Summary
Misfolded proteins accumulating in several neurodegenerative diseases (including Alzheimer’s, Parkinson’s and Huntington’s diseases) can cause aggregation of their native counterparts through a mechanism similar to the infectious prion protein’s induction of a pathogenic conformation onto its cellular isoform. Evidence for such a prion-like mechanism has now spread to the main misfolded proteins (SOD1 and TDP-43) implicated in Amyotrophic Lateral Sclerosis (ALS). The major neurodegenerative diseases may therefore have mechanistic parallels that provide a molecular pathway for non-cell autonomous disease spread within the nervous system.
Neurodegenerative disorders, such as Parkinson’s, Huntington’s, Alzheimer’s disease, frontotemporal lobar degeneration (FTLD) and Amyotrophic Lateral Sclerosis (ALS) are associated with the accumulation of misfolded proteins both inside and outside of neuronal and glial cells in the central nervous system. While the major protein component of the pathological aggregations is characteristic for each neurodegenerative disease – e.g., α-synuclein in Parkinson’s, huntingtin in Huntington’s or Aβ in Alzheimer’s disease – several proteins misfold and accumulate in multiple diseases (especially TDP-43 which mis-accumulates in ALS, FTLD, and many other conditions). Conversely, neurodegenerative conditions can be associated with the presence of more than one accumulated protein (e.g., Aβ and tau in Alzheimer’s disease). These misfolded protein aggregates are pathological hallmarks of each disease.
One widely held view is that these aggregates play a vital role in disease initiation and progression, with the misfolded versions of endogenous proteins likely to acquire toxic properties, potentially through increased hydrophobicity and/or sequestration of essential cellular components within the aggregates, generation of oxidative species, proteasome inhibition and through other pathways (reviewed in (Ilieva et al., 2009)). An alternative view is that the large aggregates detected immunohistochemically represent not the toxic species but the final product of a defensive cell response aimed at protecting cells from more toxic oligomeric species that remain undetectable by most techniques.
Prions and prion-like phenomena in neurodegeneration
Prion diseases or transmissible spongiform encephalopathies are a class of neurodegenerative diseases that, as their name suggests, can be transmitted from individual to individual through ingestion or internalization of contaminated material (reviewed in (Aguzzi et al., 2008)). The nature of the infectious agent and the transmission mechanism in prion diseases have been the subject of intense interest since the demonstration, 45 years ago, of transmissibility of human prions (Gajdusek et al., 1966). It is now widely accepted that the prion, the infectious agent of prion diseases, consists of misfolded form(s) – designated PrPSc – of a normal protein, the cellular prion protein or PrPC, as was proposed by Prusiner in the early 1980’s (Prusiner, 1982). With the amplification of infectious prions in vitro (Castilla et al., 2005) and most recently their production from purified recombinant protein (Wang et al., 2010), the evidence for the protein-only prion model is now overwhelming.
Prions replicate by recruiting PrPC in the ordered PrPSc-containing aggregates and by inducing a pathological conformation on the native endogenous prion protein. While such replication/transmission mechanisms were long thought to be uniquely associated with transmissible prion diseases, in the past decade an increasing list of neurodegenerative (and other) diseases have been shown to include “prion-like” phenomena (Table 1). We use the term “prion-like” to describe molecular events that share similarities with the infectious cycle of the mammalian prion protein’s self-perpetuating seeded aggregation and spreading. Beyond infectious prions, the notion of prion-like spreading of misfolded conformations of proteins linked to human neurodegenerative diseases arose from demonstration of Aβ plaque formation in the brains of primates after injection of brain extracts of human Alzheimer’s patients (Baker et al., 1994). Prion-like spread was then established by the work of Jucker and Walker, whose groups showed that Aβ aggregation is hastened by the presence of preformed Aβ aggregates or “seeds” in vivo. In particular, they showed that intracerebral injection of brain extracts from autopsy material of human Alzheimer’s disease patients or from aged Alzheimer’s disease model mice – both containing ordered aggregates of human Aβ – into transgenic mice expressing human amyloid precursor protein (APP), accelerated the aggregation of human Aβ produced as a proteolytic fragment of transgene encoded APP (Kane et al., 2000; Meyer-Luehmann et al., 2006). They further showed that while peripheral routes of inoculation, such as intravenous, oral, or intranasal are generally inefficient in seeding Aβ aggregation (Eisele et al., 2009), intraperitoneal administration of Aβ-containing extracts induced aggregation in the vicinity of brain blood vessels (Eisele et al., 2011) that is reminiscent of cerebral β-amyloid angiopathy associated with Alzheimer’s disease in humans (Thal et al., 2008).
Table 1.
Summary of current evidence on seeded aggregation, spreading and clinical disease induced in experimental animals by injection of aggregated proteins associated with human neurodegenerative diseases. We note that “NO” signifies that there is currently no evidence supporting the respective events.
| Native protein | Aggregated protein or peptide | Main associated diseases in humans | Acquired by infection in humans | Subcellular localization | Seeded aggregation | Cell-to-cell spreading | Inducible clinical disease in mice | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Native | Aggregates | in vitro | in cell culture | in vivo in mice | in cell culture | in vivo in mice | synthetic seed | brain | ||||
| PrPC | PrPSc | (variant, iatrogenic) Creutzfeldt-Jacob, Kuru | YES | Plasma membrane anchored | Mostly extracellular | YES | YES | YES | YES | YES | YES | YES |
| (sporadic, familial) Creutzfeldt-Jacob, Fatal familial insomnia, Gerstmann-Straussler-Scheinker | NO | |||||||||||
| Tau | Tau | Frontotemporal lobar dementia, Alzheimer’s | NO | Cytoplasmic | Cytoplasmic | YES | YES | YES | YES | YES | n.d. | NO |
| α-synuclein | α-synuclein | Parkinson’s, Lewy body dementia | NO | Nuclear and synaptic | Cytoplasmic | YES | YES | YES | YES | YES | n.d. | YES (acceleration in mutant mice) |
| APP | β-amyloid | Alzheimer’s | NO | Transmembrane | Mostly extracellular | YES | YES | YES | NO | YES | NO | NO |
| Huntingtin | Poly-Q | Huntington’s | NO | Nuclear | Nuclear | YES | YES | n.d. | YES | n.d. | n.d. | n.d. |
| Ataxins | Spinocerebellar ataxias | NO | ||||||||||
| SOD1 | SOD1 | Amyotrophic lateral sclerosis | NO | Cytoplasmic | Cytoplasmic | YES | YES | n.d. | YES | n.d. | n.d. | n.d. |
| TDP-43 | TDP-43 | Amyotrophic lateral sclerosis, Frontotemporal lobar degeneration | NO | Nuclear | Mostly cytoplasmic | YES | YES | n.d. | n.d. | n.d. | n.d. | n.d. |
| FUS/TLS | FUS/TLS | Amyotrophic lateral sclerosis, Frontotemporal lobar degeneration | NO | Nuclear | Mostly cytoplasmic | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
PrPC: cellular prion protein, PrPSc: pathologic prion protein, Tau: microtubule associated protein, Poly-Q: polyglutamine, APP: amyloid precursor protein, SOD1: superoxide dismutase 1, TDP-43: TAR DNA-binding protein 43, FUS/TLS: Fused in sarcoma, translocated in liposarcoma, n.d. not done.
Following a similar paradigm, intracerebral injection of mutant tau aggregate-containing brain extracts seed widespread aggregation of normal human tau in transgenic mice that do not otherwise develop aggregates (Clavaguera et al., 2009). Spreading and in vivo seeding of α-synuclein aggregation was first shown by induction of α-synuclein inclusions (also called Lewy bodies) within normal neuronal stem cells transplanted in Parkinson’s disease patients (Kordower et al., 2008; Li et al., 2008), a paradigm that was replicated in mice (Desplats et al., 2009; Hansen et al., 2011). Most recently, acceleration of both aggregation and, most importantly, clinical disease was shown in transgenic mice expressing human mutant α-synuclein, after intracerebral injection of brain extracts from old mice of the same transgenic line (Mougenot et al., 2011). While there is currently no experimental evidence that expanded polyglutamine (poly-Q) proteins can seed aggregation in animals, seeding and persistent, self-perpetuating cell-to-cell transmission of poly-Q aggregation has been shown in cultured cells (Ren et al., 2009). In fact, α-synuclein (Desplats et al., 2009; Hansen et al., 2011; Nonaka et al., 2010) and tau (Frost et al., 2009; Guo and Lee, 2011; Nonaka et al., 2010) also exhibit this seeding behavior in cultured cells. The propagation of the above misfolded proteins in cultured cells once again resembles PrPSc, which was initially shown to replicate in cells over 40 years ago, a feature that has been exploited to establish a quantitative cell-based assay for determination of infectious prion titers (Klohn et al., 2003; Mahal et al., 2007).
Except for PrPSc in prion disease there is currently no evidence that the other induced aggregates can spread between individuals to cause acquired disease, either in humans or in experimental animals. The latter might be due to the challenges in modeling human neurodegenerative diseases in transgenic animals, which typically do not reproduce the full pathology or clinical disease seen in humans (Jucker, 2010). Nevertheless, this crucial difference lead Aguzzi to propose the term prionoid to distinguish prion-like phenomena spreading disease within a single organism from bona fide infectious prions (Aguzzi, 2009; Aguzzi and Rajendran, 2009). This is an important distinction, with the difference between prions and prionoids probably lying in the potency of induced aggregation and spreading, rather than any difference in underlying molecular events. This augmented potency of prions to induce transmissible disease may depend on (but not restricted to) their remarkable resistance to endogenous proteases and other routes of pathogen elimination (Shorter and Lindquist, 2005).
Regardless, the prion-like replication that occurs within affected cells followed by transfer from cell to cell provides a molecular pathway for disease spread within the nervous system following focal generation of an initiating misfolding event. Indeed, the apparent spreading of pathologic changes has been described for all the major neurodegenerative diseases, e.g., Alzheimer’s (Braak and Braak, 1991), Parkinson’s (Braak et al., 2003), FTLD (Kril and Halliday, 2011), Huntington’s (Deng et al., 2004), ALS (Ravits et al., 2007a; Ravits et al., 2007b) and of course prion diseases, where in acquired cases caused by infection, the initial site of propagation may occur outside the central nervous system (Aguzzi et al., 2008).
The major misfolded proteins in ALS: from SOD1 to TDP-43 and FUS/TLS
ALS is a neurodegenerative condition that targets primarily motor neurons, resulting in progressive paralysis and death within a few years from onset. Just like Alzheimer’s, Parkinson’s and other neurodegenerative diseases, a proportion (~10%) of ALS is dominantly inherited, with the remaining 90% (referred to as sporadic) of unknown origin. The identification in 1993 of mutation in the gene encoding superoxide dismutase 1 (SOD1) as the first or second most common form of inherited ALS (Rosen et al., 1993), and subsequent generation of transgenic mice expressing ALS-causing mutants in SOD1, initiated the molecular era of deciphering disease mechanism. A flurry of approaches established that non-cell autonomous disease depends on one or more toxic properties of mutant SOD1. The latter drives disease initiation when synthesized within motor neurons, while its synthesis by glial neighbors provokes rapid disease advance (reviewed in (Ilieva et al., 2009)). Along with prion-infected mice, the ALS-linked mutant SOD1 mice are among the most faithful models of neurodegeneration, recapitulating the selective progressive loss of motor neurons that leads to the paralysis characteristic of human ALS.
In both inherited and sporadic ALS, affected neurons and glial cells contain abnormal proteinaceous accumulations, often labeled by anti-ubiquitin antibodies. The major protein component of these accumulations in familial cases with SOD1 mutations – and in mutant SOD1 mice – is SOD1 itself. An initial view that SOD1 inclusions were not found in sporadic disease (e.g., (Kerman et al., 2010)) has recently been challenged (Bosco et al., 2010; Forsberg et al., 2010). This controversy notwithstanding, over the past five years it has been established that a main component of proteinaceous cytoplasmic inclusions in essentially all sporadic ALS cases is the RNA/DNA-binding protein TDP-43, accompanied by its nuclear depletion (Arai et al., 2006; Neumann et al., 2006). Moreover, mutations in TDP-43 are causes of inherited ALS and rare instances of FTLD (Gitcho et al., 2008; Kabashi et al., 2008; Sreedharan et al., 2008; Van Deerlin et al., 2008; Yokoseki et al., 2008).
Affected neurons of patients with TDP-43 mutations also develop cytoplasmic TDP-43-positive inclusions and nuclear loss, implying that abnormal localization and aggregation of TDP-43 could represent a first mechanistic link between sporadic ALS and an inherited form caused by a known mutation. Furthermore, ALS-causing mutations were identified in a gene encoding another RNA/DNA-binding protein, called FUS/TLS for fused in sarcoma or translocated in liposarcoma (Kwiatkowski et al., 2009; Vance et al., 2009). FUS-mutant mediated disease is also accompanied by FUS/TLS-containing cytoplasmic inclusions and disturbed subcellular localization. Unresolved is whether pathogenesis in TDP-43- or FUS/TLS-mediated disease results from a loss of nuclear function of either protein, from a gain of toxic property(ies) associated (or not) with their cytoplasmic inclusions, or – perhaps most likely – from a combination of all possibilities.
Aggregation initiation of ALS-associated proteins
Within the last year, a plausible, unifying proposal for underlying disease mechanism among the inherited and sporadic ALS instances has emerged: normal SOD1, TDP-43 and possibly also FUS/TLS, can undergo seeded aggregation that can spread from cell to cell through a prion-like mechanism after an initiating event.
SOD1 is a small 153-amino acid protein, which in its native state occurs as a remarkably stable dimer that is highly resistant to proteolytic degradation. ALS-associated point mutations occur in almost every position (>140 mutations are known) with each leading to destabilization and eventually accumulation of misfolded species within affected cells of the nervous system. In vitro studies with purified SOD1 have shown that both the wild-type and several mutant versions of the protein spontaneously fibrillize under denaturing conditions (Chia et al., 2010; Grad et al., 2011; Munch et al., 2011; Prudencio et al., 2009), with a propensity to aggregate that is enhanced in the mutants (Chia et al., 2010; Prudencio et al., 2009).
TDP-43 consists of two RNA recognition motifs and a largely disorganized C-terminal domain that contains the overwhelming majority of ALS-associated mutations (reviewed in (Lagier-Tourenne et al., 2010)). This C-terminal region of TDP-43 was suspected to play a critical role in ALS pathogenesis since early analyses revealed that it is the main proteolytic fragment (designated CTF) identified in the cytoplasmic inclusions of sporadic patients (Arai et al., 2006; Igaz et al., 2008; Neumann et al., 2006). This same region was proposed to contain a glutamine/asparagine-rich (Q/N-rich) domain that shares similarities with what have been called yeast prions (Cushman et al., 2010; Fuentealba et al., 2010), proteins exhibiting ordered, self-perpetuating aggregation, and that are transmissible from an affected cell to its progeny (reviewed in (Chien et al., 2004; Cushman et al., 2010; Shorter and Lindquist, 2005)). The known yeast prion domains can switch their conformation between two states: an intrinsically unfolded one and an aggregated one that imposes its conformation to its unfolded counterpart.
Like SOD1, TDP-43 and TDP-43-derived peptides form aggregates in vitro (Furukawa et al., 2011; Guo et al., 2011; Johnson et al., 2008; Johnson et al., 2009) and ALS-causing mutations enhance this behavior (Guo et al., 2011; Johnson et al., 2009). Highlighting its critical role for TDP-43 aggregation, its C-terminal region is apparently indispensable for aggregation (Furukawa et al., 2011; Johnson et al., 2008; Johnson et al., 2009) and truncation mutants consisting solely of TDP-43 CTF show significantly increased aggregation propensities in vitro and in cells (Furukawa et al., 2011; Guo et al., 2011; Johnson et al., 2008; Johnson et al., 2009; Liu-Yesucevitz et al., 2010). Importantly, the C-terminal region of TDP-43 acquires partial resistance to proteases when packaged within full-length TDP-43 aggregates (Furukawa et al., 2011; Guo et al., 2011), a property reminiscent of the pathogenic PrPSc. Collectively, these data suggest that aggregation of TDP-43 is driven by its prion-like C-terminal region and that ALS-linked mutations are likely to promote this process.
The 526 amino acid FUS/TLS is structurally and functionally related to TDP-43 (Lagier-Tourenne et al., 2010). The ALS-causing mutations are mainly shared between the very last part of the protein containing its nuclear localization signal (NLS) (Dormann et al., 2011; Ito et al., 2011; Sun et al., 2011) and a ~100 amino acid glycine-rich domain. The latter is incorporated within a predicted Q/N-rich, yeast prion-like domain comprising the first 239 amino acids of FUS/TLS (Cushman et al., 2010). Purified FUS/TLS was shown to aggregate extremely rapidly – within a few minutes – in a cell-free system under native conditions and without the agitation (Sun et al., 2011) typically used to facilitate protein aggregation in vitro (Furukawa et al., 2011; Guo et al., 2011; Wang et al., 2010).
Compared to TDP-43 and SOD1, FUS/TLS demonstrates the highest aggregation propensity, but this property is not affected by ALS-causing mutations localized in its NLS signal (Dormann et al., 2011; Sun et al., 2011). Rather, these mutations clearly enhance the cytoplasmic accumulation/retention of FUS/TLS (Dormann et al., 2011; Ito et al., 2011). Increased cytoplasmic FUS/TLS in cultured neurons seems to eventually translate into increased aggregation that promotes its integration into RNA- and RNA-binding protein-containing foci – called stress granules – that form in response to several known stresses (Anderson and Kedersha, 2009). On the other hand, mutations residing in the prion-like domain of FUS/TLS do not enhance its cytoplasmic localization or its integration within stress granules (Dormann et al., 2011), but may directly increase its aggregation propensity (Figure 1A).
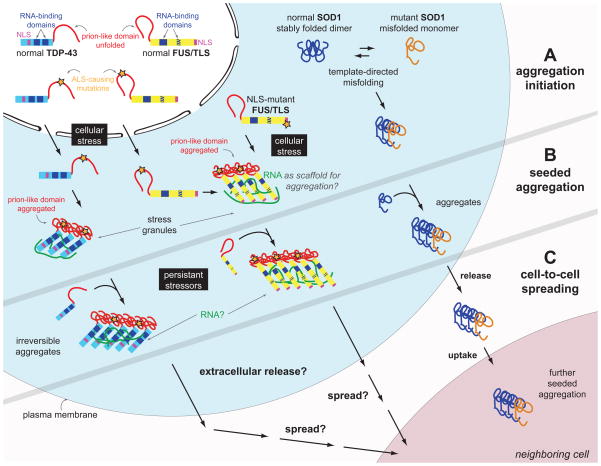
Figure 1. Prion-like phenomena in ALS: SOD1, TDP-43 and FUS/TLS seeded aggregation and cell-to-cell spreading.
A. Mutant, misfolded SOD1 was shown to induce the misfolding of its native counterpart, in a template-directed reaction, thereby forming a seed of aggregated protein. TDP-43 and FUS/TLS are both incorporated in stress granules, which form through the ordered aggregation of several RNA-binding proteins complexed with RNA molecules. This physiologic reaction to cellular stress may be the initial trigger for pathogenic inclusion formation since the increased local protein concentration and RNA scaffolding molecules may facilitate ordered aggregation of TDP-43 and/or FUS/TLS. Mutations in the prion-like domain of TDP-43 (and maybe also FUS/TLS) enhance its aggregation propensity, while mutations in the nuclear localization domain (NLS) of FUS/TLS increase its cytoplasmic localization.
B. Misfolded SOD1 follows a self-perpetuating seeding reaction in cell culture. Upon chronic cellular stress and defects in stress granule disassembly occurring with aging, the functional prion-like conformational changes of TDP-43 and FUS/TLS associated with their physiological roles in stress granule formation may transform into pathogenic self-perpetuating, irreversible aggregation. It is unknown whether cellular RNA is occasionally trapped within the cytoplasmic FUS/TLS and/or TDP-43 inclusions, thereby depleting the cell of essential RNA components.
C. SOD1 aggregates transfer from cell-to-cell to initiate misfolding and aggregation of native SOD1 in neighboring cells (shown in cell culture). It is currently not known whether TDP-43 and/or FUS/TLS can spread from cell to cell by a similar mechanism.
Filled blue boxes on TDP-43 and FUS/TLS molecules indicate RNA-recognition motifs and the striped blue box on FUS/TLS refers to the zinc finger domain that can also bind RNA.
While SOD1, TDP-43 and FUS/TLS readily aggregate in vitro, the intracellular array of protein folding chaperones must act to inhibit this. So, what triggers the initiation of aggregation in disease and the selective vulnerability of the most highly affected nervous system regions? These remain two of the most important unresolved questions in ALS (and neurodegenerative disease in general). The natural decline of proteostatic mechanisms that occurs with aging has been widely proposed to mediate the pathogenic process, with mutations likely to tip the balance by increasing the aggregation propensity of the respective proteins. In agreement with this, aggregation propensities of SOD1 mutants and the degree of cytoplasmic mislocalization of NLS-mutants of FUS/TLS inversely correlate with the age of disease onset of familial ALS patients (Dormann et al., 2011; Prudencio et al., 2009). For sporadic disease, yet undefined environmental and/or genetic triggers may converge to initiate the pathogenic process. In line with this view, recent work from cell-culture systems has suggested that a combination of two insults or “two-hits” is required for the formation of pathologic cytoplasmic inclusions of both FUS/TLS (e.g., cytoplasmic localization and cell stress (Dormann et al., 2011)) and TDP-43 (e.g., cleavage of the CTF and disruption of microtubule transport (Pesiridis et al., 2011)).
Self-propagated aggregation of ALS-associated proteins
A very provocative finding implicating a self-propagating spread for both SOD1 and TDP-43 emerged within the last year, initially from demonstrations that aggregated forms of either can seed misfolding of a much larger amount of the corresponding wild type protein in vitro (Chia et al., 2010; Furukawa et al., 2011). Moreover, in cultured cells, misfolded forms of both SOD1 and TDP-43, either exogenously applied (Furukawa et al., 2011; Munch et al., 2011), or formed within cells (Grad et al., 2011; Pesiridis et al., 2011) induced the misfolding and subsequent aggregation of the respective native proteins. Importantly, induced aggregation of endogenous SOD1 was shown to persist after removal of the misfolded seeds (Grad et al., 2011; Munch et al., 2011), suggesting that the newly formed aggregates can act as templates for the subsequent misfolding of additional native SOD1 (Figure 1B). This behavior is consistent with a self-perpetuating, cyclic reaction, analogous to that underlying the replication of infectious prion aggregates
While it remains to be determined whether FUS/TLS aggregation can be seeded, the implication of both TDP-43 and FUS/TLS in stress granule assembly (Andersson et al., 2008; Colombrita et al., 2009; Ito et al., 2011; Liu-Yesucevitz et al., 2010; McDonald et al., 2011; Moisse et al., 2009; Wang et al., 2008) offers a plausible mechanism for aggregation initiation and seeding as a response to a variety of cellular stresses (Figure 1B). Indeed, the very formation of stress granules is mediated by the ordered aggregation of TIA1, an integral stress granule protein component that possesses a Q/N-rich yeast prion-like domain (Gilks et al., 2004). Seeded aggregation of TIA1 through its prion-like domain seems to be the driving force of stress granule formation, since this domain is not only indispensable for TIA1’s nucleation, but it can even be replaced by another Q/N-rich domain from a yeast prion protein (SUP35) without visibly affecting the size or number of stress granules (Gilks et al., 2004). Aggregated TIA1 within cytoplasmic foci recruits mRNAs and other proteins, including TDP-43 and FUS/TLS (Andersson et al., 2008; Colombrita et al., 2009; Ito et al., 2011; Liu-Yesucevitz et al., 2010; McDonald et al., 2011; Moisse et al., 2009; Wang et al., 2008).
Since increased protein concentration is expected to be a main determinant for protein aggregation, the increase of local TDP-43 and FUS/TLS concentration within stress granules could facilitate the initiation of their aggregation. This may be further assisted by the presence of RNA that can act as a scaffolding molecule mediating the ordered aggregation of TDP-43 and FUS/TLS within these cytoplasmic foci. Indeed, the scaffolding capacity of RNA has been established in the in vitro aggregation of the mammalian prion protein (Deleault et al., 2003), as generation of infectious prions with purified PrP has been achieved only by the addition of RNA and phospholipids (Wang et al., 2010). While for the in vitro aggregation of PrP, the RNA is likely to mimic other, yet unknown cellular factors, in the case of RNA-binding proteins like TDP-43 and FUS/TLS, intimate interaction with RNA in stress granules may represent an early stage of inclusion formation actually occurring in neurons and glial cells.
In healthy cells, stress granule formation (and TIA1 aggregation) is dynamically regulated and reversible (Kedersha et al., 2000). In fact, TIA1 belongs to a group of proteins that undergo reversible, conformational switches leading to self-replicating aggregation, which confers a functional advantage for the aggregate-bearing cells and their neighbors (Shorter and Lindquist, 2005). The notion of such functional prion-like proteins was first shown in yeast, where several proteins were found to provide different functional roles before and after aggregation in yeast cells (including SUP35 introduced above; reviewed in (Uptain and Lindquist, 2002)). The list of such proteins is expanding and besides TIA1 and yeast prions, it currently includes the cytoplasmic polyadenylation element binding protein (CPEB), whose ordered aggregation has been proposed to facilitate long-term memory (Si et al., 2010; Si et al., 2003) and most recently, MAVS, a signaling protein that forms mitochondrial membrane-associated aggregates that trigger an RNA-dependent response to viral infection (Hou et al., 2011).
In this context, we hypothesize that the functional conformational changes of TDP-43 and FUS/TLS associated with their physiological roles in stress granule formation may transform into pathogenic, self-perpetuating, irreversible aggregation upon chronic cellular stress and defects in stress granule disassembly occurring with aging (Figure 1B). In other words, stress granules may be operating to facilitate FUS/TLS and/or TDP-43 “seeding” within the cytoplasm of diseased cells. The observation that stress granule proteins partition in the TDP-43 and FUS/TLS pathologic inclusions found in ALS patients supports this view (Dormann et al., 2011; Fujita et al., 2008; Liu-Yesucevitz et al., 2010).
Unresolved is whether specific cellular RNAs are sequestered within the cytoplasmic FUS/TLS and/or TDP-43 inclusions, thereby depleting the cell of essential RNA components. The latter, if true, could explain the observation that while the RNA-recognition motifs of TDP-43 are not required for its aggregation (Johnson et al., 2008; Pesiridis et al., 2011), binding to RNA seems to be indispensible for its cytotoxicity (Elden et al., 2010; Voigt et al., 2010).
Mutant-wild-type interactions of ALS-associated proteins
Template-directed misfolding refers to the conversion of a natively folded protein into a misfolded version of itself through direct interaction with another misfolded molecule. Such template-directed misfolding seems to occur between wild-type (native) and mutant (misfolded) proteins in ALS. Indeed, misfolded SOD1 has been shown to induce the misfolding of native, wild-type SOD1 (Figure 1A) in cultured cells (Grad et al., 2011) and mutant-wild-type co-aggregates were found in familial ALS patients (Bruijn et al., 1998), a finding replicated in mice co-expressing normal and mutant human SOD1 (Deng et al., 2006). These complexes are likely pathogenic, since co-expression of human wild-type SOD1 – which by itself is non-pathogenic in mice even when accumulated to >5 times the level of endogenous SOD1 – sharply accelerates disease onset and progression in mice from expression of various ALS-linked mutants (Deng et al., 2006). Moreover, neuron-specific expression of mutant SOD1 in mice causes dramatic aggregation in glial cells of wild-type SOD1 expressed from an ubiquitously active promoter (Jaarsma et al., 2008).
In this context, wild-type human SOD1 overexpressing mice may represent an ideal case for testing the in vivo seeding activity of misfolded SOD1 in a paradigm similar to those used for Aβ (Clavaguera et al., 2009; Meyer-Luehmann et al., 2006; Mougenot et al., 2011), tau (Clavaguera et al., 2009; Meyer-Luehmann et al., 2006; Mougenot et al., 2011) and α-synuclein (Clavaguera et al., 2009; Meyer-Luehmann et al., 2006; Mougenot et al., 2011). Since wild-type human SOD1 neither causes disease, nor aggregates in the absence of mutants, a crucial test will now be to determine if injection of pre-formed aggregated SOD1 seeds can trigger aggregation, and if so, clinical disease.
Dissimilar primary sequences between the misfolded template and the endogenous native protein substrate dramatically affects the efficiency of the template-directed misfolded reaction, as evidenced by transmission barriers of infectious prions from different species (reviewed in (Aguzzi et al., 2008)). Consistent with this, is the observation that human misfolded SOD1 is not a competent template for mouse SOD1, which was attributed to a single amino acid difference between the human and mouse protein position 32 (Grad et al., 2011), pointing to a likely interacting region for this conformational conversion. This finding explains the inactive role of mouse SOD1 in human mutant SOD1 transgenic mice, which neither affects any aspect of disease course (Bruijn et al., 1998) nor co-aggregates with the human mutant protein (Deng et al., 2006).
What about mutant wild-type interactions of TDP-43 and FUS/TLS? The cytoplasmic inclusions of TDP-43 or FUS/TLS that are accompanied by reduction of nuclear levels in ALS patients with dominant mutations in either protein strongly suggest that the wild-type isoforms are incorporated within the inclusions (assuming that expression of the wild-type allele is not silenced in these cells). The sustained expression of TDP-43 and FUS/TLS in aged motor neurons (Huang et al., 2010) may partially explain their selective vulnerability, as they provide a continuous substrate for misfolding and seeding of toxic aggregates. The latter may be further exacerbated by an autoregulation mechanism controlling TDP-43 levels (Ayala et al., 2011; Igaz et al., 2011; Polymenidou et al., 2011), which may feed-forward the seeding by elevated synthesis of new TDP-43, as more and more existing protein is incorporated into the growing aggregates.
Cross-seeding phenomena and possible contributions to disease
In some cases aggregated proteins can act as heterologous templates inducing the misfolding and aggregation of other dissimilar proteins, a phenomenon referred to as cross-seeding. So, is there any evidence for cross-seeding among the main ALS proteins? Early reports argued against the co-existence of aggregated forms of the different ALS-associated proteins, with SOD1 and FUS/TLS restricted in the respective familial ALS cases bearing mutations in each protein and TDP-43 dominating the inclusions of sporadic ALS and familial cases with TDP-43 mutations. This is now controversial: some recent studies have reported the presence of wild-type misfolded SOD1 (Bosco et al., 2010; Forsberg et al., 2010) and FUS/TLS (Deng et al., 2010) in sporadic ALS inclusions. The reported misfolded SOD1 inclusions were found to be distinct from ones containing TDP-43, while FUS/TLS was claimed to co-localize with aggregated TDP-43 in sporadic ALS patients. We note, however, that in vitro and cell culture studies do not support cross-seeding interactions between SOD1 and TDP-43 (Furukawa et al., 2011) or TDP-43 and FUS/TLS (Dormann et al., 2011; Ito et al., 2011; Sun et al., 2011).
Unexpectedly, TDP-43 and FUS/TLS can both be cross-seeded in cultured cells by poly-Q aggregates, through a mechanism that depends on the Q/N-rich prion-like domains of TDP-43 and FUS/TLS (Fuentealba et al., 2010). In fact, proteins with Q/N-rich prion like-domains may be generally suitable substrates for poly-Q aggregations, as more such proteins (for example, TIA1) were found sequestered in these inclusions (Furukawa et al., 2009). The relevance of these cross-seeding events for human disease is highlighted by the co-localization of TDP-43 and FUS/TLS with poly-Q aggregates found in patients with Huntington’s disease (Doi et al., 2008; Schwab et al., 2008; Woulfe et al., 2009) or spinocerebellar ataxia (Doi et al., 2010; Elden et al., 2010; Woulfe et al., 2009). The observation that TDP-43 and FUS/TLS aggregation occurs in many neurodegenerative diseases (summarized in (Lagier-Tourenne et al., 2010)), with occasional co-localization with other aggregated proteins, may represent unidentified cross-seeding reactions among the major misfolded proteins driving neurodegeneration. Further in vitro and in vivo studies are needed to clarify the extent of cross-seeding phenomena in ALS and other neurodegenerative diseases.
An SOD1-dependent glia-to-neuron toxic spread in familial and sporadic ALS
It is now well established that a released or secreted toxic factor produced by astrocytes or microglia mediates the killing of motor neurons in ALS-like disease in mutant SOD1 mice, a mechanism that was shown to drive rapid disease progression (Boillee et al., 2006; Yamanaka et al., 2008). This has been replicated in vitro: co-cultures of mutant SOD1 glial cells with normal motor neurons – both mouse and human – have shown that toxicity can be transferred from mutant to normal cells through an unidentified factor(s) present in the media (Di Giorgio et al., 2008; Di Giorgio et al., 2007; Marchetto et al., 2008; Nagai et al., 2007). Most recently, the in vivo toxicity of mutant SOD1-expressing astrocytes was confirmed by the transplantation of astrocyte progenitor cells in wild-type mice. In this paradigm, the grafted glial progenitor cells matured into mutant SOD1-expressing astrocytes and induced motor neuron death in the spinal cords of wild-type mice (Papadeas et al., 2011).
Most provocatively, evidence for spreading of SOD1-dependent toxicity as a general feature of human ALS has just appeared from co-cultures of neural progenitor cell-derived astrocytes from either ALS patients with disease caused by an SOD1 mutant or – in a finding that will astonish many in the neurodegenerative disease community – from seven out of seven sporadic ALS patients as well. To establish this, Kaspar and colleagues (Haidet-Phillips et al., 2011) recovered neural progenitor cells from eight ALS autopsy samples (seven sporadic and one with a SOD1A4V mutation) and then differentiated them in vitro into astrocytes. Direct co-culture of normal mouse motor neurons with these astrocytes, or conditioned media derived from these, was found to be toxic to the neurons. Of even higher impact, use of viral delivery siRNA to reduce synthesis of SOD1 in astrocytes eliminated the toxicity, demonstrating the production of a released factor(s) that is toxic to motor neurons is mediated through glial SOD1 synthesis.
One obvious possibility – whose test is now of highest importance for decoding disease mechanism in ALS – is that toxicity is transferred through a yet unidentified, misfolded SOD1 seed that may trigger prion-like aggregation of normal SOD1 in neighboring cells (Figure 1C). Indeed, mutant SOD1 has been proposed to be actively secreted with neurosecretory vesicles through an aberrant interaction with chromogranins A and B (Urushitani et al., 2006) and exogenously applied SOD1 aggregates have been shown to enter cells through macropinocytosis (Munch et al., 2011). A cautionary note, however: the transfer of toxicity from human astrocytes to mouse motor neurons in the Haidet-Phillips et al (2011) work argues against toxicity from SOD1 seeded aggregation and spreading, since mouse SOD1 is apparently not a substrate for human SOD1 seeds (Grad et al., 2011; Munch et al., 2011), as explained above.
Neurodegeneration in ALS typically begins focally and then spreads spatiotemporally until the loss of the motor neurons of the respiratory system (Ravits et al., 2007a; Ravits et al., 2007b). An attractive model for this progression of disease would be spreading of toxic aggregates from a focal site. Consequently, dissecting the molecular determinants of these processes may facilitate the construction of therapeutic agents that could interfere with disease progression.
Going forward: crucial tests now needed in ALS
Going forward, among the most crucial goals in ALS is confirmation – with replication by other teams – of the proposed SOD1-dependent, astrocyte driven toxicity that may be common to sporadic and familial ALS. So too are extensions to test whether TDP-43 or FUS/TLS plays any role in this astrocyte-mediated toxicity. With the increasing appreciation of the role of template-directed misfolding and seeded aggregation of ALS-associated proteins, the flurry of lessons learned and tools established by the prion field may become valuable for future studies in ALS. One example is to exploit protein misfolding cyclic amplification (PMCA) (Castilla et al., 2005; Wang et al., 2010), initially used to amplify pathogenic prions in vitro, and which could now be used to test the potential interplay between different proteins in seeded aggregation in ALS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Aguzzi A. Cell biology: Beyond the prion principle. Nature. 2009;459:924–925. doi: 10.1038/459924a. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10:430–436. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- Andersson MK, Stahlberg A, Arvidsson Y, Olofsson A, Semb H, Stenman G, Nilsson O, Aman P. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008;9:37. doi: 10.1186/1471-2121-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Ayala YM, De Conti L, Avendano-Vazquez SE, Dhir A, Romano M, D’Ambrogio A, Tollervey J, Ule J, Baralle M, Buratti E, et al. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011 doi: 10.1038/emboj.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Induction of beta (A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Mol Neurobiol. 1994;8:25–39. doi: 10.1007/BF02778005. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, Goolsby H, Fontaine BA, Lemay N, McKenna-Yasek D, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- Castilla J, Saa P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Chia R, Tattum MH, Jones S, Collinge J, Fisher EM, Jackson GS. Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PLoS One. 2010;5:e10627. doi: 10.1371/journal.pone.0010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien P, Weissman JS, DePace AH. Emerging principles of conformation-based prion inheritance. Annu Rev Biochem. 2004;73:617–656. doi: 10.1146/annurev.biochem.72.121801.161837. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, Silani V, Ratti A. TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem. 2009;111:1051–1061. doi: 10.1111/j.1471-4159.2009.06383.x. [DOI] [PubMed] [Google Scholar]
- Cushman M, Johnson BS, King OD, Gitler AD, Shorter J. Prion-like disorders: blurring the divide between transmissibility and infectivity. J Cell Sci. 2010;123:1191–1201. doi: 10.1242/jcs.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleault NR, Lucassen RW, Supattapone S. RNA molecules stimulate prion protein conversion. Nature. 2003;425:717–720. doi: 10.1038/nature01979. [DOI] [PubMed] [Google Scholar]
- Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HX, Zhai H, Bigio EH, Yan J, Fecto F, Ajroud K, Mishra M, Ajroud-Driss S, Heller S, Sufit R, et al. FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann Neurol. 2010;67:739–748. doi: 10.1002/ana.22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A. Differential loss of striatal projection systems in Huntington’s disease: a quantitative immunohistochemical study. J Chem Neuroanat. 2004;27:143–164. doi: 10.1016/j.jchemneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi H, Koyano S, Suzuki Y, Nukina N, Kuroiwa Y. The RNA-binding protein FUS/TLS is a common aggregate-interacting protein in polyglutamine diseases. Neurosci Res. 2010;66:131–133. doi: 10.1016/j.neures.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Doi H, Okamura K, Bauer PO, Furukawa Y, Shimizu H, Kurosawa M, Machida Y, Miyazaki H, Mitsui K, Kuroiwa Y, et al. RNA-binding protein TLS is a major nuclear aggregate-interacting protein in huntingtin exon 1 with expanded polyglutamine-expressing cells. J Biol Chem. 2008;283:6489–6500. doi: 10.1074/jbc.M705306200. [DOI] [PubMed] [Google Scholar]
- Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IR, Capell A, Schmid B, et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 2011;29:2841–2857. doi: 10.1038/emboj.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, Yan ZX, Roth K, Aguzzi A, Staufenbiel M, Walker LC, et al. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci U S A. 2009;106:12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2011;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg K, Jonsson PA, Andersen PM, Bergemalm D, Graffmo KS, Hultdin M, Jacobsson J, Rosquist R, Marklund SL, Brannstrom T. Novel antibodies reveal inclusions containing non-native SOD1 in sporadic ALS patients. PLoS One. 2010;5:e11552. doi: 10.1371/journal.pone.0011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Ollesch J, Wille H, Diamond MI. Conformational diversity of wild-type Tau fibrils specified by templated conformation change. J Biol Chem. 2009;284:3546–3551. doi: 10.1074/jbc.M805627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba RA, Udan M, Bell S, Wegorzewska I, Shao J, Diamond MI, Weihl CC, Baloh RH. Interaction with polyglutamine aggregates reveals a Q/N-rich domain in TDP-43. J Biol Chem. 2010;285:26304–26314. doi: 10.1074/jbc.M110.125039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Ito H, Nakano S, Kinoshita Y, Wate R, Kusaka H. Immunohistochemical identification of messenger RNA-related proteins in basophilic inclusions of adult-onset atypical motor neuron disease. Acta Neuropathol. 2008;116:439–445. doi: 10.1007/s00401-008-0415-x. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Kaneko K, Matsumoto G, Kurosawa M, Nukina N. Cross-seeding fibrillation of Q/N-rich proteins offers new pathomechanism of polyglutamine diseases. J Neurosci. 2009;29:5153–5162. doi: 10.1523/JNEUROSCI.0783-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of sarkosyl-insoluble TAR DNA binding protein-43 inclusions. J Biol Chem. 2011 doi: 10.1074/jbc.M111.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek DC, Gibbs CJ, Alpers M. Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature. 1966;209:794–796. doi: 10.1038/209794a0. [DOI] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, Hatanpaa KJ, White CL, 3rd, Bigio EH, Caselli R, et al. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad LI, Guest WC, Yanai A, Pokrishevsky E, O’Neill MA, Gibbs E, Semenchenko V, Yousefi M, Wishart DS, Plotkin SS, et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1102645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Lee VM. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011;286:15317–15331. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Chen Y, Zhou X, Kar A, Ray P, Chen X, Rao EJ, Yang M, Ye H, Zhu L, et al. An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity. Nat Struct Mol Biol. 2011;18:822–830. doi: 10.1038/nsmb.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011 doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS Forms Functional Prion-like Aggregates to Activate and Propagate Antiviral Innate Immune Response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Xia PY, Zhou H. Sustained expression of TDP-43 and FUS in motor neurons in rodent’s lifetime. Int J Biol Sci. 2010;6:396–406. doi: 10.7150/ijbs.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Lee EB, Chen-Plotkin A, Swanson E, Unger T, Malunda J, Xu Y, Winton MJ, Trojanowski JQ, et al. Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J Clin Invest. 2011 doi: 10.1172/JCI44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Xu Y, Truax AC, Uryu K, Neumann M, Clark CM, Elman LB, Miller BL, Grossman M, et al. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol. 2008;173:182–194. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Seki M, Tsunoda Y, Uchiyama H, Suzuki N. Nuclear transport impairment of amyotrophic lateral sclerosis-linked mutations in FUS/TLS. Ann Neurol. 2011;69:152–162. doi: 10.1002/ana.22246. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28:2075–2088. doi: 10.1523/JNEUROSCI.5258-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BS, McCaffery JM, Lindquist S, Gitler AD. A yeast TDP-43 proteinopathy model: Exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc Natl Acad Sci U S A. 2008;105:6439–6444. doi: 10.1073/pnas.0802082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem. 2009;284:20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med. 2010;16:1210–1214. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci. 2000;20:3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol. 2000;151:1257–1268. doi: 10.1083/jcb.151.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman A, Liu HN, Croul S, Bilbao J, Rogaeva E, Zinman L, Robertson J, Chakrabartty A. Amyotrophic lateral sclerosis is a non-amyloid disease in which extensive misfolding of SOD1 is unique to the familial form. Acta Neuropathol. 2010;119:335–344. doi: 10.1007/s00401-010-0646-5. [DOI] [PubMed] [Google Scholar]
- Klohn PC, Stoltze L, Flechsig E, Enari M, Weissmann C. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc Natl Acad Sci U S A. 2003;100:11666–11671. doi: 10.1073/pnas.1834432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. Pathological Staging of Frontotemporal Lobar Degeneration. J Mol Neurosci. 2011 doi: 10.1007/s12031-011-9528-0. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Bosco JD, LeClerc AD, Tamrazian E, Van den Berg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009 doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19:R46–64. doi: 10.1093/hmg/ddq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderwyde T, Citro A, Mehta T, Zaarur N, McKee A, Bowser R, Sherman M, et al. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One. 2010;5:e13250. doi: 10.1371/journal.pone.0013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahal SP, Baker CA, Demczyk CA, Smith EW, Julius C, Weissmann C. Prion strain discrimination in cell culture: the cell panel assay. Proc Natl Acad Sci U S A. 2007;104:20908–20913. doi: 10.1073/pnas.0710054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- McDonald KK, Aulas A, Destroismaisons L, Pickles S, Beleac E, Camu W, Rouleau GA, Vande Velde C. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet. 2011;20:1400–1410. doi: 10.1093/hmg/ddr021. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Moisse K, Volkening K, Leystra-Lantz C, Welch I, Hill T, Strong MJ. Divergent patterns of cytosolic TDP-43 and neuronal progranulin expression following axotomy: implications for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009;1249:202–211. doi: 10.1016/j.brainres.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Mougenot AL, Nicot S, Bencsik A, Morignat E, Verchere J, Lakhdar L, Legastelois S, Baron T. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Munch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci U S A. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. Seeded aggregation and toxicity of {alpha}-synuclein and tau: cellular models of neurodegenerative diseases. J Biol Chem. 2010;285:34885–34898. doi: 10.1074/jbc.M110.148460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadeas ST, Kraig SE, O’Banion C, Lepore AC, Maragakis NJ. Astrocytes carrying the SOD1G93A mutation induce wildtype motor neuron degeneration in vivo. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1103141108. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesiridis GS, Tripathy K, Tanik S, Trojanowski JQ, Lee VM. A “two-hit” hypothesis for inclusion formation by carboxyl-terminal fragments of TDP-43 protein linked to RNA depletion and impaired microtubule-dependent transport. J Biol Chem. 2011;286:18845–18855. doi: 10.1074/jbc.M111.231118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011 doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudencio M, Hart PJ, Borchelt DR, Andersen PM. Variation in aggregation propensities among ALS-associated variants of SOD1: correlation to human disease. Hum Mol Genet. 2009;18:3217–3226. doi: 10.1093/hmg/ddp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Ravits J, Laurie P, Fan Y, Moore DH. Implications of ALS focality: rostral-caudal distribution of lower motor neuron loss postmortem. Neurology. 2007a;68:1576–1582. doi: 10.1212/01.wnl.0000261045.57095.56. [DOI] [PubMed] [Google Scholar]
- Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007b;68:1571–1575. doi: 10.1212/01.wnl.0000260965.20021.47. [DOI] [PubMed] [Google Scholar]
- Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL. Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol. 2008;67:1159–1165. doi: 10.1097/NEN.0b013e31818e8951. [DOI] [PubMed] [Google Scholar]
- Shorter J, Lindquist S. Prions as adaptive conduits of memory and inheritance. Nat Rev Genet. 2005;6:435–450. doi: 10.1038/nrg1616. [DOI] [PubMed] [Google Scholar]
- Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell. 2010;140:421–435. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Diaz Z, Fang X, Hart MP, Chesi A, Shorter J, Gitler AD. Molecular Determinants and Genetic Modifiers of Aggregation and Toxicity for the ALS Disease Protein FUS/TLS. PLoS Biol. 2011;9:e1000614. doi: 10.1371/journal.pbio.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Griffin WS, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer’s disease. Acta Neuropathol. 2008;115:599–609. doi: 10.1007/s00401-008-0366-2. [DOI] [PubMed] [Google Scholar]
- Uptain SM, Lindquist S. Prions as protein-based genetic elements. Annu Rev Microbiol. 2002;56:703–741. doi: 10.1146/annurev.micro.56.013002.100603. [DOI] [PubMed] [Google Scholar]
- Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy DM, Wright P, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009 doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt A, Herholz D, Fiesel FC, Kaur K, Muller D, Karsten P, Weber SS, Kahle PJ, Marquardt T, Schulz JB. TDP-43-mediated neuron loss in vivo requires RNA-binding activity. PLoS One. 2010;5:e12247. doi: 10.1371/journal.pone.0012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang X, Yuan CG, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IF, Wu LS, Chang HY, Shen CK. TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. J Neurochem. 2008;105:797–806. doi: 10.1111/j.1471-4159.2007.05190.x. [DOI] [PubMed] [Google Scholar]
- Woulfe J, Gray DA, Mackenzie IR. FUS-Immunoreactive Intranuclear Inclusions in Neurodegenerative Disease. Brain Pathol. 2009 doi: 10.1111/j.1750-3639.2009.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoseki A, Shiga A, Tan CF, Tagawa A, Kaneko H, Koyama A, Eguchi H, Tsujino A, Ikeuchi T, Kakita A, et al. TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol. 2008;63:538–542. doi: 10.1002/ana.21392. [DOI] [PubMed] [Google Scholar]