Abstract
Objective To evaluate the association between non-alcoholic fatty liver disease and all cause and cause specific mortality in a representative sample of the US general population.
Design Prospective cohort study.
Setting US Third National Health and Nutrition Examination Survey (NHANES III: 1988-94) with follow-up of mortality to 2006.
Participants 11 371 adults aged 20-74 participating in the Third National Health and Nutrition Examination Survey, with assessment of hepatic steatosis.
Main outcome measure Mortality from all causes, cardiovascular disease, cancer, and liver disease (up to 18 years of follow-up).
Results The prevalence of non-alcoholic fatty liver disease with and without increased levels of liver enzymes in the population was 3.1% and 16.4%, respectively. Compared with participants without steatosis, those with non-alcoholic fatty liver disease but normal liver enzyme levels had multivariate adjusted hazard ratios for deaths from all causes of 0.92 (95% confidence interval 0.78 to 1.09), from cardiovascular disease of 0.86 (0.67 to 1.12), from cancer of 0.92 (0.67 to 1.27), and from liver disease of 0.64 (0.12 to 3.59). Compared with participants without steatosis, those with non-alcoholic fatty liver disease and increased liver enzyme levels had adjusted hazard ratios for deaths from all causes of 0.80 (0.52 to 1.22), from cardiovascular disease of 0.59 (0.29 to 1.20), from cancer of 0.53 (0.26 to 1.10), and from liver disease of 1.17 (0.15 to 8.93).
Conclusions Non-alcoholic fatty liver disease was not associated with an increased risk of death from all causes, cardiovascular disease, cancer, or liver disease.
Introduction
Non-alcoholic fatty liver disease is the leading cause of chronic liver disease in the United States and other Western countries, with a prevalence as high as 30% in the general population.1 The disease encompasses a wide spectrum of conditions, ranging from steatosis to non-alcoholic steatohepatitis, fibrosis, and cirrhosis.2 3 4 5 6 Although non-alcoholic fatty liver disease can lead to hepatocellular carcinoma7 8 and is associated with several cardiovascular risk factors,9 10 11 12 13 14 its impact on mortality is unknown. Results from studies on the association between non-alcoholic fatty liver disease and mortality have been inconsistent,15 16 17 18 19 20 but these studies were limited by the use of small, highly selected patient populations (for example, those after liver biopsy) or by the use of liver enzyme measurements as surrogate markers of non-alcoholic fatty liver disease.21 22 23 Furthermore, since non-alcoholic fatty liver disease is strongly associated with obesity and diabetes, whether it is an independent risk factor for all cause and cardiovascular death remains controversial, as many previous studies failed to account fully for adiposity.
We prospectively determined the association of non-alcoholic fatty liver disease with mortality in the US general population. We hypothesised that such disease would be associated with an increased risk of mortality from all causes, cardiovascular disease, cancer, and liver disease. We also hypothesised that the risk of death would be higher in participants with non-alcoholic fatty liver disease and increased liver enzyme levels, potentially representing the presence of non-alcoholic steatohepatitis.
Methods
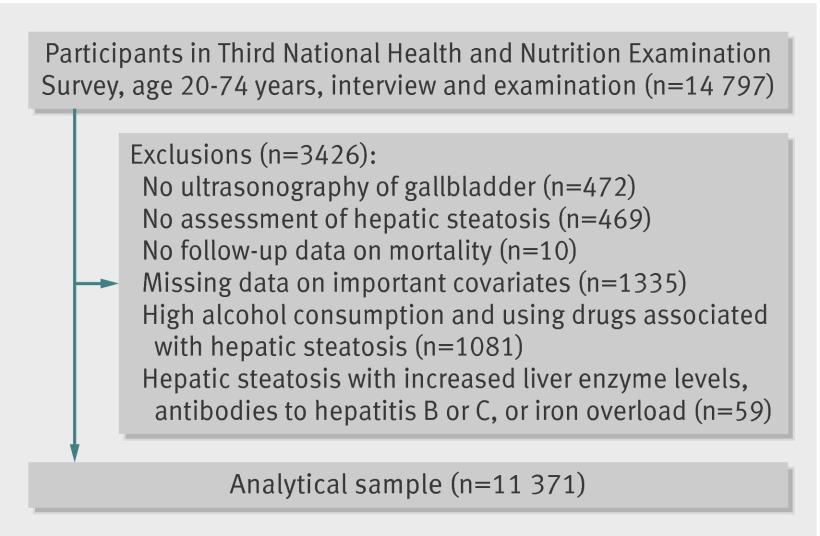
We used data from the Third National Health and Nutrition Examination Survey (NHANES III) and the Third National Health and Nutrition Examination Survey Mortality Follow-up studies. The Third National Health and Nutrition Examination Survey was carried out from 1988 to 1994 using a complex, multistage, stratified, clustered, probability sample design to obtain a representative sample of the US population living in households. The survey included an interview, physical examination, and laboratory measurements (including ultrasonography of the gallbladder) in 14 797 adults aged 20-74. The mortality follow-up study was a prospective study of the vital status of all participants aged 20 and older to December 2006. After exclusions, the analytical sample for the main analyses included 11 371 adults. All participants signed informed consent.
Data collection
The participants underwent an interview in which standardised questionnaires were used to obtain self reported data on sex, age, race or ethnicity, education, income, smoking, alcohol consumption, physical activity, prevalent medical conditions, and drug use.24 Smoking status was categorised as never, former, and current smoker. History of cardiovascular disease was defined as a self reported history of acute myocardial infarction, stroke, or heart failure. History of cancer was defined as a self reported history of any cancer. People were categorised as sedentary if they answered no to all the questions on engaging in any of the following activities over the past month: jog or run, cycle, swim, aerobics, other dancing, calisthenics, garden or yard work, weight lifting, or other sports. Average daily alcohol consumption was estimated by multiplying the number of drinking days over the past 12 months and the number of drinks, on average, on a drinking day and dividing by 365. Never drinkers replied no to the question: “In your entire life, have you had at least 12 drinks of any kind of alcoholic beverage?” Increased alcohol consumption was defined as one drink or more daily among women and two drinks or more daily among men.25
Standardised measurements of height, weight, waist circumference, and systolic and diastolic blood pressure were obtained. Body mass index was calculated.
Serum biochemistries were done using the Hitachi 737 automated multichannel chemistry analyser (Boehringer Mannheim Diagnostics, Indianapolis, IN).26 Liver tests determined levels of aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase, alkaline phosphatase, and total bilirubin. The levels of alanine aminotransferase or aspartate aminotransferase were considered raised if they were above the upper limit of normal of the National Health and Nutrition Examination Survey laboratory values (alanine aminotransferase >40 U/L for men and >31 U/L for women; aspartate aminotransferase >37 U/L for men and >31 U/L for women).26 The presence of antibodies to hepatitis C was tested using a second generation enzyme immunoassay (Abbott Laboratories, Chicago, IL) and confirmed by the MATRIX assay (Abbott Laboratories). Antibodies to hepatitis B core antigen were measured using a solid phase competitive immunoassay (Abbott Laboratories). Serum iron levels and total iron binding capacity were measured calorimetrically (Alpkem RFA analyzer, Clackamas, OR). Serum ferritin levels were measured using the BioRad Quantimmune IRMA kit (BioRad Laboratories, Hercules, CA). Serum transferrin saturation was calculated as [serum iron (μmol/L)/serum total iron binding capacity (μmol/L)]×100. Iron overload was defined as transferrin saturation greater than 45% and ferritin greater than 500 μg/L among men and greater than 400 μg/L among women.27 28
Assessment of hepatic steatosis
Ultrasound examinations on the gallbladder were carried out on the Third National Health and Nutrition Examination Survey participants aged 20 to 74 using a Toshiba (Tustin, CA) SSA-90A machine using 3.75 and 5.0 MHz transducer.29 Between 2009 and 2010, 13 856 (96.6%) of the archived videotapes on gallbladder ultrasound examinations originally obtained between 1988 and 1994 were reviewed to ascertain the presence of fat within the hepatic parenchyma. A more detailed description of the protocol can be found elsewhere.30 Briefly, information was recorded on the presence of liver to kidney contrast, degree of brightness of the liver parenchyma, presence of deep beam attenuation, presence of echogenic walls in the small intrahepatic vessels, and definition of the gallbladder walls. Based on these findings and using a standardised algorithm,30 we categorised the degree of steatosis first as a four level variable (none, mild, moderate, or severe steatosis) and then recoded as a two level variable: none to mild and moderate to severe. The intra-rater and inter-rater κ statistics for reliability of the two level variable were 0.77 (95% confidence interval 0.73 to 0.82) and 0.70 (0.64 to 0.76), respectively.30
In the absence of a standard definition, we defined non-alcoholic fatty liver disease as the presence of moderate to severe hepatic steatosis with normal liver enzymes levels. Non-alcoholic steatohepatitis was defined as the presence of moderate to severe hepatic steatosis with increased levels of liver enzymes, in the absence of antibodies to hepatitis B and hepatitis C and without evidence of iron overload. For the non-alcoholic steatohepatitis group we decided to exclude people with viral hepatitis B and hepatitis C or with iron overload because these factors are associated with increased liver enzyme levels.
Follow-up for mortality
To determine the vital status and cause of death, the National Center for Health Statistics linked all participants aged 20 years and older to the National Death Index to 31 December 2006.31 Therefore, for each participant follow-up extended from the date of the examination to the date of death or 31 December 2006.
Cause of death was determined from the underlying cause listed on the death certificate. We used the publicly available linked mortality data files, which identify the cause of death using the Underlying Cause of Death-113 (UCOD 113) groups (international classification of disease, 10th revision). The Underlying Cause of Death-113 variable was created by the National Center for Health Statistics to recode all deaths coded under ICD-9 (international classification of disease, ninth revision)—that is, deaths before 1999, into comparable ICD-10 groups to assist the researcher with analyses that span the entire survey period for mortality. The comparability of ICD-9 and ICD-10 underlying cause of death UCOD 113 recode has been validated (http://cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage.htm). We defined cardiovascular disease mortality as deaths with underlying cause of death codes: ICD-10 I00-I69. Cancer mortality was defined as deaths with underlying cause of death codes: ICD-10 C00-C-97. Finally, liver related mortality was defined as deaths with underlying cause of death codes: ICD-10 K-70, or K-73-K74.
Statistical analyses
We compared the baseline characteristics of participants by their hepatic steatosis status using Pearson χ2 statistics for categorical variables or Wald test for continuous variables. Cumulative mortality was estimated using Kaplan-Meier methods. Cox proportional hazards regression was used to estimate hazard ratios and 95% confidence intervals for deaths from all causes, cardiovascular disease, cancer, and liver disease by hepatic steatosis status, with age as the timescale and staggered entries into the study.32 We used two models with progressive degrees of adjustment: model 1 adjusted for sex and race or ethnicity; model 2 further adjusted for education, smoking, alcohol consumption, physical activity, body mass index, hypertension, hypercholesterolaemia, and diabetes.
We carried out extensive sensitivity analyses: firstly, using an alternative definition for non-alcoholic fatty liver disease that further excluded participants with hepatitis C, hepatitis B, or iron overload; secondly, including in the no steatosis group (reference) participants with high alcohol consumption or using zydovudine or didanosine but without hepatic steatosis; thirdly, excluding participants with a history of cardiovascular disease or with history of cancer from the analyses of cardiovascular death and cancer mortality; fourthly, excluding participants who died within the first year of follow-up for cancer mortality; and fifthly, using alcohol consumption defined by UK safe limits (14 drinks a week for women and 21 for men). Sixthly, to assess the potential of misclassification of exposure, we carried out another sensitivity analysis comparing the group with no hepatic steatosis (that is, excluding those with mild steatosis) against those with severe hepatic steatosis (that is, excluding those with moderate steatosis).
Finally we evaluated the association of non-alcoholic fatty liver disease and steatohepatitis with all cause mortality for subgroups defined by sex, age, race or ethnicity, smoking status, body mass index categories, diabetes, and raised γ-glutamyltransferase levels. All analyses were done using Stata 11 and using the svy commands to account for the sampling weights and the complex survey design.
Results
After exclusions (fig 1), the analytical sample for the main analyses included 11 371 adults aged 20-74 participating in the Third National Health and Nutrition Examination Survey, with assessment of hepatic steatosis. The overall prevalence of non-alcoholic fatty liver disease (excluding steatohepatitis) and steatohepatitis was 16.4% and 3.1%, respectively. Compared with people without hepatic steatosis, participants with non-alcoholic fatty liver disease were more likely to be older, men, Mexican-American, less educated, sedentary, obese, to have a high waist circumference, diabetes, hypercholesterolaemia, hypertension, and a history of cardiovascular disease, and less likely to be current smokers or to have low to moderate alcohol consumption (table 1). People with non-alcoholic fatty liver disease also had higher levels of glycated haemoglobin, triglyceride:high density lipoprotein cholesterol ratio, insulin resistance, and liver enzymes (alanine aminotransferase, aspartate aminotransferase, and γ-glutamyltransferase). People with non-alcoholic steatohepatitis were similar to those with non-alcoholic fatty liver disease, although slightly younger and with a slightly worse metabolic risk profile (table 1).
Fig 1 Flow of participants through study
Table 1.
Baseline characteristics of study sample from Third National Health and Nutrition Examination Survey (1988-94) by hepatic steatosis status. Values are percentages (standard errors) of participants unless stated otherwise
| Characteristics | No hepatic steatosis (n=8856) | Non-alcoholic fatty liver disease (n=2089) | Non-alcoholic steatohepatitis (n=426) | P value |
|---|---|---|---|---|
| Mean (SE) age (years) | 41.4 (0.4) | 48.3 (0.6) | 42.9 (1.2) | <0.001 |
| Men | 45.6 (0.7) | 52.4 (1.3) | 54.1 (4.5) | 0.001 |
| Race or ethnicity: | ≤0.001 | |||
| Non-Hispanic white | 76.4 (1.5) | 76.8 (1.7) | 71.8 (2.9) | |
| Non-Hispanic black | 10.9 (0.7) | 8.9 (0.9) | 5.9 (1.0) | |
| Mexican-American | 4.9 (0.4) | 6.2 (0.7) | 13.8 (2.0) | |
| <12 years education | 54.9 (1.2) | 64.8 (2.2) | 64.6 (4.1) | <0.001 |
| Body mass index: | <0.001 | |||
| <18.5 | 2.5 (0.3) | 1.6 (0.4) | 0.9 (0.8) | |
| 18.5-24.9 | 47.9 (1.1) | 17.6 (1.7) | 11.8 (2.7) | |
| 25-29.9 | 32.3 (0.6) | 36.1 (1.7) | 27.5 (4.6) | |
| 30-34.9 | 12.2 (0.5) | 25.8 (1.2) | 26.4 (3.4) | |
| ≥35 | 5.0 (0.6) | 19.0 (1.4) | 33.5 (4.2) | |
| High waist circumference* | 29.9 (1.0) | 66.2 (1.9) | 77.3 (4.0) | <0.001 |
| Diabetes† | 5.4 (0.4) | 15.8 (1.0) | 21.3 (3.5) | <0.001 |
| Hypertension‡ | 19.7 (0.8) | 35.7 (1.9) | 38.4 (4.4) | <0.001 |
| Hypercholesterolaemia§ | 28.5 (0.8) | 38.2 (2.0) | 48.3 (4.4) | <0.001 |
| Sedentary | 19.3 (0.9) | 26.4 (1.6) | 25.2 (3.6) | <0.001 |
| History of cardiovascular disease | 3.7 (0.3) | 8.2 (0.8) | 6.3 (1.5) | <0.001 |
| History of cancer | 6.1 (0.5) | 7.7 (0.9) | 6.3 (2.1) | 0.32 |
| Current smoking | 28.4 (0.9) | 22.7 (1.4) | 16.1 (3.8) | <0.001 |
| Alcohol consumption: | <0.001 | |||
| Never | 13.0 (0.7) | 15.9 (1.2) | 11.2 (2.1) | |
| Low-moderate | 87.0 (0.7) | 84.1 (1.2) | 88.8 (2.1) | |
| Mean (SE) glycated haemoglobin (%) | 5.3 (0.02) | 5.7 (0.04) | 6.0 (0.2) | <0.001 |
| Raised triglyceride levels¶ | 23.8 (1.1) | 52.6 (2.9) | 69.1 (5.8) | <0.001 |
| Mean (SE) triglycerides:high density lipoprotein cholesterol ratio¶ | 2.8 (0.06) | 4.7 (0.2) | 6.9 (0.9) | <0.001 |
| Insulin resistance¶** | 19.6 (0.9) | 54.4 (3.0) | 79.2 (5.7) | <0.001 |
| Mean (SE) alanine aminotransferase (U/L) | 16.3 (0.3) | 18.6 (0.4) | 54.1 (2.7) | <0.001 |
| Mean (SE) aspartate aminotransferase (U/L) | 20.4 (0.1) | 20.4 (0.1) | 44.1 (2.2) | <0.001 |
| Mean (SE) γ-glutamyltransferase (U/L††) | 25.0 (0.5) | 31.3 (1.0) | 79.1 (6.6) | <0.001 |
*>102 cm (men) and >88 cm (women).
†Self reported doctor diagnosis, drug use, fasting plasma glucose concentration ≥7.0 mmol/L or random plasma glucose concentration ≥11 mmol/L.
‡Self reported doctor diagnosis, drug use, systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.
§Self reported doctor diagnosis, drug use, or total cholesterol concentration >6.2 mmol/L.
¶Fasting (n=5066), and homeostatic model assessment-insulin resistance (HOMA-IR) >2.86 (above fourth quartile among people without diabetes).
**Among people without diabetes (n=4660).
††Among random subset of participants (n=8975).
The median follow-up was 14.5 (maximum 18.0) years. At the end of follow-up, the cumulative mortality from all causes was 22.0% (1836 deaths). For cause specific deaths the cumulative mortalities were 10.9% for cardiovascular disease (716 deaths), 6.0% for cancer (480 deaths), and 0.5% for liver disease (44 deaths).
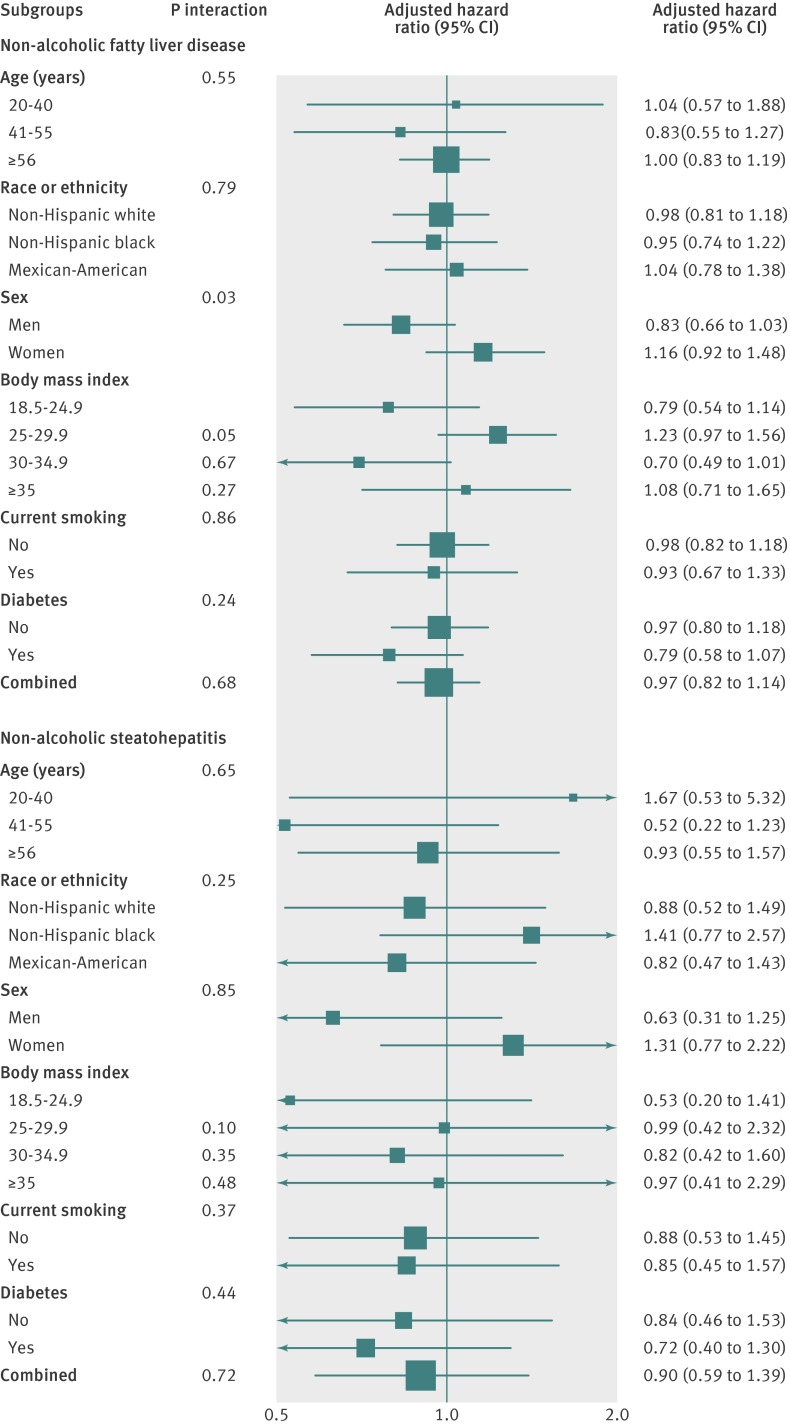
The risk of death from all causes in participants with non-alcoholic fatty liver disease or non-alcoholic steatohepatitis at baseline was not increased compared with participants without hepatic steatosis (table 2). After adjusting for sociodemographic characteristics, lifestyle risk factors, hypertension, and hypercholesterolaemia, the hazard ratios for death when participants with non-alcoholic fatty liver disease or non-alcoholic steatohepatitis were compared with those without hepatic steatosis were 0.91 (95% confidence interval 0.78 to 1.08) and 0.80 (0.53 to 1.22), respectively (table 2). In subgroups analyses, neither non-alcoholic fatty liver disease nor non-alcoholic steatohepatitis were associated with increased mortality in any subgroup (fig 2).
Table 2.
Risk of death from all causes, cardiovascular disease, cancer, and liver disease in people with non-alcoholic fatty liver disease and non-alcoholic steatohepatitis compared with those with no hepatic steatosis, Third National Health and Nutrition Examination Survey (1988-94)
| Unweighted No | No hepatic steatosis (n=8856) | Hazard ratio (95% CI) | |
|---|---|---|---|
| Non-alcoholic fatty liver disease (n=2089) | Non-alcoholic steatohepatitis (n=426) | ||
| All cause mortality: | |||
| No of events | 1310 | 469 | 57 |
| Model 1* | 1 (reference) | 1.04 (0.89 to 1.21) | 0.95 (0.62 to 1.47) |
| Model 2† | 1 (reference) | 0.92 (0.78 to 1.09) | 0.80 (0.52 to 1.22) |
| Cardiovascular disease mortality: | |||
| No of events | 508 | 192 | 16 |
| Model 1* | 1 (reference) | 1.08 (0.84 to 1.38) | 0.88 (0.43 to 1.80) |
| Model 2† | 1 (reference) | 0.86 (0.67 to 1.12) | 0.59 (0.29 to 1.20) |
| Cancer mortality: | |||
| No of events: | 350 | 116 | 14 |
| Model 1* | 1 (reference) | 0.91 (0.68 to 1.22) | 0.49 (0.25 to 0.95) |
| Model 2† | 1 (reference) | 0.92 (0.67 to 1.27) | 0.53 (0.26 to 1.10) |
| Liver disease mortality: | |||
| No of events: | 34 | 7 | 3 |
| Model 1* | 1 (reference) | 0.89 (0.20 to 4.01) | 1.67 (0.26 to 10.60) |
| Model 2† | 1 (reference) | 0.64 (0.12 to 3.59) | 1.17 (0.15 to 8.93) |
*Adjusted for sex and race.
†Further adjusted for education, smoking, alcohol consumption, physical activity, body mass index, hypertension, hypercholesterolaemia, and diabetes.
Fig 2 Subgroup analyses of association between non-alcoholic fatty liver disease and non-alcoholic steatohepatitis with all cause mortality
For cause specific mortality, the fully adjusted hazard ratios in people with non-alcoholic fatty liver disease or non-alcoholic steatohepatitis compared with those without hepatic steatosis were 0.86 (0.67 to 1.12) and 0.59 (0.29 to 1.20) for cardiovascular disease, 0.92 (0.67 to 1.27) and 0.53 (0.26 to 1.10) for cancer, and 0.64 (0.12 to 3.59) and 1.17 (0.15 to 8.93) for liver disease.
Similar findings resulted from sensitivity analyses excluding from the referent group those participants with prevalent cardiovascular disease, prevalent cancer, or deaths from cancer occurring during the first year of follow-up, further excluding participants with hepatitis C, hepatitis B, or iron overload from the non-alcoholic fatty liver disease group, or not excluding participants with high alcohol consumption or using antiretrovirals (see web extra tables 1 and 2). Sensitivity analysis with a different definition for high alcohol consumption according to the UK safe limits produced almost identical results (see web extra table 3). Finally, when participants with severe steatosis were compared with those without steatosis the fully adjusted hazard ratios for death from all causes were similar: 0.95 (0.73 to 1.25) and 0.75 (0.45 to 1.24), respectively (see web extra table 4).
Discussion
In this nationally representative study in the United States, we found no association between non-alcoholic fatty liver disease or non-alcoholic steatohepatitis and deaths from all causes, cardiovascular disease, cancer, or liver disease. These results are important given the high prevalence of non-alcoholic fatty liver disease in the general population and the concerns raised by the adverse metabolic profile associated with this disease and non-alcoholic steatohepatitis.
Comparison with other studies
Few studies have assessed the clinical course of non-alcoholic fatty liver disease and its impact on mortality. Retrospective studies of histology based non-alcoholic fatty liver disease and mortality have been inconsistent. One study reported an increased risk of death from all causes (standardised mortality ratio compared with general Danish population of 2.6) among 1804 patients who had been admitted to the hospital and had a discharge diagnosis of fatty liver; however, there was no adjustment for potential confounders.19 In contrast, two studies that used biopsy registries to identify cases of fatty liver disease found no evidence of an increased risk of death among patients with non-alcoholic fatty liver disease compared with the general population of either the United Kingdom or Denmark.16 20 These authors were not able to adjust for confounders either. More recently, a study followed up a cohort of 144 patients with biopsy confirmed non-alcoholic fatty liver disease referred for evaluation of persistently raised liver enzyme levels, thus representing a more severe spectrum of non-alcoholic fatty liver disease than the current study. Over 13.7 years of follow-up, patients with non-alcoholic fatty liver disease had similar survival to the general Swedish population (matched for age and sex), although the risk of death was increased in patients with non-alcoholic steatohepatitis.18 Finally, a study15 retrospectively identified 420 patients with non-alcoholic fatty liver disease and found a 34% increased risk of death in this group compared with the general Minnesota population over 7.6 years of follow-up. The highly selected nature of the patient population in these studies, including the use of patients with access to medical care who underwent liver biopsy as part of investigations, makes it difficult to draw firm conclusions about the impact of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis on mortality in the population as a whole.
To our knowledge only three studies have evaluated the association between non-alcoholic fatty liver disease and mortality in samples from the general population.21 22 23 These studies used data from the Third National Health and Nutrition Examination Survey, but all used liver enzyme levels as surrogate markers of non-alcoholic fatty liver disease, and follow-up extended only to 2000. These three studies used different definitions of non-alcoholic fatty liver disease (all based on liver enzyme levels) and most of them adjusted for potential confounders. One of these studies defined non-alcoholic fatty liver disease as a raised alanine aminotransferase level in the absence of high alcohol consumption or increased transferrin saturation, hepatitis B, or hepatitis C and found no increased risk for all cause mortality. Another study using a similar definition of non-alcoholic fatty liver disease further restricted the study to participants not taking a large number of potentially hepatotoxic drugs and reported a 37% increase in mortality for those with non-alcoholic fatty liver disease (P=0.07). Finally, another study found that people with raised levels of alanine aminotransferase in the absence of hepatitis B or hepatitis C were not at increased risk of death from all causes (hazard ratio of 1.2) or cardiovascular disease (hazard ratio of 0.90). All of these studies found an increased risk of liver related death for people with raised liver enzyme levels.
Contrary to our hypotheses and to current belief14 and despite a clear and strong association between non-alcoholic fatty liver disease and diabetes, insulin resistance, and obesity, our study did not find an association between non-alcoholic fatty liver disease or non-alcoholic steatohepatitis and deaths from all causes, cardiovascular disease, cancer, or liver disease. Although other outcomes related to non-alcoholic fatty liver disease, including cirrhosis, cancer, and a more rapid progression of coexistent liver diseases may be important, our findings support the suggestion that people with accumulation of fat in the liver have a good prognosis with respect to mortality. One speculation is that the ability of the liver to store triglycerides may in fact be a protection mechanism in obesity.33 In the absence of such a response, free fatty acids may accumulate and lead to more severe liver damage and systemic consequences.34
Strengths and limitations of the study
Ultrasonography is widely used to detect hepatic steatosis at the population level and, as shown in a recent meta-analysis, can accurately detect non-alcoholic fatty liver disease (pooled sensitivity of 84.8% and specificity of 93.6%).35 However, for non-alcoholic steatohepatitis in the absence of liver biopsy we used increases in the liver enzyme levels alanine aminotransferase or aspartate aminotransferase in the presence of steatosis to define non-alcoholic steatohepatitis. Although we ruled out other potential and common causes of raised liver enzyme levels, such as viral hepatitis, iron overload, and high alcohol consumption, we are aware that this definition has a limited sensitivity and specificity,36 and thus our results for non-alcoholic steatohepatitis should be interpreted with caution. Further studies with large samples and more refined diagnostic methods for non-alcoholic steatohepatitis are needed to determine the effect of steatohepatitis on mortality.
We had only a single measure of non-alcoholic fatty liver disease, which could not capture changes in hepatic steatosis over time, resulting in non-differential misclassification. We used data on education, smoking, alcohol consumption, and physical activity, but these data are generally collected based on self report. We had a limited sample size to study cause specific deaths associated with non-alcoholic fatty liver disease. Although the follow-up was longer than in previous studies, it may be too short to assess the effect of non-alcoholic fatty liver disease on liver related mortality, given the time it takes for liver disease to progress. Finally, we were unable to study the association between non-alcoholic fatty liver disease and non-fatal complications. In the future, larger multicollaborative studies, such as the Nonalcoholic Steatohepatitis-Clinical Research Network37 should be encouraged to comprehensively and extensively study the association between non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, and other non-fatal outcomes.
Our study also has notable strengths. The Third National Health and Nutrition Examination Survey and the associated mortality follow-up study represent the only nationally representative sample with results for liver imaging and long term mortality (up to 18 years). The participants are well characterised, with extensive laboratory and physical examination and measurements of hepatic steatosis. Finally, data were collected by trained staff following rigorous, standardised protocols to ensure that measurement errors were minimised.
Conclusions and implications
In conclusion, we found no association between ultrasound defined non-alcoholic fatty liver disease and deaths from all causes, cardiovascular disease, cancer, or liver disease in the US population. These results have important implications since non-alcoholic fatty liver disease is the most common liver disease in the population and is receiving considerable attention as an independent risk factor for cardiovascular disease.14 Since stage of the disease is likely to be a critical determinant of the overall prognosis, our findings need to be confirmed with improved non-invasive assessment tools and finer staging of non-alcoholic fatty liver disease in the general population. None the less, our results indicate that although non-alcoholic fatty liver disease is strongly associated with cardiovascular risk factors, such patients are not at increased risk of death from all causes or cardiovascular disease.
What is already known on this topic
Non-alcoholic fatty liver disease is highly prevalent and frequently encountered in clinical practice
There is a strong association between non-alcoholic fatty liver disease and cardiovascular risk factors such as diabetes and obesity
The association between non-alcoholic fatty liver disease and mortality remains controversial
What this study adds
Non-alcoholic fatty liver disease was not associated with an increased risk of deaths from all causes, cardiovascular disease, cancer, or liver disease in a general population sample over an average of 14.5 years of follow-up
Non-alcoholic steatohepatitis (hepatic steatosis with raised liver enzyme levels, without hepatitis B and hepatitis C antibodies or iron overload) was also not associated with mortality end points
The analysis was limited by small numbers and by potential measurement error in the definition of steatohepatitis
We thank Lisa Broitman and Mark S Eberhardt, at the National Center for Health Statistics, Center for Disease Control, Hyattsville, Maryland, for substantial administrative and logistical assistance with this project.
Contributors: ML, RH, IRK, FLB, and JMC conceived and designed the study. ML, RH, and SB acquired the data. ML, RH, EG, FLB, and JMC analysed and interpreted the data. ML and RH drafted the manuscript. ML, RH, ME, SB, EG, and JMC critically revised the manuscript for important intellectual content. ML and RH carried out the statistical analysis. ML, RH, SB, EG, and JMC obtained the funding. ML and RH provided technical or material support. EG and JMC supervised the study. ML and RH contributed equally to this work and are the guarantors.
Funding: This research was supported by grant R01 DK083393-01 from the National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases. ML, RH, and FLB were supported by the American Diabetes Association. These sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: support from the National Institutes of Health for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the Centers for Disease Control and Prevention institutional review board.
Data sharing: No additional data available.
Cite this as: BMJ 2011;343:d6891
Web Extra. Extra material supplied by the author
Results of sensitivity analyses
References
- 1.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis 2008;28:339-50. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005;42:132-8. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology 1999;29:664-9. [DOI] [PubMed] [Google Scholar]
- 4.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA 2003;289:3000-4. [DOI] [PubMed] [Google Scholar]
- 5.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol 2003;98:2042-7. [DOI] [PubMed] [Google Scholar]
- 6.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413-9. [DOI] [PubMed] [Google Scholar]
- 7.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972-8. [DOI] [PubMed] [Google Scholar]
- 8.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134-40. [DOI] [PubMed] [Google Scholar]
- 9.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol 2008;49:600-7. [DOI] [PubMed] [Google Scholar]
- 10.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005;42:473-80. [DOI] [PubMed] [Google Scholar]
- 11.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 2007;13:1579-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 2005;54:3541-6. [DOI] [PubMed] [Google Scholar]
- 13.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007;30:2119-21. [DOI] [PubMed] [Google Scholar]
- 14.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341-50. [DOI] [PubMed] [Google Scholar]
- 15.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113-21. [DOI] [PubMed] [Google Scholar]
- 16.Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sorensen TI, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut 2004;53:750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol 2009;44:1236-43. [DOI] [PubMed] [Google Scholar]
- 18.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865-73. [DOI] [PubMed] [Google Scholar]
- 19.Jepsen P, Vilstrup H, Mellemkjaer L, Thulstrup AM, Olsen JH, Baron JA, et al. Prognosis of patients with a diagnosis of fatty liver—a registry-based cohort study. Hepatogastroenterology 2003;50:2101-4. [PubMed] [Google Scholar]
- 20.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology 1995;22:1714-9. [PubMed] [Google Scholar]
- 21.Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol 2008;103:2263-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 2008;49:608-12. [DOI] [PubMed] [Google Scholar]
- 23.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009;136:477-85. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-1994. Department of Health and Human Services publication No (PHS) 94-1308. Vital and health statistics. Series 1. No 32, 1994.
- 25.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003;98:960-7. [DOI] [PubMed] [Google Scholar]
- 26.Laboratory procedures used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Environmental Health, National Center for Health Statistics, 1996.
- 27.Edwards CQ, Griffen LM, Goldgar D, Drummond C, Skolnick MH, Kushner JP. Prevalence of hemochromatosis among 11,065 presumably healthy blood donors. N Engl J Med 1988;318:1355-62. [DOI] [PubMed] [Google Scholar]
- 28.Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW. A population-based study of the clinical expression of the hemochromatosis gene. N Engl J Med 1999;341:718-24. [DOI] [PubMed] [Google Scholar]
- 29.Third National Health and Nutrition Examination Survey: gallbladder ultrasonography procedure manual. Westat, 1988.
- 30.Third National Health and Nutrition Examination Survey: hepatic steatosis assessment procedure manual. National Center for Health Statistics, 2010.
- 31.NHANES III Mortality Follow-Up. National Center for Health Statistics Center for Disease Control and Prevention. 2010. Available from www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage.htm.
- 32.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 1997;145:72-80. [DOI] [PubMed] [Google Scholar]
- 33.Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr Opin Lipidol 2008;19:295-300. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi K. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007;45:1366-74. [DOI] [PubMed] [Google Scholar]
- 35.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011; May 26. 10.1002/hep.24452. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286-92. [DOI] [PubMed] [Google Scholar]
- 37.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010;52:913-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of sensitivity analyses