72 h of treatment, 29 ± 3% of neurons were lost in the Aβ1–42-treated culture. Aβ1–42 had no significant effect on the number of necrotic or apoptotic neurons compared with the untreated cultures at any particular time point. Thus, Aβ1–42 induced neuronal loss between 2 and 3 days without changing the number of dead cells significantly.
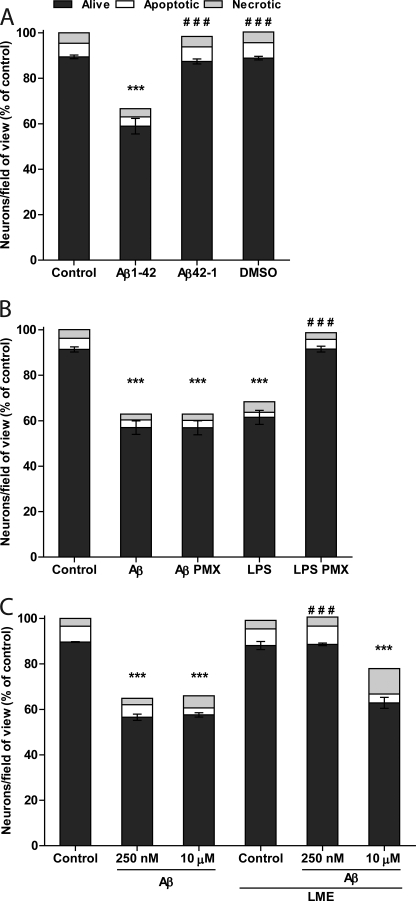
Aβ-induced Neuronal Loss Was Sequence-specific
Reverse peptide Aβ42–1, prepared in the same way, caused no loss of neurons (Fig. 4A). The solvent (dimethyl sulfoxide) added alone also did not cause any neuronal loss (Fig. 4A). Because chemical synthesis of peptides is not performed under sterile conditions and a component of bacterial cell wall (lipopolysaccharide, LPS) is known to activate microglia and cause neuronal loss in mixed neuronal/glial cultures (27), the LPS-binding antibiotic polymyxin B was added to eliminate the possibility that neuronal loss occurred due to contamination of the Aβ1–42 with LPS. Although LPS-induced loss of neurons was completely prevented by polymyxin B, pre-incubation of Aβ1–42 with polymyxin B did not inhibit Aβ1–42-induced neuronal loss (Fig. 4B), demonstrating that this loss was not mediated by LPS.
FIGURE 4.
Aβ-induced neuronal loss is sequence dependent and microglia-mediated. A, reverse sequence peptide Aβ42–1 (250 nm) or vehicle dimethyl sulfoxide (DMSO, 0.05%) does not cause neuronal loss over 3 days of treatment. B, neuronal loss is not prevented by endotoxin removal, when Aβ1–42 is preincubated for 30 min at 37 °C with polymyxin B (PMX, 1000 units/ml); however, neuronal loss induced by LPS (100 ng/ml) is completely prevented by LPS pre-incubation with polymyxin B. C, neuronal loss induced by 250 nm Aβ1–42 is completely prevented by selective depletion of microglial cells with l-leucine-methyl esther (LME), whereas neurotoxicity of 10 μm Aβ1–42 is microglia-independent. Data presented as means ± S.E. for ≥3 independent experiments. ***, p < 0.001 versus control; ###, p < 0.001 versus Aβ1–42 (A and C) or LPS (B).
Aβ-induced Neuronal Loss Was Microglia-dependent
Because Aβ1–42 may cause neuronal loss due to direct neurotoxicity as well as through activated microglia, we tested whether the observed Aβ1–42-induced neuronal loss was dependent on the presence of microglial cells in the culture. Microglia were selectively eliminated by treatment with the lysosomotropic reagent l-leucine-methyl-ester. l-Leucine-methyl-ester treatment effectively removed microglia cells without affecting astrocytes or neurons in the culture (27). When these microglia-depleted cultures were treated with 250 nm Aβ1–42, no neuronal loss occurred (Fig. 4C), thereby implicating microglia as mediators of the neurotoxicity of Aβ1–42 at low concentrations. In contrast, high concentration of Aβ1–42 killed neurons through microglia-independent mechanism because microglial cell removal did not prevent neuronal loss induced by 10 μm Aβ1–42 (Fig. 4C).
Aβ Enhanced Microglial Phagocytic Activity
Aβ1–42 is known to affect microglial phagocytic capacity. To investigate whether low concentrations of Aβ1–42 are sufficient to enhance phagocytic capacity of primary microglia, pure microglia were treated with Aβ1–42 and then presented with fluorescent carboxylate-modified microbeads. (The negative charge of carboxylate mimics that of phosphatidylserine exposed on the surface of cells.) Stimulation with 250 nm Aβ1–42 increased microglial phagocytic capacity ∼2-fold after 1 h and ∼3-fold after 24 h (Fig. 5, A and B).
FIGURE 5.
Treatment with Aβ1–42 (250 nm) for 1 or 24 hours (h) increases microglial phagocytic uptake of fluorescent carboxylate-modified microspheres (A). Data are presented as means ± S.E. for ≥3 independent experiments. ***, p < 0.001 versus control. B, microglia plasma membranes were labeled with Alexa Fluor 488-tagged isolectin B4. Scale bars, 10 μm.
Inhibition of Microglial Phagocytosis Prevented Aβ1–42-induced Neuronal Loss
Because Aβ increased microglial phagocytosis, it is possible that the Aβ-induced neuronal loss was due to microglial phagocytosis of neurons. Therefore, we investigated whether blocking phagocytosis could inhibit Aβ1–42-induced neuronal loss.
Cytochalasin D was used to inhibit F-actin polymerization, which is essential for phagocytosis to occur. When cytochalasin D was added to Aβ1–42-treated cultures, it completely prevented neuronal loss (Fig. 6A), indicating that phagocytosis (or other processes dependent on F-actin polymerization) was required for Aβ-induced loss of neurons.
FIGURE 6.
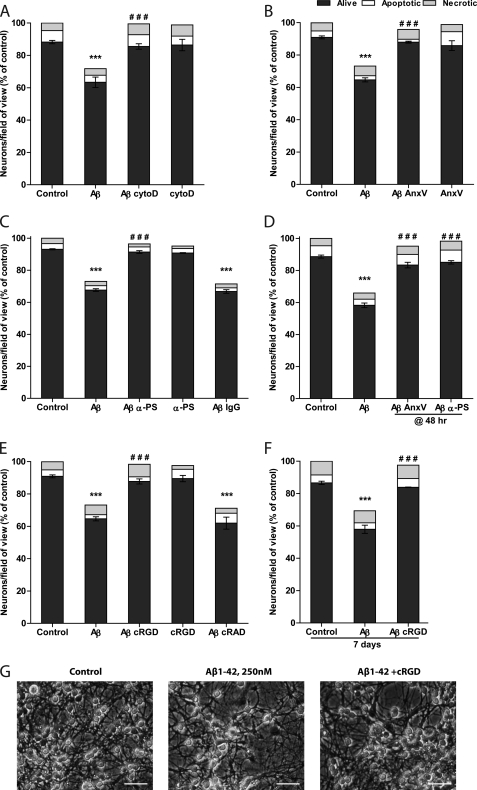
Aβ-induced neuronal loss is prevented by inhibition of phagocytosis. Mixed neuronal/glial cultures were treated for 3 days with Aβ1–42 (250 nm) with or without phagocytosis inhibitors. A, cytochalasin D (cytoD, 1 μm, added at 48 h of treatment); B, annexin V (AnxV, 100 nm, added with Aβ1–42); C, phosphatidylserine antibody (α-PS) or mouse control IgG (5 μg/ml, added with Aβ1–42); D, annexin V (AnxV, 100 nm) and phosphatidylserine antibody (α-PS) added at 48 h of treatment; E, cyclo(RGDfV) peptide (cRGD) or control peptide cyclo(RADfV) (cRAD, 50 μm, added with Aβ1–42). F and G, cultures were treated with Aβ1–42 and cRGD for 7 days. Data are presented as means ± S.E. for ≥3 independent experiments. ***, p < 0.001 versus control; ###, p < 0.001 versus Aβ1–42. Scale bars, 25 μm.
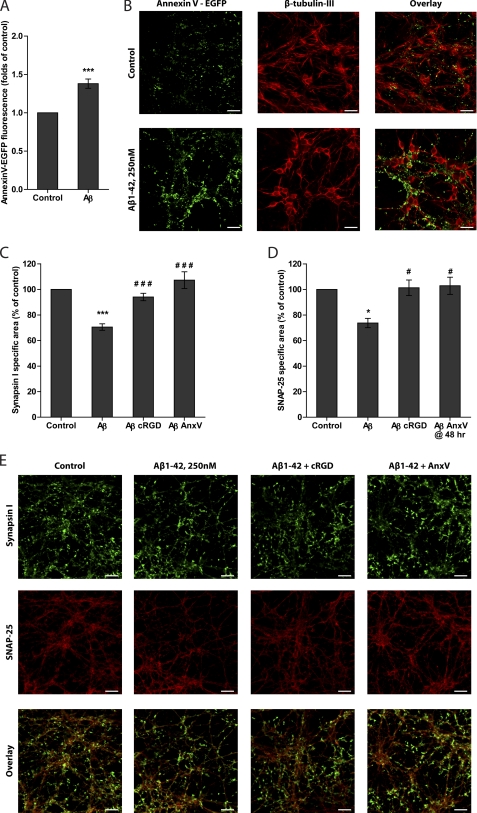
Microglial phagocytosis of cells is mediated by recognition of phosphatidylserine (PS) exposed on the external plasma membrane surface of target cell. Treatment of mixed neuronal cultures with Aβ1–42 increased PS exposure by ∼40% as determined by binding of fluorescently labeled annexin V (Fig. 7A). Most of the external PS was localized to neuronal processes and was distributed along β-tubulin III-positive neurites (Fig. 7B).
FIGURE 7.
Treatment with Aβ1–42 (250 nm) increases phosphatidylserine exposure on neurons and induces synaptic loss. A, phosphatidylserine exposure on neurons was measured by EGFP-tagged annexin V binding. B, surface PS was labeled with annexin V-EGFP (green), neuronal cytoskeleton was labeled with β-tubulin III (red). Scale bars, 30 μm. C, Aβ1–42-induced synapsin I loss can be prevented by co-treatment with phagocytosis inhibitors cyclo(RGDfV) peptide (cRGD, 50 μm) or annexin V (AnxV, 100 nm) added together with Aβ1–42. D, Aβ1–42-induced SNAP-25 loss can be prevented by co-treatment with phagocytosis inhibitors cyclo(RGDfV) peptide (cRGD, 50 μm, added with Aβ1–42) or annexin V (AnxV, 100 nm, added at 48 h of treatment). E, neurons were labeled with synaptic proteins synapsin I (green) and SNAP-25 (red). Scale bars, 10 μm. Data are presented as means ± S.E. for ≥3 independent experiments. * and ***, p < 0.05 and 0.001 versus control, respectively; # and ###, p < 0.05 and 0.001 versus Aβ1–42, respectively.
To mask surface PS, we added annexin V, which is known to bind to PS with high affinity and to block phagocytosis mediated by PS (35). When annexin V was added to Aβ1–42-treated mixed cultures, neuronal loss was completely prevented (Fig. 6B). An antibody to phosphatidylserine also prevented Aβ1–42-induced neuronal loss, whereas control immunoglobulins did not have any effect (Fig. 6C).
Cell surface phosphatidylserine is involved in Aβ binding to neurons (36), and annexin V can be protective against direct Aβ neurotoxicity (37, 38). To eliminate the possibility that annexin V and the phosphatidylserine antibody blocked Aβ binding to cells and the subsequent activation of microglia rather than phagocytosis of PS-exposing neurons, these inhibitors were added 48 h after Aβ1–42. Both annexin V and PS antibody prevented neuronal loss when added at 48 h of treatment (Fig. 6D), indicating that the neuroprotective effect was due to the inhibition of phagocytosis. These experiments indicate that PS is an essential recognition signal for the Aβ-induced removal of neuronal cells by microglia.
PS exposed on a target cell may be recognized by bridging proteins, including MFG-E8. MFG-E8, also known as lactadherin, is a secreted glycoprotein that binds to PS through C-terminal domains and to αv integrins expressed on the phagocytic cell through a RGD motif (39), thus establishing contact essential for phagocytosis. We used a specific αv/β3/5 receptor antagonist cyclo(RGDfV) (cRGD) peptide to inhibit this interaction (40). cRGD effectively prevented neuronal loss, whereas a control peptide cyclo(RADfV) (cRAD) did not have any effect on neuronal loss (Fig. 6E).
Note that these four different phagocytosis inhibitors (cytochalasin D, annexin V, PS antibody, and cyclo(RGDfV) all prevented Aβ-induced loss of live neurons, while causing no significant changes in the numbers of dead (apoptotic or necrotic) cells observed in the culture. This indicates that the neuronal loss was due to primary phagocytosis. If the neurons had been removed after they died (secondary phagocytosis), phagocytosis inhibitors would have increased the numbers of dead without changing the number of live cells. Thus, our results indicate that neuronal loss through phagocytosis was not preceded by neuronal death.
Importantly, cRGD was equally protective at 7 days of treatment with Aβ1–42 (Fig. 6, F and G) as after 3 days, demonstrating that inhibition of phagocytosis did not merely delay neuronal death but rather saved viable neurons.
Inhibition of Microglial Phagocytosis Prevented Aβ1–42-induced Loss of Synapses and Neurites
When mixed neuronal/glial cultures were treated with Aβ1–42 for 3 days, the synaptic density of the culture was reduced by ∼30% as determined by synapsin I staining (Fig. 7, C and E). Co-treatment with cRGD or annexin V blocked synaptic loss (Fig. 7, C and E). Synapsin I staining was restricted to synaptic terminals and co-localized with another synaptic protein SNAP-25. However, in addition to synapses, the SNAP-25 antibody also labeled neuronal cell bodies and axons as has been reported for developing brains and cell cultures (41, 42). Quantitatively, most of the SNAP-25 staining was on neuronal processes (neurites) (Fig. 7E). Aβ1–42 treatment for 3 days caused a decrease of SNAP-25 staining by ∼25% (Fig. 7D), and much of that loss was of neurites (Fig. 7E). Co-treatment with cRGD or treatment with annexin V 48 h after Aβ blocked loss of neuronal processes stained with SNAP-25 (Fig. 7, D and E). Hence, inhibition of different steps in microglial phagocytosis was sufficient to prevent Aβ1–42-induced microglia-dependent neuronal loss and death, leaving neurons with intact processes and synapses.
Microglia in Aβ-treated Cultures Contain Healthy NeuN+ Nuclei
When cultures were fixed during the period of neuronal loss (after 54 h of treatment with Aβ1–42), confocal imaging revealed a significant proportion of microglia containing NeuN+ nuclei (i.e. neuronal nuclei) inside the microglia in addition to a NeuN-negative microglial nucleus (supplemental Fig. S1). NeuN is a nuclear marker specific to neurons (recently indentified as Fox-3, RNA binding protein) (43), which can be lost from the nuclei of neurons undergoing stress (44). We found that indeed treatment of neurons with neurotoxic levels of glutamate (200 μm) or peroxynitrite (100 μm) caused pronounced changes in NeuN staining of neurons as soon as after 2 h of treatment. Glutamate caused pronounced nuclear condensation with condensation of nuclear NeuN staining as well as partial loss of NeuN staining from the nucleus. High peroxynitrite concentrations induced neuronal necrosis (as observed previously in Ref. 45) and caused almost complete loss of neuronal NeuN staining (supplemental Fig. S2). Many of the NeuN+ nuclei inside of microglia cells retained a healthy morphology, with no condensation or marked loss of NeuN, therefore supporting the hypothesis that the microglia phagocytosed viable neurons. Altogether, our results suggest that treatment of neuronal/glial cultures by low Aβ1–42 concentrations induced neuronal loss exclusively due to the microglial phagocytosis of neurons, which otherwise stay alive.
DISCUSSION
We found two different types of neurotoxicity of Aβ in our cultures: at high concentrations (μm), Aβ caused direct neurotoxicity independent of microglia, whereas at low concentrations (nm), Aβ caused indirect neurotoxicity requiring microglia. The concentrations of Aβ inducing indirect neurotoxicity here (EC50, 6 nm Aβ) are similar to those reported recently by Maezawa et al. (18) in hippocampal cultures. We found a similar neuronal sensitivity to Aβ when added as a monomer, oligomer, or fibrillar preparation. However, as the incubation with cells was for 3 days, and Aβ1–42 fibrillizes in less than a day, these different preparations are likely to have adopted the same conformation (or mixture of conformations) by the end of the incubation.
The neuronal loss induced by Aβ required microglia and occurred 2–3 days after Aβ addition, without any dead neurons accumulating in the cultures. Inhibition of phagocytosis by four different means (cytochalasin D, annexin V, phosphatidylserine antibody, and cyclo(RGDfV) all prevented Aβ-induced neuronal loss. Cytochalasin D blocks actin polymerization required to mechanically power phagocytosis. However, it did also cause some microglia to detach from the plate and thus does not clearly distinguish between a requirement (of the Aβ-induced neuronal loss) for microglial contact and for microglial phagocytosis. Annexin V and phosphatidylserine antibody also blocked the Aβ-induced neuronal loss, indicating that PS exposure mediated the neuronal loss. cyclo(RGDfV) is a specific inhibitor of the vitronectin receptor, which can mediate the phagocytosis of PS-exposed cells, when the adaptor protein MFG-E8 binds both to PS on the target cell (via its C2 domains) and to the vitronectin receptor (VR) on the phagocyte (via the RGD domain of MFG-E8) (39). The ability of annexin V, phosphatidylserine antibody, and cyclo(RGDfV) to block neuronal loss thus indicates a role for the PS/MFG-E8/VR phagocytic pathway in Aβ-induced neuronal loss.
Inhibition of phagocytosis by these four different means all prevented Aβ-induced neuronal loss (which is perhaps unsurprising) without increasing the numbers of dead (apoptotic or necrotic) cells observed in the culture (which is perhaps surprising), indicating that neuronal loss through phagocytosis was not preceded by neuronal death. We have shown previously that LPS- or lipoteichoic acid-activated microglia phagocytose neurons reversibly exposing PS (27). Because Aβ activates microglia via the same Toll-like receptors as lipoteichoic acid and LPS (7), it seems likely that Aβ is activating microglia to phagocytose viable PS-exposed neurons here. We and others have shown previously that PS exposure by neurons can be fully reversible without cell death (27, 46). PS exposure requires activation of the phospholipids scramblase (by calcium or oxidants) and/or inhibition of the aminophospholipid scramblase (by oxidants or ATP depletion) (12, 13). Peroxynitrite can cause PS exposure by directly activating the scramblase and inhibiting the translocase (14), and we have shown that low levels of peroxynitrite can cause reversible PS exposure by neurons that leads to microglial phagocytosis if activated microglia are present at the time of PS exposure, but the neurons revert to a healthy state if no microglia are present (27). We have also demonstrated that peroxynitrite from activated microglia mediates the reversible PS exposure of neurons (27). Futhermore, we and others (47, 48) have previously shown that Aβ activates the phagocyte NADPH oxidase of microglia, leading to inducible NO synthase induction (49), resulting in peroxynitrite production (50). Thus, Aβ might induce microglial peroxynitrite production that causes neuronal PS exposure, which induces the activated microglia to eat the viable neurons. And this may be enhanced by the Aβ-induced activation of phagocytosis in microglia shown here (Fig. 5) and elsewhere (6). However, whether phagocyte NADPH oxidase and peroxynitrite are in fact involved in the Aβ-induced neuronal loss seen here requires further study.
Phagocytosis of host cells is normally thought to be secondary to cells dying by apoptosis or necrosis. However, in principle, phagocytosis can induce death of cells, and we call this “primary phagocytosis” with the defining characteristic that death is prevented by inhibiting phagocytosis. There is increasing evidence for primary phagocytosis as a mechanism of cell death (51), including in models of inflammatory neuronal loss (27, 52), and tentative evidence that it may be involved in animal models of neurodegeneration (4, 53).
In the glial-neuronal cultures used here, Aβ induced loss of neuronal cell bodies, processes, and synapses in parallel, and this was prevented by blocking phagocytosis. In AD, synapses are lost earlier than neuronal cell bodies, which might reflect lower Aβ concentrations or other conditions in vivo. Because half-maximal neuronal loss occurred in culture at ∼5 nm Aβ, it is tempting to speculate that Aβ might have a physiological role in removal of synapses or neurons as Aβ is known to affect synaptic plasticity, which might in principle involve synaptic phagocytosis. Aβ can induce PS exposure on neurons (15) and can bind to PS (36), potentially opsonizing neurons or synapses for phagocytosis. However, we have no evidence for or against such an opsonizing role for Aβ in our cultures.
In our experiments, we have used cerebellar cultures as a model system to analyze the effects of Aβ, despite the fact that in Alzheimer disease, there is little or no neuronal loss from the cerebellum. However, the lack of cerebellar neuronal loss in AD correlates with a lack of neuritic plaques containing fibrillar amyloid and activated microglia in the cerebellum (5, 54), suggesting that the absence of cerebellar pathology in AD is due to the relative absence of toxic Aβ species, rather than a lack of sensitivity of the cerebellum to Aβ. Our experiments indeed show that cerebellar granule neurons are as sensitive to Aβ as hippocampal neurons in culture (18).
There is accumulating evidence that phagocytosis may contribute to neurodegenerative diseases. For example, mutations in the progranulin gene cause familial frontotemporal lobar degeneration, and recent evidence suggests that these inactivating mutations cause neuronal loss by promoting primary phagocytosis (53). Variants in a number of phagocytosis-related genes promote AD, including ApoE, ApoJ (clusterin), ABCA7, and CR1 (55). Microglial phagocytosis appears to contribute to neuronal loss in Alzheimer disease mouse models (4). However, testing whether phagocytosis of neurons in AD is primary or secondary to death by other means in vivo is challenging. Primary phagocytosis (unlike apoptosis or necrosis) leaves no cell corpse to diagnose the cause of death. Most mouse models expressing mutant APP lack significant neuronal loss unless they also include mutant tau. In addition, we currently lack suitable phagocytosis inhibitors that cross the blood-brain barrier. However, in principle, mouse models mutant in phagocytic genes could be used to test for primary phagocytosis.
We have found that inhibition of microglial phagocytosis of neurons prevents Aβ-induced neuronal death, which suggests the possibility that inhibition of PS/MFG-E8/vitronectin receptor-mediated phagocytosis might be used as a treatment for AD. However, (a) this system may have beneficial roles, and (b) phagocytosis of PS-exposed cells may in principle be anti-inflammatory (52, 56), so more work is required to determine whether blocking this type of phagocytosis is beneficial in models of AD.
This work was supported by Wellcome Trust Grant RG50995.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- AD
- Alzheimer disease
- Aβ
- amyloid β
- cRAD
- cyclo(Arg-Ala-Asp-D-Phe-Val) peptide
- cRGD
- cyclo(Arg-Gly-Asp-D-Phe-Val) peptide
- MFG-E8
- milk fat globule EGF factor 8
- PS
- phosphatidylserine
- EGFP
- enhanced GFP
- SNAP-25
- synaptosomal-associated protein 25.
REFERENCES
- 1. Perry G., Nunomura A., Lucassen P., Lassmann H., Smith M. A. (1998) Science 282, 1268–1269 [DOI] [PubMed] [Google Scholar]
- 2. Raina A. K., Zhu X., Monteiro M., Takeda A., Smith M. A. (2000) Prog. Cell Cycle Res. 4, 235–242 [DOI] [PubMed] [Google Scholar]
- 3. Calissano P., Matrone C., Amadoro G. (2009) Commun. Integr. Biol. 2, 163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuhrmann M., Bittner T., Jung C. K., Burgold S., Page R. M., Mitteregger G., Haass C., LaFerla F. M., Kretzschmar H., Herms J. (2010) Nat. Neurosci. 13, 411–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheng J. G., Mrak R. E., Griffin W. S. (1995) Neuropathol. Appl. Neurobiol. 21, 290–301 [DOI] [PubMed] [Google Scholar]
- 6. Wilkinson B., Koenigsknecht-Talboo J., Grommes C., Lee C. Y., Landreth G. (2006) J. Biol. Chem. 281, 20842–20850 [DOI] [PubMed] [Google Scholar]
- 7. Reed-Geaghan E. G., Savage J. C., Hise A. G., Landreth G. E. (2009) J. Neurosci. 29, 11982–11992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Witting A., Müller P., Herrmann A., Kettenmann H., Nolte C. (2000) J. Neurochem. 75, 1060–1070 [DOI] [PubMed] [Google Scholar]
- 9. Wu Y., Tibrewal N., Birge R. B. (2006) Trends Cell Biol. 16, 189–197 [DOI] [PubMed] [Google Scholar]
- 10. Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., Henson P. M. (1992) J. Immunol. 148, 2207–2216 [PubMed] [Google Scholar]
- 11. Van den Eijnde S. M., Boshart L., Reutelingsperger C. P., De Zeeuw C. I., Vermeij-Keers C. (1997) Cell Death Differ. 4, 311–316 [DOI] [PubMed] [Google Scholar]
- 12. Bratton D. L., Fadok V. A., Richter D. A., Kailey J. M., Guthrie L. A., Henson P. M. (1997) J. Biol. Chem. 272, 26159–26165 [DOI] [PubMed] [Google Scholar]
- 13. Gleiss B., Gogvadze V., Orrenius S., Fadeel B. (2002) FEBS Lett. 519, 153–158 [DOI] [PubMed] [Google Scholar]
- 14. Tyurina Y. Y., Basova L. V., Konduru N. V., Tyurin V. A., Potapovich A. I., Cai P., Bayir H., Stoyanovsky D., Pitt B. R., Shvedova A. A., Fadeel B., Kagan V. E. (2007) J. Biol. Chem. 282, 8498–8509 [DOI] [PubMed] [Google Scholar]
- 15. Mohmmad Abdul H., Butterfield D. A. (2005) Biochim. Biophys. Acta 1741, 140–148 [DOI] [PubMed] [Google Scholar]
- 16. Bader Lange M. L., Cenini G., Piroddi M., Abdul H. M., Sultana R., Galli F., Memo M., Butterfield D. A. (2008) Neurobiol. Dis. 29, 456–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bader Lange M. L., St Clair D., Markesbery W. R., Studzinski C. M., Murphy M. P., Butterfield D. A. (2010) Neurobiol. Dis. 38, 104–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maezawa I., Zimin P. I., Wulff H., Jin L. W. (2011) J. Biol. Chem. 286, 3693–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cappai R., Barnham K. J. (2008) Neurochem. Res. 33, 526–532 [DOI] [PubMed] [Google Scholar]
- 20. Liao M. Q., Tzeng Y. J., Chang L. Y., Huang H. B., Lin T. H., Chyan C. L., Chen Y. C. (2007) FEBS Lett. 581, 1161–1165 [DOI] [PubMed] [Google Scholar]
- 21. Kuo Y. M., Emmerling M. R., Vigo-Pelfrey C., Kasunic T. C., Kirkpatrick J. B., Murdoch G. H., Ball M. J., Roher A. E. (1996) J. Biol. Chem. 271, 4077–4081 [DOI] [PubMed] [Google Scholar]
- 22. McLean C. A., Cherny R. A., Fraser F. W., Fuller S. J., Smith M. J., Beyreuther K., Bush A. I., Masters C. L. (1999) Ann. Neurol. 46, 860–866 [DOI] [PubMed] [Google Scholar]
- 23. Wang J., Dickson D. W., Trojanowski J. Q., Lee V. M. (1999) Exp. Neurol. 158, 328–337 [DOI] [PubMed] [Google Scholar]
- 24. Steinerman J. R., Irizarry M., Scarmeas N., Raju S., Brandt J., Albert M., Blacker D., Hyman B., Stern Y. (2008) Arch. Neurol. 65, 906–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hellström-Lindahl E., Viitanen M., Marutle A. (2009) Neurochem. Int. 55, 243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown G. C., Neher J. J. (2010) Mol. Neurobiol. 41, 242–247 [DOI] [PubMed] [Google Scholar]
- 27. Neher J. J., Neniskyte U., Zhao J. W., Bal-Price A., Tolkovsky A. M., Brown G. C. (2011) J. Immunol. 186, 4973–4983 [DOI] [PubMed] [Google Scholar]
- 28. Jana M., Palencia C. A., Pahan K. (2008) J. Immunol. 181, 7254–7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stewart C. R., Stuart L. M., Wilkinson K., van Gils J. M., Deng J., Halle A., Rayner K. J., Boyer L., Zhong R., Frazier W. A., Lacy-Hulbert A., El Khoury J., Golenbock D. T., Moore K. J. (2010) Nat. Immunol. 11, 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Demuro A., Mina E., Kayed R., Milton S. C., Parker I., Glabe C. G. (2005) J. Biol. Chem. 280, 17294–17300 [DOI] [PubMed] [Google Scholar]
- 31. Cizas P., Budvytyte R., Morkuniene R., Moldovan R., Broccio M., Lösche M., Niaura G., Valincius G., Borutaite V. (2010) Arch. Biochem. Biophys. 496, 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kinsner A., Pilotto V., Deininger S., Brown G. C., Coecke S., Hartung T., Bal-Price A. (2005) J. Neurochem. 95, 1132–1143 [DOI] [PubMed] [Google Scholar]
- 33. Bal-Price A., Brown G. C. (2001) J. Neurosci. 21, 6480–6491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sondag C. M., Dhawan G., Combs C. K. (2009) J. Neuroinflammation 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krahling S., Callahan M. K., Williamson P., Schlegel R. A. (1999) Cell Death Differ. 6, 183–189 [DOI] [PubMed] [Google Scholar]
- 36. Simakova O., Arispe N. J. (2007) J. Neurosci. 27, 13719–13729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee G., Pollard H. B., Arispe N. (2002) Peptides 23, 1249–1263 [DOI] [PubMed] [Google Scholar]
- 38. Ciccotosto G. D., Tew D. J., Drew S. C., Smith D. G., Johanssen T., Lal V., Lau T. L., Perez K., Curtain C. C., Wade J. D., Separovic F., Masters C. L., Smith J. P., Barnham K. J., Cappai R. (2011) Neurobiol. Aging 32, 235–248 [DOI] [PubMed] [Google Scholar]
- 39. Raymond A., Ensslin M. A., Shur B. D. (2009) J. Cell. Biochem. 106, 957–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haubner R., Gratias R., Diefenbach B., Goodman S., Jonczyk A., Kessler H. (1996) J. Am. Chem. Soc. 118, 7461–7472 [Google Scholar]
- 41. Oyler G. A., Polli J. W., Wilson M. C., Billingsley M. L. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 5247–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Osen-Sand A., Catsicas M., Staple J. K., Jones K. A., Ayala G., Knowles J., Grenningloh G., Catsicas S. (1993) Nature 364, 445–448 [DOI] [PubMed] [Google Scholar]
- 43. Kim K. K., Adelstein R. S., Kawamoto S. (2009) J. Biol. Chem. 284, 31052–31061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Unal-Cevik I., Kilinç M., Gürsoy-Ozdemir Y., Gurer G., Dalkara T. (2004) Brain Res. 1015, 169–174 [DOI] [PubMed] [Google Scholar]
- 45. Bonfoco E., Krainc D., Ankarcrona M., Nicotera P., Lipton S. A. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7162–7166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Balasubramanian K., Mirnikjoo B., Schroit A. J. (2007) J. Biol. Chem. 282, 18357–18364 [DOI] [PubMed] [Google Scholar]
- 47. Bianca V. D., Dusi S., Bianchini E., Dal Prà I., Rossi F. (1999) J. Biol. Chem. 274, 15493–15499 [DOI] [PubMed] [Google Scholar]
- 48. Jekabsone A., Mander P. K., Tickler A., Sharpe M., Brown G. C. (2006) J. Neuroinflammation 3, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weldon D. T., Rogers S. D., Ghilardi J. R., Finke M. P., Cleary J. P., O'Hare E., Esler W. P., Maggio J. E., Mantyh P. W. (1998) J. Neurosci. 18, 2161–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xie Z., Wei M., Morgan T. E., Fabrizio P., Han D., Finch C. E., Longo V. D. (2002) J. Neurosci. 22, 3484–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reddien P. W., Cameron S., Horvitz H. R. (2001) Nature 412, 198–202 [DOI] [PubMed] [Google Scholar]
- 52. McArthur S., Cristante E., Paterno M., Christian H., Roncaroli F., Gillies G. E., Solito E. (2010) J. Immunol. 185, 6317–6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kao A. W., Eisenhut R. J., Martens L. H., Nakamura A., Huang A., Bagley J. A., Zhou P., de Luis A., Neukomm L. J., Cabello J., Farese R. V., Jr., Kenyon C. (2011) Proc. Natl. Acad. Sci. U.S.A. 108, 4441–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Styren S. D., Kamboh M. I., DeKosky S. T. (1998) J. Comp. Neurol. 396, 511–520 [PubMed] [Google Scholar]
- 55. Morgan K. (2011) Neuropathol. Appl. Neurobiol. 37, 353–357 [DOI] [PubMed] [Google Scholar]
- 56. De Simone R., Ajmone-Cat M. A., Tirassa P., Minghetti L. (2003) J. Neuropathol. Exp. Neurol. 62, 208–216 [DOI] [PubMed] [Google Scholar]