Abstract
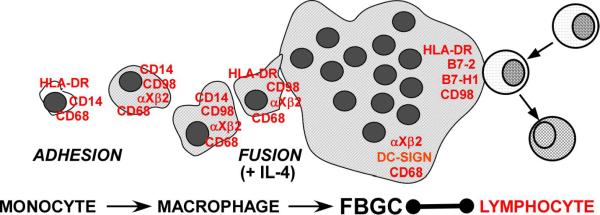
Foreign body-type multinucleated giant cells (FBGC), formed by macrophage fusion, are a prominent cell type on implanted biomaterials, although the roles they play at these and other sites of chronic inflammation are not understood. Why lymphocytes are present in this scenario and the effects of fusing macrophages/FBGC on subsequent lymphocyte responses are also unclear. To address the physiological significance of FBGC in this regard, we employed our in vitro system of interleukin (IL)-4-induced human monocyte-derived macrophage fusion/FBGC formation. Initially, we pursued the identities of lymphocyte co-stimulatory molecules on fusing macrophages/FBGC. In addition, we further compared the FBGC phenotype to that currently associated with osteoclasts and dendritic cells using recognized markers. Immunoblotting of cell lysates and immunochemistry of macrophages/FBGC in situ, revealed that IL-4-induced macrophages/FBGC strongly express HLA-DR, CD98, B7-2 (CD86), and B7-H1 (PD-L1), but not B7-1 (CD80) or B7-H2 (B7RP-1). Furthermore, molecules currently recognized to be expressed on osteoclasts (calcitonin receptor, tartrate-resistant acid phosphatase, RANK) or dendritic cells (CD1a, CD40, CD83, CD95/fas) are undetectable. In contrast, fusing macrophages/FBGC strongly express the macrophage markers αX integrin (CD11c), CD68, and dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), whereas CD14 is completely down-modulated with IL-4-induced macrophage fusion. These novel data demonstrate that IL-4-induction of macrophage multinucleation/FBGC formation features the acquisition of a CD14-negative phenotypic profile which is distinguishable from that of dendritic cells and osteoclasts, yet potentially exhibits multiple capacities for lymphocyte interactions with resultant lymphocyte down-modulation.
Keywords: Biocompatibility, chronic inflammation, lymphocyte, macrophage, multinucleated giant cell
INTRODUCTION
Foreign body-type multinucleated giant cells (FBGC)1 are formed from macrophage fusion in the chronic inflammatory setting created by the persistent presence of a non-phagocytosable biomaterial (Anderson, 2000; McNally and Anderson, 2011). Lymphocytes are present together with adherent macrophages and FBGC in this complex milieu in vivo, and, although lymphocyte responses to conventional microbial pathogens have been extensively studied, our understanding of lymphocyte responses to implanted biomaterials is relatively less illuminated (Anderson and McNally, 2011). However, the lymphokines interleukin (IL)-4 and IL-13 are each known to induce the formation of FBGC on biomaterials from adherent human monocyte-derived macrophages, which undergo fusion upon treatment with these factors in vitro and in vivo (McNally and Anderson 1995; DeFife et al, 1997; Kao et al, 1995).
Furthermore, although synthetic biomedical polymers are not immunogenic antigens in the classic adaptive sense, our efforts to date have nevertheless demonstrated that lymphocytes play a much greater role in the inflammatory and foreign body responses to implanted biomaterials than has been previously appreciated. For example, in vitro lymphocyte/macrophage interactions promote macrophage adhesion and fusion and induce lymphocyte proliferation (Brodbeck et al, 2005). These interactions are also dependent on biomaterial surface chemistry (MacEwan et al, 2005) and lead to biomaterial-dependent cytokine production (Chang et al, 2008a) and both direct and indirect interactions with lymphocytes enhance macrophage/FBGC biomaterial-dependent activation for cytokine production (Chang et al, 2008b; Chang et al, 2009). In vivo in the context of the foreign body reaction, we have demonstrated that secondary responses to biomaterial implantation are characterized by recruitment of both T lymphocytes and phagocytic cells (Rodriguez et al, 2008).
Conventionally, lymphocytes become activated through interactions with antigen-presenting cells, i.e. macrophages and dendritic cells, which present processed antigen bound to major histocompatibility complex molecules, such as human leukocyte antigen (HLA)-DR, on their cell surfaces (DeFranco and Weiss, 1998). Characteristics of lymphocyte activation include expression of cell surface markers of activation, calcium flux, and production of the classic activation lymphokines IL-2 and IFNγ. Of note, we have demonstrated the elaboration of IFNγ in lymphocyte/macrophage co-cultures on biomaterials (Chang et al, 2009). Others have reported that culture of T lymphocytes with polyurethane biomaterial induces calcium influx as well as expression of the lymphocyte activation marker CD40 ligand (Schuster et al, 2001).
Activation alone, however, is not sufficient for lymphocyte effector function, which requires additional cognate interactions with specific molecules on antigen-presenting cells; these are collectively referred to as co-stimulation (Carreno and Collins, 2002; Santana and Rosenstein, 2003). Macrophages may express multiple lymphocyte co-stimulatory molecules, e.g. B7-1 (CD80), B7-2 (CD86), B7-H1, B7-H2, and CD98 (Miyamoto et al, 2003; Khoury and Sayegh, 2004; Wang and Chen, 2004; Martin-Orozco and Dong, 2006). The nature of co-stimulation determines the functional outcome of lymphocyte activation, i.e. macrophage B7-H1 engagement with its co-receptor on lymphocytes is down-modulatory and promotes antigen tolerance, whereas B7-H2 engagement is up-regulatory and promotes immunity (Clarkson and Sayegh, 2005). Related to this, blockade of macrophage B7 interaction with its lymphocyte counter-receptor CD28 results in the generation of alternatively activated macrophages and their suppression of T lymphocytes (Tzachanos et al, 2001). In the chronic inflammatory setting at sites of biomedical material implantation, however, it is not known whether IL-4-induced FBGC possess antigen-presenting or lymphocyte co-stimulatory abilities that are linked to a particular functional outcome.
It is of further interest that the paths of development for FBGC and dendritic cells each apparently require or are supported by IL-4 signaling (McNally and Anderson, 1995; Romani et al, 1996). Phenotypic comparisons between these cell types, however, have not been made. The dendritic cell phenotype includes expression of CD1a, CD40, CD83, and CD95/fas as well as B7-1 (CD80) and B7-2 (CD86) (Romani et al, 1996; Banchereau et al, 2000). CD68 and dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) are also expressed by dendritic cells (Romani et al, 1996; Banchereau et al, 2000; Giejtenbeek et al, 2000), although their expression is also not exclusive to dendritic cells, and CD68 is commonly employed as a macrophage marker (Strobl et al, 1995; Boukour et al, 2006; Gottfried et al 2009). Similarly, the αXβ2 integrin (CD11c/CD18, complement receptor type 4) has been widely employed as a dendritic cell marker linked to antigen-presenting activity, but it is not exclusive to dendritic cells and is broadly expressed on tissue macrophages (Hume, 2008). Consistent with this, we previously found that αXβ2 integrin is strongly expressed on IL-4-induced fusing macrophages/FBGC (McNally et al, 2007), but it is not known whether this shared characteristic with dendritic cells extends to additional cell surface molecules.
Osteoclasts, like FBGC, are multinucleated macrophages that originate from blood-borne monocytes (Fujikawa et al, 1996), but they arise in normal bone tissue, not at sites of chronic inflammation. In addition, osteoclasts utilize αVβ3 and αVβ5 integrins during adhesion to bone (Teitelbaum, 2000), whereas β1 and β2 integrins, but not β3 integrins, mediate IL-4-induced FBGC adhesion (McNally et al, 2007; McNally and Anderson, 2002). Furthermore, osteoclast formation is inhibited, not promoted, by IL-4 (Moreno et al, 2003). Thus, in the absence of additional comparative data, we speculate that this type of multinucleated giant cell is also functionally distinct from the FBGC.
Therefore, in the present study, we evaluated expression of antigen-presenting (HLA-DR) and lymphocyte co-stimulatory (B7 family members and CD98) molecules. For comparison, we further addressed currently employed dendritic cell (CD1a, CD40, CD83, CD95/fas) (Banchereau et al, 2000; Lechmann et al, 2002; Savina and Amigorena, 2007) and osteoclast (tartrate-resistant acid phosphatase, TRAP; calcitonin receptor, CTR; receptor activator of nuclear factor κB, RANK) (Teitelbaum, 2000; Quinn et al, 1999; Hayman, 2008) markers as well as CD68, DC-SIGN, and αXβ2 integrin in our established system of human monocyte-to-macrophage development and IL-4-induced macrophage fusion leading to FBGC formation.
Our findings indicate strong expression of CD98 on day 10 macrophages and IL-4-induced macrophages/FBGC with selective expression of HLA-DR, B7-2, B7-H1 (lymphocyte down-modulatory) on FBGC. In contrast, we did not detect B7-1 or B7-H2 (lymphocyte up-regulatory), on macrophages or IL-4-induced fusing macrophages/FBGCs. Interestingly, CD14 is down-modulated upon FBGC formation. Further, we find distinctions between IL-4-induced FBGC and dendritic cells or osteoclasts. Whereas FBGC, like dendritic cells, express αX integrin (CD11c), CD68, and DC-SIGN, we do not detect other widely employed dendritic cell markers (CD1a, CD40, CD83, or CD95/fas) in whole cell lysates of these multinucleated giant cells. Comparisons with the osteoclast in terms of CTR, TRAP, or RANK expression indicate additional phenotypic distinctions between these multinucleated cell types.
METHODS
Monocyte/macrophage culture and FBGC formation
Human monocytes were obtained and isolated as previously described (McNally and Anderson, 2002) and suspended in serum-free medium for macrophages (SFM, Invitrogen, Grand Island, NY) supplemented with 20% autologous serum (AS) and antibiotics/antimycotic (PenStrepFungizone, Invitrogen). All culture surfaces were pre-coated with an RGD (arginine-glycine-aspartate) polymer reagent (fibronectin-like protein polymer, Sigma-Aldrich, St. Louis, MO), 25 μg per ml, for 30 minutes followed by two washes with phosphate-buffered saline containing calcium and magnesium (PBS++, Invitrogen) (McNally et al, 2007; McNally and Anderson 2002). For immunoblotting lysates, monocytes were added to 6-well culture plates at 3 × 106 per well. For immunochemistry, monocytes were seeded onto 8-well chamber slides at a concentration of 5 × 105 monocytes per well. After 1.5 hr at 37°C in a humidified atmosphere of 5% CO2 and 95% air, non-adherent cells were removed by washing with pre-warmed (37°C) PBS++. At this point, wells designated for the day 0 time point were processed for immunoblotting or immunochemistry (see below). The remaining cultures were replenished with pre-warmed SFM without AS and incubated until day 3. On day 3, cultures were either processed or the culture medium was changed to pre-warmed SFM with 5% AS and without or with 15 ng per ml of recombinant human IL-4 (R and D Systems, Minneapolis, MN) to induce macrophage fusion (McNally and Anderson, 1995). On day 7, this step was repeated, and the cultures were terminated on day 10. For each time point of each experiment, culture wells were designated for May-Grünwald/Giemsa staining, adherent cell counting, and determinations of percent fusion as previously described (McNally et al, 2007; McNally and Anderson, 2002; McNally et al, 2008).
Preparation of whole cell lysates for immunoblotting
Cultures were washed twice with warm (37°C) PBS++, cooled on ice, and then solubilized in RIPA solution with Halt protease inhibitors (Thermo Scientific, Rockford, IL) for 30 minutes on an orbital shaker at 4°C. Lysates were transferred to microcentrifuge tubes and centrifuged at 10,000 × g for 30 minutes at 4°C. Supernatants were stored at −80°C. Prior to analysis, lysate pools were prepared by combining equal volumes of supernatant from 5 donors for each time point: day 0, day 3, day 10 (no IL-4) and day 10 (plus IL-4). For day 10 (plus IL-4), three separate lysate pools were prepared for repeat testing with identical results; results from one representative lysate pool are shown in all cases.
Immunoblotting
Lysate pools for each time point were diluted 1:1 with Laemmli sample buffer containing 2-mercaptoethanol (Bio-Rad Laboratories, Hercules, CA), loaded on to Tris-HCl Ready Gels, 4–15% acrylamide (Bio-Rad) in parallel with positive control cell lysates (Table 1), and subjected to SDS-PAGE under standard conditions. Proteins were transferred to polyvinylidine difluoride membranes at 100 v for 45 minutes using standard protocols. All remaining steps were carried out at on a rocking platform at room temperature. Membranes were incubated in tris-buffered saline with 0.05% Tween-20 (TTBS) for 5 minutes, and non-specific sites were blocked with 5% Blotto (Santa Cruz Biotechnology, Santa Cruz, CA) in TTBS for 2 hr at room temperature. Primary antibodies (Table 1) were applied overnight in 1% Blotto/TTBS, and membranes were washed thrice in TTBS for 10 minutes each. Secondary antibody conjugates, either goat anti-mouse IgG or donkey anti-goat IgG conjugated to horseradish peroxidase (Bio-Rad) were diluted 1:25,000 in 1% Blotto/TTBS and applied for 1 hr.
TABLE 1.
Antibodies and positive control lysates for immunoblotting.
| Antigen | Antibody Source | Catalog Number | Positive control lysate(s)a |
|---|---|---|---|
| HLA-DR | Santa Cruz | sc-25614 | Namalwa, Ramos |
| B7-1 | Santa Cruz | sc-73721 | B7-l(h):293T, BJAB, Namalwa |
| B7-2 | Santa Cruz | sc-19617 | BJAB, Ramos |
| B7-H1 | R & D Systems | AF156 | Daudi |
| B7-H2 | R & D Systems | AF165 | B7-H2(h):293T |
| CD98 | Santa Cruz | sc-7095 | HL-60, U937 |
| CTR | Santa Cruz | sc-8859 | K562 |
| TRAP | Santa Cruz | sc-30833 | TRAP(h):293T |
| RANK | Santa Cruz | sc-59981 | RANK(m):293T, SJRH30 |
| CD la | Santa Cruz | sc-18885 | CDla(h):293T |
| CD40 | Santa Cruz | sc-13128 | CD40(h):293T, BJAB |
| CD83 | Santa Cruz | sc-19677 | CD83(h):293T |
| CD95 | Santa Cruz | sc-8009 | Fas(m):293T |
| CD68 | Santa Cruz | sc-17832 | THP-1 |
| DC-SIGN | Santa Cruz | sc-74589 | DC-SIGN(h):293T |
| αX integrin | Santa Cruz | sc-46676 | αX(h):293T, HL-60 |
| CD 14 | Santa Cruz | sc-58951 | HL-60 |
| Actin | Santa Cruz | sc-1616 | (ubiquitous) |
Santa Cruz Biotechnology, employed as per manufacturer's instructions.
Membranes were then washed thrice with TTBS and once with TBS for 15 minutes each and then developed with a substrate kit (Immobilon Western Chemiluminescent HRP, Millipore Corporation, Billerica, MA).
Immunochemistry
Cultures were washed twice with warm (37°C) PBS++, fixed with cold (−20°C) acetone for 2 minutes, and air dried. Staining was carried out as previously described ((McNally et al, 2007; McNally and Anderson, 2002) using primary antibodies to B7-1 (Santa Cruz Biotechnology, sc-1634), B7-2 (sc-1635), B7-H1 (R and D Systems, Minneapolis, MN, AF-156), B7-H2 (AF-165), CD98 (sc-7095) and HLA-DR (sc-18875). Components of interest were detected with secondary antibodies conjugated to AlexaFluor568 (Molecular Probes, Eugene, OR). Where indicated, nuclei were stained with YO-YO-1 (Molecular Probes). Confocal scanning laser microscopic imaging (MRC-600, Bio-Rad, Richmond, CA) of samples was carried out after any residual background fluorescence from corresponding nonspecific antibody controls was blackened. Shown images are representative results from at least 3 different monocyte donors.
RESULTS
To investigate the phenotypic profile of IL-4-induced FBGCs, we utilized our established in vitro system of human monocyte/macrophage culture for preparation of whole cell lysates at time points corresponding to adherent monocytes (day 0), short-term monocyte-to-macrophage development (day 3), long-term cultured macrophages (day 10), and IL-4-induced fusing macrophages/FBGC (day 10 following treatments with IL-4 on days 3 and 7). Figure 1 and Table 2 depict representative morphological and numerical data, respectively, corresponding to these culture time points. It can be seen that approximately 50% of the adherent monocyte population on day 0 progresses to macrophage development at later time points. In otherwise unstimulated day 10 macrophages, there is marked monocyte-to-macrophage morphological development in terms of increased cytoplasmic to nuclear ratios; very little macrophage fusion occurs in these cultures. In contrast, following treatment with IL-4 on days 3 and 7, a high degree of fusion is observed (≈70%), resulting in the formation of large FBGC. These multinucleated giant cells exhibit characteristic random arrangements of tens of nuclei within an extensively spread, irregular cytoplasm. Each cell lysate utilized for lysate pools was similarly representative at each time point in terms of morphology, adherent cell numbers, and degrees of macrophage fusion. Thus, by applying equal volumes of lysate pools for immunoblotting analysis, the absence or presence and relative expression of a particular antigen could be qualitatively compared between time points.
Figure 1.
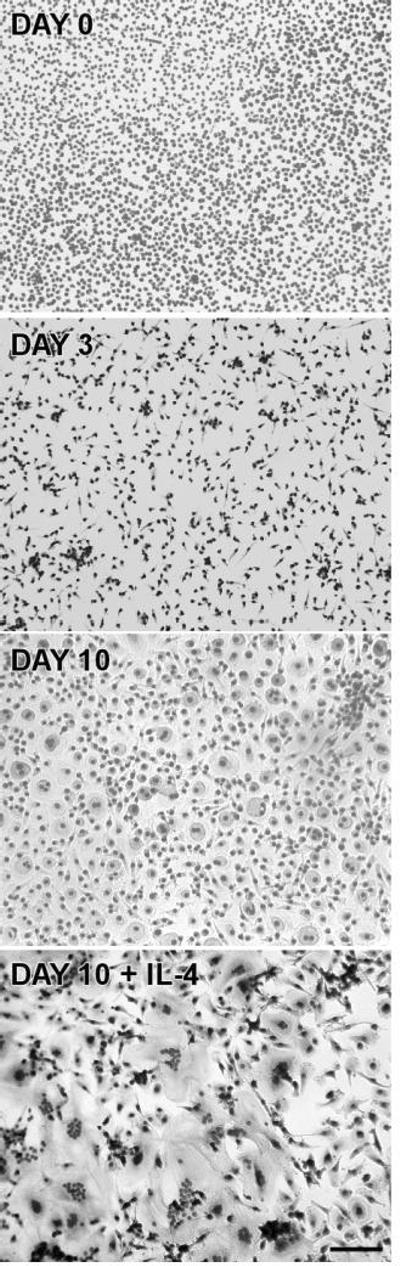
Monocyte-to-macrophage development and IL-4-induced FBGC formation in vitro. Monocytes were cultured on RGD-adsorbed culture surfaces for 1.5 hr (Day 0), or otherwise unstimulated macrophage development was allowed to proceed until Day 3 or Day 10. To induce macrophage fusion and FBGC formation, IL-4 was added on days 3 and 7, and the cultures also terminated on day 10. (May-Grünwald/Giemsa, scale bar = 50 μm)
TABLE 2.
Monocyte/macrophage adhesion and fusion to form FBGC in vitro.
| Morphology | Culture Time | Adherent Cells (Nuclei per mm2 )a | Macrophage Fusion(%)b |
|---|---|---|---|
| Monocytes | Day 0 | 2013 ± 214 | n.a.c |
| Macrophages (short-term) | Day 3 | 1056 ± 50 | n.a. |
| Macrophages (long-term) | Day 10 | 1067 ± 40 | 6 ± 2 |
| Fusing macrophages/FBGC | Day 1O + IL-4 | 1073 ± 42 | 71 ± 3 |
Nuclei per mm2 in May-Grünwald/Giemsa-stained cells were counted at 20× magnification on an inverted stage microscope
Percent macrophage fusion represents the mean number of nuclei ± SEM within FBGC (cells with > 2 nuclei) compared to total nuclei counted (n = 3).
not applicable
In Figure 2, results of immunoblotting analyses to probe for the expression of HLA-DR and lymphocyte co-stimulatory molecules are shown and compared with expression on adherent monocytes (day 0) and macrophages (day 3 and day 10 of culture). The expression patterns of HLA-DR and the lymphocyte co-stimulator CD98, reveal initial expression of HLA-DR on day 0 which decreases with culture time. HLA-DR very strongly recurs in IL-4-induced FBGC at day 10. CD98 is not expressed at Day 0 but is apparently up-regulated with macrophage development on days 3 and 10, and expression is maintained in IL-4-induced FBGC.
Figure 2.
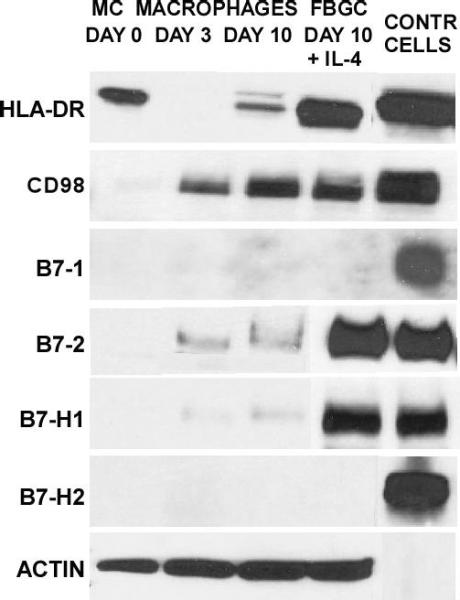
Selective expression of lymphocyte co-stimulatory molecules. Whole cell lysate pools were prepared for each culture time point and probed for the indicated molecules (left) by immunoblotting. In the right-most column, corresponding positive control cell lysates are presented for: HLA-DR, Namalwa; B7-1, B7-1:293T; B7-2. BJAB; B7-H1, Daudi; B7-H2, B7-H2:293T; CD98, HL-60.
Although we utilized several different B7-1 antibodies which reacted positively with a human B7-1-transfected fibroblast control lysate, Figure 2 also demonstrates that B7-1 is not detectable in either lysate pool. In contrast, IL-4-induced fusing macrophages/FBGC very strongly express the lymphocyte co-stimulator B7-2, which is not present in adherent monocytes and only moderately detectable in otherwise unstimulated macrophages.
We further evaluated B7-H1 and B7-H2 at each time point and discovered an additional distinctive expression pattern in that B7-H1 was clearly present only in fusing macrophages/FBGC but not in untreated day 10 macrophages, day 3 macrophages, or adherent monocytes. In contrast, B7-H2 was not detectable in either cell type. B7-H2-transfected control cell lysate samples demonstrated that our detection methods were appropriate and effective, thus confirming the selective expression of B7-H1 but not B7-H2 in fusing macrophages/FBGC. For comparison, the expression of a cytoskeletal protein, actin, was consistently high in all lysate pools, particularly following macrophage development as expected.
We further explored the expression of lymphocyte co-stimulatory molecules with immunocytochemistry and confocal microscopy of cells fixed in situ at each time point. Figures 3 and 4 confirm our immunoblotting data as evidenced by strong signals for B7-2 and B7-H1 but not B7-1 or B7-H2. Simultaneous nuclear staining (blue) indicates that there are comparable numbers of adherent macrophages and FBGC within the representative microscopic fields. Likewise, Figure 5 depicts confirmation staining for CD98, not in adherent monocytes but appearing in day 3 macrophages with continued expression in fusing macrophages/FBGC. Again, HLA-DR is observed by its initial expression at day 0, loss of expression at day 3, and strong reappearance on day 10 in fusing macrophages/FBGC.
Figure 3.
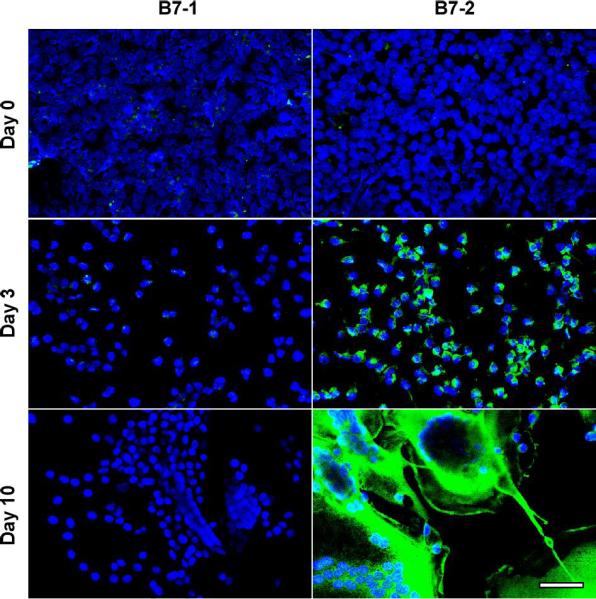
Selective expression of B7-2, but not B7-1, on FBGC. Samples were fixed and stained at the indicated time points and imaged with confocal microscopy as described in Methods. B7-1 or B7-2 (green), nuclei (blue). (scale bar = 50μm)
Figure 4.
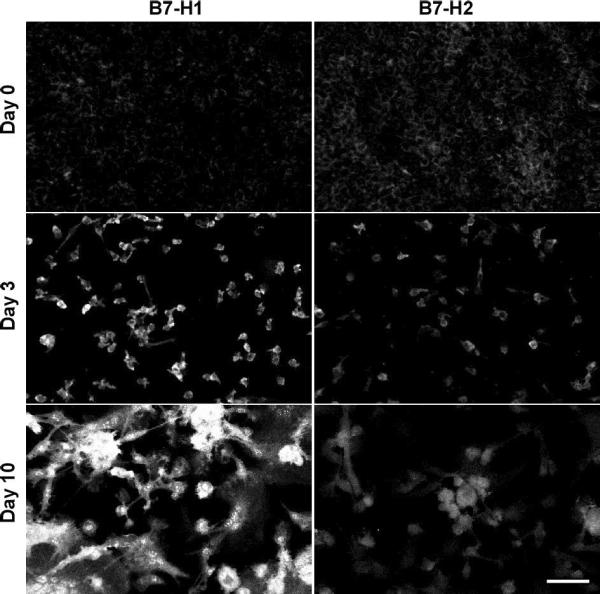
Selective expression of B7-H1, but not B7-H2, on FBGC. Samples were fixed and stained at the indicated time points and imaged with confocal microscopy as described in Methods. (scale bar = 50μm)
Figure 5.
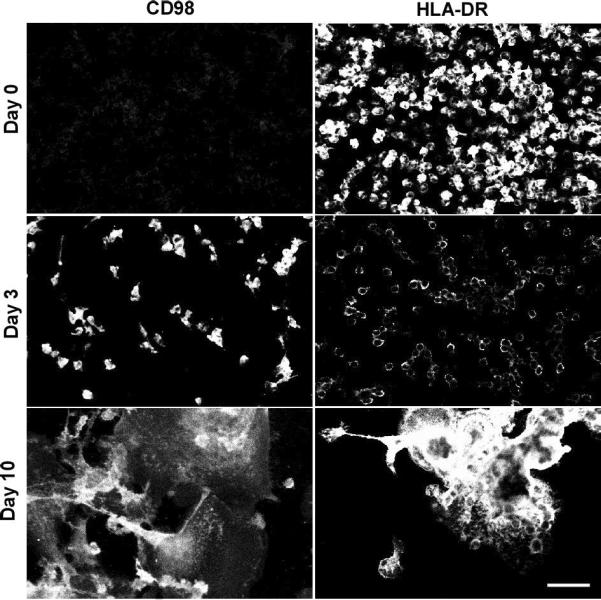
Expression of CD98 and HLA-DR on FBGC. Samples were fixed and stained at the indicated time points and imaged with confocal microscopy as described in Methods. (scale bar = 50μm)
To ask whether fusing macrophages/FBGCs share phenotypic characteristics with osteoclasts, the multinucleated macrophages of normal bone physiology, we performed immunoblotting analyses at each time point for several currently described osteoclast markers. Compared to actin detection and positive control cells, FBGC do not express CTR, TRAP, or RANK (Fig. 6). These antigens are not apparent at any point in our in vitro FBGC-generating system, although they are clearly detectable in positive control cell lysates.
Figure 6.
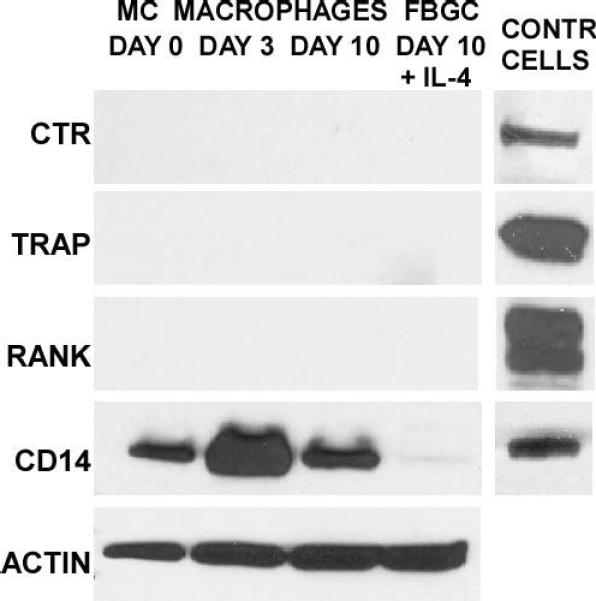
FBGC do not express widely employed osteoclast markers or the macrophage marker CD14. Whole cell lysate pools were prepared for each culture time point and probed for the indicated molecules (left) by immunoblotting. In the right-most column, corresponding positive control cell lysates are presented for: CTR, K562; TRAP, TRAP:293T; RANK, RANK:293T; CD14, CD14:293T.
For a further comparison, we evaluated expression of the widely employed macrophage marker and scavenger receptor, CD14. This confirmed that CD14 is relatively strongly present throughout the entire culture period on otherwise untreated monocytes/macrophages, particularly on day 3. Interestingly, however, following treatment with IL-4, CD14 expression is apparently completely down-regulated and is not detectable in fusing macrophages/FBGC on day 10.
Finally, we compared the FBGC phenotype with that of dendritic cells as they are currently described in the literature. Among currently held dendritic cell markers are CD1a, CD40, CD83, and CD95/fas. In addition, the macrophage marker CD68, DC-SIGN, and the leukocyte integrin αXβ2 (or CD11c/CD18) are reported on dendritic cells (Romani et al, 1996; Banchereau et al, 2000; Hume, 2008). Our expression analyses indicate that neither CD1a, CD40, CD83, nor CD95 are detectable at either time point, and importantly, they are also absent in IL-4-induced fusing macrophages/FBGC (Fig. 7). Control cell lysates were found to be positive in all cases. In contrast, CD68 is present throughout the culture period and is strongly expressed in day 10 macrophages as well as in IL-4-induced macrophages/FBGC. DC-SIGN, however, appears only upon IL-4 induction of macrophage fusion and FBGC formation and is not present in otherwise unstimulated day 10 macrophages. Further, we previously reported detection of αXβ2 integrin by immunocytochemistry on IL-4-induced FBGC (McNally and Anderson, 2007). We extend this to immunoblotting detection of αX integrin in day 10 macrophages in the absence or presence of IL-4, in contrast to the absence of dendritic cell markers (Fig. 7).
Figure 7.
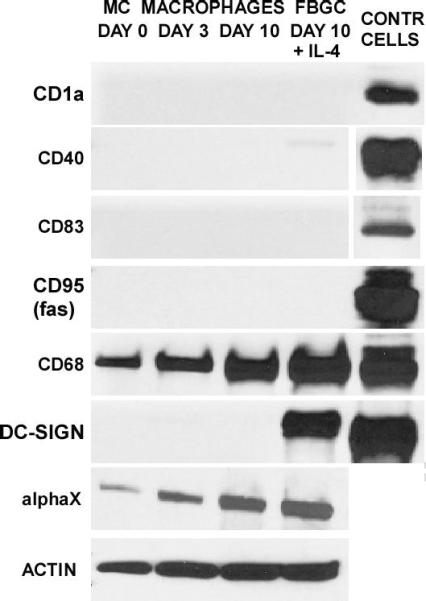
FBGC do not express dendritic cell markers. Whole cell lysate pools were prepared for each culture time point and probed for the indicated molecules (left) by immunoblotting. In the right-most column, corresponding positive control cell lysates are presented for: CD1a, CD1a:293T; CD40, CD40:293T; CD83, CD83:293T; CD95, FAS:293T; CD68, THP-1; DC-SIGN, DC-SIGN:293T.
DISCUSSION
In the present study, our aims were two-fold. First, we sought to illuminate the physiological significance of IL-4-induced FBGC by evaluating their potential for interactions with lymphocytes through the expression of lymphocyte co-stimulatory molecules. Whereas it is widely believed that the progression of FBGC formation on implanted biomaterials appears to be associated with biomaterial degradation (Anderson, 2000), the effects of multinucleated macrophages on the activities of multiple other cell types and surrounding host tissues remain important unknowns. Secondly, we questioned whether the emerging phenotypic profile of FBGC would share notable characteristics with or distinguish itself from that of osteoclasts or dendritic cells. Table 3 summarizes our current and novel findings on the phenotypic characteristics of IL-4-induced fusing macrophages/FBGC. This study is the first of its kind to address the potential host physiological significance of IL-4-induced FBGC formation on implanted biomaterials or at other sites of chronic inflammation.
TABLE 3.
Summary of phenotypic expressiona during adherent monocyte-to-macrophage development and IL-4-induced macrophage fusion into FBGC in vitro.
| Monocytes | Macrophages | Macrophages | Fusing Macrophages/FBGC | |
|---|---|---|---|---|
| Antigen | Day O | Day 3 | Day 10 No IL-4 | Day 10 + IL-4 |
| HLA-DR | +++ | 0 | ++ | ++++ |
| B7-1 | 0 | 0 | 0 | 0 |
| B7-2 | 0 | + | ++ | ++++ |
| B7-H1 | 0 | 0 | 0 | ++++ |
| B7-H2 | 0 | 0 | 0 | 0 |
| CD98 | 0 | ++ | +++ | +++ |
| CTR | 0 | 0 | 0 | 0 |
| TRAP | 0 | 0 | 0 | 0 |
| RANK | 0 | 0 | 0 | 0 |
| CDla | 0 | 0 | 0 | 0 |
| CD40 | 0 | 0 | 0 | 0 |
| CD83 | 0 | 0 | 0 | 0 |
| CD95 | 0 | 0 | 0 | 0 |
| CD68 | ++ | ++ | +++ | ++++ |
| DC-SIGN | 0 | 0 | 0 | +++ |
| αX integrin | + | ++ | +++ | ++++ |
| CD14 | +++ | ++++ | +++ | 0 |
| Actin | +++ | ++++ | ++++ | ++++ |
Relative expression as detected by immunoblotting is graded as 0, not detected
+, detectable but weak
++, moderate
+++, strong
++++, very strong.
These combined data indicate that the IL-4 induction of multinucleation leading to FBGC formation is accompanied by the acquisition of a CD14-negative phenotypic profile that is distinct from monocytes, otherwise untreated but morphologically developed macrophages, and at least two other differentiated cell types of monocytic origin. CD14 is widely employed as a monocyte/macrophage marker, but, consistent with an earlier study on IL-4-treated human monocytes (Lauener et al, 1990), our data demonstrate that CD14 is not useful to detect IL-4-induced FBGC in tissues. By several criteria, the FBGC expression profile does not match that of osteoclasts, which are multinucleated macrophages of normal bone, or dendritic cells, which are antigen-presenting cells of monocytic origin in normal tissue.
Furthermore, as illustrated in Figure 8, we have discovered that FBGC exhibit multiple capacities for interactions with lymphocytes, including expression of HLA-DR and select lymphocyte co-stimulatory molecules. CD98 is up-regulated with macrophage development, and its expression is maintained upon IL-4-induced macrophage fusion. However, there is expression of B7-2 in the apparent absence of B7-1, particularly upon macrophage fusion and FBGC formation. Likewise, the IL-4 induction of macrophage fusion and FBGC formation is accompanied by the selective up-regulation of B7-H1 without B7-H2, which indicates potential for lymphocyte down-modulation (Carreno and Collins, 2002; Clarkson and Sayegh, 2005; Tzachanis et al, 2001; Krozek et al, 2004). Therefore, fusing macrophages/FBGC at sites of biomaterial implantation may undergo interactions with lymphocytes that promote lymphocyte anergy, inactivation, or tolerance. We further speculate that, analogously, wherever they arise at chronic inflammatory sites due to the persistent presence of microorganisms or nonphagocytosable foreign bodies, FBGC may be clinical indicators of these or related processes.
Figure 8.
IL-4-induced macrophage fusion and FBGC formation features maintained expression of CD68, CD98 and αXβ2 integrin, de novo appearance of DC-SIGN, and down-modulation of CD14. Significantly, FBGC formation is accompanied by re-expression of HLA-DR and de novo expression of B7-2 and B7-H1, which may provide opportunities for interactions with lymphocytes that, in the case of B7-H1, result in lymphocyte down-modulation.
The relationships between FBGC and osteoclasts, which are each multinucleated, or between FBGC and dendritic cells, the development of which is each influenced by IL-4, have been unclear. In the case of dendritic cells, their formation in vitro is apparently promoted by treatment of a non-adherent population of blood monocytes with a combination of IL-4 and GMCSF to induce an immature state of dendritic cell, which is then matured by exposure to a second signal, such as bacterial lipopolysaccharide (Romani et al, 1996; Banchereau et al, 2000). Through the expression of appropriate co-stimulatory molecules (HLA, CD1a, CD40, CD83, B7-1, and B7-2), mature dendritic cells are capable of antigen presentation to resting T lymphocytes, thus initiating adaptive immune responses.
In contrast, FBGC formation in vitro requires an adherent monocyte population and an adhesion substrate that promotes continued adherence and/or macrophage development, e.g. adsorbed vitronectin or RGD-modified cell culture polystyrene (McNally and Anderson, 1995; McNally and Anderson, 2000; McNally et al, 2008). Mannose receptor-mediated fusion of adherent macrophages is then initiated by IL-4, IL-13, or α-tocopherol (McNally and Anderson, 1995; DeFife et al, 1997; McNally and Anderson, 2003), and the resultant FBGC phenotype, which features HLA-DR but lacks CD1a, CD40, CD83, CD95/fas, and B7-1, is apparently distinct and can thus be distinguished from that of dendritic cells.
The osteoclast found in normal bone tissue is a multinucleated giant cell that is also formed from monocyte precursors (Fujikawa et al, 1996). However, in addition to the present findings that FBGC do not exhibit osteoclast markers (CTR, TRAP, or RANK), a further distinction between these cell types is that the latter are known to utilize β3 integrins as adhesion receptors (Teitelbaum, 2000; Boissy et al, 1998). Previously, we reported that IL-4-induced FBGC adhesion is mediated by β1 and β2 integrins ((McNally and Anderson, 2002). In that study, we did not detect β3 integrin on FBGC, nor did function-blocking antibodies to β3 integrin restrict FBGC adhesion to substrate. In additional studies to address this, we concluded that adhesion to adsorbed vitronectin (McNally et al, 2008) is mediated by αvβ1 integrin (McNally et al, 2007) as opposed to αvβ3-mediated osteoclast adhesion to bone. In further contrast to FBGC, osteoclast formation is inhibited by IL-4 (Moreno et al, 2003) and is instead promoted by TNFα, which does not support FBGC formation (McNally and Anderson, 1995).
These findings provide new perspective on multiple pathological scenarios in which FBGC have long been regarded as hallmark, albeit poorly understood, features of chronic inflammation. The physiological significance of these distinct patterns of expression of costimulatory molecules in the context of biomaterials and their effects on lymphocyte and macrophage/FBGC behaviors at sites of biomaterial implantation remain to be further elucidated.
ACKNOWLEDGMENTS
The authors thank Erica Colton for monocyte isolations. These studies were supported by the National Institutes of Health, Grant EB000282.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: AS, autologous serum; CTR, calcitonin receptor; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; FBGC, foreign body giant cell; TRAP, tartrate-resistant acid phosphatase; RANK, receptor activator of nuclear factor B; SFM, serum-free medium.
REFERENCES
- Anderson JM. Multinucleated giant cells. Curr. Opin. Hematol. 2000;7:40–47. doi: 10.1097/00062752-200001000-00008. [DOI] [PubMed] [Google Scholar]
- Anderson JM, McNally AK. Biocompatibility of implants: lymphocyte/macrophage interactions. Semin. Immunopathol. 2011;33:221–233. doi: 10.1007/s00281-011-0244-1. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y, Pulendran B, Palucka K. Immunobiology of dendritic cells. Ann. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Boissy P, Machuca I, Pfaff M, Ficheux D, Jurdic P. Aggregation of mononucleated precursors triggers cell surface expression of alphavbeta3 integrin essential to formation of osteoclast-like multinucleated cells. J. Cell Sci. 1998;111:2563–2574. doi: 10.1242/jcs.111.17.2563. [DOI] [PubMed] [Google Scholar]
- Boukour S, Masse JM, Benit L, Dubart-Kupperschmidt A, Cramer EM. Lentivirus degradation and DC-SIGN expression by human platelets and megakaryocytes. J. Thromb. Haemost. 2006;4:426–435. doi: 10.1111/j.1538-7836.2006.01749.x. [DOI] [PubMed] [Google Scholar]
- Brodbeck WG, MacEwan M, Colton E, Meyerson H, Anderson JM. Lymphocytes and the foreign body response: lymphocyte enhancement of macrophage adhesion and fusion. J. Biomed. Mater. Res. 2005;74A:222–229. doi: 10.1002/jbm.a.30313. [DOI] [PubMed] [Google Scholar]
- Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Ann. Rev. Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- Chang DT, Jones JA, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Lymphocyte/macrophage interactions: Biomaterial surface-dependent cytokine, chemokine, and matrix protein production. J. Biomed. Mater. Res. 2008a;87A:676–687. doi: 10.1002/jbm.a.31630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Colton E, Anderson JM. Paracrine and juxtacrine lymphocyte enhancement of adherent macrophage and foreign body giant cell activation. J. Biomed. Mater. Res. 2008b;89A:490–498. doi: 10.1002/jbm.a.31981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Colton E, Matsuda T, Anderson JM. Lymphocyte adhesion and interactions with biomaterial adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. 2009;91A:1210–1220. doi: 10.1002/jbm.a.32218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson MR, Sayegh MH. T cell costimulatory pathways in allograft rejection and tolerance. Transplantation. 2005;80:555–563. doi: 10.1097/01.tp.0000168432.60022.99. [DOI] [PubMed] [Google Scholar]
- DeFife KM, Jenney CR, McNally AK, Colton E, Anderson JM. Interleukin-13 induces monocyte macrophage fusion and mannose receptor expression. J. Immunol. 1997;158:3385–3390. [PubMed] [Google Scholar]
- DeFranco AL, Weiss A. Lymphocyte activation and effector functions. Curr. Opin. Immunol. 1998;10:243–267. doi: 10.1016/s0952-7915(98)80159-3. [DOI] [PubMed] [Google Scholar]
- Fujikawa Y, Quinn JM, Sabokbar A, McGee JO, Althanasou NA. The human osteoclast precursor circulates in the monocyte fraction. Endocrinology. 1996;137:4058–4060. doi: 10.1210/endo.137.9.8756585. [DOI] [PubMed] [Google Scholar]
- Giejtenbeek TB, Torensma R, Van Vliet SJ, Van Duijnhoven GC, Adema GJ, Van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Gottfried , Kunz-Schughart LA, Weber A, Rehli M, Peuker A, Muller A, Kastenberger M, Brockhoff G, Andreesen R, Kreutz M. Expression of CD68 in non-myeloid cell types. Scand. J. Immunol. 2009;70:161–162. doi: 10.1111/j.1365-3083.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- Hayman AR. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity. 2008;41:218–223. doi: 10.1080/08916930701694667. [DOI] [PubMed] [Google Scholar]
- Hume DA. Macrophages as APC and the dendritic cell myth. J. Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- Kao WJ, McNally AK, Hiltner A, Anderson JM. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J. Biomed. Mater. Res. 1995;29:1267–1275. doi: 10.1002/jbm.820291014. [DOI] [PubMed] [Google Scholar]
- Khoury SJ, Sayegh MH. The roles of new negative T cell co-stimulatory pathways in regulating autoimmunity. Immunity. 2004;20:529–538. doi: 10.1016/s1074-7613(04)00116-5. [DOI] [PubMed] [Google Scholar]
- Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr. Opin. Immunol. 2004;16:321–327. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Lauener RP, Goyer SM, Geha RS, Vercelli D. Interleukin-4 down-regulates the expression of CD14 in normal human monocytes. Eur. J. Immunol. 1990;20:2375–2381. doi: 10.1002/eji.1830201103. [DOI] [PubMed] [Google Scholar]
- Lechmann M, Berchtold S, Hauber J, Steinkasserer A. CD83 on dendritic cells: more than just a marker for maturation. Trends in Immunol. 2002;23:273–275. doi: 10.1016/s1471-4906(02)02214-7. [DOI] [PubMed] [Google Scholar]
- MacEwan MR, Brodbeck WG, Matsuda T, Anderson JM. Monocyte/lymphocyte interactions and the foreign body response: in vitro effects of biomaterial surface chemistry. J. Biomed. Mater. Res. 2005;74A:285–293. doi: 10.1002/jbm.a.30316. [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N, Dong C. New battlefields for co-stimulation. J. Exp. Med. 2006:817–820. doi: 10.1084/jem.20060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally AK, Anderson JM. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am. J. Pathol. 1995;147:1487–1499. [PMC free article] [PubMed] [Google Scholar]
- McNally AK, Anderson JM. β1 and β2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am. J. Pathol. 2002;160:621–630. doi: 10.1016/s0002-9440(10)64882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally AK, Anderson JM. Foreign body-type multinucleated giant cell formation is potently induced by α-tocopherol and prevented by the diacylglycerol kinase inhibitor R59022. Am. J. Pathol. 2003;163:1147–1156. doi: 10.1016/s0002-9440(10)63474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally AK, Anderson JM. Macrophage fusion and multinucleated giant cells of inflammation. In: Dittmar T, Zanker KS, editors. Cell Fusion in Health and Disease, Advances in Experimental Medicine and Biology. Springer; New York: 2011. pp. 97–111. [DOI] [PubMed] [Google Scholar]
- McNally AK, Jones JA, MacEwan SR, Colton E, Anderson JM. Vitronectin is a critical protein adhesion substrate for IL-4-induced foreign body giant cell formation. J. Biomed. Mater. Res. 2008;86A:535–543. doi: 10.1002/jbm.a.31658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally AK, MacEwan SR, Anderson JM. α-subunit partners to β1 and β2 integrins during IL-4-induced foreign body giant cell formation. J. Biomed. Mater. Res. 2007;82A:568–574. doi: 10.1002/jbm.a.31161. [DOI] [PubMed] [Google Scholar]
- Miyamoto YJ, Mitchell JS, McIntyre BW. Physical association and functional interaction between beta1 integrin and CD98 on human T lymphocytes. Mol. Immunol. 2003;39:739–751. doi: 10.1016/s0161-5890(02)00255-9. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Kaczmarek M, Keegan AD, Tondravi M. IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: Irreversible inhibition of the differentiation program activated by RANKL. Blood. 2003;102:1078–1086. doi: 10.1182/blood-2002-11-3437. [DOI] [PubMed] [Google Scholar]
- Quinn JMW, Morfis M, Lam MHC, Elliot J, Kartsogiannis V, Williams ED, Gillespie MT, Martin TJ, Sexton PM. Calcitonin receptor antibodies in the identification of osteoclasts. Bone. 1999;25:1–8. doi: 10.1016/s8756-3282(99)00094-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Voskerician G, Meyerson H, MacEwan S, Anderson JM. T cell subset distributions following primary and secondary implantation at subcutaneous biomaterial implant sites. J. Biomed. Mater. Res. 2008;85A:556–565. doi: 10.1002/jbm.a.31562. [DOI] [PubMed] [Google Scholar]
- Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Meth. 1996;96:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- Santana MA, Rosenstein Y. What it takes to become an effector T cell: the process, the cells involved, and the mechanisms. J. Cell Physiol. 2003;195:392–401. doi: 10.1002/jcp.10258. [DOI] [PubMed] [Google Scholar]
- Savina A, Amigorena S. Phagocytois and antigen presentation in dendritic cells. Immunol. Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Schuster M, Kocher A, Lietz K, Ankersmit J, John R, Edwards N, Oz M, Itescu S. Induction of CD40 ligand expression in human T cells by biomaterials derived from left ventricular assist device. Transplantation Proceedings. 2001;33:1960–1961. doi: 10.1016/s0041-1345(00)02754-8. [DOI] [PubMed] [Google Scholar]
- Strobl H, Scheinecker C, Csmarits B, Majdic O, Knapp W. Flow cytometric analysis of intracellular CD68 molecule expression in normal and malignant haemopoiesis. Immunobiol. 1995;90:774–782. doi: 10.1111/j.1365-2141.1995.tb05195.x. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Tzachanis D, Berezovskaya A, Nadler LM, Boussiotis VA. Blockade of B7/CD28 in mixed lymphocyte reaction cultures results in the generation of alternatively activated macrophages, which suppress T-cell responses. Blood. 2001;99:1465–14. doi: 10.1182/blood.v99.4.1465. [DOI] [PubMed] [Google Scholar]
- Wang S, Chen L. T Lymphocyte co-signaling pathways of the B7-CD28 family. Cell. Mol. Immunol. 2004;1:37–42. [PubMed] [Google Scholar]