Abstract
Objective
Abdominal aortic aneurysms (AAAs) are associated with fragmentation of extracellular matrix (ECM) during development of aortic dilation and rupture. Therefore, it is important to identify specific protease systems involved in ECM degradation during AAA formation. The present study determined the contribution of the urokinase system to AAA formation and rupture.
Methods and Results
AngII-induced AAAs were associated with increased aortic abundance of both uPAR and uPA proteins. However, this increased presence was unrelated to AAA formation since deficiencies of either uPAR or uPA had no effect on either the incidence or size of AngII-induced AAAs in both normolipidemic mice and LDL receptor −/− mice fed a saturated fat-enriched diet. Although uPA deficiency did not affect development of AAAs, there was an effect to increase mortality rate from AAA rupture in hypercholesterolemic mice. Bone marrow transplantation demonstrated enhanced aneurysmal rupture was attributable to deficiency of uPA in leukocytes. uPA deficiency led to an increased propensity for impaired resolution of the thrombotic material within the aneurysmal tissue. Neither uPAR nor uPA deficiency had any effect on AngII-induced atherosclerosis in LDL receptor −/− mice.
Conclusion
The uPA-uPAR axis has no effect on the formation of AngII-induced AAAs, but uPA deficiency promotes aneurysmal rupture.
Keywords: aneurysm, angiotensin II, urokinase type plasminogen activator, fibrinogen, rupture
INTRODUCTION
Abdominal aortic aneurysms (AAAs) are permanent dilations that are associated with marked changes in the arterial tissue including accumulation of several types of leukocytes and fragmentation of extracellular matrix (ECM).1 Destruction of elastin and collagen is required for aortas to dilate and rupture. Several classes of proteases have been inferred in the degradation of matrix fibers, including metalloproteinases (MMPs), cathepsins, and serine proteases.2 Many of these proteases have been detected in aneurysmal tissue from humans and experimental animal models.3–5 However, there is uncertainty regarding the functional roles of these proteases in the disease process and whether they differ in the initiation, progression, and rupture phases of aneurysmal development.
Many laboratories have demonstrated that chronic subcutaneous AngII infusion into mice promotes AAAs.5–7 The prominent aortic lumen expansion that occurs during AngII infusion is due to medial rupture within the first 10 days of infusion.8 Days following the medial rupture, some mice succumb to AAA rupture.8 Thrombi form at the site of medial rupture and provoke pronounced macrophage recruitment. This region becomes highly heterogeneous in gross appearance, with large variances of cellular and extracellular matrices within the aneurysmal region. Remodeling of the aneurysmal tissue occurs with thickening of aortic walls in regions that are distal and proximal to the pronounced lumen expansion. Therefore, like the human disease, development of AngII-induced AAAs requires destruction of elastin and collagen to enable lumen expansion.9 Furthermore, it is likely that proteases are required to enable the compositional changes that are characteristic of the progression and rupture of AAAs during AngII infusion.
Urokinase-type plasminogen activator (uPA) is a serine protease that is initially secreted as an inactive single chain polypeptide. Although macrophages are a major source of uPA, it is also secreted by many other cell types present in AAAs, including endothelial cells, smooth muscle cells, and fibroblasts. uPA is cleaved by plasmin to form an active two-chain molecule that is linked by two disulphide bridges. This active form promotes the cleavage of plasminogen to plasmin. This activation may be localized and enhanced by uPA interaction with a specific GPI-linked receptor, uPAR, although uPA can be activated independent of interaction with uPAR.10 The generated plasmin may cleave extracellular matrix both directly and indirectly via MMP activation.
A single study has reported that uPA deficiency attenuates AngII-induced AAAs in both apoE −/− mice and wild type C57BL/6 mice.11 Currently, no study has addressed either the role of uPAR or the source of uPA and uPAR involved in AngII-induced AAAs. We found that AngII infusion promoted an increased abundance of uPAR in aneurysmal tissue. Therefore, we hypothesized that deficiency of uPAR would attenuate AngII-induced AAAs in LDL receptor −/− mice. However, uPAR deficiency had no measurable effects on any facet of the aneurysmal process. Moreover, contrary to the previous publication,11 uPA deficiency had no effect on the rate of formation of AAAs, but enhanced death due to aneurysmal rupture. Deficiency of uPA in bone marrow-derived cells also enhanced mortality due to AAA rupture. Therefore, the uPA-uPAR axis had no discernable effect on formation of AngII-induced AAAs, but uPA deficiency in leukocytes enhanced the fatal consequences of AAA formation through a uPAR-independent process.
METHODS
For detailed Materials and Methods please see Supplemental Materials that are provided online at atvb.ahajournals.org.
Mice
LDL receptor −/− , apoE −/−, uPA −/− , uPAR −/− and C57BL/6 mice were purchased from The Jackson Laboratory. All study procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Mouse Genotyping
Genotypes of mice were confirmed by PCR of genomic DNA.
Diet and AngII Infusion
Mice were fed a saturated fat-enriched diet and infused with saline or AngII (1,000 ng/kg/min) as described previously.6
Bone Marrow Transplantation
Bone marrow transplantation was performed as described previously.12,13
Blood Pressure Measurements
Systolic blood pressure was measured using a tail-cuff system as described previously.14
Cholesterol and uPA Concentration Measurements
Serum cholesterol concentrations and plasma uPA concentrations were measured as described in the Online Supplemental Materials.
Atherosclerosis and Aneurysm Analysis
Atherosclerosis and aneurysm analysis was quantified as described previously.15,16
Gene Array
Gene array was performed according to manufacturer’s protocol as described in the Online Supplemental Materials.
Zymography
Zymography of MMP-2, MMP-9, and uPA was performed as described previously.5
Western Blot Analyses
Western blotting of uPAR, uPA, and β-actin was performed as described previously.17
Histology and Immunostaining
Abdominal aortic sections were stained for elastin or collagen, or immunostained for fibrinogen and macrophages as described previously.18
Statistics
Detailed statistic analyses are described in the Online Supplemental Materials.
RESULTS
Relative Aortic Abundance of uPAR and uPA in Hypercholesterolemic Mice
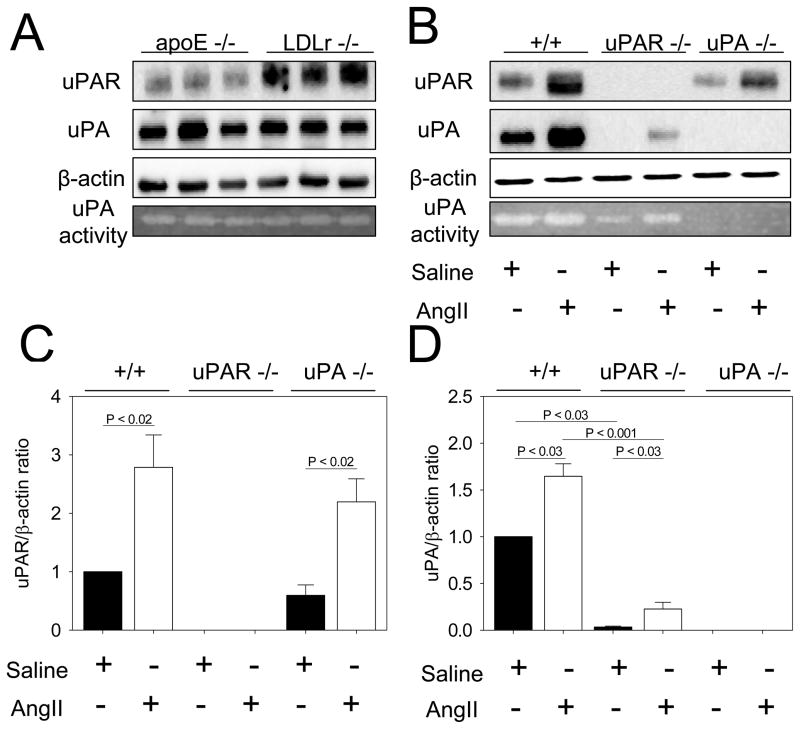
To examine the relative expression of uPAR and uPA, aortic tissues were extracted from LDL receptor −/− and apoE −/− mice prior to AngII infusion. Interestingly, Western blots demonstrated that apoE −/− mice contained less uPAR protein in aortas as compared to LDL receptor −/− mice (Figure 1A). uPAR and apoE genes are closely linked on chromosome 7 that provides an impediment to the generation of mice with compound deficiencies of the two genes. Therefore, we used mice in an LDL receptor −/− background to define the role of uPAR in AngII-induced AAA formation in the present study. Accordingly, the effect of uPA in AngII-induced AAAs was determined in the LDL receptor −/− mice. A previous publication has described the role of uPA in apoE −/−mice rather than LDL receptor −/− mice used in this study.11 We found there was no difference in expression and activity of uPA between apoE−/− and LDL receptor −/− mice (Figure 1A).
Figure 1. Aortic abundance of uPAR and uPA.
A. uPA and uPAR proteins were detected by Western blotting of suprarenal aortic extracts from apoE −/− and LDL receptor −/− mice. β-actin was shown as loading control. Plasminogen zymography was performed to detect uPA activity. B. uPA and uPAR proteins were detected by Western blotting in protein extracts from aneurysmal tissues from LDL receptor −/− mice infused with either saline or AngII for 14 days that were wild type for both uPAR and uPA, uPAR −/−, or uPA −/−. β-actin was shown as loading control. Plasminogen zymography was performed to detect uPA activity after 14 days of AngII infusion. C. Quantification of uPAR and uPA protein abundances in suprarenal aortic tissue excised from LDL receptor −/− mice infused with AngII for 14 days that were wild type for uPAR or uPA, uPAR −/−, or uPA −/−. Images in A and B are representative of more than 4 independent experiments.
AngII Increased uPAR and uPA in Aneurysmal Tissue
To determine whether uPAR and uPA were increased in aneurysmal tissue of AngII-infused LDL receptor −/− mice, aortic tissue was extracted after 14 days of infusion with either saline or AngII. Western blots detected the presence of uPA and uPAR protein after 14 days of either saline or AngII infusion (Figure 1B).
Genotypes of uPAR and uPA deficient mice were phenotypically confirmed using Western blotting of aortic extracts. In addition, uPA genotypes were confirmed by the absence of uPA in plasma using an ELISA (data not shown) and by zymography for uPA activity in aortic extracts (Figure 1B). Quantification of Western blots demonstrated that uPA deficiency did not affect aortic uPAR protein abundance (Figure 1C); while AngII infusion equivalently increased uPAR abundance in both uPA +/+ and −/− mice. In contrast, the uPA protein in aortas was more abundant in uPAR +/+ than in uPAR −/− mice (Figure 1D). There was an increase of uPA in aortas extracted from mice infused with AngII compared to those infused with saline in both uPAR +/+ and −/−mice. Consistent with the finding in aortic tissues, uPA protein in macrophages was more abundant in uPAR +/+ mice than in uPAR −/− mice (supplemental Figure I). In contrast to the increase in aortic tissues, uPA protein abundance was not changed in macrophages after AngII infusion in uPAR −/− mice.
uPAR Deficiency Had No Effect on AngII-induced AAAs
To determine whether uPAR had a functional role in AAA development, either saline or AngII was infused for 28 days into age-matched male LDL receptor −/− mice that were either uPAR +/+ or −/−. uPAR deficiency imparted a modest decrease of serum cholesterol concentrations and had no effect on systolic blood pressure during AngII infusion (supplemental Table II).
uPAR deficiency had no effect on diameter of the suprarenal aorta in mice infused with either saline or AngII (Figure 2A). Also, there was no change in the incidence of AngII-induced AAAs in uPAR +/+ mice (50%; 9/18) versus −/− mice (61%; 11/18). The incidence of aortic rupture was 24% (5/21) in uPAR +/+ and 11% (2/19) in −/− mice (Figure 2B) and the percent survival rate over time of infusions did not attain statistical significance during the 28 days of AngII infusion (Figure 2C). In addition to AAA formation, we determined the role of uPAR in atherosclerosis and found that deficiency of uPAR had no effect on the area of aortic atherosclerotic lesions (Figure 2D).
Figure 2. uPAR deficiency had no effect on formation of AngII-induced AAAs.
A. Maximal widths of suprarenal aortas were measured after 28 days of infusion of either saline or AngII. Circles and inverted triangles represent values from individual mice, diamonds represent means, and bars are SEMs. B. Mortality due to AAA rupture in AngII-infused uPAR +/+ or −/−mice. C. uPAR deficiency had no effect on survival rates during 28 days of AngII infusion. Percent survival curve with closed and open diamonds represent uPAR +/+ and −/− mice, respectively. D. Aortic atherosclerosis was not influenced by uPAR deficiency. The areas of grossly discernable atherosclerotic lesions were determined on aortic arches of LDL receptor −/− mice that were either uPAR +/+ or −/−. The areas for each individual mouse are represented by inverted triangles; diamonds represent means, and bars are SEM.
uPA Deficiency Did Not Influence AngII-induced AAAs Formation, but Increased AAA Rupture
uPA deficiency had no effect on diet-induced increases in serum cholesterol concentration or AngII-induced increases in SBP in age-matched male LDL receptor -/-mice (supplemental Table III).
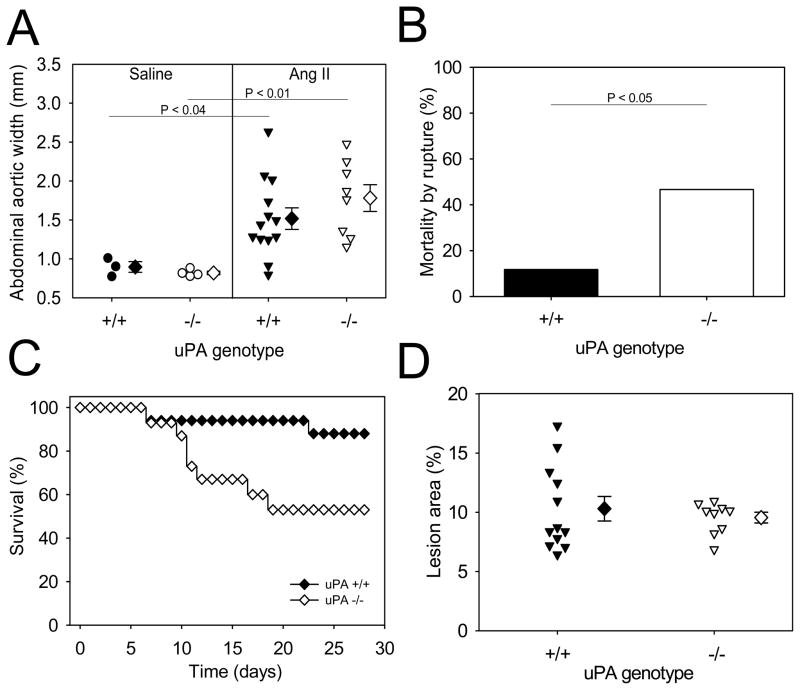
uPA deficiency did not alter the diameters of suprarenal aortas of saline-infused mice. Of the mice that survived 28 days of AngII infusion, there was no significant difference in diameters of suprarenal aortas between uPA +/+ and −/− mice (Figure 3A). However, a significantly greater number of uPA deficient mice (7/15, or 47%) succumbed to AAA rupture compared to uPA wild type mice (2/17, or 12%) during 28 days of AngII infusion (P< 0.05; Figure 3B). There was no significant difference between the groups of mice in the combined endpoint of aneurysm, or death due to aneurysmal rupture in uPA+/+ (88%; 15/17) and uPA−/− mice (93%;14/15). However, there was a significant difference in survival rates between uPA +/+ and −/− mice during 28 days of AngII infusion (P<0.05; Figure 3C). In addition, uPA deficiency had no effect on areas of atherosclerotic lesions (Figure 3D). Our data from uPA deficient mice that were deficient in LDL receptors contrast markedly with a previous study reporting a protective effect of uPA deficiency against AngII-induced AAAs in apoE −/−mice.11
Figure 3. uPA deficiency had no effect on development of AngII-induced AAAs, but increased mortality by AAA rupture.
A. Maximal widths of suprarenal aortas were measured after 28 days of infusion of either saline or AngII. Circles and inverted triangles represent values from individual mice, diamonds represent means, and bars are SEMs. B. Mortality due to AAA rupture in AngII-infused uPA +/+ or −/− mice. C. uPA deficiency significantly decreased the survival rate during 28 days of AngII infusion (P<0.05). Percent survival curves with closed and open diamonds represented wild type and uPA −/−, respectively. D. Aortic atherosclerosis was not influenced by uPA deficiency. The areas of grossly discernable atherosclerotic lesions were determined in aortic arches of LDL receptor −/− mice that were either uPAR +/+ or −/−. Areas for each individual mouse are represented by inverted triangles; diamonds represent means, and bars are SEM.
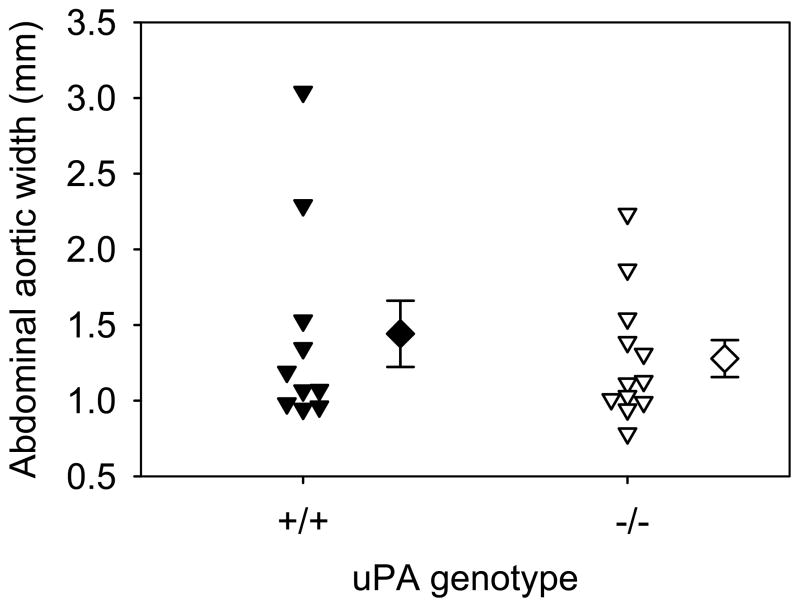
The previous study also studied AngII-induced AAAs in normocholesterolemic C57BL/6 mice.11 AAAs that form from AngII-infusion in normocholesterolemic mice appear grossly similar to those formed in either apoE −/− or LDL receptor −/− mice, although with much lower incidence. uPA deficiency was shown previously to reduce AngII-induced AAAs in normocholesterolemic mice.11 Therefore, we infused AngII into male C57BL/6 mice that were either uPA +/+ or −/−. AngII infusion generated an equivalent increase in SBP in the 2 groups (supplemental Table IV). Unlike the previous publication, uPA deficiency had no significant effect on AngII-induced increases of suprarenal diameter (Figure 4). There was also no significant difference in the incidence of AngII-induced AAA formation in uPA +/+ (45%;5/11) and uPA−/− mice (42%; 5/12), or death due to AAA rupture (+/+ = 0 versus −/− = 1 mouse).
Figure 4. uPA deficiency in normocholesterolemic mice had no effect on the development of AngII-induced AAAs.
Maximal widths of suprarenal aortas after 28 days of AngII infusion. Circles and inverted triangles represents values from individual mice, diamonds represent means, and bars are SEMs.
uPA deficiency in Bone Marrow-derived Cells Increased AAA Rupture
Since uPA is highly expressed in macrophages, we performed a bone marrow transplantation procedure to ablate uPA expression in cells of this origin to determine their role in aneurysmal rupture. LDL receptor −/− x uPA −/− recipient mice were irradiated and repopulated with bone marrow-derived stem cells harvested from either uPA +/+ or −/− mice that were deficient in LDL receptors.
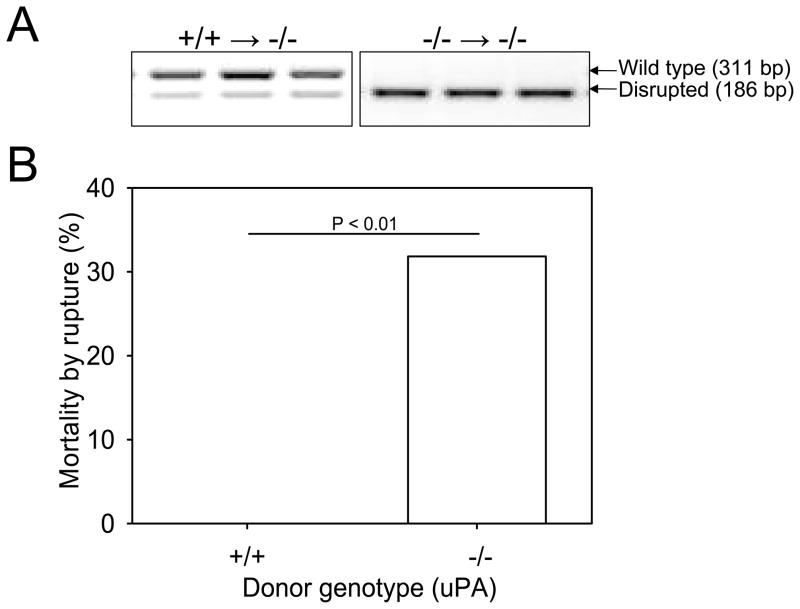
The chimerism was confirmed by PCR of bone marrow-derived DNA at the termination of the study (Figure 5A). Plasma concentrations of uPA were undetectable in irradiated uPA −/− recipient mice, irrespective of the uPA donor genotype. Also, the uPA genotype of donor cells did not influence the blood leukocyte count, serum cholesterol concentrations, or SBP (supplemental Table V).
Figure 5. uPA deficiency in bone marrow-derived cells was responsible for increased mortality by AAA rupture.
A. PCR confirmed the genotypes of uPA gene in bone marrow-derived cells harvested from chimeric mice. PCR on bone marrow-derived DNA yielded amplicons of 311 and 186 bp for wild-type and disrupted uPA alleles, respectively. B. Percent mortality due to AAA rupture in uPA −/− x LDL receptor −/− recipient mice was increased in mice repopulated with donor cells from uPA −/−mice (0%; 0/24 versus 32%; 7/22, for uPA +/+ versus −/− donor cells, respectively: P=0.009).
Bone marrow transplantation led to a lower incidence of AngII-induced AAAs compared to non-irradiated mice, as noted previously.12 Rupture was not observed in mice repopulated with cells from uPA +/+ mice (0/24, or 0%). However, there was a significant increase in death due to AAA rupture in mice repopulated with cells from uPA −/− mice (7/22, or 32%; P= 0.009; Figure 5B).
Compositional Analyses of Aortic Tissue
To define the cellular and molecular mechanisms of increased rupture of AngII-induced AAAs in uPA −/− mice, first we examined the histological features of the aneurysm-prone region of the suprarenal aorta from wild type, uPAR −/−, and uPA −/−mice prior to AngII infusion. There were no discernable differences in dimensions or compositions in serial sections of aortas prior to AngII infusion (supplemental Figure II).
The enhanced aortic rupture in uPA −/− mice may relate to changes in proteolysis during the early phase of AAA formation that affect tissue stability or thrombus resolution. To determine whether there was any difference in AAA tissues that would provide insight into enhanced aortic rupture during AngII infusion in uPA −/− mice, we acquired suprarenal aortas in the early stage of AAA development (7 days). For some of the tissues, RNA was isolated and a gene array was performed to determine mRNA abundance for several genes related to extracellular matrix and cellular adhesion. However, we were unable to discern any overt differences in mRNA abundances of these genes in suprarenal aortic tissues from uPA +/+ versus −/− mice (supplemental Table VI). The wide variances of mRNAs abundance is likely linked to the heterogeneity of AngII-induced AAA tissues. Furthermore, the necessity to analyze whole tissue compromises the ability to detect localized changes that are likely to underlie the mechanisms of rupture. Hence, we also performed immunostaining on suprarenal aortic tissues after 7 days of AngII infusion. Since macrophage presence has been implicated in thrombi resolution, we immunostained for this cell type. We were unable to discern any overt differences in macrophages present in aneurysmal thrombi from tissues between uPA +/+ and −/− mice. The predominance of thrombi at this early interval of AngII infusion provided an impediment to serial sectioning of aneurysmal tissue that might have negated quantitative assessment of macrophages as we reported recently.19
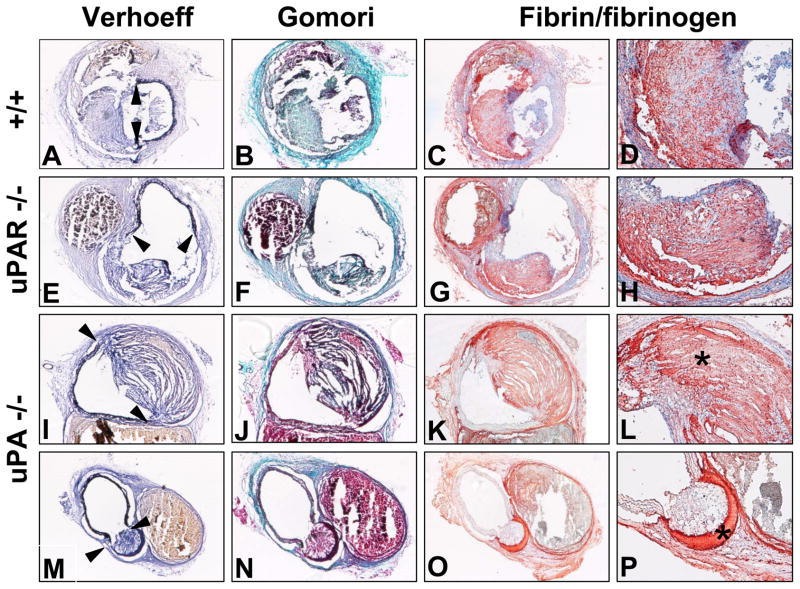
Since we were unable to discern any differences in the early phase of AAA development, we also determined whether there were any morphological differences of AAAs between uPA +/+ or −/− mice after 28 days of AngII infusion. We examined the elastin fragmentation and media rupture occurring in LDL receptor −/− mice that were wild type, uPA −/−, or uPAR −/− genotypes, (indicated by arrows), that resulted in marked luminal dilation (Figure 6A,E,I,M). This area included thrombi surrounded by ECM with a predominant collagen content that was similar among all groups (Figure 6B,F,J,N). There was strong immunostaining for fibrin/fibrinogen in all AAAs. Counterstaining with hematoxylin demonstrated infiltration of cells into thrombi of wild type and uPAR −/− mice (Figure 6C,D,G,H). In contrast, thrombi in AAAs of uPA −/− mice were acellular with minimal nuclei staining (Figure 6K,L,O,P). Despite these compositional changes, neither uPA nor uPAR deficiency led to significant changes in latent or active forms of both MMP-2 and MMP-9 in aneurysmal tissue (supplemental Figure III).
Figure 6. Deficiency of uPA altered the composition of aneurysmal tissue.
Examples are shown for mice with the following genotypes: A-D. LDL receptor −/−. E-H. uPAR −/− x LDL receptor −/−. I-P. uPA −/− x LDL receptor −/−. A, E, I, M. Verhoeff staining (40X). B, F, J, N. Gomori staining (40X). C, G, K, O. Fibrin/fibrinogen staining (40X). D, H, L, P. Fibrin/fibrinogen staining (100X). * Represents a typical acellular fibrin-rich thrombi area.
DISCUSSION
AngII-induced AAAs form rapidly and exhibit large luminal expansions due to transmural fragmentation of ECM.8,20 The mechanisms of the proteolytic events involved in the initiation and propagation of AngII-induced AAAs are undefined. However, one candidate system has been the uPA-uPAR axis. Despite the hypothetical involvement of this system in AngII-induced AAAs, we found that deficiency of either uPA or uPAR had no discernable effect on development of the pathology, as defined by the incidence of the disease. In contrast, uPA deficiency in leukocytes led to increases in adverse consequences of aneurysm formation with increased mortality due to enhanced rupture of AAAs.
Both the initial dilation and rupture phase of AAA formation involve degradation of ECM. Several proteases that degrade ECM have been detected in aneurysmal tissue promoted by AngII infusion. These include uPA, MMP-2, -9, and -14.5,21,22 uPA is the only one of these proteases to have been studied in deficient mice.11 uPAR facilitates the localized activation of uPA to enable plasminogen to be cleaved to plasmin that may promote ECM degradation. The presence of uPAR facilitates macrophage infiltration into arterial walls of apoE −/− mice.23 However, it is clear that uPAR is not an essential requirement for uPA activity. For example, unlike uPA deficiency, deletion of uPAR failed to influence healing in response to electrical arterial injury.10 In addition, uPAR deficiency does not affect the response of uPA to vascular injury, indicating that uPA has a uPAR-independent role on vascular remodeling after arterial injury.10 In the present study, we demonstrated a lack of effect of uPAR deficiency on AngII-induced AAAs in LDL receptor −/− mice.
Given the failure of uPAR deficiency to influence development of AngII-induced AAAs in LDL receptor −/− mice, we assessed the effects of uPA deficiency that have been reported previously.11 The present study failed to demonstrate a significant difference of uPA deficiency on AAA development in LDL receptor −/− mice that were strain-, gender-, and age-matched with wild type mice. Additionally, we verified the presence or absence of uPA protein to confirmed the genotype of the mice. We also confirmed the previous observation that uPA increased in AngII-induced aneurysmal tissue.5 Our finding that uPA had no effect on development of AngII-induced AAAs contrasts sharply with the previous publication of Deng et al.11 In their study, uPA deficiency in apoE −/− mice led to a reduction of AngII-induced AAA incidence from 90% (9 of 10) to 22% (2 of 9). One obvious difference is the contrast of apoE versus LDL receptor deficiency to induce hypercholesterolemia. While there have not been many direct comparisons between the mechanisms of AngII-induced AAA formation in the 2 strains, there is no obvious difference in the pathological characteristics of the aneurysmal tissue.5,6,8,24 Also, there are no overt differences of AngII-induced AAAs between LDL receptor −/− and apoE −/− mice with respect to location of formation, incidence, rate of onset, rupture rate, size, and gender preference.6,12 Although, apoE-uPA compound deficient mice were not available, we were able to repeat the study of Deng et al 11 using uPA deficient mice in normolipidemic C57BL/6 background. AngII infusion causes AAAs in normocholesterolemic male mice, albeit with a much lesser incidence than strain-matched hypercholesterolemic mice.11,25 Nevertheless, we failed to detect any significant reduction in the incidence and size of AAAs in uPA −/−mice. Therefore, discrepancy in findings between our studies and those of Deng et al.11 cannot be explained on the basis of differences in the hyperlipidemic background.
Proteases mediating AngII-induced dilation may differ from those that regulate development of aortic rupture. Consistent with this notion, uPA deficiency had no effect on either the incidence or maximal external diameter of AngII-induced AAAs. However, uPA deficiency increased mortality due to rupture of AAAs. Although uPA can be synthesized by several cell types,26 the bone marrow transplantation study demonstrated deficiency of uPA in infiltrating leukocytes also promoted rupture. We have demonstrated previously that marked luminal dilation of AngII-induced AAAs precedes aortic rupture in this region by up to several days.8,20 Therefore, the process of proteolytic destruction of medial ECM is distinct from events leading to aortic rupture. We have also previously demonstrated that transmural thrombi occur in the early stage of luminal dilation that coincides with development of aortic rupture.8 Therefore, it is likely that increased aortic rupture rates are determined by alterations in thrombi characteristics. We attempted to characterize aneurysmal tissues after 7 days of AngII infusion to provide insight into the increased rate of rupture. However, a combination of the difficulty in serially sectioning thrombotic material and the heterogeneity of the aneurysmal tissue presented a challenge to determine thrombus characteristics by immunostaining.19 We also performed gene array of AAA tissues in the early phase of AngII infusion, but were unable to distinguish differences in the selected extracellular matrix and adhesion molecule genes examined in aneurysmal tissue between uPA +/+ or −/− mice. Because of this inherent difficulty, we determined characteristics of aneurysmal tissue after 28 days of AngII infusion. Although these tissues were extracted from mice that survived the 28 days of AngII infusion, there were marked differences in the nature of aneurysmal tissue that included increased deposition of fibrin/fibrinogen and decreased nuclei in the remodeled region in uPA −/− mice. These changes in aneurysmal tissue are consistent with changes that have been noted previously in uPA deficient mice subjected to ferric chloride-induced carotid lesions in apoE −/− mice.27 Future studies will address the nature of the transmural thrombus and the effect of uPA deficiency on its characteristics.
MMPs have been detected in AngII-induced aneurysmal tissue, with consistent demonstrations of MMP-9 and MMP-2. Using zymography, MMP-9 is usually detected almost exclusively in the latent form, while MMP-2 is routinely detected in both latent and activated forms.28 No studies have used genetic deficiencies of these MMPs to demonstrate a direct role in AngII-induced AAA formation, although pharmacological studies using a broad MMP inhibitor, doxycycline, showed reduced severity of the aortic pathology.29 To the contrary, mice with MMP resistance to collagen degradation have increased AngII-induced AAAs.22 Although the direct effects of MMPs on AngII-induced AAAs are unclear, similar to previous studies, we demonstrated the presence of the latent form of MMP-9 and both latent and active forms of MMP-2 in extracts of AngII-induced AAAs. However, the deficiency of uPA or uPAR did not change the presence and activation of these MMPs. This lack of effect on MMP abundance in uPA and uPAR deficient mice is consistent with the lack of effect on AAA formation.
Although atherosclerosis was not a primary end-point of the current studies, in addition to AAAs, AngII infusion also augments lesion development in both apoE −/− and LDL receptor −/− mice.6,12,30 Although not previously described, we were unable to demonstrate any effect of uPAR on size of atherosclerotic lesions. There have been a few studies defining the role of uPA on atherosclerosis that have demonstrated no effect of uPA deficiency on atherosclerosis in apoE −/− and apoE3-Leiden mice fed diets enriched in saturated fat, cholesterol, and cholate.31,32 In agreement with data derived using these other models, deficiency of uPA also had no effect on AngII-induced atherosclerosis in LDL receptor −/− mice. Conversely, several studies have reported that overexpression of uPA in macrophages accelerates atherosclerosis, promotes cardiac fibrosis and macrophage accumulation.33–35 The present study provided evidence that lack of uPA was associated with less fibrosis in thrombi. Similar to uPAR deficiency, lack of uPA deficiency had no effect on atherosclerosis. Overall, this study failed to demonstrate any significant effects of deficiency of the uPA-uPAR axis on AngII-augmented atherosclerotic lesions.
In conclusion, our study has failed to demonstrate an effect of the uPA-uPAR axis on development of AngII-induced AAAs under either normo- or hyper-cholesterolemic conditions. Unexpectedly, uPA deficiency leads to profoundly increased death due to AAA rupture. The potential mechanism of this increased rupture rate may be related to the inability of uPA deficient mice to resolve the transmural thrombi that are formed in the early phase of AngII-induced AAAs.
Supplementary Material
Acknowledgments
We acknowledge the skilled technical assistance of Debra L. Rateri, Deborah A. Howatt, Jessica J. Moorleghen and Anju Balakrishnan, and editing by Dr. Hong Lu and Debra L. Rateri.
SOURCE OF FUNDING
This work was supported by a grant from the NHLBI (P01HL80100). Haruhito A. Uchida was partially supported by a fellowship grant from the Sumitomo Life Social Welfare Services Foundation, Japan.
Footnotes
DISCLOSURES
None.
References
- 1.Powell JT, Brady AR. Detection, management, and prospects for the medical treatment of small abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:241–245. doi: 10.1161/01.ATV.0000106016.13624.4a. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 3.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, Ridker PM, Libby P, Chapman HA. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YX, Martin-McNulty B, Freay AD, Sukovich DA, Halks-Miller M, Li WW, Vergona R, Sullivan ME, Morser J, Dole WP, Deng GG. Angiotensin II increases urokinase-type plasminogen activator expression and induces aneurysm in the abdominal aorta of apolipoprotein E-deficient mice. Am J Pathol. 2001;159:1455–1464. doi: 10.1016/S0002-9440(10)62532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas M, Gavrila D, McCormick ML, Miller FJ, Jr, Daugherty A, Cassis LA, Dellsperger KC, Weintraub NL. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 9.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm. Pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P, Moons L, Dewerchin M, Rosenberg S, Herbert JM, Lupu F, Collen D. Receptor-independent role of urokinase-type plasminogen activator in pericellular plasmin and matrix metalloproteinase proteolysis during vascular wound healing in mice. J Cell Biol. 1998;140:233–245. doi: 10.1083/jcb.140.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, Loskutoff DJ, Carmeliet P, Dole WP, Wang YX. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 12.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 13.Lu H, Rateri DL, Feldman DL, Charnigo RJ, Jr, Fukamizu A, Ishida J, Oesterling EG, Cassis LA, Daugherty A. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. doi: 10.1172/JCI32970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daugherty A, Rateri D, Lu H, Balakrishnan A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J Vis Exp. 2009:1291. doi: 10.3791/1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]
- 16.Wang YX, Cassis LA, Daugherty A. Angiotensin II-induced abdominal aortic aneurysms. In: Xu Q, editor. A Handbook of Mouse Models for Cardiovascular Disease. John Wiley & Sons; 2006. pp. 125–136. [Google Scholar]
- 17.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, Rateri DL, Daugherty A. Immunostaining of mouse atherosclerosis lesions. Methods Mol Med. 2007;139:77–94. doi: 10.1007/978-1-59745-571-8_4. [DOI] [PubMed] [Google Scholar]
- 19.Rateri DL, Howatt DA, Moorleghen JJ, Charnigo R, Cassis LA, Daugherty A. Prolonged infusion of angiotensin II in apoE−/− mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysms. Am J Pathol. 2011 doi: 10.1016/j.ajpath.2011.05.049. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barisione C, Charnigo R, Howatt DA, Moorleghen JJ, Rateri DL, Daugherty A. Rapid dilation of the abdominal aorta during infusion of angiotensin II detected by noninvasive high-frequency ultrasonography. J Vasc Surg. 2006;44:372–376. doi: 10.1016/j.jvs.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 21.Findeisen HM, Gizard F, Zhao Y, Cohn D, Heywood EB, Jones KL, Lovett DH, Howatt DA, Daugherty A, Bruemmer D. Telomerase Deficiency in Bone Marrow-Derived Cells Attenuates Angiotensin II-Induced Abdominal Aortic Aneurysm Formation. Arterioscler Thromb Vasc Biol. 2011;31:253–260. doi: 10.1161/ATVBAHA.110.218545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deguchi JO, Huang H, Libby P, Aikawa E, Whittaker P, Sylvan J, Lee RT, Aikawa M. Genetically engineered resistance for MMP collagenases promotes abdominal aortic aneurysm formation in mice infused with angiotensin II. Lab Invest. 2009;89:315–326. doi: 10.1038/labinvest.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu JM, Johns A, Morser J, Dole WP, Greaves DR, Deng GG. Urokinase plasminogen activator receptor promotes macrophage infiltration into the vascular wall of ApoE deficient mice. J Cell Physiol. 2005;204:73–82. doi: 10.1002/jcp.20262. [DOI] [PubMed] [Google Scholar]
- 24.Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann NY Acad Sci. 1999;892:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- 25.King VL, Trivedi D, Gitlin JM, Loftin CD. Selective cyclooxygenase-2 inhibition with celecoxib decreases angiotensin II-induced abdominal aortic aneurysm formation in mice. Arterioscler Thromb Vasc Biol. 2006;26:1137–1143. doi: 10.1161/01.ATV.0000216119.79008.ac. [DOI] [PubMed] [Google Scholar]
- 26.Fay WP, Garg N, Sunkar M. Vascular functions of the plasminogen activation system. Arterioscler Thromb Vasc Biol. 2007;27:1231–1237. doi: 10.1161/ATVBAHA.107.140046. [DOI] [PubMed] [Google Scholar]
- 27.Dellas C, Schremmer C, Hasenfuss G, Konstantinides SV, Schafer K. Lack of urokinase plasminogen activator promotes progression and instability of atherosclerotic lesions in apolipoprotein E-knockout mice. Thromb Haemost. 2007;98:220–227. [PubMed] [Google Scholar]
- 28.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 30.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 31.Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nature Genet. 1997;17:439–444. [Google Scholar]
- 32.Rezaee F, Gijbels M, Offerman E, Verheijen J. Genetic deletion of tissue-type plasminogen activator (t-PA) in APOE3-Leiden mice reduces progression of cholesterol-induced atherosclerosis. Thromb Haemost. 2003;90:710–716. doi: 10.1160/TH03-03-0160. [DOI] [PubMed] [Google Scholar]
- 33.Cozen AE, Moriwaki H, Kremen M, DeYoung MB, Dichek HL, Slezicki KI, Young SG, Veniant M, Dichek DA. Macrophage-targeted overexpression of urokinase causes accelerated atherosclerosis, coronary artery occlusions, and premature death. Circulation. 2004;109:2129–2135. doi: 10.1161/01.CIR.0000127369.24127.03. [DOI] [PubMed] [Google Scholar]
- 34.Kremen M, Krishnan R, Emery I, Hu JH, Slezicki KI, Wu A, Qian K, Du L, Plawman A, Stempien-Otero A, Dichek DA. Plasminogen mediates the atherogenic effects of macrophage-expressed urokinase and accelerates atherosclerosis in apoE-knockout mice. Proc Natl Acad Sci USA. 2008;105:17109–17114. doi: 10.1073/pnas.0808650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnan R, Kremen M, Hu JH, Emery I, Farris SD, Slezicki KI, Chu T, Du L, Dichek HL, Dichek DA. Level of macrophage uPA expression is an important determinant of atherosclerotic lesion growth in Apoe−/− mice. Arterioscler Thromb Vasc Biol. 2009;29:1737–1744. doi: 10.1161/ATVBAHA.109.195529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.