Abstract
People have been consuming brewed tea from the leaves of the Camellia sinensis plant for almost 50 centuries. Although health benefits have been attributed to tea, especially green tea consumption since the beginning of its history, scientific investigations of this beverage and its constituents have been underway for less than three decades. Currently, tea, in the form of green or black tea, next to water, is the most widely consumed beverage in the world. In vitro and animal studies provide strong evidence that polyphenols derived from tea may possess the bioactivity to affect the pathogenesis of several chronic diseases. Among all tea polyphenols, epigallocatechin-3-gallate has been shown to be responsible for much of the health promoting ability of green tea. Tea and tea preparations have been shown to inhibit tumorigenesis in a variety of animal models of carcinogenesis. However, with increasing interest in the health promoting properties of tea and a significant rise in scientific investigation, this review covers recent findings on the medicinal properties and health benefits of tea with special reference to cancer and cardiovascular diseases.
Keywords: cancer prevention, health effects, medicinal properties, tea polyphenols
Introduction
Tea is one of the most popular beverages consumed worldwide. About three billion kilograms of tea is produced and consumed yearly. Tea, brewed from the plant Camellia sinensis is consumed in different parts of the world as green, black or Oolong tea. Green tea is favored in Japan and China, and initial research on the benefits of green tea was carried out in these countries because of the local customs. Of the tea produced worldwide, 78% is black tea, which is usually consumed in the Western countries, 20% is green tea, which is commonly consumed in Asian countries, and 2% is Oolong tea which is produced (by partial fermentation) mainly in southern China. Brewed tea contains many compounds, especially polyphenols, and several studies show that polyphenolic compounds present in tea reduce the risk of a variety of diseases (Yang and Wang, 1993; Mukhtar and Ahmad, 1999, 2000). Research findings suggest that the polyphenolic compounds, (−)-epigallocatechin-3-gallate found primarily in green tea, and theaflavin-3,3′-digallate, a major component of black tea, are the two most effective anti-cancer factors found in tea. The possible beneficial health effects of tea are being extensively investigated and have received a great deal of attention in recent times. This review examines the available scientific information concerning tea and health.
Green and black teas are processed differently during manufacturing. To produce green tea, freshly harvested leaves are steamed to prevent fermentation, yielding a dry, stable product. Tea polyphenols, known as catechins, usually account for 30–42% of the dry weight of the solids in brewed green tea. Catechins are characterized by di-or tri-hydroxyl group substitution of the B ring and the meta-5,7-dihydroxy substitution of the A ring. The structures of the four major catechins, (−)-epigallocatechin gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), and (−)-epicatechin (EC) are shown in Figure 1. EGCG is the major catechin in tea and may account for 50–80% of the total catechin in tea. Catechin, gallocatechin, epigallocatechin digallates, epicatechin digallate, 3-O-methyl EC and EGC, catechin gallate, and gallocatechin gallate are present in smaller quantities. 3′-O-methyl-EGCG has been isolated from Oolong tea as a minor component. Flavonols, including quercetin, kaempferol, myricitin and their glycosides are also present in tea. A typical tea beverage, prepared in a proportion of 1 g leaf to100 ml water in a 3-min brew, usually contains 250–350 mg tea solids, comprised of 30–42% catechins and 3–6% caffeine (Mukhtar and Ahmad, 1999).
Figure 1.
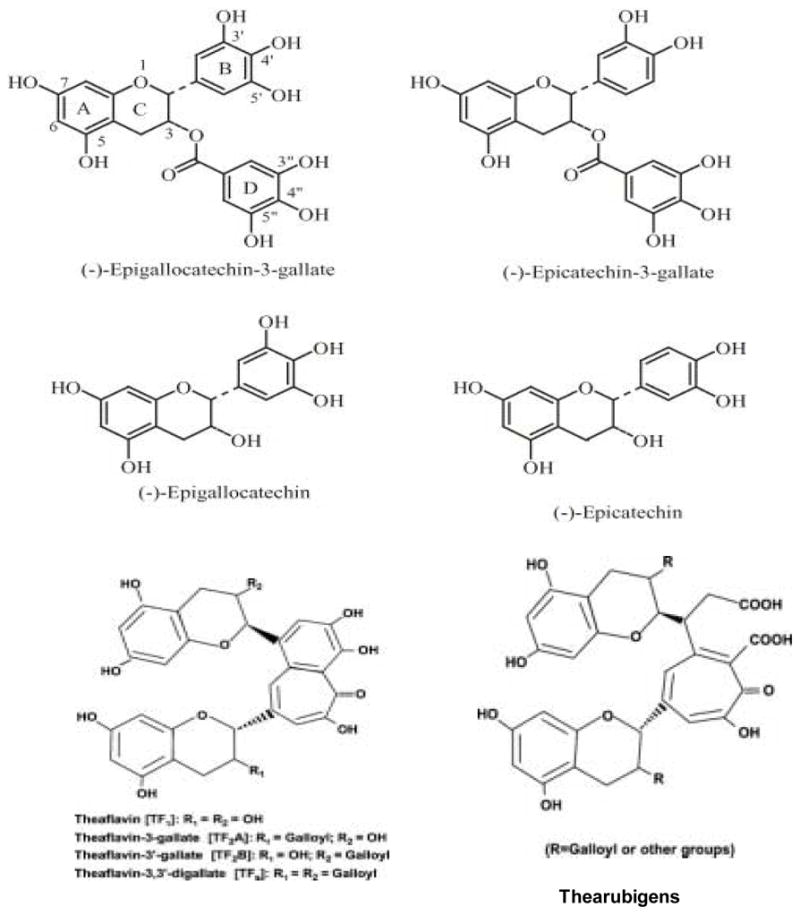
Structures of the major tea polyphenols
In the production of black tea, about 75% of catechins contained in the tea leaves undergo enzymatic transformation consisting of oxidation and partial polymerization. In manufacturing black tea, the tea leaves are crushed to allow the polyphenol oxidase to catalyze the oxidation, leading to polymerization of catechins. The resulting black tea composition depends on the technological process of its production. It is difficult to state a definitive composition for black tea beverage, as it varies with different preparations. The approximate mean percentages of components of solid extracts in black tea are: catechins (10–12%), theaflavins (3–6%), thearubigins (12–18%), flavonols (6–8%), phenolic acids (10–12%), amino acids (13–15%), methylxanthines (8–11%), carbohydrates (15%), proteins (1%), mineral matter (10%), and volatiles (<0.1%). Tea leaves contain about 2–5% caffeine and much smaller quantities of theobromine and theophyline. Most of the catechin mass in the process of black tea manufacture is transformed to less structurally defined compounds called as thearubigins. They are water soluble, acidic, and some, though not necessarily all, are rust-brown in color and all display similar ill-defined chromatographic behaviour. Little progress has been made towards an understanding of the chemical nature of the thearubigins. The structures and the formation process of these compounds are incompletely known. The high oxidation potential of some of the catechin quinones generated at fermentation stage is probably responsible for the generation of theaflavins, bisflavanols, and epitheaflavic acids and the subsequent incorporation of their oxidation products into the thearubigin formation. The proposed structures of theaflavins and thearubigins are shown in Figure 1. Thearubigins, which have higher molecular weights, are poorly characterized chemically. Theaflavins are characterized by the benzotropolone ring structure and bright red-orange color, and contribute to the unique taste of black tea. The amount of caffeine in tea beverage is determined by the leaf size, the brewing time and the temperature (Luczaj and Skrzydlewska, 2005).
Biotransformation
The catechins are subject to extensive biotransformation including methylation, glucuronidation, sulfation and ring-fission metabolism. Recent studies on the enzymology of EGC and EGCG methylation have shown that EGC is methylated to form 4′-O-methyl-(−)-EGC and EGCG is methylated to form 4″-O-methyl-(−)-EGCG and 4′,4″-O-dimethyl-(−)-EGCG. It has been reported in studies of EGCG and EGC glucuronidation that EGCG-4″-O-glucuronide is the major metabolite formed by human, mouse and rat microsomes (Lambert and Yang, 2003). The greatest catalytic efficiency for glucuronidation is in mouse intestinal microsomes followed in decreasing order by mouse liver, human liver, rat liver and rat small intestine. EC undergoes sulfation catalyzed by human and rat intestinal and liver cytosol with the human liver being the most efficient (Vaidyanathan, and Walle, 2002). Tea catechins undergo metabolism in the gut to form the ring fission products 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone (M4), 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (M6) and 5-(3′,5′-dihydroxyphenyl)-γ-valerolactone (M6′). These metabolic intermediates are further broken down by gut flora to phenylacetic and phenylpropionoic acids (Li et al., 2000).
Pharmacokinetics
The potential health effects of catechins depend on the amount consumed and on their bioavailability. Following oral administration of tea catechins to rats, the four principal catechins (EC, ECG, EGC, and EGCG) have been identified in the portal vein, indicating that tea catechins are absorbed intestinally (Okushio et al., 1996). The plasma concentrations of EGCG were much lower than those of EGC or EC in rats given 0.6% GTP in their drinking water over a period of 28 days, even though the ratio of EGCG to EGC was 5:1 in the GTP solution. The plasma levels of EGCG were much higher than those of EGC and EC when the same GTP preparation was given to mice. (Kim et al., 2000). In a study in humans which compared the pharmacokinetics of equimolar doses of pure EGC, ECG, and EGCG in 10 healthy volunteers, average peak plasma concentrations after a single dose of 1.5 mmol were 5.0 μmol/L for EGC, 3.1 μmol/L for ECG, and 1.3 μmol/L for EGCG. The plasma EGC and EGCG returned to baseline, but plasma ECG remained elevated after 24 hours (Higdon et al., 2003). Substantial amounts of EGC and EC were found in the esophagus, large intestine, kidney, bladder, lung, and prostate; EGC and EC concentrations were relatively low in liver, spleen, heart, and thyroid; EGCG levels were higher in the esophagus and large intestine, but lower in other organs, when rats were given 0.6% GTP in their drinking water over a period of 28 days (Kim et al., 2000). Studies in rats indicated that EGCG is mainly excreted through the bile, while EGC and EC are excreted through urine and bile, indicating that catechins are rapidly and extensively metabolized. A Tmax in the plasma of 1.5 to 2.5 h after consumption of decaffeinated green tea solids (1.5, 3.0, 4.5 g) has been demonstrated. These levels decreased and were not detectable by 24 h. While EGCG was not detected in the urine, 90% of the total EC and EGC were excreted in the urine by 8 h. The bioavailability of EGCG was found to be less than that of EGC (Yang et al., 1998).
Antioxidant Properties of tea
The potential health benefits associated with tea consumption have been partially attributed to the antioxidative property of tea polyphenols (Mukhtar and Ahmad, 2000). Recently, it has been reported that green tea consumed within a balanced controlled diet improve the overall antioxidative status and protect against oxidative damage in humans (Erba et al., 2005). Tea preparations have been shown to trap reactive oxygen species, such as superoxide radical, singlet oxygen, hydroxyl radical, peroxyl radical, nitric oxide, nitrogen dioxide and peroxynitrite, reducing their damage to lipid membranes, proteins and nucleic acids in cell-free systems. The radical quenching ability of green tea is usually higher than that of black tea. Among tea catechins, EGCG is most effective in reacting with most reactive oxygen species. The chemical structures contributing to effective antioxidant activity of catechins include the vicinal dihydroxy or trihydroxy structure, which can chelate metal ions and prevent the generation of free radicals. This structure also allows electron delocalization, conferring high reactivity to quench free radicals. Under certain conditions, however, catechins may undergo autooxidation and behave like prooxidants. During the reactions of tea polyphenols with free radicals, several oxidation products are formed. Reactions of EGCG and other catechins with peroxyl radicals lead to the formation of anthocyanin-like compounds as well as seven-membered B ring anhydride dimers and ring-fission compounds. The B ring appears to be the principal site of antioxidant reactions (Kondo et al., 1999). Green and black tea can inhibit the oxidation of lipoproteins induced by Cu2+ in vitro. The protective activities of tea and tea polyphenols against the oxidation of lipoproteins have been proposed to contribute to the prevention of atherosclerosis and other cardiovascular diseases.
The antioxidant capacity of tea and tea polyphenols has been assessed by several methods. Using the Oxygen Radical Absorbance Capacity (ORAC) assay, it has been found both green and black tea have much higher antioxidant activity against peroxyl radicals than vegetables such as garlic, spinach and Brussels sprouts (Cao et al., 1996). Several clinical trials have demonstrated that a single dose of tea improves plasma antioxidant capacity of healthy adults within 30 to 60 minutes after ingestion. A significant rise in plasma antioxidant capacity was detected after brewed green tea or black tea solids were consumed (Leenen et al., 2000). In general, the rise in plasma antioxidant capacity peaks about one to two hours after tea ingestion and subsides shortly thereafter. Repeated consumption of tea and encapsulated tea extracts for one to four weeks has been demonstrated to decrease biomarkers of oxidative status. In a trial of 40 male smokers in China and 27 men and women (smokers and non-smokers) in the United States, oxidative DNA damage, lipid peroxidation and free radical generation were reduced after consuming ~6 cups a day of green tea for seven days (Klaunig et al., 1999). Plasma malondialdehyde, another indicator of lipid peroxidation, was reduced in 20 healthy women, 23 to 50 years of age, consuming a high linoleic acid diet and administered an encapsulated tea extract (equivalent to 10 cups a day of green tea) for four weeks (Freese et al., 1999).
Epidemiological observations
Many epidemiological studies have been conducted to investigate the effects of tea consumption on human cancer incidence. Most of the studies showing an inverse relationship between tea consumption and development of cancer were conducted on gastrointestinal cancers in Japan and China where green tea is the main form of tea consumed. Studies in northern Italy have suggested a protective effect of tea against oral, pharyngeal and laryngeal cancer. In a case-control study in Shanghai, frequent consumption of green tea has been shown to be associated with a lower incidence of esophageal cancer, especially among those who neither smoke nor consume alcohol. A protective effect against gastric cancer by tea has also been suggested from studies in Japan, northern Turkey and central Sweden. In Japan, women consuming >10 cups of tea daily have been shown to have lower risk for all cancers and increased tea consumption was associated with lower risk for breast cancer metastasis and recurrence. Tea drinking was shown to be associated with a lower risk for digestive tract cancers and urinary tract cancers in women in a prospective cohort study in Iowa (Lambert and Yang, 2003). A prospective cohort study with 8,552 individuals in Yoshimi town in Saitama Prefecture was conducted with green tea. During an 11-year follow-up, a total of 488 cancer patients (285 males and 203 females) were determined. Respondents were divided into three groups according to daily consumption of green tea: below 3 cups, from 4 to 9 cups, and over 10 cups. Individuals who consumed over 10 cups of green tea per day showed remarkable reduction of relative risk for lung, colon, and liver cancers. The relative risk of stomach cancer was also low, although not statistically significant (Sueoka et al., 2001). A case-control study conducted in south-east China assessed 130 patients with histologically confirmed incidental prostate cancer and 274 patients without cancer matched by age, showed that the risk of prostate cancer declined with increasing frequency, duration and quantity of green tea consumed. This reduction was statistically significant, suggesting the preventive effect of green tea against prostate cancer (Jian et al., 2004).
Anticarcinogenic effects of tea
Evidence for the anticarcinogenic potential of tea polyphenols has been provided by numerous in vitro and experimental studies describing their action to bind directly to carcinogens, induce Phase II enzymes such as UDP-glucuronosyl transferase and inhibit heterocyclic amine formation. Molecular mechanisms, including catechin-mediated induction of apoptosis and cell cycle arrest, inhibition of transcription factors NF-κB and AP-1 and reduction of protein tyrosine kinase activity and c-jun mRNA expression have also been suggested as relevant chemopreventive pathways for tea (Mukhtar and Ahmad, 2000; Khan et al., 2006). Some epidemiological studies also support a protective role of tea against the development of cancer. Studies conducted in Asia, where green tea is consumed frequently and in large amounts, tend to show a beneficial effect on cancer prevention. For example, a prospective nine year study among 8,552 Japanese adults observed consumption of ten or more cups of green tea a day delayed cancer onset by 8.7 years in females and three years in males when compared to patients consuming fewer than three cups a day (Wiseman et al., 1997). Protective effects appear to be observed less frequently in European populations where intake of black tea predominates. Importantly, the putative chemopreventive effect of tea also varies by the specific type of cancer.
Skin Cancer
The activity of tea and tea polyphenols on the inhibition of skin tumorigenesis has been widely studied. We reported the first topical application of green tea polyphenols (GTP) for protection from skin cancer in a complete skin tumorigenesis protocol using 3-methylcholanthrene on BALB/c mice, and a two-stage skin tumorigenesis protocol using DMBA as the initiating agent and TPA as tumor promoter with Sencar mice (Wang et al., 1989). It has been demonstrated that topical application or ingestion of GTP or EGCG inhibit tumor initiation and promotion by chemical carcinogens and UV light in mice (Yang and Wang, 1993; Mukhtar and Ahmad, 2000). Oral administration of green tea polyphenols (GTP) reduced UVB-induced skin tumor incidence, tumor multiplicity and tumor growth in SKH-1 mice. There was also reduced expression of the matrix metalloproteinases (MMP)-2 and MMP-9, CD31, vascular endothelial growth factor (VEGF) and proliferating cell nuclear antigen (PCNA) in the GTP treated group. Additionally, there were more cytotoxic CD8(+) T cells and greater activation of caspase-3 in the tumors of the orally administered GTP group indicating the apoptotic death of the tumor cells (Mantena et al., 2005). Oral administration of green tea, black tea or EGCG inhibits the growth of well-established skin tumors and, in some cases, tumor regression was also observed. Complete regression was observed in papilloma-bearing mice (Conney et al., 1999). The growth of nonmalignant tumors, squamous cell carcinomas and tumor volume decreased significantly when tumor-bearing mice were fed with black tea. Inhibition of DNA synthesis and enhancement of apoptosis have been observed. Recently, EGCG treatment was found to result in a dose-dependent decrease in the viability and growth of A-375 amelanotic malignant melanoma and Hs-294T metastatic melanoma cell lines (Nihal et al., 2005). In a study from our laboratory, we have shown that topical application of a green tea polyphenol fraction (GTP) to the skin of DMBA-initiated mice, prior to that of 12-O-tetradecanoylphorbol-13-acetate (TPA) or mezerein, resulted in protection against skin tumor promotion as judged by the decrease in tumor incidence, multiplicity and tumor volume. GTP also protected against the malignant conversion of papillomas to squamous cell carcinomas (SCC) (Katiyar et al., 1997). Oral administration of green tea as the sole source of drinking fluid starting immediately after discontinuation of UVB treatment enhanced the rate and extent of disappearance of the mutant p53-positive patches in SKH-1 mice (Lu et al., 2005).
In a study, SKH-1 female mice were treated with UVB light twice weekly for 22 weeks, and allowed to develop tumors during the following 13 weeks, and then treated with oral administration of black tea for 11 weeks. The tea treatment markedly decreased the number and volume of nonmalignant and malignant tumors (by 54% to 84%). In CD1 mice with established papillomas (initiated with DMBA and promoted with TPA), oral administration of black tea for 11 to 15 weeks inhibited the growth of papillomas (by 35% to 48%), but decaffeinated black tea gave inconsistent results (Lu et al., 1997). We have also shown that topical application of green tea polyphenols resulted in significant decrease in UVB-induced bifold-skin thickness, skin edema, infiltration of leukocytes and inhibition of MAPKs and NFκB pathways in SKH-1 hairless mice (Afaq et al., 2003). Consumption of tea had protective effect on cutaneous malignant melanoma in a case-control study conducted in Italy (Naldi et al., 2004). In a population-based case-control study, subjects who reported consumption of both hot black tea and citrus peel had a significant marked decrease in the development of SCC (Hakim and Harris, 2001).
Lung Cancer
Studies of the effects of green and black teas on the development of lung cancer in mice could have promising implications for humans. Brewed black tea and theaflavins have been shown to protect against NNK-induced pulmonary hyperproliferation and tumorigenesis in mice initiated with NNK. In mice that already developed lung adenomas (16 weeks after the NNK dose), oral administration of brewed black tea inhibited the progression of adenoma to adenocarcinoma. Administration of theaflavins (0.1%) as the sole source of drinking fluid, starting 2 days after the NNK treatment until the termination of the experiment, significantly reduced the tumor multiplicity and volume by 23% and 34% respectively (Yang et al., 1997). Recently, patterns of gene expressions were identified and offer clues for green tea’s potential mechanisms of action and provide a molecular signature specific for green tea exposure (Lu et al., 2006). Black tea has been reported to suppress cell proliferation and induce apoptosis in benzo(a)pyrene-induced lung carcinogenesis in mice (Banerjee et al., 2005). Black tea and green tea infusions have also been shown to inhibit the spontaneous formation of lung tumors and rhabdomyosarcomas in A/J mice. Treatment with black tea or green tea for 60 weeks significantly reduced lung tumor incidence from 52–27%, multiplicity from 0.72–0.33 tumors/mouse and volume from 38.3–4.27mm3. The incidence and multiplicity of rhabdomyosarcomas were also reduced significantly by green and black tea. The body weights and particularly the body fat weights (as measured by the retroperitoneal fat pad weight) of the mice in the tea-treated groups were significantly lower than in the control group (Landau et al., 1998). The effects of black tea and caffeine on lung tumorigenesis in F344 rats induced by NNK in a two-year bioassay were examined. After 20 weeks of NNK administration, the animals were given either 2% black tea infusions or caffeine for 22 weeks. Black tea caused a significant reduction of the total lung tumor (adenomas, adenocarcinomas, and adenosquamous carcinomas) incidence (from 47–19%). Caffeine treatment also caused a remarkable reduction of the lung tumor incidence (from 47–10%). It appears that caffeine could account for the inhibitory activity of black tea. In a previous study with A/J mice, however, pure EGCG was shown to be slightly more effective than caffeine in inhibiting lung tumorigenesis when both compounds were used at concentrations corresponding to those present in 2% green tea (Xu et al., 1992). The above results clearly demonstrated the inhibition of lung tumorigenesis by different tea preparations. In a population-based case-control study in Shanghai, China, consumption of green tea was associated with a reduced risk of lung cancer among nonsmoking women and the risks decreased with increasing consumption (Zhong et al., 2001). In a case control study in Uruguay, tea drinking is associated with reduced risk of lung cancer in male cigarette smokers (Mendilaharsu et al., 1998). A protective effect of frequent (daily or several times per week) black tea drinking appeared among non-smoking women in a case control study (Kubik et al., 2004).
Liver cancer
A significant decrease in the incidence of NNK-induced liver tumors (from 34–12%) in rats receiving 2% black tea was reported (Chung et al., 1998). Inhibitory effects of individual tea catechins, black tea extract, and Oolong tea extract (0.05 or 0.1%) on hepatic preneoplastic GST-positive foci formation were demonstrated in rats treated with a single dose of NDEA and then phenobarbital in the drinking water for a period of six weeks (Matsumoto et al., 1996). In a study, GTP inhibited the development of hetrocyclic amine, NDMA and NDEA-induced preneoplastic GST-positive hepatic foci (Hirose et al., 1995). Recent findings suggest that regular intake of green tea may reduce the carcinogenic risk posed by an environmental pollutant, pentachlorophenol (Umemura et al., 2003). A population based case-control study was conducted in Taixing, Jiangsu province to explore the role of green tea in decreasing the risks of liver cancer among alcohol drinkers or cigarette smokers. Green tea drinking decreased the risk for the development of liver cancer by 78% among alcohol drinkers and 43% among cigarette smokers (Mu et al., 2003). The administration of tea polyphenols and tea pigments in drinking water caused decrease in the number and area of GST-P-positive foci, cyclin D1, cdk4 and induction of p21 in liver of Wistar rats (Jia et al., 2002).
Pancreatic and bladder cancer
EGCG treatment caused significant suppression of the invasive ability of pancreatic carcinoma cells PANC-1, MIA PaCa-2, and BxPC-3 (Takada et al., 2002). It has been reported that EGCG exert a growth-suppressive effect on human pancreatic cancer cells in vitro (Qanungo et al., 2005). In another study, the incidence and multiplicity of pancreatic tumors in hamsters decreased when green tea extract was administered after the initiation of pancreatic tumorigenesis. They also observed the inhibitory effect of green tea extract on the growth of transplanted pancreatic cancer (Hiura et al., 1997). A combination of low dose tea polyphenol (50 ppm) and β-carotene also inhibited the lesion development. Inhibition of N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder tumors in rats by green tea has been reported, when administered either as an infusion in the drinking fluid or in a solid form in the diet (Sato, 1999). In a large population-based case-control study conducted in Shanghai, China, an inverse association with pancreatic cancer was observed with increasing amount of green tea consumption (Ji et al., 1997). GTP caused decrease in the numbers of hyperplasia and total pancreatic duct lesions in Syrian hamsters (Majima et al., 1998).
Gastrointestinal tract cancer
It was demonstrated that oral administration of 1.5% green tea or other tea preparations as drinking fluid, starting two weeks before initiation with DMBA and proceeding until the end of the experiment, significantly reduced the incidence of oral dysplasia and carcinoma in Syrian golden hamsters. The treatment also reduced the frequency of micronucleated cells, the proliferation index and the level of epidermal growth factor (EGF) receptor expression in oral mucosal cells (Li et al., 1999). The inhibitory effect of EGCG on N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced glandular stomach carcinogenesis in rats and on N-ethyl-N′-nitro-N-nitrosoguanidine (ENNG)-induced duodenal carcinogenesis in mice has been reported (Yamane et al., 1996). Green tea extract administration also inhibited intestinal tumor formation in the Apc mutant Min mice, and the effect was enhanced synergistically when combined with sulindac (Suganuma et al., 2001). The protective effect of black tea extract on AOM-induced intestinal carcinogenesis in F344 rats has been shown (Caderni et al., 2000). EGCG was found to inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells (Shimizu et al., 2005). In a population based case-control study conducted in Taixing, Jiangsu province, green tea decreased risk for the development of stomach cancer by 81% and esophageal cancer by 39% in alcoholics and development of stomach cancer by 16% and esophageal cancer by 31% among cigarette smokers (Mu et al., 2003). In a case-control study conducted in Jiangsu, China, drinking green tea was found to decrease the risk of esophageal and gastric cancers (Wang et al., 1999). It has been reported that small intestinal tumorigenesis was inhibited in a dose-dependent manner by oral administration of EGCG which was accompanied by increased levels of E-cadherin and decreased levels of nuclear β-catenin, c-myc, phospho-Akt, and phospho-ERK1/2 in small intestinal tumors in Apc (min/+) mice (Ju et al., 2005).
Breast Cancer
Since the 1980s, the incidence of late-onset breast cancer has been increasing in the United States. Known risk factors, such as genetic modifications, have been estimated to account for approximately 5–10% of breast cancer cases. Dietary habits may have a role in breast cancer risk and prevention as well. Recently, EGCG was found to suppress Wnt signaling in invasive breast cancer cells (Kim et al., 2006). GTPs have been reported to have effect on growth and metastasis of highly metastatic mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Treatment of EGCG-rich GTP in drinking water to 4T1 cells bearing BALB/c mice resulted in reduction of tumor growth accompanied with increase in Bax/Bcl-2 ratio, reduction in PCNA and activation of caspase 3 in tumors. Metastasis of tumor cells to lungs was inhibited and survival period of animals was increased after treatment with GTP (Baliga et al., 2005). Green tea or EGCG exhibited chemopreventive action on DMBA-induced mammary carcinogenesis only when given in the postinitiation stage, and the effect was not dose dependent. Indeed, green tea ingestion markedly increased the mean latency of tumors and reduced the tumor burden and the number of invasive tumors in rats with DMBA-induced mammary carcinogenesis (Kavanagh et al., 2001). Recently, a specific formulation of lysine, proline, arginine, ascorbic acid and green tea extract significantly reduced the incidence and growth of MNU-induced mammary tumors in Sprague-Dawley rats (Roomi et al., 2005). In a case-control study conducted among Asian-American women in Los Angeles County, a significant inverse relationship between intake of green tea and risk of breast cancer was reported (Wu et al., 2003a). In a population-based case-control study of breast cancer in Chinese, Japanese and Filipino-American women, and the risk of breast cancer was found to be significantly decreased by green tea intake (Wu et al., 2003b). Increased consumption of green tea was closely associated with decreased numbers of axillary lymph node metastases among patients with stage I and II breast cancer in Japan (Nakachi et al., 2000). Recently, a meta-analysis which included 13 papers examined populations in eight countries and provided data on consumption of either green tea or black tea, or both in relation to breast cancer risk. The results indicated a lower risk for breast cancer with green tea consumption (Sun et al., 2006).
Prostate Cancer
Our interest in a potential preventive effect of green tea on prostate cancer stemmed from the epidemiological observations of the low incidence of prostate cancer among Japanese and Chinese populations with a high intake of green tea. In studies using the transgenic adenocarcinoma of the mouse prostate (TRAMP), an animal model that mimics progressive forms of human prostatic disease, GTP consumption was found to significantly inhibit prostate cancer development and metastasis. This was achieved by an oral infusion of GTP equivalent to six cups of green tea a day, i.e. at an achievable dose in humans (Gupta et al., 2001). Studies on cell-culture systems using human prostate cancer cells DU145 (androgen-insensitive) and LNCaP (androgen-sensitive) showed that EGCG induces apoptosis, cell-growth inhibition and cell-cycle dysregulation (Adhami et al., 2003). EGCG was also reported to reduce the expression of PSA and inhibits its activity in a dose-dependant way, thereby reducing prostate cancer aggressiveness (Pezzato et al., 2004). In addition, elevated levels of IGF-1, coupled with lowering of IGF-binding protein-3 (IGFBP-3) are associated with a greater risk of developing prostate cancer. GTP infusion has been shown to reduce the levels of IGF-1 and concomitantly increase IGFBP-3 with marked inhibition of markers of angiogenesis and metastasis. This leads to a reduction in the downstream signaling and inhibition of protein expression, hence inhibiting prostate cancer development and progression. (Adhami et al., 2004; 2006). We have shown that GTP feeding to TRAMP mice resulted in marked inhibition of prostate cancer progression which was associated with reduction of S100A4 and restoration of E-cadherin (Saleem et al., 2005). Evidence from a case-control study conducted in south-east China assessing 130 patients with histologically confirmed incidental prostate cancer and 274 patients without cancer matched by age, showed that the prostate cancer risk declined with increasing frequency, duration and quantity of green tea consumed. This reduction was statistically significant, suggesting that green tea protects against prostate cancer (Jian et al., 2004). It is also shown that green and black tea significantly reduced prostate tumors. The combination of soy phytochemical concentrate (SPC) and green tea synergistically inhibited final tumor weight and metastasis, and significantly reduced serum concentrations of both testosterone and dihydrotestosterone in vivo. Inhibition of tumor progression was associated with reduced tumor cell proliferation and tumor angiogenesis (Zhou et al., 2003). Recently, we have shown that green and black tea inhibits CWR22Rυ1 tumor growth and PSA secretion in athymic nude mice (Siddiqui et al., 2005). In a recent study in Italy, green tea given in the form of capsules demonstrated cancer preventive activity against prostate cancer in patients who had premalignant lesions and were at high risk to develop prostate cancer (Bettuzzi et al., 2006). We have reported that EGCG induced apoptosis in human prostate carcinoma LNCaP cells via stabilization of p53 by phosphorylation on critical serine residues and p14ARF-mediated downregulation of murine double minute 2(MDM2) protein, negative regulation of NF-κB activity, activation of caspases, causing a change in the ratio of Bax/Bcl-2 in a manner that favors apoptosis and poly (ADP-ribose) polymerase (PARP) cleavage (Hastak et al., 2003). EGCG treatment of LNCaP and DU145 cells resulted in induction of G1 phase ckis, which inhibits the cyclin-cdk complexes operative in the G0/G1 phase of the cell cycle, thereby causing an arrest which ultimately leads to apoptotic cell death (Gupta et al., 2003). Using isogenic cell lines, we have demonstrated that EGCG activates growth arrest and apoptosis in prostate carcinoma cells primarily via p53-dependent pathway that involves the function of both p21 and Bax such that down-regulation of either molecule confers a growth advantage to the cells (Hastak et al., 2005). Recently, we have shown that treatment of human prostate cancer cells LNCaP, PC-3, and CWR22Rυ1 with combination of EGCG and Cox-2 inhibitor resulted in enhanced cell growth inhibition, apoptosis induction and inhibition of NF-κB. In athymic nude mice, implanted with CWR22Rυ1 cells, combination treatment with GTP and celecoxib resulted in enhanced tumor growth inhibition, lowering of PSA and IGF-1 levels, and increase in IGFBP-3 levels (Adhami et al., 2007). Based on all evidences, green tea clearly has a marked effect by reducing the development of prostate cancer.
Cardiovascular Diseases
The onset of cardiovascular disease (CVD) depends on numerous factors that can be modulated by components in the diet. In coronary heart disease, atherosclerotic plaques protrude from the inner surface of the arteries, narrow the lumen, and reduce blood flow. At the first stage, low-density lipoprotein (LDL) deposits at lesion sites of the arterial wall and is subjected to oxidation when protectors such as tocopherols are depleted. Later, oxidation of LDL induces modification in lipoproteins, stimulates inflammatory reactions, causes monocytes and monocyte-derived macrophages to accumulate in large amounts of oxidized LDL, and forms lipid-laden foam cells and atherosclerotic plaques. The intake of saturated fat and smoking habits accelerate these events. Underlying mechanisms for the beneficial effects of tea include vasculoprotective, antioxidative, antithrombogenic, anti-inflammatory and lipid-lowering properties of tea flavonoids (Stangl et al., 2006). The intake of green tea has been inversely associated with the development and progression of atherosclerosis and it has been reported that dietary green tea intake preserves and improves arterial compliance and endothelial function (Murakami and Oshato, 2003). In a prospective cohort study in Saitama Prefecture, Japan, a decreased relative risk of death from CVD was found for people consuming over 10 cups of green tea a day, and green tea also had life-prolonging effects on cumulative survival (Sueoka et al., 2001). Recent studies conducted in vitro, with animal models, and with humans suggest that tea and its components can play a protective role in the development of CVD. Epidemiological evidence, particularly from a 10–15 year follow-up of cohorts of 550–800 men from the Zutphen Study in Netherlands, reveals a strong inverse association between flavonol intake and coronary heart disease (CHD) mortality (Hertog et al., 1997). Rats fed with 2.5% green tea leaves in the diet for a long time had a reduction in blood triglycerides and total cholesterol contents, enhancement in the superoxide dismutase and phase II enzyme activities in the liver without any liver or kidney damage (Lin et al., 1998). Green tea also increases the activity of SOD in serum and the expression of catalase in the aorta, enzymes implicated in cellular protection against reactive oxygen species. This action is combined with direct action on oxygen species by a decrease in the nitric oxide plasma concentration (Negishi et al., 2004). Green tea catechins affect lipid metabolism by different pathways and prevent the appearance of atherosclerotic plaque. Its intake decreases the absorption of triglycerides and cholesterol and these findings are in accordance with the fact that it increases excretion of fat (Raederstorff et al., 2003).
In a case-control study, the relation between tea and coffee consumption and myocardial infarction was studied. There was 44% reduction in cardiovascular risk in the individuals drinking more than a cup of tea per day. There was no significant relation between coffee consumption and cardiovascular disease (Sesso et al., 1999). A recent study examined the effect of tea consumption on recurrent myocardial infarction in patients. Using a prospective cohort design, Mukamal and colleagues examined tea consumption in 1900 patients in the Myocardial Infarction Onset Study, a study that examined patients with myocardial infarction presenting to community hospitals in the United States (Mukamal et al., 2002). Tea consumption was assessed by questionnaire and the patients were followed for 3.8 years. This study demonstrated a 31% and 39% reduction in cardiovascular risk in moderate and heavy tea drinkers after adjustment for other risk factors. In a study with a follow-up duration of two to three years, aortic atherosclerosis via X-ray measurement of calcified deposits in the abdominal aorta of humans was measured. The odds ratio for drinking 1–2 cups of black tea daily was less when compared with those drinking more than four cups (Geleijnse et al., 1999). In a cross sectional study of 512 coronary patients (302 men and 210 women), it was shown that green tea may be protective against coronary atherosclerosis in men but not in women (Sasazuki et al., 2000). In a prospective cohort study of 8522 men and women, it was reported that consuming ≥10 cups/day of green tea is linked with a decreased relative risk of death from cardiovascular disease in men and women (Nakachi et al., 1998). An inverse correlation was demonstrated between catechin intake and coronary heart disease mortality after a 25-year follow-up of 12763 men from seven different countries (Hertog et al., 1995). A meta-analysis suggested a decrease in the rate of cardiovascular disease outcomes with increasing green tea consumption (Peters et al., 2001). An inverse association of green tea intake and myocardial infarction and its genetic variation has also been reported (Hirano et al., 2002). Research on both overall cholesterol status and the mechanisms which regulate cholesterol metabolism indicate that tea itself and various tea ingredients such as EGCG, polyphenols and catechins can prevent or reduce cholesterol related events that could lead to CVD. The use of tea to control hypertension and obesity may also impact on the incidence of CVD.
Diabetes
Type II diabetes is a heterogeneous disorder that involves resistance of glucose and lipid metabolism in peripheral tissues to the biological activity of insulin and inadequate insulin secretion by pancreatic β-cells. Various animal models and treatments mimic diabetes: Zucker rats (which are genetically obese), injection of streptozotocin or alloxan (which destroys pancreatic β-cells), and treatment with sucrose-rich diets (which induces obesity and insulin resistance). The effects of tea on obesity and diabetes have received increasing attention. Tea catechins, especially EGCG, appear to have antiobesity and antidiabetic effects (Kao et al., 2006). African black tea extract has been shown to possess suppressive effect on the elevation of blood glucose during food intake and the body weight in KK-A(y)/TaJcl diabetic mice (Shoji Y and Nakashima, 2006). While few epidemiological and clinical studies show the health benefits of EGCG on obesity and diabetes, the mechanisms of its actions are emerging based on the various laboratory data. These mechanisms may be related to certain pathways, such as through the modulations of energy balance, endocrine systems, food intake, lipid and carbohydrate metabolism and the redox status (Yang et al., 2001). These results suggest that green tea catechins could act as preventive agents and could have a beneficial effect against lipid and glucose metabolism disorders implicated in type II diabetes.
Obesity
Recently, it has been shown in a double-blind, placebo-controlled, cross-over design study that consumption of a beverage containing green tea catechins, caffeine, and calcium increases 24-hour energy expenditure by 4.6%, but the contribution of the individual ingredients cannot be distinguished. It was suggested that such modifications were sufficient to prevent weight gain (Rudelle et al., 2007). It has been reported that the body weights of rats and their plasma triglyceride, cholesterol and LDL-cholesterol have been significantly reduced by feedings of Oolong, black, and green tea leaves to the animals. It is also suggested that the inhibition of growth and suppression of lipogenesis in MCF-7 breast cancer cells may be through down-regulation of fatty acid synthase gene expression in the nucleus and stimulation of cell energy expenditure in the mitochondria (Lin and Lin Shiau, 2006). EGCG purified from green tea when given to mice in diet decreased diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation (Klaus et al., 2005). Supplementation with tea catechins resulted in a significant reduction of high-fat diet-induced body weight gain, visceral and liver fat accumulation, and the development of hyperinsulinemia and hyperleptinemia in C57BL/6J mice (Murase et al., 2002). Intraperitoneal injection of EGCG caused acute body weight loss in male and female Sprague-Dawley rats within 2–7 days of treatment. EGCG also significantly reduced or prevented an increase in body weight in lean and obese male and female Zucker rats (Kao et al., 2000). On the basis of the in vivo effects of EGCG on body weight loss, body fat, serum lipid nutrients, thermogenesis, and fat oxidation and of the in vitro effects of EGCG on fat cell functions, long-term consumption of green tea may decrease the incidence of obesity and, perhaps, green tea components such as EGCG may be useful for treating obesity. It has been reported that green tea extract rich in catechins and caffeine has thermogenic properties and promotes fat oxidation beyond than those explained by its caffeine content per se; the green tea extract may play a role in the control of body composition via sympathetic activation of thermogenesis, fat oxidation, or both (Dulloo et al., 1999). The thermogenic properties of green tea could reside primarily in an interaction between its high content in catechins and the presence of caffeine with sympathetically released noradrenaline, since polyphenols are known to be capable of inhibiting catechol-o-methyl-transferase, and caffeine of inhibiting trancellular phosphodiesterases. The increased and prolonged sympathetic stimulation of thermogenesis by the interaction between polyphenols and caffeine could be of value in assisting the management of obesity (Dulloo et al., 2000).
Longevity
In a prospective cohort study of a Japanese population with 13-year follow-up data, there was found an apparent delay of cancer onset/death and all cause deaths associated with increased consumption of green tea, specifically in persons less than 79 years of age. This is consistent with analyses of age-specific cancer death rate and cumulative survival, indicating a significant slowing of the increase in cancer death and all cause death with aging. These results indicate that daily consumption of green tea in sufficient amounts will help to prolong life by avoiding premature death, particularly death caused by cancer (Nakachi et al., 2003). An 8-year follow-up survey was conducted by Saitama Cancer Center in Japan concerning the effects of green tea on the prolongation of human life using 8500 participants in Saitama Prefecture. The average life span was 66 years for males who had more than 3 cups of green tea every day and 68 years for females. The average life span was 70 years for males who had more than 10 cups of green tea per day and 74 years for females. In this study, a decreased relative risk of death from cardiovascular disease was also found for people consuming over 10 cups of green tea a day and, importantly, green tea consumption also had life-prolonging effects on cumulative survival (Fujiki et al., 1996). We have also shown that consumption of 0.1% GTP to TRAMP mice prolongs their overall survival more than two fold (Gupta et al., 2001).
Antihistaminic and anti-arthritic effects
Tea consumption was identified as an independent factor protecting against the risk of hip fractures in women and seven men, respectively, over age 50 in the Mediterranean Osteoporosis Study (Johnell et al., 1995; Kanis et al., 1999) Consistent with this observation, in a study, 1,256 British women 65 to 76 years of age, were studied and it was found that those who drank tea had greater bone mineral density than those who did not drink tea (Hegarty et al., 2000). The release of histamine from mast cells is associated with inflammation, dermatis, urticaria, mastocytosis and asthma in allergic responses triggered by environment antigens. Beside its anti-inflammatory properties, tea extract exhibits an antihistaminic effect on rat peritoneal mast cells and inhibits hyaluronidase activity (Toyoda et al., 1997). EGCG can inhibit histamine release up to 90% in rat cell culture. The inhibitory effect of the histamine release is likely related to the triphenol moiety of the molecule. Quercetin produces an anti-inflammatory activity in cells activated by an antigen and causes a concentration-dependant inhibition of histamine release (Alexis et al., 1999). Quercetin also inhibits protein kinase C, an enzyme important in the activation of the secretory process, by blocking the ATP binding site in the catalytic portion of the enzyme. GTP produce a significant reduction in arthritis incidence with a marked reduction of inflammatory mediators, of neutral endopeptidase activity, of IgG and type II collagen-specific IgG levels in arthritic joints in mice (Haqqi et al., 1999).
Neurological and psychological effects
There are reports that indicate that tea can improve neurologic and psychologic functions. Recently, it has been reported that tea catechins possesses divalent metal chelating, antioxidant and anti-inflammatory activities to penetrate the brain barrier and protect neuronal death in a wide array of cellular and animal models of neurological diseases (Mandel et al., 2006). It has been shown that EGCG reduces focal ischemia/reperfusion-induced brain injury in a rat model (Choi et al., 2004). Theanine acts as a neurotransmitter in the brain and decreases blood pressure significantly in hypertensive rats. Alpha-waves are a relaxation index indicating relaxation without drowsiness. In volunteers, alpha-waves are generated in the occipital and parietal regions of the brain surface within 40 minutes after administration of theanine in a dose-dependent manner. Theanine is absorbed, modulates brain serotonin and dopamine levels, improving memory, learning ability and affects emotions (Juneja et al., 1999). Investigations were carried out in mice with experimentally induced convulsions to evaluate the effect of acute and chronic administration of green or black tea. Tea appears to accelerate the onset of convulsion, to increase duration and mortality. Tea may be acting on Ca2+ channels and not through GABA in brain (Gomes et al., 1999). Recent studies have indicated that in addition to the known antioxidant activity of catechins, other mechanisms such as modulation of signal transduction pathways, cell survival/death genes and mitochondrial function, contribute significantly to the induction of cell viability (Mandel et al., 2005). The promising future treatment of neurodegenerative diseases and aging depends on availability of effective brain permeable, iron-chelatable/radical scavenger neuroprotective drugs that would prevent the progression of neurodegeneration. Tea catechins have been reported to possess potent iron-chelating, radical-scavenging and anti-inflammatory activities and to protect neuronal death in a wide array of cellular and animal models of neurological diseases.
Antibacterial and Antiviral effects
The methanolic extract of tea leaves was screened for antimicrobial property against 111 bacteria comprising 2 genera of Gram positive and 7 genera of Gram negative bacteria. Most of these strains were inhibited by the tea extract. The antibacterial activity of tea extract was also confirmed in vivo. It protected the Swiss strain of white mice challenged with Salmonella typhimurium at different dosages (Bandyopadhyay et al., 2005). Recently, it has been reported that EGCG has an effect on the inhibition of HIV infection and Staphylococcus aureus infections (Nance and Shearer, 2003). Drinking tea leads to a reduction of enterobacteria which produce ammonia and other harmful amines and a beneficial increase in the level of lactobacilli and bifidobacteria which produce organic acids and lower the intestinal pH (Weisburger, 1999). Tea can have a beneficial effect against viral infection. Tea polyphenols strongly inhibit rotavirus propagation in monkey cell culture and influenza A virus in animal cell culture. It is reported that several flavonoids including EGCG and ECG inhibit retrovirus human immunodeficiency virus (HIV) propagation by inhibiting reverse transcriptase, an enzyme allowing the establishment of the virus in host cells (Yamamoto et al., 1997).
Conclusion and future prospects
Tea is a pleasant, popular, socially accepted, economical and safe drink that is enjoyed every day by hundreds of millions of people across all continents. Tea also provides a dietary source of biologically active compounds that help prevent a wide variety of diseases. Recent research in last 30 years identified tea as a Nature’s gift for promoting human health. The amount of experimental evidence documenting the properties of tea and its constituents continues to increase. At the same time the factors both endogenous and exogenous, that influence the incidence and progression of many chronic diseases are becoming better defined and understood. It is apparent that the tea is a source of a wide range of phytochemicals that are digested, absorbed and metabolized by the body, and that tea constituents exert their effects at the cellular level. Tea’s status as a functional food lends credibility to what has been believed by tea drinkers for centuries. The past five years have been rich in information coming from laboratories all around the world concerning the positive impact of food on human health. More and more emphasis is being placed to define events at the cellular level. Much interest has been centered on the role of oxidant/antioxidant activity in regards to the aging process and degenerative diseases like cancer, cardiovascular disease and diabetes. Epidemiological surveys, though inconclusive, have associated tea drinking with reduced risk of cardiovascular diseases and cancer, while studies in cell cultures and animal models indicate a potentially beneficial effect of tea on Phase I and II hepatic enzymes, gene transcription, cell proliferation and other molecular functions. Within the last few years, clinical studies have revealed several physiological responses to tea which may be relevant to the promotion of health and the prevention or treatment of some chronic diseases. For the promotion of health, there are various factors that matters like type of tea (i.e., green, Oolong and black) and preparation (e.g., short vs. long brew time and hot vs. iced) be important, but so will the frequency and timing of intake as these factors directly affect the pharmacokinetics and ultimate disposition of the polyphenols within tissues.
Future research needs to define the actual magnitude of health benefits, establish the safe range of tea consumption associated with these benefits and elucidate potential mechanisms of action. Further progress in the evaluation of the effects of tea on humans depends on the development of new experimental systems. Exploration at the cellular level allows a better understanding of the underlying mechanisms regulating functions in normal and pathologic states. Development of more specific and sensitive methods with more representative models along with the development of good predictive biomarkers will give a better understanding of how tea interacts with endogenous systems and other exogenous factors. In order to understand the effect of tea consumption on cancer in humans, additional research on the pharmacokinetics of tea constituents as well as their mechanisms of action is needed. Definitive conclusions concerning the protective effect of tea have to come from well-designed observational epidemiological studies and intervention trials. The development of biomarkers for tea consumption, as well as molecular markers for its biological effects, will contribute to better future studies in this area. As tea is already one of the most popular beverages worldwide, further studies, designed to accurately assess tea consumption and tea polyphenol status, should be directed to quantifying its role in the primary and secondary prevention of chronic diseases.
Table 1.
Preventive effects of tea and tea polyphenols against cancer and cardiovascular diseases in experimental animals and humans.
| Disease | Preventive effects reported in laboratory animals | Preventive effects reported in human epidemiological or case-control studies |
|---|---|---|
| Skin cancer | Prevention of photocarcinogenesis (Mukhtar and Ahmad, 2000; Yang and Wang, 1993) Decrease in progression of papillomas to SCC (Katiyar et al., 1997) Reduction in tumor incidence or multiplicity and tumor growth (Nihal et al., 2005; Lu et al., 1997) Decrease in papilloma formation and size (Conney et al., 1999) Decrease in UVB-induced bifold-skin thickness, skin edema and infiltration of leukocytes, suppression of UV-induced MAPKs and NFκB (Afaq et al., 2003) Increase in the rate and extent of disappearance of the mutant p53-positive patches (Lu et al., 2005) Reduction in skin tumor incidence, tumor multiplicity, tumor growth, inhibition of MMPs, VEGF and PCNA, increase in CD(8+) T cells and caspase-3 activity (Mantena et al., 2005) |
In a population-based case-control study, black tea intake was found to be associated with decreased risk of skin SCC (Hakim and Harris, 2001) Protective effect of tea on cutaneous malignant melanoma in a case-control study (Naldi et al., 2004) |
| Liver cancer | Decrease in GST positive hepatic foci (Xu et al., 1992; Zhong et al., 2001) Inhibition of hepatocarcinogenesis, reduction in tumor multiplicity and tumor incidence (Landau et al., 1998) Decrease in number and area of GST-P-positive foci, induction of p21, inhibition of cyclin D1 and cdk4 (Jia et al., 2002) |
Inhibition of liver cancer by green tea (Sueoka et al., 2001) In a population based case-control study conducted in Taixing, Jiangsu province, green tea drinking decreased risk for the development of gastric cancer by 78% in alcoholics and by 48% among cigarette smokers (Mu et al., 2003). |
| Lung cancer | Reduction in tumor volume (Landau et al., 1998) Reduction in tumor incidence and multiplicity (Landau et al., 1998; Banerjee et al., 2005) Reduction in progression of adenoma to adenocarcinoma (Naldi et al., 2004) |
In a case-control study in Uruguay, heavy drinkers of tea were associated with a reduced risk (Mendilaharsu et al., 1998) In a population-based case-control study in Shanghai, among nonsmoking women, green tea was associated with a decreased risk of lung cancer and the risks decreased with increased consumption (Zhong et al., 2001) In a case control study, a protective effect of frequent (daily or several times per week) black tea drinking against risk of lung cancer appeared among non-smoking women (Kubik et al., 2004) |
| Gastrointestinal tract cancer | Reduction in tumor incidence and multiplicity, inhibition of tumor promotion (Hiura et al., 1997; Takada et al., 2002; Qanungo et al., 2005) Inhibition of small intestinal tumorigenesis, increase in E-cadherin and decrease in nuclear beta-catenin, c-myc, phospho-Akt, and phospho-ERK1/2 in small intestinal tumors (Ju et al., 2005) |
In a population based study in Japan, green tea decreased development of gastric cancer and esophageal cancer (Wang et al., 1999) Green tea caused reduction in risk for the development of stomach cancer by 81% in alcoholics and by 16% among cigarette smokers in a case control study in Taixing, China (Mu et al., 2003) In same study, there was decrease in esophageal cancer by 39% in alcoholics and by 31% among cigarette smokers (Mu et al., 2003) |
| Pancreatic and bladder cancer | Inhibition of pancreatic tumor initiation, hyperplasia and total duct lesion (Hirose et al., 1995) Decrease in the numbers of hyperplasia and total pancreatic duct lesions (Majima et al., 1998) Inhibition of formation of bladder tumors (Umemura et al., 2003) |
In a large population-based case-control study conducted in Shanghai, China, decrease in pancreatic cancer with increasing amount of green tea consumption (Ji et al., 1997) Decrease in urinary bladder cancer (Lambert and Yang, 2003) |
| Breast cancer | Increase in mean latency of tumors (Li et al., 1999) Decrease in tumor incidence, multiplicity and tumor size (Suganuma et al., 2001) Reduction of tumor growth accompanied with increase in Bax/Bcl-2 ratio, reduction in PCNA and activation of caspase 3 (Baliga et al., 2005) |
Increased consumption of green tea was closely associated with decreased numbers of axillary lymph node metastases in patients with stage I and II breast cancer (Nakachi et al., 2000) In a case control study, significant decrease in risk of breast cancer (Wu et al., 2003) In a population-based case-control study of breast cancer in Chinese, Japanese, and Filipino-American women in Los Angeles County, green tea drinkers showed a significantly reduced risk of breast cancer (Wu et al., 2003) In Japan, women consuming >10 cups of tea had lower risk for breast cancer metastasis and recurrence (Lambert and Yang, 2003) A meta-analysis examined populations in eight countries indicate a lower risk for breast cancer with green tea consumption (Sun et al., 2006) |
| Prostate cancer | Delay in primary tumor incidence and tumor burden, decrease in PCNA, inhibition of CaP development and increase in survival time (Gupta et al., 2001) Reduction in IGF-I and increase in IGFBP-3, inhibition of markers of angiogenesis and metastasis (Adhami et 2006) Decrease in tumor size (Kavanagh et al., 2001) Decrease in PSA secretion (Wang et al., 1999; Roomi et al., 2005) Inhibition of CaP development and metastasis (Mantena et al., 2005) Inhibition of CaP progression with reduction of S100A4 and restoration of E-cadherin (Saleem et al., 2005) Inhibition in growth of implanted level PSA, induction of apoptosis prostate tumors, reduction in the level of serum with upregulation in Bax and decrease in Bcl-2, decrease in VEGF (Siddiqui et al., 2006) |
In a case-control study conducted in South-east China, prostate cancer risk declined with increasing frequency, duration and quantity of green tea consumed (Baliga et al., 2005) Chemopreventive effect of green tea in patients of HG-PIN in Italy (Bettuzzi et al., al., 2004, 2006) |
| Cardiovascular disease | Vasculoprotective, antioxidative, antithrombogenic, anti-inflammatory lipid-lowering effects (Wu et al., 2003) EGCG caused decrease in cholesterol in plasma, hepatic total cholesterol, triglyceride and cholesterol absorption and LDL cholesterol Increase in fat excretion and HDL cholesterol (Raederstroff et al., 2003) Green tea caused reduction in systolic and diastolic blood pressure, increase in catalase expression (aorta) Decrease in nitric oxide plasma concentration (Negishi et al., 2004) Green tea leaves in diet caused in reduction in blood triglycerides and total cholesterol contents (Stangl et al., 2006) |
Consuming ≥ 10 cups/day of green tea is linked with decreased relative risk of death from CVD in men and women (Nakachi et al., 1998) In a case control study, 44% decrease in cardiovascular risk in the individuals drinking more than a cup of tea per day (Sesso et al., 1999) Inverse association between tea intake and coronary heart disease mortality (Nakachi et al., 2000) Decrease in aortic atherosclerosis in a cross sectional study of 512 coronary patients (Sasazuki et al., 2000) Decrease in rate of CVD with increased green tea consumption(Peters et al., 2001) Decrease in relative risk of death from CVD for people consuming over 10 cups of green tea a day (Sueoka et al., 2001) In a prospective cohort study, 31 and 39% reduction in cardiovascular risk in moderate and heavy tea drinkers (Mukamal et al., 2002) Inverse association of green tea intake and myocardial infarction (Hirano etal., 2002) Inverse association of green tea with atherosclerosis (Wu et al., 2003) Green tea Improves arterial compliance and endothelial function (Wu et al., 2003) |
Acknowledgments
The original work from the author’s (HM) laboratory outlined in this review was supported by United States Public Health Service Grants R01 CA 78809, R01 CA 101039, R01 CA 120451 and P50 DK065303.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhami VM, Afaq F, Mukhtar H. Insulin-like growth factor-I axis as a pathway for cancer chemoprevention. Clinical Cancer Research. 2006;12(19):5611–5614. doi: 10.1158/1078-0432.CCR-06-1564. [DOI] [PubMed] [Google Scholar]
- Adhami VM, Ahmad N, Mukhtar H. Molecular targets for green tea in prostate cancer prevention. Journal of Nutrition. 2003;133 (7 Suppl):2417S–2424S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, Afaq F, Pasha FS, Saleem M, Mukhtar H. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clinical Cancer Research. 2007;13(5):1611–1619. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signalling in an autochthonus mouse model of prostate cancer. Cancer Research. 2004;64 (23):8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- Afaq F, Ahmad N, Mukhtar H. Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice. Oncogene. 2003;22(58):9254–64. doi: 10.1038/sj.onc.1207035. [DOI] [PubMed] [Google Scholar]
- Alexis AF, Jones VA, Stiller MJ. Potential therapeutic applications of tea in dermatology. International Journal of Dermatology. 1999;38 (10):735–743. doi: 10.1046/j.1365-4362.1999.00796.x. [DOI] [PubMed] [Google Scholar]
- Baliga MS, Meleth S, Katiyar SK. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Clinical Cancer Research. 2005;11(5):1918–1927. doi: 10.1158/1078-0432.CCR-04-1976. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay D, Chatterjee TK, Dasgupta A, Lourduraja J, Dastidar SG. In vitro and in vivo antimicrobial action of tea: the commonest beverage of Asia. Biological and Pharmaceutical Bulletin. 2005;28 (11):2125–2127. doi: 10.1248/bpb.28.2125. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Manna S, Saha P, Panda CK, Das S. Black tea polyphenols suppress cell proliferation and induce apoptosis during benzo(a)pyrene-induced lung carcinogenesis. European Journal of Cancer Prevention. 2005;14(3):215–221. doi: 10.1097/00008469-200506000-00004. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Research. 2006;66(2):1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- Caderni G, De Filippo C, Luceri C, Salvadori M, Giannini A, Biggeri A, Remy S, Cheynier V, Dolara P. Effects of black tea, green tea and wine extracts on intestinal carcinogenesis induced by azoxymethane in F344 rats. Carcinogenesis. 2000;21 (11):1965–1969. doi: 10.1093/carcin/21.11.1965. [DOI] [PubMed] [Google Scholar]
- Cao G, Sofic E, Prior R. Antioxidant capacity of tea and common vegetables. Journal of Agriculture and Food Chemistry. 1996;44 (11):3426–3431. [Google Scholar]
- Choi YB, Kim YI, Lee KS, Kim BS, Kim DJ. Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Research. 2004;1019 (1–2):47–54. doi: 10.1016/j.brainres.2004.05.079. [DOI] [PubMed] [Google Scholar]
- Chung FL, Wang M, Rivenson A, Iatropoulos MJ, Reinhardt JC, Pittman B, Ho CT, Amin SG. Inhibition of lung carcinogenesis by black tea in Fischer rats treated with a tobacco-specific carcinogen: caffeine as an important constituent. Cancer Research. 1998;58 (18):4096–101. [PubMed] [Google Scholar]
- Conney AH, Lu YP, Lou YR, Xie JG, Huang MT. Inhibitory effect of green and black tea on tumor growth. Proceedings of Society of Experimental Biology and Medicine. 1999;220 (4):229–233. doi: 10.1046/j.1525-1373.1999.d01-39.x. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J. Efficacy of a green tea extracts rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. American Journal of Clinical Nutrition. 1999;70 (6):1040–1045. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J. Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. International Journal of Obesity Related Metabolic Disorders. 2000;24 (2):252–258. doi: 10.1038/sj.ijo.0801101. [DOI] [PubMed] [Google Scholar]
- Erba D, Riso P, Bordoni A, Foti P, Biagim PL, Testolin G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. Journal of Nutritional Biochemistry. 2005;16 (3):144–149. doi: 10.1016/j.jnutbio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Freese R, Basu S, Hietanen E, Nair J, Nakachi K, Bartsch H, Mutanen M. Green tea extract decreases plasma malondialdehyde concentration but does not affect other indicators of oxidative stress, nitric oxide production, or hemostatic factors during a high-linoleic acid diet in healthy females. European Journal of Nutrition. 1999;38 (3):149–157. doi: 10.1007/s003940050056. [DOI] [PubMed] [Google Scholar]
- Fujiki H, Suganuma M, Okabe S, Komori A, Sueoka E, Sueoka N, Kozu T, Sakai Y. Japanese green tea as a cancer preventive in humans. Nutrition Review. 1996;54 (2):S67–S70. doi: 10.1111/j.1753-4887.1996.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Geleijnse J, Launer L, Hofman A, Pols H, Witteman J. Tea flavonoids may protect against atherosclerosis: The Rotterdam Study. Archives of Internal Medicine. 1999;159 (18):2170–2174. doi: 10.1001/archinte.159.18.2170. [DOI] [PubMed] [Google Scholar]
- Gomes A, Das M, Vedasiromoni JR, Ganguly DK. Proconvulsive effect of tea (Camellia sinensis) in mice. Phytotherapy Research. 1999;13 (5):376–379. doi: 10.1002/(sici)1099-1573(199908/09)13:5<376::aid-ptr465>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proceedings of the National Academy of Sciences USA. 2001;98(18):10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Hussain T, Mukhtar H. Molecular pathway for (−)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Archives of Biochemistry and Biophysics. 2003;410(1):177–185. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- Hakim IA, Harris RB. Joint effects of citrus peel use and black tea intake on the risk of squamous cell carcinoma of the skin. BMC Dermatology. 2001;1:3. doi: 10.1186/1471-5945-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqqi TM, Anthony DD, Gupta S, Ahmad N, Lee MS, Kumar GK, Mukhtar H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proceedings of the National Academy of Sciences USA. 1999;96 (8):4524–4529. doi: 10.1073/pnas.96.8.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22(31):4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- Hastak K, Agarwal MK, Mukhtar H, Agarwal ML. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB Journal. 2005;19(7):789–791. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- Hegarty V, May H, Khaw K. Tea drinking and bone mineral density in older women. American Journal of Clinical Nutrition. 2000;71 (4):1003–1007. doi: 10.1093/ajcn/71.4.1003. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Gianpaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, Simic B, Toshima H, Feskens E, Hollman P, Katan M. Flavonoid intake and long-term risk of coronary heart disease and cancer in the Seven Countries Study. Archives of Internal Medicine. 1995;155 (4):381–386. [PubMed] [Google Scholar]
- Hertog MG, Feskens E, Kromhout D. Antioxidant flavonols and coronary heart disease risk. Lancet. 1997;349 (9053):699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Critical Reviews in Food Science and Nutrition. 2003;43 (1):89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Hirano R, Momiyama Y, Takahashi R, Taniguchi H, Kondo K, Nakamura H, Ohusuzu F. Comparison of green tea intake in Japanese patients with and without angiographic coronary artery disease. American Journal of Cardiology. 2002;90 (10):1150–1153. doi: 10.1016/s0002-9149(02)02787-x. [DOI] [PubMed] [Google Scholar]
- Hirose M, Hasegawa R, Kimura J, Akagi K, Yoshida Y, Tanaka H, Miki T, Satoh T, Wakabayashi K, et al. Inhibitory effects of 1-O-hexyl-2,3,5-trimethylhydroquinone (HTHQ), green tea catechins and other antioxidants on 2-amino6-methyldipyrido[1,2-a:3′,2′-d]imidazole (Glu-P-1)-induced rat hepatocarcinogenesis and dose-dependent inhibition by HTHQ of lesion induction by Glu-P-1 or 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) Carcinogenesis. 1995;16 (3049):3049–3055. doi: 10.1093/carcin/16.12.3049. [DOI] [PubMed] [Google Scholar]
- Hiura A, Tsutsumi M, Satake K. Inhibitory effect of green tea extract on the process of pancreatic carcinogenesis induced by N-nitrosobis-(2-oxypropyl) amine (BOP) and on tumor promotion after transplantation of N-nitrosobis-(2-hydroxypropyl)amine (BHP)-induced pancreatic cancer in Syrian hamsters. Pancreas. 1997;15(3):272–277. doi: 10.1097/00006676-199710000-00009. [DOI] [PubMed] [Google Scholar]
- Ji BT, Chow WH, Hsing AW, McLaughlin JK, Dai Q, Gao YT, Blot WJ, Fraumeni JF., Jr Green tea consumption and the risk of pancreatic and colorectal cancers. International Journal of Cancer. 1997;70(3):255–258. doi: 10.1002/(sici)1097-0215(19970127)70:3<255::aid-ijc1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Jia X, Han C, Chen J. Effects of tea on preneoplastic lesions and cell cycle regulators in rat liver. Cancer Epidemiology Biomarkers and Prevention. 2002;11(12):1663–1667. [PubMed] [Google Scholar]
- Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. International Journal of Cancer 2004. 2004;108(1):130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. International Journal of Cancer. 2004;108(1):130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- Johnell O, Gullberg B, Kanis J, Allander E, Elffors L, Dequeker J, Dilsen G, Gennari C, Lopes Vaz A, Lyritis G. Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. Journal of Bone and Mineral Research. 1995;10 (11):1802–1815. doi: 10.1002/jbmr.5650101125. [DOI] [PubMed] [Google Scholar]
- Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, Yang GY, Liu YY, Hou Z, Lin Y, Ma J, Shih WJ, Carothers AM, Yang CS. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Research. 2005;65(22):10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- Juneja R, Chu DC, Okubo T, Nagato Y, Yokogoshi H. L-theanine-a unique amino acid of green tea and its effect in humans. Trends in Food Science and Technology. 1999;10(6):199–204. [Google Scholar]
- Kanis J, Johnell O, Gullberg B, Allander E, Elffors L, Ranstam J, Dequeker J, Dilsen G, Gennari C, Vaz AL, Lyritis G, Mazzuoli G, Miravet L, Passeri M, Perez Cano R, Rapado A, Ribot C. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporos International. 1999;9(1):45–54. doi: 10.1007/s001980050115. [DOI] [PubMed] [Google Scholar]
- Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Molecular Nutrition and Food Research. 2006;50(2):188–210. doi: 10.1002/mnfr.200500109. [DOI] [PubMed] [Google Scholar]
- Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141 (3):980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Mohan RR, Agarwal R, Mukhtar H. Protection against induction of mouse skin papillomas with low and high risk of conversion to malignancy by green tea polyphenols. Carcinogenesis. 1997;18 (3):497–502. doi: 10.1093/carcin/18.3.497. [DOI] [PubMed] [Google Scholar]
- Kavanagh KT, Hafer LJ, Kim DW, Mann KK, Sherr DH, Rogers AE, Sonenshein GE. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. Journal of Cellular Biochemistry. 2001;82 (3):387–398. doi: 10.1002/jcb.1164. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Research. 2006;66 (5):2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. Suppression of WNT signaling by the green tea compound EGCG in invasive breast cancer cells: Requirement of the transcriptional repressor HBP1. Journal of Biological Chemistry. 2006;281 (16):10865–10875. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee MJ, Hong J. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutrition and Cancer. 2000;37 (1):41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- Klaunig J, Xu Y, Han C, Kamendulis L, Chen J, Heiser C, Gordon M, Mohler E. The effect of tea consumption on oxidative stress in smokers and nonsmokers. Proceedings of Society of Experimental Biology and Medicine. 1999;220 (4):249–254. doi: 10.1046/j.1525-1373.1999.d01-43.x. [DOI] [PubMed] [Google Scholar]
- Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. International Journal of Obesity. 2005;29 (6):615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kurihara M, Miyata N, Suzuki T, Toyoda M. Scavenging mechanisms of (−)-epigallocatechin gallate and (−)-epicatechin gallate on peroxyl radicals and formation of superoxide during the inhibitory action. Free Radical Biology and Medicine. 1999;27 (7–8):855–863. doi: 10.1016/s0891-5849(99)00133-1. [DOI] [PubMed] [Google Scholar]
- Kubik AK, Zatloukal P, Tomasek L, Pauk N, Havel L, Krepela E, Petruzelka L. Dietary habits and lung cancer risk among non-smoking women. European Journal of Cancer Prevention. 2004;13(6):471–480. doi: 10.1097/00008469-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutatation Research. 2003;523–524:201–208. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. Journal of Nutrition. 2003;133 (10):3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- Landau JM, Wang ZY, Yang GY, Ding W, Yang CS. Inhibition of spontaneous formation of lung tumors and rhabdomyosarcomas in A/J mice by black and green tea. Carcinogenesis. 1998;19 (3):501–507. doi: 10.1093/carcin/19.3.501. [DOI] [PubMed] [Google Scholar]
- Leenen R, Roodenburg A, Tijburg L, Wiseman SA. A single dose of tea with or without milk increases plasma antioxidant activity in humans. European Journal of Clinical Nutrition. 2000;54 (1):87–92. doi: 10.1038/sj.ejcn.1600900. [DOI] [PubMed] [Google Scholar]
- Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, Huang B, Chung JY, Yan S, Ho CT, Yang CS. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chemical Research in Toxicology. 2000;13(3):177–184. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- Li N, Han C, Chen J. Tea preparations protect against DMBA-induced oral carcinogenesis in hamsters. Nutrition and Cancer. 1999;35 (1):73–79. doi: 10.1207/S1532791473-79. [DOI] [PubMed] [Google Scholar]
- Lin JK, Lin-Shiau SY. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Molecular Nutrition and Food Research. 2006;50 (2):211–217. doi: 10.1002/mnfr.200500138. [DOI] [PubMed] [Google Scholar]
- Lin YL, Cheng CY, Lin YP, Lau YW, Juan IM, Lin JK. Hypolipidemic effect of green tea leaves through induction of antioxidant and phase II enzymes including superoxide dismutase, catalase, and glutathione S-transferase in rats. Journal of Agriculture and Food Chemistry. 1998;46 (5):1893–1899. [Google Scholar]
- Lu Y, Yao R, Yan Y, Wang Y, Hara Y, Lubet RA, You MA. Gene expression signature that can predict green tea exposure and chemopreventive efficacy of lung cancer in mice. Cancer Research. 2006;66 (4):1956–1963. doi: 10.1158/0008-5472.CAN-05-3158. [DOI] [PubMed] [Google Scholar]
- Lu YP, Lou YR, Liao J, Xie JG, Peng QY, Yang CS, Conney AH. Administration of green tea or caffeine enhances the disappearance of UVB-induced patches of mutant p53 positive epidermal cells in SKH-1 mice. Carcinogenesis. 2005;26(8):1465–72. doi: 10.1093/carcin/bgi086. [DOI] [PubMed] [Google Scholar]
- Lu YP, Lou YR, Xie JG, Yen P, Huang MT, Conney AH. Inhibitory effect of black tea on the growth of established skin tumors in mice: effects on tumor size, apoptosis, mitosis and bromodeoxyuridine incorporation into DNA. Carcinogenesis. 1997;18 (11):2163–2169. doi: 10.1093/carcin/18.11.2163. [DOI] [PubMed] [Google Scholar]
- Luczaj W, Skrzydlewska E. Antioxidative properties of black tea. Preventive Medicine. 2005;40(6):910–918. doi: 10.1016/j.ypmed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Majima T, Tsutsumi M, Nishino H, Tsunoda T, Konishi Y. Inhibitory effects of beta-carotene, palm carotene, and green tea polyphenols on pancreatic carcinogenesis initiated by N-nitorsobis(2-oxopropyl)amine in Syrian golden hamsters. Pancreas. 1998;16(1):13–8. doi: 10.1097/00006676-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Mandel S, Amit T, Reznichenko L, Weinreb O, Youdim MB. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Molecular Nutrition and Food Research. 2006;50 (2):229–234. doi: 10.1002/mnfr.200500156. [DOI] [PubMed] [Google Scholar]
- Mandel SA, Avramovich-Tirosh Y, Reznichenko L, Zheng H, Weinreb O, Amit T, Youdim MB. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. Neurosignals. 2005;14 (1–2):46–60. doi: 10.1159/000085385. [DOI] [PubMed] [Google Scholar]
- Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T-cells and inhibition of angiogenesis in tumors. Journal of Nutrition. 2005;135 (12):2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Kohri T, Okushio K, Hara Y. Inhibitory effects of tea catechins, black tea extract and Oolong tea extract on hepatocarcinogenesis in rat. Japanese Journal of Cancer Research. 1996;87 (10):1034–1038. doi: 10.1111/j.1349-7006.1996.tb03106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendilaharsu M, De Stefani E, Deneo-Pellegrini H, Carzoglio JC, Ronco A. Consumption of tea and coffee and the risk of lung cancer in cigarette-smoking men: a case-control study in Uruguay. Lung Cancer. 1998;19(2):101–117. doi: 10.1016/s0169-5002(97)00075-5. [DOI] [PubMed] [Google Scholar]
- Mu LN, Zhou XF, Ding BG, Wang RH, Zhang ZF, Chen CW, Wei GR, Zhou XM, Jiang QW, Yu SZ. A case-control study on drinking green tea and decreasing risk of cancers in the alimentary canal among cigarette smokers and alcohol drinkers. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24(3):192–195. [PubMed] [Google Scholar]
- Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Tea consumption and mortality after acute myocardial infarction. Circulation. 2002;105 (21):2476–2481. doi: 10.1161/01.cir.0000017201.88994.f7. [DOI] [PubMed] [Google Scholar]
- Mukhtar H, Ahmad N. Cancer chemoprevention: future holds in multiple agents. Toxicology and Applied Pharmacology. 1999;158 (3):207–210. doi: 10.1006/taap.1999.8721. [DOI] [PubMed] [Google Scholar]
- Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. American Journal of Clinical Nutrition. 2000;71 (6 Suppl):1698S–1702S. doi: 10.1093/ajcn/71.6.1698S. [DOI] [PubMed] [Google Scholar]
- Murakami T, Oshato K. Dietary green tea intake preserves and improves arterial compliance and endothelial function. Journal of the American College of Cardiology. 2003;l41:271–274. [Google Scholar]
- Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. International Journal of Obesity Related Metabolic Disorders. 2002;26(11):1459–1464. doi: 10.1038/sj.ijo.0802141. [DOI] [PubMed] [Google Scholar]
- Nakachi K, Eguchi H, Imai K. Can teatime increase one’s lifetime? Ageing Research Review. 2003;2 (1):1–10. doi: 10.1016/s1568-1637(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Nakachi K, Matsuyama S, Miyake S, Suganuma M, Imai K. Preventive effects of drinking green tea on cancer and cardiovascular disease: Epidemiological evidence for multiple targeting prevention. Biofactors. 2000;13 (1–4):49–54. doi: 10.1002/biof.5520130109. [DOI] [PubMed] [Google Scholar]
- Nakachi K, Suemasu K, Suga K, Takeo T, Imai K, Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Japanese Journal of Cancer Research. 1998;89(3):254–261. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldi L, Gallus S, Tavani A, Imberti GL, La Vecchia C. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. European Journal of Cancer Prevention. 2004;13(6):503–508. doi: 10.1097/00008469-200412000-00007. [DOI] [PubMed] [Google Scholar]
- Nance CL, Shearer WT. Is green tea good for HIV-1 infection? Journal of Allergy and Clinical Immunology. 2003;112 (5):851–853. doi: 10.1016/j.jaci.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Negishi H, Xu JW, Ikeda K, Njelekela M, Nara Y, Yamori Y. Black and green tea polyphenols attenuate blood pressure I ncreases in stroke-prone spontaneously hypertensive rats. Journal of Nutrition. 2004;134 (1):38–42. doi: 10.1093/jn/134.1.38. [DOI] [PubMed] [Google Scholar]
- Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (−)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. International Journal of Cancer. 2005;114 (4):513–521. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- Okushio K, Matsumoto N, Kohri T, Suzuki M, Nanjo F, Hara Y. Absorption of tea catechins into rat portal vein. Biological and Pharmaceutical Bulletin. 1996;19(2):326–329. doi: 10.1248/bpb.19.326. [DOI] [PubMed] [Google Scholar]
- Peters U, Poole C, Arab L. Does tea affect cardiovascular disease? A meta-analysis. American Journal of Epidemiology. 2001;154 (6):495–503. doi: 10.1093/aje/154.6.495. [DOI] [PubMed] [Google Scholar]
- Pezzato E, Sartor L, Dell’Aica I, Dittadi R, Gion M, Belluco C, Lise M, Garbisa S. Prostate carcinoma and green tea: PSA-triggered basement membrane degradation and MMP-2 activation are inhibited by (−) epigallocatechin-3-gallate. International Journal of Cancer. 2004;112 (5):787–792. doi: 10.1002/ijc.20460. [DOI] [PubMed] [Google Scholar]
- Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26 (5):958–967. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]
- Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. Journal of Nutritional Biochemistry. 2003;14(6):326–332. doi: 10.1016/s0955-2863(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Roomi MW, Roomi NW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Modulation of N-methyl-N-nitrosourea induced mammary tumors in Sprague-Dawley rats by combination of lysine, proline, arginine, ascorbic acid and green tea extract. Breast Cancer Research. 2005;7 (3):R291–295. doi: 10.1186/bcr989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudelle S, Ferruzzi MG, Cristiani I, Moulin J, Mace K, Acheson KJ, Tappy L. Effect of a thermogenic beverage on 24-hour energy metabolism in humans. Obesity (Silver Spring) 2007;15(2):349–355. doi: 10.1038/oby.2007.529. [DOI] [PubMed] [Google Scholar]
- Saleem M, Adhami VM, Ahmad N, Gupta S, Mukhtar H. Prognostic significance of metastasis-associated protein S100A4 (Mts1) in prostate cancer progression and chemoprevention regimens in an autochthonous mouse model. Clinical Cancer Research. 2005;11(1):147–53. [PubMed] [Google Scholar]
- Sasazuki S, Kodama H, Yoshimasu K, Liu Y, Washio M, Tanaka K, Tokunaga S, Kono S, Arai H, Doy Y, Kawano T, Nakagaki O, Takada K, Koyanagi S, Hiyamuta K, Nii T, Shirai K, Ideishi M, Arakawa K, Mohri M, Takeshita A. Relation between green tea consumption and the severity of coronary atherosclerosis among Japanese men and women. Annals of Epidemiology. 2000;10 (6):401–408. doi: 10.1016/s1047-2797(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Sato D. Inhibition of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine in rats by green tea. International Journal of Urology. 1999;6 (2):93–99. doi: 10.1046/j.1442-2042.1999.06239.x. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Gaziano JM, Buring JE, Hennekens CH. Coffee and tea intake and the risk of myocardial infarction. American Journal of Epidemiology. 1999;149 (2):162–167. doi: 10.1093/oxfordjournals.aje.a009782. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clinical Cancer Research. 2005;11 (7):2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- Shoji Y, Nakashima H. Glucose-lowering effect of powder formulation of African black tea extract in KK-A(y)/TaJcl diabetic mouse. Archives of Pharmacal Research. 2006;29(9):786–794. doi: 10.1007/BF02974080. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Zaman N, Aziz MH, Reagan-Shaw SR, Sarfaraz S, Adhami VM, Ahmad N, Raisuddin S, Mukhtar H. Inhibition of CWR22R{nu}1 tumor growth and PSA secretion in athymic nude mice by green and black teas. Carcinogenesis. 2005;27(4):833–839. doi: 10.1093/carcin/bgi323. [DOI] [PubMed] [Google Scholar]
- Stangl V, Lorenz M, Stangl K. The role of tea and tea flavonoids in cardiovascular health. Molecular Nutrition and Food Research. 2006;50(2):218–228. doi: 10.1002/mnfr.200500118. [DOI] [PubMed] [Google Scholar]
- Sueoka N, Suganuma M, Sueoka E, Okabe S, Matsuyama S, Imai K, Nakachi K, Fujiki H. A new function of green tea: prevention of lifestyle-related diseases. Annals of New York Academy of Sciences. 2001;928:274–280. doi: 10.1111/j.1749-6632.2001.tb05656.x. [DOI] [PubMed] [Google Scholar]
- Suganuma M, Ohkura Y, Okabe S, Fujiki H. Combination cancer chemoprevention with green tea extract and sulindac shown in intestinal tumor formation in Min mice. Journal of Cancer Research in Clinical Oncology. 2001;127 (1):69–72. doi: 10.1007/s004320000189. [DOI] [PubMed] [Google Scholar]
- Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and breast cancer risk: a meta-analysis of epidemiological studies. Carcinogenesis. 2006;27(7):1310–1315. doi: 10.1093/carcin/bgi276. [DOI] [PubMed] [Google Scholar]
- Takada M, Nakamura Y, Koizumi T, Toyama H, Kamigaki T, Suzuki Y, Takeyama Y, Kuroda Y. Suppression of human pancreatic carcinoma cell growth and invasion by epigallocatechin-3-gallate. Pancreas. 2002;25 (1):45–48. doi: 10.1097/00006676-200207000-00012. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Tanaka K, Hoshino K, Akiyama H, Tanimura A, Saito Y. Profiles of potentially antiallergic flavonoids in 27 kinds of health tea and green tea infusions. Journal of Agriculture and Food Chemistry. 1997;45 (7):2561–2564. [Google Scholar]
- Umemura T, Kai S, Hasegawa R, Kanki K, Kitamura Y, Nishikawa A, Hirose M. Prevention of dual promoting effects of pentachlorophenol, an environmental pollutant, on diethylnitrosamine-induced hepato- and cholangiocarcinogenesis in mice by green tea infusion. Carcinogenesis. 2003;24 (6):1105–1109. doi: 10.1093/carcin/bgg053. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan JB, Walle T. Glucuronidation and sulfation of the tea flavonoid (−)-epicatechin by the human and rat enzymes. Drug Metabolism and Disposition. 2002;30 (8):897–903. doi: 10.1124/dmd.30.8.897. [DOI] [PubMed] [Google Scholar]
- Wang M, Guo C, Li M. A case-control study on the dietary risk factors of upper digestive tract cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 1999;20(2):95–97. [PubMed] [Google Scholar]
- Wang ZY, Khan WA, Bickers DR, Mukhtar H. Protection against polycyclic aromatic hydrocarbon-induced skin tumor initiation in mice by green tea polyphenols. Carcinogenesis. 1989;10 (2):411–415. doi: 10.1093/carcin/10.2.411. [DOI] [PubMed] [Google Scholar]
- Weisburger JH. Tea and health: the underlying mechanisms. Proceedings of Society of Experimental Biology and Medicine. 1999;220 (4):271–275. doi: 10.1046/j.1525-1373.1999.d01-46.x. [DOI] [PubMed] [Google Scholar]
- Wiseman SA, Balentine DA, Frei B. Antioxidants in tea. Critical Reviews in Food Science and Nutrition. 1997;37(8):705–718. doi: 10.1080/10408399709527798. [DOI] [PubMed] [Google Scholar]
- Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Research. 2003b;63(21):7526–7529. [PubMed] [Google Scholar]
- Wu AH, Yu MC, Tseng CC, Hankin J, Pike MC. Green tea and risk of breast cancer in Asian Americans. International Journal of Cancer. 2003a;106(4):574–579. doi: 10.1002/ijc.11259. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ho CT, Amin SG, Han C, Chung FL. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Research. 1992;52 (14):3875–3879. [PubMed] [Google Scholar]
- Yamamoto T, Juneja LR, Chu DC, Kim M. Chemistry and Applications of Green Tea. CRC Press LLC; Boca Raton, USA: 1997. [Google Scholar]
- Yamane T, Nakatani H, Kikuoka N, Matsumoto H, Iwata Y, Kitao Y, Oya K, Takahashi T. Inhibitory effects and toxicity of green tea polyphenols for gastrointestinal carcinogenesis. Cancer. 1996;77 (8 Suppl):1662–1667. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1662::AID-CNCR36>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Yang CS, Chen L, Lee MJ, Balentine D, Kuo M, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiology Biomarkers and Prevention. 1998;7 (4):351–354. [PubMed] [Google Scholar]
- Yang CS, Wang ZY. Tea and cancer: review. Journal of the National Cancer Institute. 1993;85 (13):1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- Yang GY, Wang ZY, Kim S, Liao J, Seril DN, Chen X, Smith TJ, Yang CS. Characterization of early pulmonary hyperproliferation, tumor progression and their inhibition by black tea in a 4-(methylnitrosamino)-1-(3-pyridyl)-1 butanone (NNK)-induced lung tumorigenesis model with A/J mice. Cancer Research. 1997;57 (10):1889–1894. [PubMed] [Google Scholar]
- Yang M, Wang C, Chen H. Green, Oolong and black tea extracts modulate lipid metabolism in hyperlipidemia rats fed high-sucrose diet. Journal of Nutritional Biochemistry. 2001;12 (1):14–20. doi: 10.1016/s0955-2863(00)00140-6. [DOI] [PubMed] [Google Scholar]
- Zhong L, Goldberg MS, Gao YT, Hanley JA, Parent ME, Jin F. A population-based case-control study of lung cancer and green tea consumption among women living in Shanghai, China. Epidemiology. 2001;12(6):695–700. doi: 10.1097/00001648-200111000-00019. [DOI] [PubMed] [Google Scholar]
- Zhou JR, Yu L, Zhong Y, Blackburn GL. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. Journal of Nutrition. 2003;133 (2):516–521. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]


