Abstract
Pancreatic cancer (PC) has the worst prognosis among all cancers due to its late diagnosis and lack of effective therapies. Therefore, identification of novel gene targets, which are differentially expressed in PC and functionally involved in malignant phenotypes, is critical to achieve early diagnosis and development of effective therapeutic strategies. We have shown previously that MUC4, an aberrantly overexpressed transmembrane mucin, promotes growth, invasion and metastasis of PC cells, thus underscoring its potential as a clinical target. Here, we report a novel microRNA (miRNA)-mediated mechanism underlying aberrant expression of MUC4 in PC. We demonstrate that the 3′ untranslated region of MUC4 contains a highly conserved miRNA-150 (miR-150) binding motif and its direct interaction with miR-150 downregulates endogenous MUC4 protein levels. We also show that miR-150-mediated MUC4 downregulation is associated with a concomitant decrease in human epidermal growth factor receptor 2 and its phosphorylated form, leading to reduced activation of downstream signaling. Furthermore, our findings demonstrate that miR-150 overexpression inhibits growth, clonogenicity, migration and invasion and enhances intercellular adhesion in PC cells. Finally, our data reveal a downregulated expression of miR-150 in malignant pancreatic tissues, which is inversely associated with MUC4 protein levels. Altogether, these findings establish miR-150 as a novel regulator of MUC4 and a tumor suppressor miRNA in PC.
Introduction
Pancreatic cancer (PC) is a highly lethal malignancy and has the worst prognosis among all cancers. Currently, it is the fourth leading cause of cancer-related deaths in the USA (1). The collective median survival for all patients with PC is 2–8 months, and only 1–4% of all patients survive 5 years after diagnosis (2). Such a grim prognosis of PC is explained by the fact that at the time of diagnosis, majority of patients have already developed an aggressive form of the disease thus limiting the potential for therapeutic intervention (3). Even small adenocarcinoma of pancreas at diagnosis is genetically advanced and carries numerous genetic and epigenetic aberrations that co-operatively act to confer aggressive malignant phenotypes (4,5). Recent years have witnessed important advances in our understanding of the molecular progression of PC, and several important targets have been identified and experimentally tested for their functional participation in the disease processes (4,6,7).
MUC4 is a high-molecular weight glycoprotein that belongs to the family of membrane-bound mucins (8). It is overexpressed in pancreatic adenocarcinomas and tumor cell lines while remains undetectable in the normal pancreas (9). Expression analysis of MUC4 in increasing grade pancreatic intraepithelial neoplasias and malignant lesions demonstrated a positive correlation of MUC4 with disease progression (10). Importantly, in our earlier studies, we have shown a pathogenic role of MUC4 in promoting pancreatic tumor growth and metastasis (11,12). Furthermore, aberrant MUC4 expression is also reported in other malignancies indicating its clinical relevance as a target for therapeutic intervention (8,13). However, there is still little known about the molecular mechanisms that regulate MUC4 expression and whose perturbation ultimately leads to its aberrant expression during cancer initiation and progression (8,14).
Recently, a novel class of endogenous small non-coding gene regulatory RNAs, termed as microRNAs (miRNAs or miRs), has gained significant attention (15). These small molecules exert their regulatory effects by base pairing with partially complementary messenger RNAs (mRNAs) and function by two mechanisms: degrading target mRNA or inhibiting their translation (16,17). It is now well established that miRNAs play critical roles in the development of cancer by altering the expression of oncogenes and tumor suppressor genes (15,16). In the present study, we have investigated the role of microRNA-150 (miR-150) in the regulation of MUC4 expression in PC cells. Our findings demonstrate that 3′ untranslated region (UTR) of MUC4 contains putative binding site for miR-150, which is highly conserved across several mammalian species. Furthermore, we experimentally show that miR-150 directly targets the 3′ UTR of MUC4 to suppress its expression. Downregulation of MUC4 by miR-150 also leads to a concomitant decrease in human epidermal growth factor receptor 2 (HER2), an interacting partner of MUC4 (18), and its phosphorylated form leading to reduced activation of downstream signaling molecules. Our findings also demonstrate that miR-150 overexpression leads to reduced growth, clonogenicity, migration and invasion in PC cells. Finally, our data reveal a discordant expression of MUC4 at the transcript and protein levels, which is inversely associated with miR-150 expression in malignant clinical specimens. Altogether, our study characterizes a novel miRNA-mediated mechanism of MUC4 regulation and suggests tumor suppressive actions of miR-150 in PC cells.
Materials and methods
Cell lines and pancreatic tissue specimens
HPAF, Panc10.05 and Colo357 PC cell lines were maintained as monolayer cultures in RPMI 1640 media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and 100 μM each of penicillin and streptomycin (Invitrogen) in a humidified atmosphere of 5% CO2 at 37°C. All the cells were tested and determined to be free from mycoplasma contamination every alternate month. Frozen pancreatic tissue samples (normal and malignant) were obtained through co-operative human tissue network (CHTN) at the University of Alabama at Birmingham (UAB) under an Institutional Review Board (IRB)-approved protocol.
Transfection
HPAF, Panc10.05 and Colo357 cells were seeded at 3 × 105 cells per well in six-well plates. After the cells reached 60–70% confluence, they were transfected with miR-150 mimics (Catalog # AM17100) or non-targeting control (miR-NC) mimics (Catalog # AM17111) (Ambion, Austin, TX) at the concentrations ranging from 50 to 150 nM using Lipofectamine 2000 (Invitrogen) as transfection reagent. As per the supplier, these miRNA mimics are small interfering RNA (siRNA)-like structures. The mature miRNA is paired with its complement to form a 21-mer antiparallel duplex with 3′ overhangs similar to a siRNA and there is no hairpin. The passenger strand contains ‘proprietary modifications’ that drive the loading of the mature miRNA onto the RNA-induced silencing complex. The structural difference between the miRNA mimics and the endogenous miRNA is the complement of the miRNA, whereas the actual miRNA sequence is identical between both of them. After 16 h of transfection, media was replaced with complete media and cells were further cultured for 48 h. For MUC4 silencing, cells were transiently transfected for 48 h with 50 nM of human MUC4-specific or scrambled sequence control-siRNAs (SCR) using DharmaFECT transfection reagent (Dharmacon, Lafayette, CO) as per the manufacturer’s protocol.
RNA isolation and reverse transcription–quantitative real-time polymerase chain reaction assay
Total RNA was extracted using TRIzol reagent (Invitrogen). Complementary DNA was synthesized using 1 μg of total RNA and the High Capacity complementary DNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) in a 10 μl reaction volume following manufacturer’s instruction. Quantitative real-time polymerase chain reaction (PCR) was performed using 5 μl (for MUC4) and 2.5 μl (for GAPDH) of 1:10 dilution first-strand complementary DNA in 96-well plates using SYBR Green Master Mix (Applied Biosystems) on an iCycler system (Bio-Rad Laboratories, Hercules, CA). To examine the expression level of mature miR-150, we followed the strategy developed by Chen et al. (19). In brief, we first designed stem–loop RT primers by incorporating a stem–loop extension (at the 3′ end) to the six nucleotides reverse complementary sequence of the 3′ end of miRNA. Later, 5′ sequence of this extension was used to design a universal reverse primer. Forward primer was designed by excluding the last six nucleotides at the 3′ end of miR-150. We added six nucleotides at the 5′ end of forward primer to increase the melting temperature. U6 small nuclear RNA was used as an internal control. Threshold cycle (Ct) values for MUC4 and miR-150 were normalized against Ct values for GAPDH and U6 small nuclear RNA, respectively, and a relative fold change in expression with respect to a reference sample was calculated by the 2−ΔΔCt method. Sequence detail for all the primers used is provided in supplementary Table 1, available at Carcinogenesis Online. The thermal conditions for the real-time PCR were as follows: cycle 1: 95°C for 10 min, cycle 2 (×40): 95°C for 10 s and 58°C for 45 s.
Western blot analysis
Protein extraction and western blotting were performed as described earlier (2,12). In brief, 15–80 μg of protein lysates were resolved by electrophoresis on 10% sodium dodecyl sulfate–polyacrylamide gels. For immunodetection of MUC4, we resolved protein lysates on 2% sodium dodecyl sulfate–agarose gel due to its large size (12). Resolved proteins were transferred onto polyvinylidene difluoride membranes and subjected to standard immunodetection procedure using specific antibodies against: MUC4 (8G7), pY1248-HER2 (mouse monoclonal) (Santa Cruz Biotechnology, Santa Cruz, CA), HER2, pERK1/2 (mouse monoclonal), extracellular signal-regulated kinase (ERK) 1/2, focal adhesion kinase (FAK) and pFAK (rabbit monoclonal) (Epitomics, Burlingame, CA) and β-actin (mouse monoclonal) (Sigma–Aldrich, St Louis, MO). All secondary antibodies (Santa Cruz Biotechnology) were used at 1:2500 dilutions. Proteins were visualized with the SuperSignal West Femto Maximum sensitivity substrate kit (Thermo Scientific, Logan, UT) and the signal detected using an LAS-3000 image analyzer (Fuji Photo Film Co., Tokyo, Japan).
Dual-luciferase 3′ UTR-reporter assay
For the validation of MUC4 as a direct target of miR-150, we performed miRNA target luciferase reporter assay using a pEZX-MT01 target reporter plasmid containing MUC4 3′ UTR region (Genecoepia, Rockville, MD). Additionally, we generated mutant MUC4 3′ UTR (MUT-MUC4 3′ UTR) reporter construct by site-directed mutagenesis in the putative target site of miR-150 in the wild-type MUC4 3′ UTR (WT-MUC4 3′ UTR) using Quickchange XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). Cells were transiently cotransfected for 24 h with reporter plasmids (200 ng) and 100 nM of miR-150 or miR-NC mimic as described in earlier section and harvested in reporter lysis buffer. Both firefly luciferase and Renilla luciferase activities were measured using the Dual-Luciferase assay kit (Promega, Madison, WI) according to manufacturer’s instructions. The luciferase activity normalized against protein concentration was expressed as a ratio of firefly luciferase to Renilla luciferase units.
Cell growth and clonogenicity assays
For cell growth assay, cells (2 × 103 cells per well) were transfected with miR-150 or miR-NC or MUC4-specific or scrambled (SCR) sequence siRNAs in 96-well plates, as described above and cultured up to 5 days. Cell growth was then monitored using WST-1 assay kit (Roche Diagnostics, Mannheim, Germany) as per manufacturer’s instructions. The absorbance was measured at a wavelength of 450 nm, using a Bio-Rad Benchmark microplate reader (Bio-Rad Laboratories). For clonogenicity assay, cells were cultured and subsequently transfected with 100 nM of miR mimics (miR-150 or miR-NC) and 50 nM of MUC4-specific siRNA or SCR siRNAs. Following 48 h transfection, cells were trypsinized and plated in six-well plates at a density of 1 × 103 cells per well in a regular media for colony formation. After 2 weeks, colonies were fixed with methanol, stained with crystal violet, photographed and counted using Image analysis software (Gene Tools; Syngene, Frederick, MD).
Motility and invasion assays
Cells were transfected with 100 nM of miR mimics (miR-150 or miR-NC) for 48 h, trypsinized and plated for motility and invasion assays. For motility assay, cells i.e. HPAF (1 × 106), Colo357 (1 × 106) and Panc10.05 (2 × 105) were plated in the top chamber of monocoated polyethylene teraphthalate membrane (six-well insert, pore size 8 μ; BD Biosciences, Bedford, MA). For the invasion assay, HPAF (2.5 × 105), Colo357 (2.5 × 105) and Panc10.05 cells (5 × 104) were plated in the top chamber of the transwell with a Matrigel-coated polycarbonate membrane (24 wells 0.8 μm, BD Biosciences). Respective medium with 10% fetal bovine serum was added to the lower chamber as a chemoattractant. After 16 h of incubation, cells remaining on the upper surface of the insert membrane were removed by cotton swab. Cells that had migrated or invaded through the membrane/Matrigel to the bottom of the insert were fixed and stained with Diff-Quick cell staining kit (Dade Behring, Newark, DE) and mounted on slide.
Aggregation assay
Cells were transfected with miR-150 and miR-NC mimics and tested for their ability to aggregate in hanging drop suspension cultures as previously demonstrated (12). In brief, drops of cell suspension (20 μl each containing 20 000 cells) were placed onto the inner surface of the lid of a Petri dish. The lid was then placed on the Petri dish so that the drops were hanging from the lid with the cells suspended within them. To eliminate evaporation, 8 ml of serum-free culture medium were placed in the bottom of the Petri dish. After overnight incubation at 37°C, the lid of the Petri dish was inverted and photographed using Nikon Eclipse microscope (Nikon Instruments, Melville, NY).
Statistical analysis
Each experiment was performed at least three times independently. All the values were expressed as mean ± SD. The downregulation trend of miR-150 in human pancreatic adenocarcinoma tissue specimens (PCs) and normal pancreatic (NP) tissues was tested using unpaired one-tailed Student’s t-test. Throughout this study, the level of significance was taken to be <0.05. A pairwise comparison was performed to check whether miR-150 had lower expression level in PCs than in NPs. To analyze an association of discordant expression of MUC4 at mRNA and protein levels with miR-150 expression levels, we first computed the MUC4 mRNA and protein ratios (R = MUC4 protein/MUC4 mRNA) and then we regressed R on the expression level of miR-150. We also performed the comparison by standardizing the expression level of MUC4 at mRNA and protein levels, respectively, and then we computed the difference between each pair of Z-scores, D = Z (protein)-Z (mRNA). Finally, we regressed D on the expression level of miR-150. In addition, the correlation coefficients are computed to study the association as well.
Results
miR-150 is a novel posttranscriptional regulator of MUC4
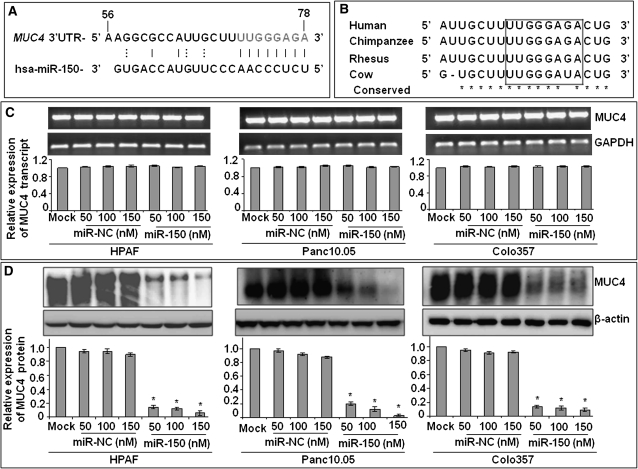
To investigate whether the expression of MUC4 is regulated by miRNAs, we searched the human MUC4 for potential miRNA-binding sites using TargetScan (www.targetscan.org) and miRanda (www.microrna.org). As shown in Figure 1A, in silico analysis revealed a putative 8-mer-binding site for miR-150 in the 3′ UTR of MUC4 transcript. We also observed that miR-150 complementary site in the MUC4 3′ UTR is highly conserved across several mammalian species (Figure 1B), which further suggested the MUC4 targeting ability of miR-150. Next, we experimentally tested the role of miR-150 in MUC4 regulation in three aggressive and metastatic pancreatic adenocarcinoma cell lines, i.e. HPAF, Panc10.05 and Colo357, which express high levels of MUC4. The cells were transiently transfected with miR-150 mimic and the expression of MUC4 was examined. Our data revealed no apparent change in MUC4 expression at the transcript level (Figure 1C), whereas it was significantly downregulated at the protein level (Figure 1D) in miR-150 mimic transfected cells as compared with non-targeting control (miR-NC) mimic transfected cells. Our data suggest that miR-150 downregulates MUC4 expression through a posttranscriptional mechanism.
Fig. 1.
miR-150 negatively regulates the expression of MUC4. (A) Identification of a putative miR-150-binding site in the MUC4 3′ UTR at position. Eight bases (71 through 78) of the MUC4 3′ UTR are perfect matches (seed sequence) for the miR-150. (B) Comparison of the MUC4-binding element among mammals demonstrates a high degree of conservation. (C and D) Posttranscriptional regulation of MUC4 by miR-150. HPAF, Panc10.05 and Colo357 PC cells treated with different concentration of miR-150 or non-targeting control (miR-NC) mimic for 48 h. Mock-transfected cells represent cells treated with Lipofectamine 2000 alone. Expression of MUC4 was examined at mRNA (C) and protein (D) levels by quantitative reverse transcription–PCR and western blot analyses, respectively. GAPDH (for RNA) and β-actin (for protein) were used as internal controls. Amplified products from one of replicate wells of MUC4 and GAPDH quantitative PCR were also run on 1% agarose gel (C). Intensities of the immunoreactive bands in western blots were quantified by densitometry (D). Bars represent relative MUC4 expression after normalization with the relative internal control ± SD, *P < 0.05.
miR-150 directly targets 3′ UTR of MUC4
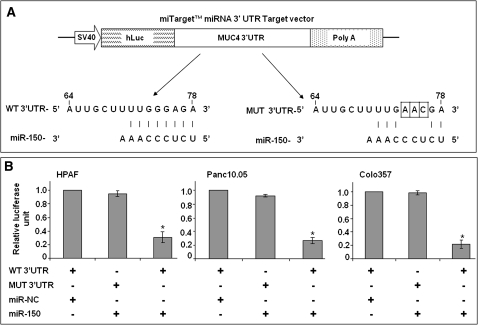
We next determined whether MUC4 is a direct target of miR-150 and if the repression of MUC4 occurs due to the interaction of miR-150 with the predicted binding site in its 3′ UTR. For this, we cotransfected the PC cells with miR-150 or miR-NC (non-targeting control mimic) and a firefly luciferase reporter plasmid containing a region of MUC4 3′ UTR harboring miR-150 target site. As a control, we also generated MUC4 3′ UTR mutant in the miR-150 target region to disrupt its binding (Figure 2A) and used in cotransfection of PC cells with miR-150 or miR-NC. Luciferase activity was measured after 24 h of transfection (Figure 2B). Our data demonstrated that relative luciferase unit was decreased (>70%) in wild-type 3′ UTR transfected PC cells that were cotransfected with miR-150 mimic as compared with that cotransfected with miR-NC. Furthermore, cells transfected with MUT 3′ UTR were resistant to the suppressor activity of miR-150 (Figure 2B). Thus, our data strongly suggest that miR-150 negatively regulates the expression of MUC4 by directly targeting the 3′ UTR of MUC4 transcript.
Fig. 2.
MUC4 is a direct target of miR-150. (A) Schematic representation of firefly luciferase reporter construct containing MUC4 3′ UTR with either wild-type (WT) or mutant (MUT) miR-150 target site. MUT-3′ UTR construct carries three nucleotides (74–76) variation in the seed matching region of the target site to disrupt binding of miR-150. (B) Luciferase reporter assay to examine the miR-150-mediated control of gene expression. HPAF, Panc10.05 and Colo357 cells (0.5 × 106 cells per well) were transiently cotransfected for 24 h with reporter plasmids (200 ng, WT or MUT) and 100 nM of miR-150 or miR-NC mimic. Subsequently, protein lysates were made and luciferase (Firefly; test and Renilla, transfection efficiency control) activity assessed using a dual-luciferase assay system. Data are presented as normalized fold change in luciferase activity (mean ± SD; n = 3, *P < 0.05).
miR-150 represses the expression of HER2, an interacting partner of MUC4, and its downstream signaling
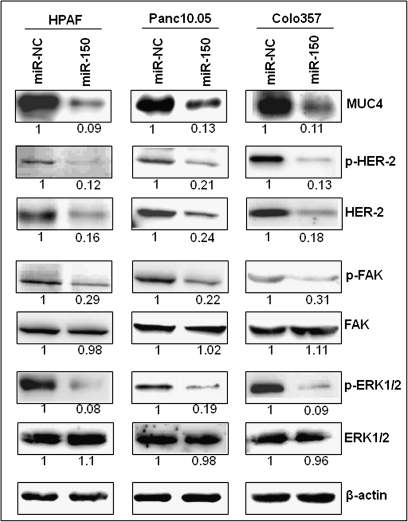
Earlier, it has been shown that MUC4 interacts with HER2 and positively regulates its expression by increasing its stability (18). Thus, we investigated the effect of miR-150 restoration on the expression of HER2. Our immunoblot data demonstrate that the expression of HER2 and its phosphorylated form (pY1248-HER2) was decreased following restoration of miR-150 in all the three PC cell lines (Figure 3). We next examined the activation status of ERK or p42/44 MAPK and FAK, which are among the downstream mediators of HER2 signaling (18). Our data demonstrated a decreased phosphorylation of ERK and FAK in miR-150-transfected cells as compared with controls, whereas there was no change in the expression of total ERK and FAK. In parallel findings, we observed similar effects on the expression of HER2 and its downstream targets following MUC4 silencing (supplementary Figure 1 is available at Carcinogenesis Online). Altogether, our data suggest that miR-150 represses HER2 and its downstream signaling through MUC4 downregulation in PC cells.
Fig. 3.
miR-150 represses HER2 expression and its downstream signaling in PC cells. HPAF, Panc10.05 and Colo357 cells were treated with either miR-150 mimic or non-targeting control (miR-NC) mimic for 48 h. Immunoblotting was performed for MUC4, HER2, p-HER2, ERK1/2, pERK1/2, FAK, pFAK and β-actin (as loading control) proteins followed by densitometry of immunoreactive bands. Normalized densitometric values are indicated at the bottom of the bands. Data show a parallel decrease in HER2 and its phosphorylated form (pY1248-HER2) with MUC4 and consequently decreased phosphorylation of its downstream effector molecules.
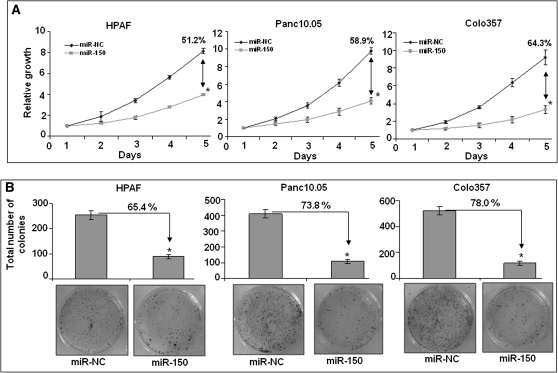
miR-150 overexpression inhibits growth and clonogenicity of PC cells
MUC4 overexpression has been associated with enhanced tumorigenic potential of PC cells (11,12). Therefore, we first studied the effect of miR-150 on the growth of three PC cell lines. Our data demonstrated that relative cell growth was significantly inhibited in miR-150 mimic transfected HPAF (∼51.2%), Panc10.05 (∼58.9%) and Colo357 (∼64.3%) PC cells on day 5 as compared with their respective control (miR-NC transfected) cells (Figure 4A). We next examined the effect of miR-150 restoration on the anchorage-dependent clonogenic ability of the PC cells. In our assay, we observed that the clonogenic ability was decreased by ∼65.4, 73.8 and 78.0% in miR-150-transfected HPAF, Panc10.05 and Colo357 cells, respectively, as compared with their respective controls (Figure 4B). In parallel, we also investigated the effect of MUC4 silencing on growth and clonogenicity of PC cells to validate if the effect of miR-150 was indeed mediated through MUC4 downregulation. Although we observed a decrease in the growth and clonogenicity of PC cells following silencing of MUC4 (supplementary Figure 2A and B is available at Carcinogenesis Online), it was relatively less as compared with the effects caused by miR-150. Our findings, thus suggest that miR-150-mediated inhibition of growth and clonogenicity in PC cells may, in part, be due to downregulation of MUC4 expression.
Fig. 4.
miR-150 decreases growth and clonogenicity of PC cells. (A) HPAF, Panc10.05 and Colo357 cells (2 × 103 per well) were seeded in 96-well plates. After 24 h, cells were treated with either miR-150 mimic or non-targeting control (miR-NC) mimic and growth was monitored by WST-1 assay every day for next 5 days. After analysis, data were presented as relative growth induction (in folds) compared with growth at day 1. Growth inhibition of miR-150-transfected PC cells was calculated in comparison with miR-NC transfected PC cell on fifth day and shown as percentage. Bars represent mean ± SD; (n = 3); *P < 0.05. (B) Cells were transfected with miR-150 or miR-NC mimic and 48 h later, cells were trypsinized and seeded in six-well plate (1 × 103 cells per well) for clonogenicity assay. After 2 weeks, colonies were stained with 0.1% crystal violet, photographed and counted using imaging system. Data were presented as percent inhibition of clonogenic ability of miR-150-transfected cells as compared with their respective controls. Bars represent the mean of total number of colonies ± SD (n = 3); *P < 0.05.
Ectopic expression of miR-150 suppresses the malignant behavior of PC cells
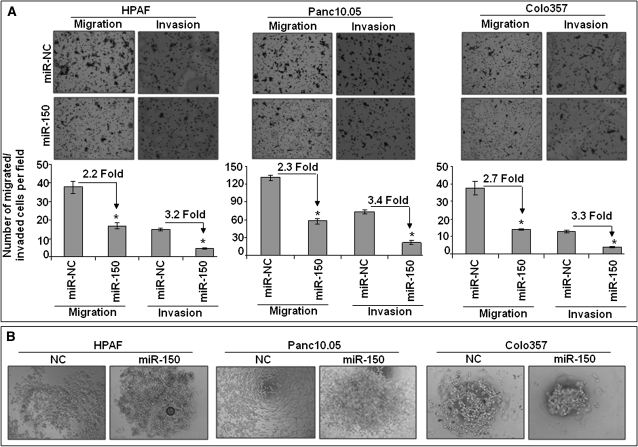
Aggressiveness of a cancer cell is determined by its capacity to invade through the basement membrane. As MUC4 was shown previously to potentiate migration and invasion (11,12), we investigated the role of miR-150 on these malignant behavioral properties in PC cells. Cell motility assay was performed by following the migration of tumor cells under chemotactic drive in a Boyden’s chamber. We observed that the number of migrated cells was significantly decreased in the miR-150-transfected HPAF (2.2-fold), Panc10.05 (2.3-fold) and Colo357 (2.7-fold) cells as compared with their respective controls (Figure 5A). To determine the effect of miR-150 on invasive capacity of PC cells, an in vitro Matrigel invasion assay was performed. Our data demonstrated decreased invasiveness of the HPAF (3.2-fold), Panc10.05 (3.4-fold) and Colo357 (3.3-fold) PC cells transfected with miR-150 as compared with the cells transfected with miR-NC (Figure 5A). Another important characteristic of cancer cells is the loss of homotypic interactions that facilitate their dissemination. As MUC4 downregulation was shown previously to promote cell–cell interaction (12), we examined the effect of miR-150 on PC cells. As speculated, we observed enhanced cell–cell interaction in all the miR-150-transfected PC cell lines as compared with their respective controls (Figure 5B). Altogether, these findings indicate that miR-150 may also act as a metastasis suppressor in PC cells.
Fig. 5.
miR-150 decreases motility, invason and homotypic cell–cell interaction. (A) HPAF, Panc10.05 and Colo357 cells were transfected with miR mimics (miR-150 or miR-NC). After 48 h of transfection, cells were trypsinized and seeded in serum-deprived media on non-coated or Matrigel-coated membranes for motility and invasion assays, respectively, and incubated for 16 h in transwell plates. Media containing 10% fetal bovine serum in the lower chamber was used as a chemoattractant. Cells that had migrated/invaded through the membrane/Matrigel to the bottom of the insert were fixed, stained and counted in 10 random view fields. Bars represent the mean ± SD (n = 3) of number of migrated/invaded cells per field; *P < 0.05. Results show decreased number of migrated and invaded cells after miR-150 overexpression as compared with control cells in all the three cell lines. (B) Effect on cell–cell interaction was determined in a hanging drop assay. Restoration of miR-150 was associated with enhanced cell–cell interaction in HPAF, Panc10.05 and Colo357 PC cells.
miR-150 is downregulated and inversely correlated with MUC4 in PC
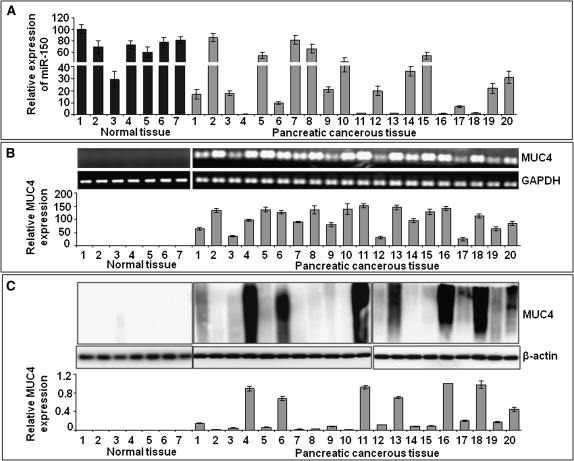
To obtain a clinical evidence of an inverse correlation between miR-150 and MUC4, we examined their expression in a set of 20 human PC tissue specimens along with seven NP tissues. We observed that majority of PCs exhibited downregulated expression of miR-150 as compared with NPs, except one (NP3) as examined in an reverse transcription–quantitative PCR assay (Figure 6A). A t-test analysis showed that the mean expression level of miR-150 in the NPs (69.93) was significantly higher than that of the PCs (21.68) with P value = 0.000662. When we kept NP3 out of the comparison (outlier), we observed a downtrend of miR-150 expression in 15 (75%) PC samples and ∼70% exhibited at least 2-fold downregulation. In contrast, MUC4 expression was not detected in any of the NPs both at transcript and protein levels, whereas it was expressed in malignant pancreatic tissues (Figure 6B and C). Interestingly, we observed a discordant expression of MUC4 at the transcript and protein levels (Figure 6B and C). In further analysis, our data indicated a correlation of miR-150 levels with the discordant expression of MUC4 at mRNA and protein levels (the Pearson correlation coefficient is −0.80 with P value <0.00001). As shown in supplementary Figure 3A (available at Carcinogenesis Online), miR-150 levels are inversely correlated with the ratio R (MUC4 protein/transcript). Consistent results were obtained when we compared the data using Z-scores (the Pearson correction coefficient is −0.80 with P-value <0.00001, supplementary Figure 3B is available at Carcinogenesis Online). Our study confirms the clinical relevance of our experimental data on miR-150-mediated MUC4 regulation in PC cells.
Fig. 6.
Inverse correlation of expression of miR-150 and MUC4 in PC. (A) Expression of mature miR-150 was examined in normal and pancreatic cancerous tissues by quantitative reverse transcription–PCR. U6 small nuclear RNA was used as an internal control for relative quantitation. A reduced expression of miR-150 (>2.0-fold) was observed in 14 of 20 cancer tissues, whereas one normal tissue (#3) also exhibited decreased expression. (B and C) Expression analyses of MUC4 in normal and cancerous pancreatic tissue at mRNA (B) and protein (C) levels. An aberrant expression of MUC4 was detected in malignant pancreatic tissues, whereas it was not expressed in any of the NP sample even at the transcript level. Furthermore, we observed discordance in the expression of MUC4 at transcript and protein levels that correlated with dysregulated expression of miR-150.
Discussion
In the present study, we investigated the role of miR-150 as a novel regulator of MUC4 mucin and determined the functional consequences of its overexpression on the phenotype of PC cells. In silico analysis revealed that 3′ UTR of human MUC4 gene contains an 8-mer target site of miR-150 and miR-150/MUC4 target relationship is conserved across several mammalian species. We also demonstrated that ectopic expression of miR-150 in MUC4-expressing HPAF, Panc10.05 and Colo357 PC cell lines resulted in MUC4 downregulation at the protein level. Furthermore, our study provided experimental data for a role of miR-150 in PC cell growth and malignant behavior and presented evidence for an inverse correlation of miR-150 and MUC4 expression in clinical specimens.
miRNAs are posttranscriptional regulators of gene expression exerting their action through partial complementary elements in the 3′ UTR of their target mRNAs (15,17). Most animal miRNAs are evolutionarily conserved and often exist in clusters (20). Numerous miRNAs have been reported to be differentially expressed in PC as compared with the normal pancreas (21,22), suggesting their involvement in PC pathogenesis. In this study, we identified miR-150 as an evolutionarily conserved novel regulator of MUC4 mucin, which is downregulated in a significant proportion of pancreatic tumors. An aberrant expression of miR-150 has also been reported in other malignancies. Although miR-150 is downregulated in lymphoma and leukemia (23,24), its expression is upregulated in gastric and colorectal malignancies (25,26). Furthermore, miR-150 is shown to promote gastric cell proliferation (26) while it acts as a tumor suppressor in malignant melanoma (24). Among the important targets of miR-150 that has been experimentally validated are c-Myb, P2X7 and EGR2, of which latter two mediate its tumor promoting functions (26–28). Therefore, these studies along with our findings indicate cell type-specific and/or context-dependent functions of miR-150.
MUC4 is frequently deregulated in a wide variety of cancers (29), and its overexpression has been associated with pancreatic tumor growth and metastasis (11,12). Furthermore, recent data also indicate its pathological involvement in other malignancies and inflammatory diseases (30–33). MUC4 and its rat homolog (SMC/Muc4) have been shown to regulate a variety of cell processes such as proliferation, apoptosis, migration, invasion and differentiation, sometimes in a context-dependent manner (8,11,13,14). In majority of studies, MUC4/Muc4 has been shown to modulate cell signaling by either acting as an intramembrane ligand for ErbB2 (rat Muc4) or by modulating HER2 expression through enhanced stability (18,29). However, an indirect role of MUC4 in cell phenotype has also been predicted through surface interference (steric hindrance) due to its large molecular size (13,34). MUC4 may thus alter intercellular interaction of surface adhesion proteins and impact cell signaling by indirect mechanism. In other instance, MUC4 may also facilitate tumor cell–endothelial cell interactions through presence of selectin ligands (Sialyl Lewis x/a glycotopes) on the core protein (13,34). Thus, identification of miR-150 as a novel regulator of MUC4 may have important therapeutic implications in pancreatic and other malignancies.
miRNAs are potentially able to target multiple RNAs through imperfect binding; therefore, a single miRNA is believed to exert diverse, more potent and context-dependent functions (16,17). Growing body of evidence now suggests that miRNAs act either as oncogenes or tumor suppressors in a variety of cancers (35). miRNAs have been implicated in a broad range of biological processes including cell proliferation, apoptosis, differentiation, metabolism and migration and invasion (27,36–39). Our investigation revealed that miR-150 overexpression decreases pancreatic tumor cell growth, clonogenicity and suppresses the malignant behavioral properties. These effects are consistent with previously reported role of MUC4 and thus downregulation of MUC4 may underlie such functional consequences of miR-150 overexpression. However, in other studies, several additional targets of miR-150 have also been established (26,28), and some may still be pending experimental validation, these targets along with MUC4 may also mediate miR-150 action in PC. In fact, this notion is further substantiated by the fact that we observed a more potent effect of miR-150 restoration as compared with that observed upon silencing of MUC4 alone (supplementary Figure 2A and B is available at Carcinogenesis Online). Therefore, it will be of interest to identify novel targets of miR-150 and/or examine the functional role of already characterized targets in PC progression. Nonetheless, our study provides a clear evidence for a role of miR-150 as a tumor suppressor and establishes MUC4 downregulation as a plausible mechanism. In our earlier studies, we have shown that MUC4 expression is associated with the activation of FAK and ERK pathways through modulation of HER2 expression in pancreatic and ovarian cancer cells (18,32). Consistent with this, we observed inhibition of HER2 and its downstream signaling (FAK and ERK) in miR-150 overexpressing PC cells and this effect could partly mediate the tumor suppressive action of miR-150.
In summary, we have validated the role of miR-150 as a negative regulator of MUC4 in three PC cell lines. As a consequence, we also observe repression of HER2 and its downstream signaling. These molecular changes, at least in part, are responsible for the decreased growth, clonogenicity, migration and invasion and enhanced homotypic interactions of PC cells. Furthermore, our results provide an evidence for a clinical correlation of miR-150 and MUC4 expression in a small subset of malignant pancreatic tissues, whereas MUC4 in normal pancreas primarily seemed to be regulated by transcriptional mechanisms. In conclusion, we postulate that restoring miR-150 levels may have therapeutic effects in PC.
Supplementary material
Supplementary Table 1 and Figures 1–3 can be found at http://carcin.oxfordjournals.org/
Funding
NIH/NCI (CA137513), DOD/US Army (W81XWH-09-1-0137) and USAMCI.
Supplementary Material
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- HER2
human epidermal growth factor receptor 2
- miRNA
microRNA
- miR-150
miRNA-150
- mRNA
messenger RNA
- PC
pancreatic cancer
- PCR
polymerase chain reaction
- siRNA
small interfering RNA
- UTR
untranslated region
References
- 1.Jemal A, et al. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, et al. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br. J. Cancer. 2010;103:1671–1679. doi: 10.1038/sj.bjc.6605968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D, et al. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 4.Bardeesy N, et al. Pancreatic cancer biology and genetics. Nat. Rev. Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 5.Rhim AD, et al. Molecular biology of pancreatic ductal adenocarcinoma progression: aberrant activation of developmental pathways. Prog. Mol. Biol. Transl. Sci. 2010;97:41–78. doi: 10.1016/B978-0-12-385233-5.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koorstra JB, et al. Pancreatic carcinogenesis. Pancreatology. 2008;8:110–125. doi: 10.1159/000123838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mimeault M, et al. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas. 2005;31:301–316. doi: 10.1097/01.mpa.0000175893.04660.1b. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi P, et al. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrianifahanana M, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin. Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- 10.Swartz MJ, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am. J. Clin. Pathol. 2002;117:791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi P, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol. Cancer Res. 2007;5:309–320. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 12.Singh AP, et al. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–630. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 13.Singh AP, et al. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67:433–436. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- 14.Carraway KL, et al. Muc4/MUC4 functions and regulation in cancer. Future Oncol. 2009;5:1631–1640. doi: 10.2217/fon.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhardwaj A, et al. MicroRNA-based cancer therapeutics: big hope from small RNAs. Mol. Cell. Pharmacol. 2010;2:213–219. doi: 10.4255/mcpharmacol.10.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garzon R, et al. MicroRNAs in cancer. Annu. Rev. Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 17.Iorio MV, et al. MicroRNAs in cancer: small molecules with a huge impact. J. Clin. Oncol. 2009;27:5848–5856. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi P, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glazov EA, et al. Origin, evolution, and biological role of miRNA cluster in DLK-DIO3 genomic region in placental mammals. Mol. Biol. Evol. 2008;25:939–948. doi: 10.1093/molbev/msn045. [DOI] [PubMed] [Google Scholar]
- 21.Lee EJ, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int. J. Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szafranska AE, et al. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26:4442–4452. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, et al. miRNA analysis in B-cell chronic lymphocytic leukaemia: proliferation centres characterized by low miR-150 and high BIC/miR-155 expression. J. Pathol. 2008;215:13–20. doi: 10.1002/path.2333. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe A, et al. The role of microRNA-150 as a tumor suppressor in malignant lymphoma. Leukemia. 2011;25:1324–1334. doi: 10.1038/leu.2011.81. [DOI] [PubMed] [Google Scholar]
- 25.Lin M, et al. MicroRNA expression profiles in human colorectal cancers with liver metastases. Oncol. Rep. 2011;25:739–747. doi: 10.3892/or.2010.1112. [DOI] [PubMed] [Google Scholar]
- 26.Wu Q, et al. MiR-150 promotes gastric cancer proliferation by negatively regulating the pro-apoptotic gene EGR2. Biochem. Biophys. Res. Commun. 2010;392:340–345. doi: 10.1016/j.bbrc.2009.12.182. [DOI] [PubMed] [Google Scholar]
- 27.Xiao C, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Zhou L, et al. MicroRNAs miR-186 and miR-150 down-regulate expression of the pro-apoptotic purinergic P2X7 receptor by activation of instability sites at the 3'-untranslated region of the gene that decrease steady-state levels of the transcript. J. Biol. Chem. 2008;283:28274–28286. doi: 10.1074/jbc.M802663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carraway KL, et al. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog. Nucleic Acid Res. Mol. Biol. 2002;71:149–185. doi: 10.1016/s0079-6603(02)71043-x. [DOI] [PubMed] [Google Scholar]
- 30.Hamada T, et al. MUC4 is a novel prognostic factor of oral squamous cell carcinoma. Int. J. Cancer. 2011 doi: 10.1002/ijc.26187. doi:10.1002/ijc.26187. [DOI] [PubMed] [Google Scholar]
- 31.Hoebler C, et al. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig. Dis. Sci. 2006;51:381–389. doi: 10.1007/s10620-006-3142-y. [DOI] [PubMed] [Google Scholar]
- 32.Ponnusamy MP, et al. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br. J. Cancer. 2008;99:520–526. doi: 10.1038/sj.bjc.6604517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh AP, et al. MUC4 expression is regulated by cystic fibrosis transmembrane conductance regulator in pancreatic adenocarcinoma cells via transcriptional and post-translational mechanisms. Oncogene. 2007;26:30–41. doi: 10.1038/sj.onc.1209764. [DOI] [PubMed] [Google Scholar]
- 34.Hollingsworth MA, et al. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 35.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N. Engl. J. Med. 2005;353:1768–1771. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 36.Calin GA, et al. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 37.Sachdeva M, et al. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sikand K, et al. miR 488* inhibits androgen receptor expression in prostate carcinoma cells. Int. J. Cancer. 2011;129:810–819. doi: 10.1002/ijc.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trajkovski M, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.