Abstract
The endocrine system is highly susceptible to damage by high-dose chemotherapy and/or irradiation prior to hematopoietic cell transplantation (HCT) during childhood. The specific endocrine organs most affected by HCT include the thyroid gland, the pituitary, and the gonads. In addition, hormones that support development and stability of the skeletal system are also affected. Insufficiency of thyroid hormone is one of the most common late sequelae of HCT, and occurs more often in young children. Deficiency in the pituitary’s production of growth hormone is a problem of unique concern to the pediatric population. The reproductive risks of HCT depend on the patient’s gender and pubertal status at the time of HCT. Pubertal or gonadal failure frequently occurs, especially in females. Infertility risks for both genders remain high, while methods of fertility preservation are limited in all but post-pubertal males. Bone health post-HCT can be compromised by low bone mineral density as well as avascular necrosis, but the data on both problems in the pediatric HCT population are limited. In this paper, the current state of knowledge, gaps in that knowledge, and recommendations for future research are addressed in detail for each of these systems.
INTRODUCTION
The endocrine system is commonly affected by high-dose chemotherapy and/or irradiation prior to hematopoietic cell transplantation (HCT) during childhood (1). The risks for the development of endocrine dysfunction depend on a variety of factors, including age at HCT, the type of conditioning regimen utilized, and the gender of the patient. The specific endocrine organs most affected by HCT include the thyroid gland, the pituitary, gonads, and hormones that support development and stability of the skeletal system.
In April 2011 the NCI/NHLBI along with the Pediatric Blood and Marrow Transplant Consortium (PBMTC) sponsored a consensus conference of international experts in clinical and biological research into late effects after HCT convened to review the state of the science of pediatric studies and identify key areas for future research. This manuscript will describe the conclusions shared at that conference relating to key endocrine systems affected by HCT. Although there is a large body of research evaluating endocrinologic late effects after HCT in adults, the pediatric literature is relatively limited. Children, especially those who are pre-pubertal and still growing, are a unique population in which the data regarding sequelae in adults after HCT are not directly relevant. Therefore, endocrinologic late effects in children after HCT is an important field of research to both better understand the epidemiology and risk factors for the development of a particular endocrine dysfunction, but also to begin to develop strategies by which the incidence of these late effects can be minimized.
THYROID DYSFUNCTION
Thyroid dysfunction (TD) is a commonly encountered problem following HCT. TD can be screened for with serum free T4 (FT4) and thyroid-stimulating hormone (TSH) levels. There are several distinct patterns of TD, including overt hypothyroidism (low FT4), subclinical compensated hypothyroidism (high TSH with normal FT4), hyperthyroidism (high FT4), and rare classic autoantibody-mediated thyroiditis.
Current State of Knowledge
Centers have reported incidences of TD in pediatric patients undergoing HCT between 0 to 52%, depending upon the size of the cohort and the type of transplants performed (Table 1), with the larger series generally confirming a ~30% incidence (2–11). Of note, this is much higher than generally reported for adult patients, where rates are generally around 15% for patients receiving fractionated total body irradiation (TBI), and even lower for chemotherapy-based preparative regimens (12). Since a major risk factor for the development of TD post-HCT is undergoing HCT before the age of 10 years (2, 4, 8), this suggests that the developing thyroid gland may be more susceptible to damage. The group of children with the highest risk of TD were those patients undergoing HCT for treatment of Hodgkin lymphoma, with a very high cumulative incidence of 73% (2).
Table 1.
Studies of Thyroid Dysfunction (TD) Following Pediatric HCT*
| Reference | Incidence of TD | Number | Disease | Conditioning | F/U Median (range) | Risk Factors | |
|---|---|---|---|---|---|---|---|
| Michel et al. 1997 | 5 | 9% | 26 | AML | BU + CY | 5.9 yrs (1–9) | None |
| Afify et al. 2000 | 6 | 0% | 23 | AML only | BU + CY | 4.9 yrs (2–10) | None |
| Slatter et al. 2004 | 7 | 11% | 83 | PID only | BU + CY | NR (2–4.5) | Autoimmunity? |
| Ishiguro et al. 2004 | 4 | 30% | 147 | Varied | Varied | 11.1 yrs (5.8–21.5) | Age <10 yrs |
| Berger et al. 2005 | 8 | 27% 29% |
101 14 |
ALL only ALL only |
FTBI + Chemo BU + Chemo |
8.5 yrs (5–16.5) | Age <10 yrs; Transplant in >CR1 |
| Leung et al. 2007 | 9 | 34% | 155 | Varied | Varied | 9 yrs (3.1–15.9) | Young age; higher dose TBI |
| Sanders et al. 2008 | 2 | 32% 23% |
538 108 |
Varied Varied |
FTBI + CY BU + Chemo |
NR (1–30) | Age <10 yrs; Malignancy |
| Dvorak et al. 2008 | 10 | 8% | 25 | Varied | Varied | 6.5 yrs (1–15) | Only seen in FTBI recipients |
| Bailey et al. 2008 | 3 | 52% | 33 | Mainly malignancy | FTBI + Chemo | 4 yrs (0.5–8.5) | Unrelated Donors |
| Sanders et al. 2011 | 11 | 12% | 137 | SAA only | CY/CY + TBI | 21.8 yrs (1–38.1) | TBI |
Studies including only unfractionated TBI were excluded
AML Acute Myeloblastic Leukemia
ALL Acute Lymphoblastic Leukemia
SAA Severe Aplastic Anemia
PID Primary Immunodeficiency
BU Busulfan
CY Cyclophosphamide
FTBI Fractionated Total Body Irradiation
NR Not Reported
In general, radiation-based preparative regimens have been shown to be one of the most significant risk factors for the development of TD (12). Of note, fractionated TBI in combination with cyclophosphamide (CY) is not significantly more likely to cause thyroid dysfunction than busulfan (BU) in combination with CY or other alkylating agents. Since CY on its own as part of the preparative regimen for the transplant of patients with severe aplastic anemia (SAA) only induced a 7% incidence of thyroid dysfunction (2), it appears that the administration of BU may also be a significant risk factor, though other reports have challenged this (5–7).
Gaps in Current Knowledge
It remains to be determined if reduced-toxicity regimens incorporating BU in combination with the non-alkylating fludarabine produce a lower risk of TD than seen with classic BU plus CY regimens.
In children with SAA, single-agent GVHD prophylaxis was associated with extremely high rates of hypothyroidism 5 years post-HCT (82%) compared to those receiving 3-drug GVHD prophylaxis (16%) (13). Also children undergoing unrelated donor HCT are more likely to develop hypothyroidism than those receiving transplants from matched siblings (36% vs. 9%) (3). This suggests that a sub-clinical GVHD-like phenomena may play a role in the development of some cases of thyroid dysfunction following allogeneic HCT (7, 14).
Recommendations for Future Research
The majority of TD seen post-HCT is primary hypothyroidism, with central hypothyroidism being less common (2, 3). Therefore, local damage, especially from TBI, appears to be a common causal agent. To the best of our knowledge, an attempt to shield the thyroid during TBI has never been reported. However, this approach has been successfully applied to the gonads, thymus, eyes, and lungs (15–18). Obviously this approach would be easiest to implement in the rare patients receiving TBI for the treatment of a non-malignant condition. In patients with leukemia, care would need to be taken to ensure that the marrow cavities in the vicinity of the thyroid were properly treated, though actual recurrence of leukemia in the thyroid gland itself appears to be vanishingly rare (19). Another possibility would be to provide physiologic TSH-suppressive doses of exogenous thyroxine, in order to induce a metabolic quiescence in the thyroid gland. A small pilot trial in 14 patients undergoing irradiation for HL suggests that this method may be effective (20), and it would potentially be translatable to patients undergoing TBI. However, the delayed nature of TD post-HCT does present a significant barrier to adequately measuring the effect of an intervention, and TD is one of the easiest late effects to monitor for and manage, making prevention of TD a relatively lower priority topic to study.
GROWTH IMPAIRMENT
Problems obtaining final predicted adult height are a post-HCT complication unique to the pediatric population. Although pituitary production of growth hormone (GH) plays an important role in determining final height, many other factors play a role, including nutritional status, thyroid function, corticosteroid therapy, and the production of sex hormones during the pubertal growth spurt. One mechanism by which GH functions is via the stimulation of production of both insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 (IGF-BP3), a carrier molecule which enhances the plasma half-life of IGF-1. In patients with decreased linear growth, measurement of serum levels of these two molecules is thus an easy method to screen for true decreased production of GH, as opposed to other etiologies for growth impairment.
Current State of Knowledge
There are major difficulties in interpreting the literature on growth impairment following pediatric HCT due to differences in how growth impairment is defined. As seen in Table 2, the exact incidence of GI post-HCT varies widely between reports, likely due to differences in the age of the patients at the time of HCT, the type of preparative regimen utilized, and the inclusion of patients who did or did not receive additional cranial irradiation (CI). Nevertheless, the reports demonstrate incidences ranging from 20–85% (9, 21–24).
Table 2.
Studies of Growth Impairment Following Pediatric HCT*
| Reference | Incidence of Growth Impairment | Number | Disease | Conditioning | F/U Median (range) | Risk Factors | |
|---|---|---|---|---|---|---|---|
| Huma, et al. 1995 | 21 | 20% | 72 | Leukemia | FTBI + Chemo | 7.1 yrs (1.2–12.9) | CI, HCT for ALL |
| Giorgiani, et al. 1995 | 22 | 54% 9% |
37 22 |
Varied Varied |
FTBI + Chemo BU + Chemo |
> 2 yrs (NR) | Prior CI |
| Cohen, et al. 1996 | 23 | 82% | 28 | Varied | Varied | 7.9 yrs (3.2–11.4) | TBI, CI |
| Sanders, et al. 2005 | 24 | 84% | 107 | Varied | FTBI + CY + CI | 11 yrs (2.7–23) | CI, HCT for ALL |
| Leung, et al. 2007 | 9 | 39% | 155 | Varied | Varied | 9 yrs (3.1–15.9) | Young age; higher dose TBI |
| Dvorak, et al. 2008 | 10 | 24% | 24 | Varied | Varied | 6.5 yrs (1–15) | Only seen in FTBI recipients |
Studies including unfractionated TBI were excluded
BU Busulfan
CY Cyclophosphamide
FTBI Fractionated Total Body Irradiation
CI Cranial Irradiation
NR Not Reported
Clearly, irradiation-based conditioning regimens play the largest role in the development of growth impairment post-HCT (5, 9). However, the exact role of BU or other alkylators in the development of growth impairment is less clear. Several groups have reported low rates of growth impairment with BU-based regimens (5, 6, 22, 25), unless they also received CI. In patients with genetic diseases undergoing HCT, growth is usually not affected by BU-based preparative regimens (26), unless the HCT is performed during the adolescent growth spurt (27), HCT may actually accelerate growth in patients where the underlying disease (such as thalassemia) was inhibiting it (28).
Similar to TD, age less than 10 years at the time of HCT is associated with the highest risk of growth impairment (24, 25). Fortunately, younger patients also show the best response to GH administration (24).
Gaps in Current Knowledge
It is still unknown what the impact of GH deficiency is on aspects of metabolism unrelated to linear growth, such as muscle and lean body mass. Similarly, we do not yet understand what happens to GH deficient patients that are treated with recombinant GH during adolescence, but then discontinue GH replacement once epiphyseal fusion occurs and final adult height is obtained. Another major gap in current knowledge regarding growth impairment is what the impact of non-TBI-based conditioning regimens, especially newer reduced intensity and non-myeloablative regimens. It is possible that as we move away from TBI-containing regimens growth impairment due to biochemical GH deficiency following HCT may become a thing of the past, or only occur rarely such as in patients who have significant pre-HCT radiation exposures.
Recommendations for Future Research
Since irradiation likely plays the largest role in the development of this late effect, given the practical difficulties of shielding the pituitary gland the easiest step will be the development of preparative regimens that avoid the use of full-dose TBI or preferably even no TBI, while still providing optimal engraftment and protection from malignant relapse.
BONE HEALTH: LOW BONE MINERAL DENSITY
Current State of Knowledge
Only seven studies have been published that address bone loss in pediatric HCT recipients (Table 3). Only one of these studies was prospective (29), the remainder were cross-sectional, and most of them were limited by small sample size (10–66 participants) (30–35). Bone mineral density (BMD) was determined by a dual-energy X-ray absorptiometry (DXA) scan in most studies (33). A mild reduction in mean BMD Z-score (either total body or lumbar) was observed (mean about −1.0, range −5.2 to +2.3). A significant proportion of pediatric HCT recipients transplanted between 0.6 and 18 years of age had a Z-score between −1 and −2 (18–33%), and even below −2 (6–21%).
Table 3.
Studies of Low Bone Mineral Density (BMD) Following Pediatric HCT*
| Reference | Number | Disease | Age at HCT (years) | Age at Study (years) | F/U Median (range) | Z-score | Z-score −1 to −2 | Z-score <−2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total body | Lumbar | |||||||||
| Bhatia et al. 1998 | 30 | 10 | AML, CML | 5.0Mdn (3.0–18.0) | 12.0Mdn (4.0–22.0) | 2.0 yrsMdn (1.0–10.0) | −0.5Mdn (−2.0 to 1.0) | NR | NR | NR |
| Nysom et al. 2000 | 31 | 25 | ALL, AML, CML, NHL | 11.3Mdn (5.7–17.6) | 17.2Mdn (11.3–26.5) | 7.5 yrsMdn (3.6–12.6) | −0.5M | −0.5M | NR | NR |
| Daniels et al. 2003 | 32 | 15 | Varied | NR | 15.2M (9.4–17.7) | 6.3 yrsM (1.0–12.2) | −0.7 to −0.9M | −0.7 to −0.9M | NR | NR |
| Kaste et al. 2004 | 33 | 43 | Varied | 10.3Mdn (1.6–20.4) | 15.8Mdn (4.4–27.2) | 5.1 yrsMdn (1.0–10.2) | NR | −0.9Mdn,^ (−3.3 to 2.3) | 26% | 21% |
| Petryk et al. 2006 | 29 | 21 | Varied | 10.2M (5.1–17.9) | 10.2M (5.1–17.9) | ≤1.0 yrs | NR | −0.9M (−2.9 to 1.1) | 33% | 19% |
| Perkins et al. 2007 | 34 | 17 | ALL, AML | 1.7M (0.6–3.0) | 13.2M (3.8–23.8) | 11.6 yrsM (3.3–22.3) | NR | −0.3M (−2.4 to 2.0) | 18% | 6% |
| Carpenter et al. 2007 | 35 | 66 | ALL, others | (1.1–17.9) | (2.0–18.2) | NR | NR | −3.6 to −2.5Mdn (−5.2 to −0.9) | NR | NR |
Studies without data on lumbar and/or total body BMD were excluded
ALL Acute Lymphoblastic Leukemia
AML Acute Myeloblastic Leukemia
CML Chronic Myelogenous Leukemia
MDS Myelodysplastic Syndrome
NHL Non-Hodgkins Lymphoma
^ by QCT
Mmean
Mdnmedian
NR Not Reported
The pathogenesis of bone loss after HCT is multifactorial. Some of the risk factors are general (gender, age, physical inactivity, poor nutritional status, inadequate intake of calcium and/or vitamin D, Caucasian or Asian race, family history), and some are specific to cancer treatment and/or HCT (chemotherapy, TBI/craniospinal irradiation, the malignancy itself, corticosteroids, cyclosporine, G-CSF, endocrine deficiencies, including growth hormone deficiency and hypogonadism, GVHD or its treatment, direct effects of conditioning regimens on bone marrow stromal cells, cytokine release after HCT, and reduced production of growth factors) (36–46). Notably, however, many of the purported risk factors are only presumed as such based on their mode of action and effects on bone formation and/or resorption in various diseases, animal models, or in vitro experiments.
Only one prospective study in children has been published that longitudinally assessed changes in BMD before and after HCT (29). Most significant bone loss occurred within the first 6 months after transplantation. The number of patients with a Z-score below −1 increased from 34% at baseline to 52% one year after HCT. Several prospective studies in adult HCT recipients have demonstrated that a decrease in BMD is preceded by changes in the markers of bone turnover. A consistent finding in adult HCT is a decrease in bone formation and an increase in bone resorption.
There is insufficient data in pediatric HCT recipients to determine whether similar changes in bone turnover occur in children. Based on one prospective study, which examined the levels of serum bone specific alkaline phosphatase, osteocalcin (OCN), and urinary N-telopeptide before and up to 12 months after HCT (29), it appears that bone formation in children may be similarly reduced.
The mechanisms through which changes in bone turnover are evoked by the various risk factors have been previously reviewed (36, 40, 47). Of particular relevance to HCT recipients are direct effects of myeloablative regimens and the acute release of cytokines on osteoblastic and osteoclastic activity after HCT. Myeloablative therapy can directly damage the recipient’s osteoprogenitor cells within the bone marrow stroma independently of secondary effects on gonadal function and growth hormone secretion, negatively affecting bone formation (41, 48, 49). Moreover, a marked “cytokine storm” (IL-6, IL-7, G-CSF, M-CSF, TNF-α) occurs within the first 3 weeks after HCT, which stimulates osteoclasts and increases bone resorption (43, 47, 48, 50, 51). The main mediator of these pro-resorptive effects on bone is thought to be the RANK/RANKL/OPG pathway (52, 53). Receptor activator of the nuclear factor-κB ligand (RANKL) and RANKL/OPG (osteoprotegerin) ratio increase after HCT, reaching a peak at 3 weeks, stimulating osteoclastogenesis (48, 54). It is currently unknown whether similar changes occur in children after HCT and whether OPG, a decoy receptor that competes with RANKL can counterbalance these effects.
Some of these biomarkers could be used as predictors of bone recovery. In children, the OCN level at 100 days after HCT predicted recovery from the initial bone loss by 1 year (29). In adult HCT recipients, lower IGF-I levels and higher cytokine levels after HCT correlated with lower BMD at 1 year (43, 48, 54).
Studies in adult HCT recipients have shown that BMD can improve years after HCT (39, 47, 55). Since peak bone mineral accretion occurs during adolescence and young adulthood, children who undergo HCT at a very young age would presumably still have time to regain BMD. Data to support this presumption is limited. Some studies have shown that, the potential for stromal reconstitution after HCT may be greater young children (34), especially those younger than 5 years compared to older than 8 years (49). However, Bhatia et al. found a positive correlation between age at HCT and BMD (30). Thus, it remains to be determined whether there is an age effect and how it affects BMD outcome.
Gaps in Current Knowledge
The limitations of the current studies in pediatric HCT recipients are several-fold. The first is that the majority of the studies have small sample sizes and are cross-sectional. Furthermore, it is unclear whether there are age-related differences in predisposition to bone loss and/or subsequent BMD recovery after HCT. In addition, markers of bone turnover have been insufficiently studied to determine if bone resorption is increased after HCT and there is a potential for identifying biomarkers that would help identify patients at risk who would require closer follow up and an appropriate intervention to prevent or reverse bone loss.
Recommendations for Future Research
Prospective controlled studies are needed to define the time course of changes in bone turnover and BMD prior to and following HCT, incidence and severity of bone loss, and whether age at HCT is a factor in BMD recovery. By identifying risk factors, these studies should better define a population at risk. Future studies should also have multiple biologic aims: to identify the prevalence and the degree of vitamin D deficiency (which may be a significant contributor in certain geographic regions), to define biological predictors of BMD recovery, and to decide which markers of bone formation and resorption are most informative in children. Finally, we need to identify the time period and suitable mode of intervention to provide patients with weight-bearing exercise after HCT, as this intervention has been clearly shown to improve BMD.
BONE HEALTH: OSTEONECROSIS
Current State of Knowledge
Osteonecrosis (ON) was first recognized as a complication of HCT in 1987 (56). Only a few papers have addressed the occurrence of ON in pediatric HCT recipients where the prevalence ranges from 1.3% to 14% (9, 57–59), with even higher occurrence (44%) being found in pediatric allogeneic HCT recipients who underwent routine screening for ON by MRI (33). The true prevalence, however, is unknown as it can only be determined by prospective screening with MR, which is a much more sensitive method of detection of ON than plain radiographs (12, 58, 60–62).
In children, knees (31–40%) are the most frequent site of ON, followed by hips (19–24%), shoulders (9%), and other sites (33, 63–65). The majority of patients manifest ON in two or more joints (12, 59, 63, 65). Typically, ON occurs within 3 years after HCT, the earliest time point being 1–6 months after the onset of steroid therapy, particularly if MRI is used for detection (45, 59, 62, 63, 65–68). A median interval for the development of ON is 11 months after HCT in children (57).
Patients usually present with either vague, diffuse bone pain, presumably due to increased intraosseous pressure, or joint-related pain due to an effusion. Hip involvement is typically manifested by groin pain. Once subchondral collapse and articular deformity occur, arthritic-type joint pain predominates accompanied by functional limitation (limp, reduced range of motion) (66, 69) However, during early stages of ON, patients may have mild transient bone pain during treatment or they may be completely asymptomatic and not necessarily progress to symptomatic disease (33, 62). If left untreated, joint destruction usually occurs within 1–5 years after the onset of symptoms (66, 70). Once disease progresses beyond a certain point, collapse of necrotic bone is inevitable. The reparative processes are usually ineffective, and actually counterproductive, leading to further separation of acellular necrotic bone tissue from viable tissue by a fibrous layer, preventing revascularization (68, 69, 71). The risk of subchondral fracture of the necrotic bone leading to joint collapse is determined by the size (best assessed using the necrotic arc index) and location of the necrotic lesion (72). For example, involvement of less than 10–15% of the femoral head and less than a third of the weight-bearing portion carries a good prognosis, while involvement of more than 25% of the femoral head or more than two thirds of the weight-bearing portion carries a poor prognosis (68, 69, 73).
The pathogenesis of ON is multifactorial. Several mechanisms have been proposed, including increased intraosseous pressure or intraluminal obliteration that compromise intramedullary blood flow, leading to marrow ischemia, and ultimately necrosis (65) (Figure 1). The likely contributing mechanisms are defective bone repair due to damage to the bone marrow stroma, immunosuppression as well as radiation and drug induced injury to the vessel wall and vasculitis (59, 66, 68, 69, 71, 74–77).
Figure 1.
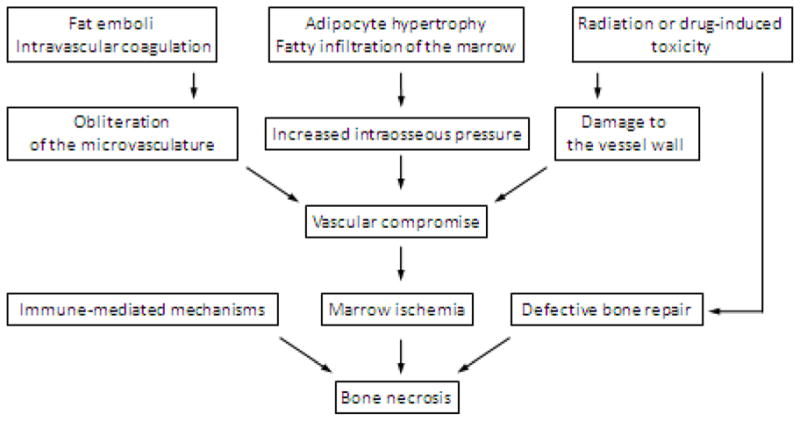
Pathophysiology of AVN after HCT
There are multiple risk factors for the development of ON which occurs in pediatric HCT recipients at a median age of 14.4 years (57). Higher incidence of ON has been reported in patients exposed to TBI-based conditioning regimens (57, 59, 78), presumably due to radiation-induced microvascular damage (79), in adult patients with acute leukemia and aplastic anemia compared to AML, CML, and other diagnoses (59), and in recipients of allogeneic HCT (particularly unrelated) compared to autologous HCT (66, 74, 78). The latter likely explains an increase in risk in patients transplanted after 1985 when unrelated donor HCTs became more common and newer immunosuppressive agents were introduced (74). The data about the association between gender and the incidence of ON have been inconsistent. While GVHD, both acute and chronic, has been associated with ON, it is unclear whether it plays an independent pathogenic role since it is strongly correlated with the use of steroids (57–59, 66, 74, 80). An argument for an additional independent role of GVHD is that it increases the risk for microangiopathy (81–83). Corticosteroids are the strongest risk factor for ON, with both the cumulative dose and duration of treatment playing a role (57, 58, 65, 66, 77, 78, 84–86). Several mechanisms for this effect have been proposed, including altered lipid metabolism, adipocyte hypertrophy, stimulation of adipogenic differentiation of bone marrow stem cells at the expense of osteogenic differentiation, leading to the formation of fat emboli and fatty infiltration of the bone marrow, or a direct effect on osteocyte apoptosis (67, 68). The risk of ON increases with the number of drugs used for immunosuppression, including prednisone, cyclosporine (CSA), tacrolimus (FK506), and mycophenolate mofetil (MMF) (74, 78), due to their thrombogenic effects, as well as through vascular damage and dyslipidemia (87–90)
An association between low BMD and ON has not been characterized beyond the observation that both can coexist in pediatric HCT patients (65). While the causative link is absent, it is conceivable that higher BMD would likely improve the biomechanical properties of the bone, and perhaps delay the collapse of the necrotic bone. There is a significant overlap between the risk factors for low BMD and ON. In addition, the two conditions may share pathogenic pathways, and therefore have additive effects. For example, impaired osteoblast activity contributes to reduced BMD in both pediatric HCT and adult HCT patients (29, 43, 48, 91–93). Reduced number of osteoblast precursors may in turn adversely affect the regenerative potential of the osteogenic compartment and the course of ON (66, 77, 94, 95)
Gaps in Current Knowledge
The major limitation of current studies in pediatrics is the lack of an animal model, an insufficient number of patients to allow identification of patients at high risk for progression of ON, and inadequate assessment of asymptomatic disease. Furthermore, there is no consensus regarding optimal screening and treatment of early stage ON. Clearly MRI is the most sensitive screening modality, however, its cost effectiveness, especially in view of the lack of a reliably effective intervention in early stage disease, remains an obstacle.
Recommendations for Future Research
Prospective studies with screening MRIs are needed to identify the incidence of ON after pediatric HCT, the natural history of asymptomatic ON detected by MRI, and appropriate preventive interventions. Given the significant prevalence of ON in non-HCT ALL patients, as part of a prospective study, a baseline MRI would need to be performed on all patients prior to HCT. Only then will progress be made in discerning the effectiveness and safety of pharmacologic interventions in preventing post-HCT ON.
REPRODUCTIVE RISKS
The reproductive risks of HCT include gonadal failure, infertility, and pubertal failure.
Current State of Knowledge
Post-Pubertal Individuals
Females
The ovary is particularly sensitive to the adverse effects of cancer treatments because of the finite number of germ cells present in the post-natal ovary (96, 97). Since reproductive lifespan is determined by the size of the follicular pool, cancer treatments that cause follicular depletion accelerate the onset of menopause (98). The irreversible gonadotoxic effects of some chemotherapeutic agents are well documented, particularly for alkylating agents such as CY (99, 100). In women 30–39 years of age, a dose of 9 gm/m2 of CY results in ovarian failure, while in women who are a decade younger, 20 gm/m2 causes a similar effect. In contrast, the prepubescent female has been shown to tolerate as much as 25–30 gm/m2 of CY and still retain ovarian function (101).
Ovarian failure after HCT has been observed in 65–84% of pediatric transplant recipients (Table 4) (165–170). Exposure to CY, BU, and TBI are associated with gonadal failure while younger age at transplant is associated preservation of menstrual function (102, 103). It is important to recognize that studies assessing fertility after HCT are limited by the fact that they have not accounted for whether patients were actually trying to conceive. One of the most comprehensive studies of pregnancy in pediatric and adult HSC survivors reported that 32/708 (4.5%) of post-pubertal females became pregnant after HCT (104). Pregnancies were most likely to be reported in patients who had been exposed to CY only conditioning regimens (56/103, 54% reported pregnancy), compared to BU/CY (0/73, 0% reported pregnancy) or TBI (7/532, 1.3%). In general, studies indicate that fertility is most likely to be preserved in patients who undergo transplant as young adults (15–30 years) and receive non-TBI based conditioning regimens. Most pregnancies occur 5–10 years post-transplant. Nonetheless, pregnancy has been reported in patients who received high dose alkylator-based conditioning and TBI, and even in patients who underwent more than one transplant.
Table 4.
Studies of Fertility Following HCT
| Reference | Type of Study | Number of Patients | Summary of Findings | |
|---|---|---|---|---|
| Sanders et al. 1996 | 104 | Retrospective Cohort | 1326 (708 females, 618 males) | 15.5% of females had ovarian function & 4.5% conceived 24.4% of males had testicular function & 5.7% of partners conceived 79% of pregnancies resulted in a live birth Females had an increased risk of PTD and LBW infants |
| Salooja et al. 2001 | 132 | Retrospective Cohort | 37,362 (all females) | 0.6% HCT recipients or female partners conceived 87% of pregnancies resulted in a live birth Female HCT recipients had an increased risk of PTD, LBW, and Cesarian Deliveries |
| Carter et al. 2006 | 131 | Retrospective Cohort | 619 (292 females, 327 males) >2 years post-HCT Compared to sibling controls |
5.5% HCT recipients or female partners conceived 85% of pregnancies resulted in a live birth Risk of miscarriage and stillbirth was no different that sibling controls Older age, female sex, exposure to TBI = less conception |
| Loren et al. 2011 | 133 | Case Series | 178 HCT (83 females, 95 males) | Fertility is most likely to be preserved in patients: undergo HCT as young adults (15–30 years) receive non-TBI conditioning regimens males |
PTD Preterm Deliveries
LBW Low Birth Weight
Males
Unlike the female, germ cells in the testes normally continue to produce sperm during adulthood. However, conditioning therapies such as CY and TBI destroy germ cells within the testes leading to low or absent sperm production which can subsequently lead to infertility (105). Impairment of spermatogenesis may be permanent or temporary following chemotherapy (105, 106). The chance of recovery of spermatogenesis following cytotoxic therapy and the extent and speed of recovery are related to the agent used and the dose received. Azoospermia develops in the majority of post-pubertal males exposed to over of 300 mg/kg of cyclophosphamide (105). Moreover, spermatogenesis is exquisitely sensitive to radiation and low doses (over 2–3 Gy) can cause significant impairment in function. Many (48–85%) males who undergo HCT will experience testicular failure with azoospermia (104, 105, 107). Similar to females, the risk of gonadal failure appears to be dependent on the type of therapy and the doses administered. Fertility data in the transplant population are limited and summarized in Table 4. A large study of HCT survivors found that 32/618 post-pubertal males fathered a child. Pregnancies were most likely to be reported in patients who had been exposed to CY only conditioning regimens (26/109, 24% reported pregnancy), compared to BU/CY (3/46, 6.5% reported pregnancy) or TBI (6/463, 1.3%) (104).
Pre-Pubertal Individuals
Normal pubertal development requires a functioning hypothalamic-pituitary-gonadal axis. HCT can result in pubertal delay or failure in both sexes. Incomplete pubertal development or pubertal failure has been reported to occur in approximately 57% of prepubescent females following HCT (2). However the risk of delayed puberty is dependent on the conditioning regimen administered (16% after 200 mg/kg CY alone, 72% after 16 mg/kg BU plus 120–200 mg/kg CY, 71% after 10Gy single-exposure TBI, 57% after 12–15.75 Gy TBI) (11, 108). In males, incomplete pubertal development or pubertal failure has been reported to occur in approximately 53% of prepubescent males exposed to HCT (109). Similar to females, the risk of delayed puberty is dependent on the conditioning regimen administered (14% after 200 mg/kg CY alone, 48% after 16 mg/kg BU plus 120–200 mg/kg CY, 81% after 10Gy single-exposure TBI, 58% after 12–15.75 Gy TBI) (11, 108). Boys who receive high dose (>24 Gy) testicular irradiation for testicular relapse have a very high risk of pubertal failure requiring testosterone replacement to develop secondary sexual characteristics (109).
Fertility Preservation in Females
The ability to lead full reproductive lives is very important to both female and male HCT survivors. Indeed, there is evidence that HCT survivors have persistently elevated concerns about their fertility even 10 years after treatment (110). Therefore, there has been increasing interest in methods to expand the reproductive options for patients facing fertility-threatening treatments. While embryo cryopreservation remains the standard option for adult females with a committed sexual partner, oocyte cryopreservation and ovarian tissue cryopreservation (OTC) technologies have become clinically-available experimental options for females without a partner. These fertility preservation technologies have gained traction, particularly after the publication of the ASCO fertility preservation recommendations in 2006 (111). However, embryo and oocyte cryopreservation are limited by the need for ovarian stimulation and oocyte retrieval, which can delay treatment 2–4 weeks. This delay in treatment is usually not an option for patients with leukemia, who tend to be quite ill with impaired blood counts at initial presentation. OTC eliminates the need for ovarian stimulation and does not require a sperm source. While investigational, live births have been reported following OTC and transplantation in cancer patients (112). Currently, this is the only method available for fertility preservation in pre-pubertal girls (113–116). There is a significant concern regarding the potential for reseeding tumor cells following ovarian transplantation procedures in cancers that involve the ovary, such as leukemia. A recent study of 18 patients with leukemia (CML or ALL) showed that leukemic tumors occurred (4/18 cases) after thawed human ovarian cortical tissue was xenografted into mice (117). Therefore, transplantation of ovarian tissue is not recommended in patients with a history of leukemia. In order to achieve pregnancy without transplantation, it would be necessary to mature and fertilize oocytes from ovarian tissue in vitro for embryo transfer. This has only been possible in the mouse and ongoing studies are being conducted to move this technology forward (118).
Ovarian Suppression
The observation that cancer therapies were less gonadotoxic in pre-pubertal girls led to speculation that ovarian suppression in post-pubertal females might reduce the negative impact of cancer therapies on the ovary. Ovarian suppression with GNRH analogues administered during chemotherapy is the most common method of ovarian suppression employed. Several small short-term studies comparing GNRHa plus chemotherapy to chemotherapy alone have demonstrated that menstrual function is more likely to be preserved in women who receive GNRHa during treatment (119–121). However, there are insufficient data to support the use of GNRH agonists in transplant recipients for the purpose of fertility preservation (121). Nonetheless, GNRH analogues have been shown to reduce menstrual bleeding during cancer therapy and may be useful for that purpose in the HCT population (122).
Fertility Preservation in Males
Sperm cryopreservation remains the best option for fertility preservation in adolescent and adult males diagnosed with cancer. All adolescents and young adults facing cancer therapy should be offered sperm cryopreservation as a way to preserve future fertility (123). Ideally, multiple samples should by cryopreserved before cancer treatment has begun. In situations where self-stimulation is unsuccessful, vibratory stimulation, electroejaculation, or surgical sperm extraction may be used to obtain sperm (124–127). Even though sperm banking is a relatively simple process, there is evidence that oncologists do not routinely discuss this option with their patients (128). Fertility preservation in pre-pubertal boys remains problematic and is an active area of investigation. Extracting and cryopreserving spermatogonial stem cells from boys in order to later autograft or to mature sperm in vitro are promising avenues of investigation. While transplantation of cryopreserved testicular tissue has been successful in mice and rats, data in humans are lacking (129, 130).
Pregnancy Outcomes
Overall, pregnancy outcomes appear to be reassuring in survivors of HCT (104, 131–133). Most pregnancies reported by HCT survivors and their partners result in a live birth. However, in female HCT survivors who were exposed to TBI, there appears to be an increased risk of preterm delivery and delivery of low birth weight infants. This is consistent with literature in childhood cancer survivors and is thought to be related to radiation-induced structural changes in the uterus (134, 135). In addition, female HCT survivors are at higher risk of cesarean section compared to the normal population (42% vs. 16%). This observation may be related to the perception that transplant survivors are higher risk and therefore pregnancies are managed differently than the general population. While pregnancy outcomes of male survivors of HCT have been reported to be reassuring overall, one study of childhood cancer survivors reported that the likelihood of having a live birth was lower among survivors compared to siblings (RR 0.77, p = 0.007) (136). Nonetheless, offspring of male and female HCT recipients do not appear to be at increased risk for birth defects, developmental delay, or cancer (132).
Gaps in Current Knowledge
While data from retrospective cohort studies exist estimating the risk of pubertal problems and gonadal failure after HSC, there are no accurate estimates of fertility in this population. It must be recognized that retrospective reports of pregnancy after HCT are limited by ascertainment bias and do not determine whether patients have actually tried to conceive and have experienced difficulty, or whether HCT survivors simply are less likely to attempt pregnancy. Moreover, there are limited data assessing the reproductive risk of newer conditioning regimens prior to HCT. While lower-intensity conditioning regimens without exposure to CY and TBI appear to be less deleterious to reproductive function, more research is needed to determine which regimens are least gonadotoxic but equally effective for treatment. Such information would be useful in order to adequately counsel patients regarding their reproductive horizon and target fertility preservation technologies to those at highest risk.
After HCT, some females will resume menstrual function. While measures of ovarian reserve are likely to be impaired post treatment, it is not clear whether these measures predict fertility and age at menopause in cancer survivors. Understanding the significance of measures of ovarian reserve would greatly improve counseling about fertility, contraception, and long-term ovarian function post-HCT.
There are also significant gaps in knowledge in the areas of contraception and hormone replacement therapy after HCT. While the risk profile of hormonal contraceptives may be less favorable in HCT survivors with concomitant medical problems compared to the general population, the safety and efficacy of contraception has not been studied in this population. Moreover, in female HCT survivors with premature ovarian failure, the optimal regimen for hormone replacement therapy is not known. Data on the long-term benefits and risks to the reproductive system of various regimens used for HCT are lacking.
Recommendations for Future Research
As outlined above, major gaps in knowledge regarding the reproductive risks, fertility preservation options, and long term contraceptive and endocrine needs of the HSC population exist. Therefore, there is an urgent need to conduct research in various aspects of reproductive health in the transplant population. Large prospective cohort studies assessing clinically meaningful reproductive outcomes in HCT recipients receiving newer preparative regimens are needed to better define the reproductive risks associated with these therapies. Furthermore, more data are needed to determine whether ovarian suppression during HCT decreases risk of reproductive and/or gonadal failure.
Additional research is necessary to determine whether measures of ovarian reserve predict fertility and time to menopause in menstruating transplant survivors. This would greatly improve post treatment counseling regarding fertility, contraception and anticipated timing of menopause. Studies assessing the safety and efficacy of contraceptives and hormone replacement regimens may transform care and could have a major impact on the long health and quality of life of HCT recipients.
CONCLUSIONS
The developing child is significantly more likely to be affected by endocrinopathies or poor bone health than a fully mature adult, therefore, the need for research and interventions in this unique patient population is significant. A large prospective trial which evaluates pre-HCT endocrine function and then follows the same tests on a routine basis post-HCT would significantly add to our current knowledge and assist in defining interventions.
Although thyroid dysfunction and growth impairment can be managed with replacement hormones, this damage can be permanent and result in life-long need for medications. Similarly, low bone mineral density or avascular necrosis following HCT can lead to significant long-term problems with ambulation and quality of life. Finally, for many parents of young children being considered for HCT, the thought that their child may be sterile can be emotionally devastating and may prohibit them from proceeding with HCT. Advances that could minimize this risk would likely increase the acceptance of HCT as a therapeutic alternative, particularly for some non-malignant conditions. Thus, in order to maximize both access to HCT and the quality of life post-HCT for our pediatric patients, further research is urgently needed in the field of endocrinopathies, bone health, and fertility.
Acknowledgments
Funding for this work was made possible in part by the following National Institute of Health grants: 1R13CA159788-01 (MP, KSB), U01HL069254 (MP), R01 CA112530-05 (KSB). The views expressed in this manuscript do not reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government. Further support was provided by a generous grant from the St. Baldrick’s Foundation and the Lance Armstrong Foundation, as well as the following pharmaceutical companies: Genzyme, Otsuka America Pharmaceutical, Inc., and Sigma-Tau Pharmaceuticals, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of those that provided funding. A.P. would like to thank Dr. Lynda Polgreen for her helpful comments.
Financial Disclosures: CCD, AP, JS, and CG have nothing to disclose. EYC would like to disclose a limited financial relationship (<$5000) with Innomed and Biomet, and past research funding from Aastrom Biosciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher C. Dvorak, Email: dvorakc@peds.ucsf.edu.
Clarisa R. Gracia, Email: cgracia@obgyn.upenn.edu.
Jean E. Sanders, Email: jsanders@fhcrc.org.
Edward Y. Cheng, Email: cheng002@umn.edu.
K. Scott Baker, Email: ksbaker@fhcrc.org.
Michael A. Pulsipher, Email: michael.pulsipher@hsc.utah.edu.
Anna Petryk, Email: petry005@umn.edu.
References
- 1.Baker K, Ness K, Weisdorf D, Francisco L, Sun C, Forman S, et al. Late effects in survivors of acute leukemia treated with hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Leukemia. 2010;24(12):2039–47. doi: 10.1038/leu.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders J, Hoffmeister P, Woolfrey A, Carpenter P, Storer B, Storb R, et al. Thyroid function following hematopoietic cell transplantation in children: 30 years’ experience. Blood. 2009;113(2):306–8. doi: 10.1182/blood-2008-08-173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey H, Kappy M, Giller R, Gralla J. Time-course and risk factors of hypothyroidism following allogeneic hematopoietic stem cell transplantation (HSCT) in children conditioned with fractionated total body irradiation. Pediatr Blood Cancer. 2008;51(3):405–9. doi: 10.1002/pbc.21634. [DOI] [PubMed] [Google Scholar]
- 4.Ishiguro H, Yasuda Y, Tomita Y, Shinagawa T, Shimizu T, Morimoto T, et al. Long-term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescence. J Clin Endocrinol Metab. 2004;89(12):5981–6. doi: 10.1210/jc.2004-0836. [DOI] [PubMed] [Google Scholar]
- 5.Michel G, Socié G, Gebhard F, Bernaudin F, Thuret I, Vannier J, et al. Late effects of allogeneic bone marrow transplantation for children with acute myeloblastic leukemia in first complete remission: the impact of conditioning regimen without total-body irradiation--a report from the Société Française de Greffe de Moelle. J Clin Oncol. 1997;15(6):2238–46. doi: 10.1200/JCO.1997.15.6.2238. [DOI] [PubMed] [Google Scholar]
- 6.Afify Z, Shaw P, Clavano-Harding A, Cowell C. Growth and endocrine function in children with acute myeloid leukaemia after bone marrow transplantation using busulfan/cyclophosphamide. Bone Marrow Transplant. 2000;25(10):1087–92. doi: 10.1038/sj.bmt.1702384. [DOI] [PubMed] [Google Scholar]
- 7.Slatter M, Gennery A, Cheetham T, Bhattacharya A, Crooks B, Flood T, et al. Thyroid dysfunction after bone marrow transplantation for primary immunodeficiency without the use of total body irradiation in conditioning. Bone Marrow Transplant. 2004;33(9):949–53. doi: 10.1038/sj.bmt.1704456. [DOI] [PubMed] [Google Scholar]
- 8.Berger C, Le-Gallo B, Donadieu J, Richard O, Devergie A, Galambrun C, et al. Late thyroid toxicity in 153 long-term survivors of allogeneic bone marrow transplantation for acute lymphoblastic leukaemia. Bone Marrow Transplant. 2005;35(10):991–5. doi: 10.1038/sj.bmt.1704945. [DOI] [PubMed] [Google Scholar]
- 9.Leung W, Ahn H, Rose SR, Phipps S, Smith T, Gan K, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore) 2007;86(4):215–24. doi: 10.1097/MD.0b013e31812f864d. Epub 2007/07/17. [DOI] [PubMed] [Google Scholar]
- 10.Dvorak C, Wright N, Wong W, Kristovich K, Matthews E, Weinberg K, et al. Safety of hematopoietic stem cell transplantation in children less than three years of age. Pediatr Hematol Oncol. 2008;25(8):705–22. doi: 10.1080/08880010802243524. [DOI] [PubMed] [Google Scholar]
- 11.Sanders JE, Woolfrey AE, Carpenter PA, Storer BE, Hoffmeister PA, Deeg HJ, et al. Late effects among pediatric patients followed for nearly 4 decades after transplantation for severe aplastic anemia. Blood. 2011;118(5):1421–8. doi: 10.1182/blood-2011-02-334953. Epub 2011/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Socie G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101(9):3373–85. doi: 10.1182/blood-2002-07-2231. Epub 2003/01/04. [DOI] [PubMed] [Google Scholar]
- 13.Katsanis E, Shapiro R, Robison L, Haake R, Kim T, Pescovitz O, et al. Thyroid dysfunction following bone marrow transplantation: long-term follow-up of 80 pediatric patients. Bone Marrow Transplant. 1990;5(5):335–40. [PubMed] [Google Scholar]
- 14.Savani B, Koklanaris E, Le Q, Shenoy A, Goodman S, Barrett A. Prolonged chronic graft-versus-host disease is a risk factor for thyroid failure in long-term survivors after matched sibling donor stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2009;15(3):377–81. doi: 10.1016/j.bbmt.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishiguro H, Yasuda Y, Tomita Y, Shinagawa T, Shimizu T, Morimoto T, et al. Gonadal shielding to irradiation is effective in protecting testicular growth and function in long-term survivors of bone marrow transplantation during childhood or adolescence. Bone Marrow Transplant. 2007;39(8):483–90. doi: 10.1038/sj.bmt.1705612. [DOI] [PubMed] [Google Scholar]
- 16.MacMillan M, Blazar B, DeFor T, Dusenbery K, Wagner J. Thymic shielding (TS) in recipients of total body irradiation (TBI) and alternative donor hematopoietic stem cell transplant (AD-HSCT): reduced risk of opportunistic infection in patients with Fanconi anemia (FA) Blood. 2006;108:Abstract #3134. [Google Scholar]
- 17.van Kempen-Harteveld M, van Weel-Sipman M, Emmens C, Noordijk E, van der Tweel I, Révész T, et al. Eye shielding during total body irradiation for bone marrow transplantation in children transplanted for a hematological disorder: risks and benefits. Bone Marrow Transplant. 2003;31(12):1151–6. doi: 10.1038/sj.bmt.1704076. [DOI] [PubMed] [Google Scholar]
- 18.Savani B, Montero A, Wu C, Nlonda N, Read E, Dunbar C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(3):223–30. doi: 10.1016/j.bbmt.2004.12.328. [DOI] [PubMed] [Google Scholar]
- 19.Byrd J, Dow N, Gaertner E, Hargis J, Raber T, Burrell L, et al. Leukemic thyroiditis as the initial relapsing sign in a patient with acute lymphocytic leukemia and blast expression of the neural cell adhesion molecule. Am J Hematol. 1997;55(4):212–5. doi: 10.1002/(sici)1096-8652(199707)55:4<212::aid-ajh9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Massimino M, Gandola L, Pignoli E, Seregni E, Marchianò A, Pecori E, et al. TSH suppression as a possible means of protection against hypothyroidism after irradiation for childhood Hodgkins lymphoma. Pediatr Blood Cancer. 2011;57(1):166–8. doi: 10.1002/pbc.22915. [DOI] [PubMed] [Google Scholar]
- 21.Huma Z, Boulad F, Black P, Heller G, Sklar C. Growth in children after bone marrow transplantation for acute leukemia. Blood. 1995;86(2):819–24. [PubMed] [Google Scholar]
- 22.Giorgiani G, Bozzola M, Locatelli F, Picco P, Zecca M, Cisternino M, et al. Role of busulfan and total body irradiation on growth of prepubertal children receiving bone marrow transplantation and results of treatment with recombinant human growth hormone. Blood. 1995;86(2):825–31. [PubMed] [Google Scholar]
- 23.Cohen A, Rovelli A, Van-Lint M, Uderzo C, Morchio A, Pezzini C, et al. Final height of patients who underwent bone marrow transplantation during childhood. Arch Dis Child. 1996;74(5):437–40. doi: 10.1136/adc.74.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders J, Guthrie K, Hoffmeister P, Woolfrey A, Carpenter P, Appelbaum F. Final adult height of patients who received hematopoietic cell transplantation in childhood. Blood. 2005;105(3):1348–54. doi: 10.1182/blood-2004-07-2528. [DOI] [PubMed] [Google Scholar]
- 25.Cohen A, Rovelli A, Bakker B, Uderzo C, van Lint M, Esperou H, et al. Final height of patients who underwent bone marrow transplantation for hematological disorders during childhood: a study by the Working Party for Late Effects-EBMT. Blood. 1999;93(12):4109–15. [PubMed] [Google Scholar]
- 26.Brachet C, Heinrichs C, Tenoutasse S, Devalck C, Azzi N, Ferster A. Children with sickle cell disease: growth and gonadal function after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2007;29(7):445–50. doi: 10.1097/MPH.0b013e31806451ac. [DOI] [PubMed] [Google Scholar]
- 27.Eggleston B, Patience M, Edwards S, Adamkiewicz T, Buchanan G, Davies S, et al. Effect of myeloablative bone marrow transplantation on growth in children with sickle cell anaemia: results of the multicenter study of haematopoietic cell transplantation for sickle cell anaemia. Br J Haematol. 2007;136(4):673–6. doi: 10.1111/j.1365-2141.2006.06486.x. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Chik K, Wong G, Cheng P, Lee V, Shing M. Growth and endocrine function following bone marrow transplantation for thalassemia major. Pediatr Hematol Oncol. 2004;21(5):411–9. doi: 10.1080/08880010490457132. [DOI] [PubMed] [Google Scholar]
- 29.Petryk A, Bergemann TL, Polga KM, Ulrich KJ, Raatz SK, Brown DM, et al. Prospective study of changes in bone mineral density and turnover in children after hematopoietic cell transplantation. J Clin Endocrinol Metab. 2006;91(3):899–905. doi: 10.1210/jc.2005-1927. Epub 2005/12/15. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia S, Ramsay NK, Weisdorf D, Griffiths H, Robison LL. Bone mineral density in patients undergoing bone marrow transplantation for myeloid malignancies. Bone Marrow Transplant. 1998;22(1):87–90. doi: 10.1038/sj.bmt.1701275. [DOI] [PubMed] [Google Scholar]
- 31.Nysom K, Holm K, Michaelsen KF, Hertz H, Jacobsen N, Muller J, et al. Bone mass after allogeneic BMT for childhood leukaemia or lymphoma. Bone Marrow Transplant. 2000;25(2):191–6. doi: 10.1038/sj.bmt.1702131. [DOI] [PubMed] [Google Scholar]
- 32.Daniels MW, Wilson DM, Paguntalan HG, Hoffman AR, Bachrach LK. Bone mineral density in pediatric transplant recipients. Transplantation. 2003;76(4):673–8. doi: 10.1097/01.TP.0000076627.70050.53. [DOI] [PubMed] [Google Scholar]
- 33.Kaste SC, Shidler TJ, Tong X, Srivastava DK, Rochester R, Hudson MM, et al. Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33(4):435–41. doi: 10.1038/sj.bmt.1704360. [DOI] [PubMed] [Google Scholar]
- 34.Perkins JL, Kunin-Batson AS, Youngren NM, Ness KK, Ulrich KJ, Hansen MJ, et al. Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatr Blood Cancer. 2007;49(7):958–63. doi: 10.1002/pbc.21207. Epub 2007/05/03. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter P, Hoffmeister P, Chesnut C, Storer B, Charuhas P, Woolfrey A, et al. Bisphosphonate therapy for reduced bone mineral density in children with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(6):683–90. doi: 10.1016/j.bbmt.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Weilbaecher KN. Mechanisms of osteoporosis after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2000;6(2A):165–74. doi: 10.1016/s1083-8791(00)70039-5. Epub 2000/05/18. [DOI] [PubMed] [Google Scholar]
- 37.Rodgers C, Monroe R. Osteopenia and osteoporosis in pediatric patients after stem cell transplant. J Pediatr Oncol Nurs. 2007;24(4):184–9. doi: 10.1177/1043454207303942. Epub 2007/06/26. [DOI] [PubMed] [Google Scholar]
- 38.McClune BL, Polgreen LE, Burmeister LA, Blaes AH, Mulrooney DA, Burns LJ, et al. Screening, prevention and management of osteoporosis and bone loss in adult and pediatric hematopoietic cell transplant recipients. Bone Marrow Transplant. 2011;46(1):1–9. doi: 10.1038/bmt.2010.198. Epub 2010/08/24. [DOI] [PubMed] [Google Scholar]
- 39.Schulte CM, Beelen DW. Bone loss following hematopoietic stem cell transplantation: a long-term follow-up. Blood. 2004;103(10):3635–43. doi: 10.1182/blood-2003-09-3081. [DOI] [PubMed] [Google Scholar]
- 40.Schimmer AD, Minden MD, Keating A. Osteoporosis after blood and marrow transplantation: clinical aspects. Biol Blood Marrow Transplant. 2000;6(2A):175–81. doi: 10.1016/s1083-8791(00)70040-1. Epub 2000/05/18. [DOI] [PubMed] [Google Scholar]
- 41.Banfi A, Podesta M, Fazzuoli L, Sertoli MR, Venturini M, Santini G, et al. High-dose chemotherapy shows a dose-dependent toxicity to bone marrow osteoprogenitors: a mechanism for post-bone marrow transplantation osteopenia. Cancer. 2001;92(9):2419–28. doi: 10.1002/1097-0142(20011101)92:9<2419::aid-cncr1591>3.0.co;2-k. Epub 2001/12/18. [DOI] [PubMed] [Google Scholar]
- 42.Castaneda S, Carmona L, Carvajal I, Arranz R, Diaz A, Garcia-Vadillo A. Reduction of bone mass in women after bone marrow transplantation. Calcif Tissue Int. 1997;60(4):343–7. doi: 10.1007/s002239900240. Epub 1997/04/01. [DOI] [PubMed] [Google Scholar]
- 43.Lee WY, Baek KH, Rhee EJ, Tae HJ, Oh KW, Kang MI, et al. Impact of circulating bone-resorbing cytokines on the subsequent bone loss following bone marrow transplantation. Bone Marrow Transplant. 2004;34(1):89–94. doi: 10.1038/sj.bmt.1704535. Epub 2004/06/01. [DOI] [PubMed] [Google Scholar]
- 44.Baker KS, Gurney JG, Ness KK, Bhatia R, Forman SJ, Francisco L, et al. Late effects in survivors of chronic myeloid leukemia treated with hematopoietic cell transplantation: results from the Bone Marrow Transplant Survivor Study. Blood. 2004;104(6):1898–906. doi: 10.1182/blood-2004-03-1010. [DOI] [PubMed] [Google Scholar]
- 45.Stern JM, Sullivan KM, Ott SM, Seidel K, Fink JC, Longton G, et al. Bone density loss after allogeneic hematopoietic stem cell transplantation: a prospective study. Biol Blood Marrow Transplant. 2001;7(5):257–64. doi: 10.1053/bbmt.2001.v7.pm11400947. [DOI] [PubMed] [Google Scholar]
- 46.Ebeling PR, Thomas DM, Erbas B, Hopper JL, Szer J, Grigg AP. Mechanisms of bone loss following allogeneic and autologous hemopoietic stem cell transplantation. J Bone Miner Res. 1999;14(3):342–50. doi: 10.1359/jbmr.1999.14.3.342. [DOI] [PubMed] [Google Scholar]
- 47.Tauchmanova L, Colao A, Lombardi G, Rotoli B, Selleri C. Bone loss and its management in long-term survivors from allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2007;92(12):4536–45. doi: 10.1210/jc.2006-2870. Epub 2007/10/04. [DOI] [PubMed] [Google Scholar]
- 48.Baek KH, Lee WY, Oh KW, Kim HS, Han JH, Kang MI, et al. Changes in the serum growth factors and osteoprotegerin after bone marrow transplantation: impact on bone and mineral metabolism. J Clin Endocrinol Metab. 2004;89(3):1246–54. doi: 10.1210/jc.2003-031206. Epub 2004/03/06. [DOI] [PubMed] [Google Scholar]
- 49.Galotto M, Berisso G, Delfino L, Podesta M, Ottaggio L, Dallorso S, et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999;27(9):1460–6. doi: 10.1016/s0301-472x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 50.Withold W, Wolf HH, Kollbach S, Heyll A, Schneider W, Reinauer H. Relationship between bone metabolism and plasma cytokine levels in patients at risk of post-transplantation bone disease after bone marrow transplantation. Eur J Clin Chem Clin Biochem. 1996;34(4):295–9. doi: 10.1515/cclm.1996.34.4.295. Epub 1996/04/01. [DOI] [PubMed] [Google Scholar]
- 51.Lee WY, Cho SW, Oh ES, Oh KW, Lee JM, Yoon KH, et al. The effect of bone marrow transplantation on the osteoblastic differentiation of human bone marrow stromal cells. J Clin Endocrinol Metab. 2002;87(1):329–35. doi: 10.1210/jcem.87.1.8135. [DOI] [PubMed] [Google Scholar]
- 52.Vega D, Maalouf NM, Sakhaee K. CLINICAL Review #: the role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: clinical implications. J Clin Endocrinol Metab. 2007;92(12):4514–21. doi: 10.1210/jc.2007-0646. Epub 2007/09/27. [DOI] [PubMed] [Google Scholar]
- 53.Boyce BF, Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5(3):98–104. doi: 10.1007/s11914-007-0024-y. Epub 2007/10/11. [DOI] [PubMed] [Google Scholar]
- 54.Baek KH, Oh KW, Lee WY, Tae HJ, Rhee EJ, Han JH, et al. Changes in the serum sex steroids, IL-7 and RANKL-OPG system after bone marrow transplantation: influences on bone and mineral metabolism. Bone. 2006;39(6):1352–60. doi: 10.1016/j.bone.2006.06.011. Epub 2006/08/15. [DOI] [PubMed] [Google Scholar]
- 55.Kananen K, Volin L, Tahtela R, Laitinen K, Ruutu T, Valimaki MJ. Recovery of bone mass and normalization of bone turnover in long-term survivors of allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002;29(1):33–9. doi: 10.1038/sj.bmt.1703317. Epub 2002/02/13. [DOI] [PubMed] [Google Scholar]
- 56.Atkinson K, Cohen M, Biggs J. Avascular necrosis of the femoral head secondary to corticosteroid therapy for graft-versus-host disease after marrow transplantation: effective therapy with hip arthroplasty. Bone Marrow Transplant. 1987;2(4):421–6. Epub 1987/12/01. [PubMed] [Google Scholar]
- 57.Faraci M, Calevo MG, Lanino E, Caruso S, Messina C, Favr C, et al. Osteonecrosis after allogeneic stem cell transplantation in childhood. A case-control study in Italy. Haematologica. 2006;91(8):1096–9. Epub 2006/08/04. [PubMed] [Google Scholar]
- 58.Enright H, Haake R, Weisdorf D. Avascular necrosis of bone: a common serious complication of allogeneic bone marrow transplantation. Am J Med. 1990;89(6):733–8. doi: 10.1016/0002-9343(90)90214-x. Epub 1990/12/01. [DOI] [PubMed] [Google Scholar]
- 59.Socie G, Cahn JY, Carmelo J, Vernant JP, Jouet JP, Ifrah N, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: analysis of risk factors for 4388 patients by the Societe Francaise de Greffe de Moelle (SFGM) Br J Haematol. 1997;97(4):865–70. doi: 10.1046/j.1365-2141.1997.1262940.x. Epub 1997/06/01. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell DG, Rao VM, Dalinka MK, Spritzer CE, Alavi A, Steinberg ME, et al. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology. 1987;162(3):709–15. doi: 10.1148/radiology.162.3.3809484. Epub 1987/03/01. [DOI] [PubMed] [Google Scholar]
- 61.Petrigliano FA, Lieberman JR. Osteonecrosis of the hip: novel approaches to evaluation and treatment. Clin Orthop Relat Res. 2007;465:53–62. doi: 10.1097/BLO.0b013e3181591c92. Epub 2007/10/02. [DOI] [PubMed] [Google Scholar]
- 62.Ojala AE, Lanning FP, Paakko E, Lanning BM. Osteonecrosis in children treated for acute lymphoblastic leukemia: a magnetic resonance imaging study after treatment. Med Pediatr Oncol. 1997;29(4):260–5. doi: 10.1002/(sici)1096-911x(199710)29:4<260::aid-mpo5>3.0.co;2-k. Epub 1997/10/01. [DOI] [PubMed] [Google Scholar]
- 63.Burger B, Beier R, Zimmermann M, Beck JD, Reiter A, Schrappe M. Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)--experiences from trial ALL-BFM 95. Pediatr Blood Cancer. 2005;44(3):220–5. doi: 10.1002/pbc.20244. Epub 2004/10/30. [DOI] [PubMed] [Google Scholar]
- 64.Strauss AJ, Su JT, Dalton VM, Gelber RD, Sallan SE, Silverman LB. Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol. 2001;19(12):3066–72. doi: 10.1200/JCO.2001.19.12.3066. Epub 2001/06/16. [DOI] [PubMed] [Google Scholar]
- 65.Mattano LA, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: A report from the children’s cancer group. Journal of Clinical Oncology. 2000;18(18):3262–72. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 66.Tauchmanova L, De Rosa G, Serio B, Fazioli F, Mainolfi C, Lombardi G, et al. Avascular necrosis in long-term survivors after allogeneic or autologous stem cell transplantation: a single center experience and a review. Cancer. 2003;97(10):2453–61. doi: 10.1002/cncr.11373. Epub 2003/05/07. [DOI] [PubMed] [Google Scholar]
- 67.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;(386):173–8. doi: 10.1097/00003086-200105000-00022. Epub 2001/05/12. [DOI] [PubMed] [Google Scholar]
- 68.Lafforgue P. Pathophysiology and natural history of avascular necrosis of bone. Joint Bone Spine. 2006;73(5):500–7. doi: 10.1016/j.jbspin.2006.01.025. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 69.Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32(2):94–124. Epub 2002/11/14. [PubMed] [Google Scholar]
- 70.Hungerford DS, Jones LC. Asymptomatic osteonecrosis: should it be treated? Clin Orthop Relat Res. 2004;429:124–30. Epub 2004/12/04. [PubMed]
- 71.Jones KB, Seshadri T, Krantz R, Keating A, Ferguson PC. Cell-based therapies for osteonecrosis of the femoral head. Biol Blood Marrow Transplant. 2008;14(10):1081–7. doi: 10.1016/j.bbmt.2008.06.017. Epub 2008/09/23. [DOI] [PubMed] [Google Scholar]
- 72.Cherian S, Laorr A, Saleh K, Kuskowski M, Bailey R, Cheng E. Reliability and prognostic ability of quantifying the extent of femoral head involvement in osteonecrosis. J Bone Joint Surg Am. 2003;85A(2):309–15. doi: 10.2106/00004623-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 73.Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop Relat Res. 1998;355(Suppl):S314–35. Epub 1999/01/26. [PubMed] [Google Scholar]
- 74.Campbell S, Sun CL, Kurian S, Francisco L, Carter A, Kulkarni S, et al. Predictors of avascular necrosis of bone in long-term survivors of hematopoietic cell transplantation. Cancer. 2009;115(18):4127–35. doi: 10.1002/cncr.24474. Epub 2009/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang CC, Greenspan A, Gershwin ME. Osteonecrosis: current perspectives on pathogenesis and treatment. Semin Arthritis Rheum. 1993;23(1):47–69. doi: 10.1016/s0049-0172(05)80026-5. Epub 1993/08/01. [DOI] [PubMed] [Google Scholar]
- 76.Kerachian MA, Harvey EJ, Cournoyer D, Chow TY, Seguin C. Avascular necrosis of the femoral head: vascular hypotheses. Endothelium. 2006;13(4):237–44. doi: 10.1080/10623320600904211. Epub 2006/09/23. [DOI] [PubMed] [Google Scholar]
- 77.Schulte CM, Beelen DW. Avascular osteonecrosis after allogeneic hematopoietic stem-cell transplantation: diagnosis and gender matter. Transplantation. 2004;78(7):1055–63. doi: 10.1097/01.tp.0000138026.40907.38. Epub 2004/10/14. [DOI] [PubMed] [Google Scholar]
- 78.Fink JC, Leisenring WM, Sullivan KM, Sherrard DJ, Weiss NS. Avascular necrosis following bone marrow transplantation: a case-control study. Bone. 1998;22(1):67–71. doi: 10.1016/s8756-3282(97)00219-6. Epub 1998/01/23. [DOI] [PubMed] [Google Scholar]
- 79.Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(1 Suppl):15S–21S. doi: 10.1016/j.jvs.2010.06.175. Epub 2010/09/17. [DOI] [PubMed] [Google Scholar]
- 80.Socie G, Selimi F, Sedel L, Frija J, Devergie A, Esperou Bourdeau H, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: clinical findings, incidence and risk factors. Br J Haematol. 1994;86(3):624–8. doi: 10.1111/j.1365-2141.1994.tb04795.x. Epub 1994/03/01. [DOI] [PubMed] [Google Scholar]
- 81.Holler E, Kolb HJ, Hiller E, Mraz W, Lehmacher W, Gleixner B, et al. Microangiopathy in patients on cyclosporine prophylaxis who developed acute graft-versus-host disease after HLA-identical bone marrow transplantation. Blood. 1989;73(7):2018–24. Epub 1989/05/15. [PubMed] [Google Scholar]
- 82.Pettitt AR, Clark RE. Thrombotic microangiopathy following bone marrow transplantation. Bone Marrow Transplant. 1994;14(4):495–504. Epub 1994/10/01. [PubMed] [Google Scholar]
- 83.Willems E, Baron F, Seidel L, Frere P, Fillet G, Beguin Y. Comparison of thrombotic microangiopathy after allogeneic hematopoietic cell transplantation with high-dose or nonmyeloablative conditioning. Bone Marrow Transplant. 2010;45(4):689–93. doi: 10.1038/bmt.2009.230. Epub 2009/09/01. [DOI] [PubMed] [Google Scholar]
- 84.Cruess RL. Cortisone-induced avascular necrosis of the femoral head. J Bone Joint Surg Br. 1977;59(3):308–17. doi: 10.1302/0301-620X.59B3.893509. Epub 1977/08/01. [DOI] [PubMed] [Google Scholar]
- 85.Fryer JP, Granger DK, Leventhal JR, Gillingham K, Najarian JS, Matas AJ. Steroid-related complications in the cyclosporine era. Clin Transplant. 1994;8(3 Pt 1):224–9. Epub 1994/06/01. [PubMed] [Google Scholar]
- 86.Tang S, Chan TM, Lui SL, Li FK, Lo WK, Lai KN. Risk factors for avascular bone necrosis after renal transplantation. Transplant Proc. 2000;32(7):1873–5. doi: 10.1016/s0041-1345(00)01471-8. Epub 2000/12/20. [DOI] [PubMed] [Google Scholar]
- 87.Rosenthal J, Pawlowska A, Bolotin E, Cervantes C, Maroongroge S, Thomas SH, et al. Transplant-associated thrombotic microangiopathy in pediatric patients treated with sirolimus and tacrolimus. Pediatr Blood Cancer. 2010 doi: 10.1002/pbc.22861. Epub 2010/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fortin MC, Raymond MA, Madore F, Fugere JA, Paquet M, St-Louis G, et al. Increased risk of thrombotic microangiopathy in patients receiving a cyclosporin-sirolimus combination. Am J Transplant. 2004;4(6):946–52. doi: 10.1111/j.1600-6143.2004.00428.x. Epub 2004/05/19. [DOI] [PubMed] [Google Scholar]
- 89.Pizzolato GP, Sztajzel R, Burkhardt K, Megret M, Borisch B. Cerebral vasculitis during FK 506 treatment in a liver transplant patient. Neurology. 1998;50(4):1154–7. doi: 10.1212/wnl.50.4.1154. Epub 1998/05/05. [DOI] [PubMed] [Google Scholar]
- 90.Vathsala A, Weinberg RB, Schoenberg L, Grevel J, Goldstein RA, Van Buren CT, et al. Lipid abnormalities in cyclosporine-prednisone-treated renal transplant recipients. Transplantation. 1989;48(1):37–43. doi: 10.1097/00007890-198907000-00009. Epub 1989/07/01. [DOI] [PubMed] [Google Scholar]
- 91.Valimaki MJ, Kinnunen K, Volin L, Tahtela R, Loyttyniemi E, Laitinen K, et al. A prospective study of bone loss and turnover after allogeneic bone marrow transplantation: effect of calcium supplementation with or without calcitonin. Bone Marrow Transplant. 1999;23(4):355–61. doi: 10.1038/sj.bmt.1701586. [DOI] [PubMed] [Google Scholar]
- 92.Carlson K, Simonsson B, Ljunghall S. Acute effects of high-dose chemotherapy followed by bone marrow transplantation on serum markers of bone metabolism. Calcif Tissue Int. 1994;55(6):408–11. doi: 10.1007/BF00298552. Epub 1994/12/01. [DOI] [PubMed] [Google Scholar]
- 93.Kang MI, Lee WY, Oh KW, Han JH, Song KH, Cha BY, et al. The short-term changes of bone mineral metabolism following bone marrow transplantation. Bone. 2000;26(3):275–9. doi: 10.1016/s8756-3282(99)00265-3. [DOI] [PubMed] [Google Scholar]
- 94.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999;81(2):349–55. doi: 10.1302/0301-620x.81b2.8818. Epub 1999/04/16. [DOI] [PubMed] [Google Scholar]
- 95.Tauchmanova L, Serio B, Del Puente A, Risitano AM, Esposito A, De Rosa G, et al. Long-lasting bone damage detected by dual-energy x-ray absorptiometry, phalangeal osteosonogrammetry, and in vitro growth of marrow stromal cells after allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2002;87(11):5058–65. doi: 10.1210/jc.2002-020800. [DOI] [PubMed] [Google Scholar]
- 96.Forabosco A, Sforza C, De Pol A, Vizzotto L, Marzona L, Ferrario VF. Morphometric study of the human neonatal ovary. Anat Rec. 1991;231(2):201–8. doi: 10.1002/ar.1092310208. Epub 1991/10/01. [DOI] [PubMed] [Google Scholar]
- 97.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–50. doi: 10.1038/nature02316. Epub 2004/03/12. [DOI] [PubMed] [Google Scholar]
- 98.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–6. doi: 10.1093/oxfordjournals.humrep.a137570. Epub 1992/11/01. [DOI] [PubMed] [Google Scholar]
- 99.Hensley ML, Reichman BS. Fertility and pregnancy after adjuvant chemotherapy for breast cancer. Crit Rev Oncol Hematol. 1998;28(2):121–8. doi: 10.1016/s1040-8428(98)00013-4. Epub 1998/10/13. [DOI] [PubMed] [Google Scholar]
- 100.Damewood MD, Grochow LB. Prospects for fertility after chemotherapy or radiation for neoplastic disease. Fertil Steril. 1986;45(4):443–59. doi: 10.1016/s0015-0282(16)49268-x. Epub 1986/04/01. [DOI] [PubMed] [Google Scholar]
- 101.Shalet SM. Effects of cancer chemotherapy on gonadal function of patients. Cancer Treat Rev. 1980;7(3):141–52. doi: 10.1016/s0305-7372(80)80028-4. Epub 1980/09/01. [DOI] [PubMed] [Google Scholar]
- 102.Shalet SM, Didi M, Ogilvy-Stuart AL, Schulga J, Donaldson MD. Growth and endocrine function after bone marrow transplantation. Clin Endocrinol (Oxf) 1995;42(4):333–9. doi: 10.1111/j.1365-2265.1995.tb02640.x. Epub 1995/04/01. [DOI] [PubMed] [Google Scholar]
- 103.Schubert MA, Sullivan KM, Schubert MM, Nims J, Hansen M, Sanders JE, et al. Gynecological abnormalities following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1990;5(6):425–30. Epub 1990/06/01. [PubMed] [Google Scholar]
- 104.Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87(7):3045–52. Epub 1996/04/01. [PubMed] [Google Scholar]
- 105.Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005;(34):12–7. doi: 10.1093/jncimonographs/lgi003. Epub 2005/03/24. [DOI] [PubMed] [Google Scholar]
- 106.Viviani S, Santoro A, Ragni G, Bonfante V, Bestetti O, Bonadonna G. Gonadal toxicity after combination chemotherapy for Hodgkin’s disease. Comparative results of MOPP vs ABVD. Eur J Cancer Clin Oncol. 1985;21(5):601–5. doi: 10.1016/0277-5379(85)90088-4. Epub 1985/05/01. [DOI] [PubMed] [Google Scholar]
- 107.Anserini P, Chiodi S, Spinelli S, Costa M, Conte N, Copello F, et al. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant. 2002;30(7):447–51. doi: 10.1038/sj.bmt.1703651. Epub 2002/10/09. [DOI] [PubMed] [Google Scholar]
- 108.Sanders J. Growth and Development after Hematopoietic Cell Transplantation. In: Appelbaum FRSJF, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation: Stem Cell Transplantation. Oxford, UK: Wiley-Blackwell; 2009. [Google Scholar]
- 109.Sanders JE, Flournoy N, Thomas ED, Buckner CD, Lum LG, Clift RA, et al. Marrow transplant experience in children with acute lymphoblastic leukemia: an analysis of factors associated with survival, relapse, and graft-versus-host disease. Med Pediatr Oncol. 1985;13(4):165–72. doi: 10.1002/mpo.2950130402. Epub 1985/01/01. [DOI] [PubMed] [Google Scholar]
- 110.Hammond C, Abrams JR, Syrjala KL. Fertility and risk factors for elevated infertility concern in 10-year hematopoietic cell transplant survivors and case-matched controls. J Clin Oncol. 2007;25(23):3511–7. doi: 10.1200/JCO.2007.10.8993. Epub 2007/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–31. doi: 10.1200/JCO.2006.06.5888. Epub 2006/05/03. [DOI] [PubMed] [Google Scholar]
- 112.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43(6):437–50. doi: 10.3109/07853890.2010.546807. Epub 2011/01/14. [DOI] [PubMed] [Google Scholar]
- 113.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23(10):2266–72. doi: 10.1093/humrep/den244. Epub 2008/07/08. [DOI] [PubMed] [Google Scholar]
- 114.Demeestere I, Simon P, Buxant F, Robin V, Fernandez SA, Centner J, et al. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod. 2006;21(8):2010–4. doi: 10.1093/humrep/del092. Epub 2006/04/06. [DOI] [PubMed] [Google Scholar]
- 115.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–10. doi: 10.1016/S0140-6736(04)17222-X. Epub 2004/10/19. [DOI] [PubMed] [Google Scholar]
- 116.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94(6):2191–6. doi: 10.1016/j.fertnstert.2009.12.073. Epub 2010/02/23. [DOI] [PubMed] [Google Scholar]
- 117.Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116(16):2908–14. doi: 10.1182/blood-2010-01-265751. Epub 2010/07/03. [DOI] [PubMed] [Google Scholar]
- 118.West ER, Zelinski MB, Kondapalli LA, Gracia C, Chang J, Coutifaris C, et al. Preserving female fertility following cancer treatment: current options and future possibilities. Pediatr Blood Cancer. 2009;53(2):289–95. doi: 10.1002/pbc.21999. Epub 2009/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clowse ME, Behera MA, Anders CK, Copland S, Coffman CJ, Leppert PC, et al. Ovarian preservation by GnRH agonists during chemotherapy: a meta-analysis. J Womens Health (Larchmt) 2009;18(3):311–9. doi: 10.1089/jwh.2008.0857. Epub 2009/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bedaiwy MA, El-Nashar SA, El Saman AM, Evers JL, Sandadi S, Desai N, et al. Reproductive outcome after transplantation of ovarian tissue: a systematic review. Hum Reprod. 2008;23(12):2709–17. doi: 10.1093/humrep/den301. Epub 2008/08/12. [DOI] [PubMed] [Google Scholar]
- 121.Beck-Fruchter R, Weiss A, Shalev E. GnRH agonist therapy as ovarian protectants in female patients undergoing chemotherapy: a review of the clinical data. Hum Reprod Update. 2008;14(6):553–61. doi: 10.1093/humupd/dmn041. Epub 2008/09/30. [DOI] [PubMed] [Google Scholar]
- 122.Meirow D, Rabinovici J, Katz D, Or R, Shufaro Y, Ben-Yehuda D. Prevention of severe menorrhagia in oncology patients with treatment-induced thrombocytopenia by luteinizing hormone-releasing hormone agonist and depo-medroxyprogesterone acetate. Cancer. 2006;107(7):1634–41. doi: 10.1002/cncr.22199. Epub 2006/09/01. [DOI] [PubMed] [Google Scholar]
- 123.Quinn B, Kelly D. Sperm banking and fertility concerns: enhancing practice and the support available to men with cancer. Eur J Oncol Nurs. 2000;4(1):55–8. doi: 10.1054/ejon.2000.0078. Epub 2003/07/10. [DOI] [PubMed] [Google Scholar]
- 124.Ohl DA, Wolf LJ, Menge AC, Christman GM, Hurd WW, Ansbacher R, et al. Electroejaculation and assisted reproductive technologies in the treatment of anejaculatory infertility. Fertil Steril. 2001;76(6):1249–55. doi: 10.1016/s0015-0282(01)02895-3. Epub 2001/12/04. [DOI] [PubMed] [Google Scholar]
- 125.Wheeler JS, Jr, Walter JS, Culkin DJ, Canning JR. Idiopathic anejaculation treated by vibratory stimulation. Fertil Steril. 1988;50(2):377–9. doi: 10.1016/s0015-0282(16)60092-4. Epub 1988/08/01. [DOI] [PubMed] [Google Scholar]
- 126.Schwarzer JU, Fiedler K, Hertwig Iv, Krusmann G, Wurfel W, Schleyer M, et al. Sperm retrieval procedures and intracytoplasmatic spermatozoa injection with epididymal and testicular sperms. Urol Int. 2003;70(2):119–23. doi: 10.1159/000068185. Epub 2003/02/20. [DOI] [PubMed] [Google Scholar]
- 127.Schrader M, Muller M, Sofikitis N, Straub B, Krause H, Miller K. “Onco-tese”: testicular sperm extraction in azoospermic cancer patients before chemotherapy-new guidelines? Urology. 2003;61(2):421–5. doi: 10.1016/s0090-4295(02)02264-1. Epub 2003/02/25. [DOI] [PubMed] [Google Scholar]
- 128.Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Oncologists’ attitudes and practices regarding banking sperm before cancer treatment. J Clin Oncol. 2002;20(7):1890–7. doi: 10.1200/JCO.2002.07.174. Epub 2002/03/29. [DOI] [PubMed] [Google Scholar]
- 129.Brook PF, Radford JA, Shalet SM, Joyce AD, Gosden RG. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil Steril. 2001;75(2):269–74. doi: 10.1016/s0015-0282(00)01721-0. Epub 2001/02/15. [DOI] [PubMed] [Google Scholar]
- 130.Wyns C, Curaba M, Vanabelle B, Van Langendonckt A, Donnez J. Options for fertility preservation in prepubertal boys. Hum Reprod Update. 2010;16(3):312–28. doi: 10.1093/humupd/dmp054. Epub 2010/01/06. [DOI] [PubMed] [Google Scholar]
- 131.Carter A, Robison LL, Francisco L, Smith D, Grant M, Baker KS, et al. Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant. 2006;37(11):1023–9. doi: 10.1038/sj.bmt.1705364. Epub 2006/04/11. [DOI] [PubMed] [Google Scholar]
- 132.Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358(9278):271–6. doi: 10.1016/s0140-6736(01)05482-4. Epub 2001/08/11. [DOI] [PubMed] [Google Scholar]
- 133.Loren AW, Chow E, Jacobsohn DA, Gilleece M, Halter J, Joshi S, et al. Pregnancy after hematopoietic cell transplantation: a report from the late effects working committee of the Center for International Blood and Marrow Transplant Research (CIBMTR) Biol Blood Marrow Transplant. 2010;17(2):157–66. doi: 10.1016/j.bbmt.2010.07.009. Epub 2010/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Green DM, Whitton JA, Stovall M, Mertens AC, Donaldson SS, Ruymann FB, et al. Pregnancy outcome of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol. 2002;187(4):1070–80. doi: 10.1067/mob.2002.126643. Epub 2002/10/22. [DOI] [PubMed] [Google Scholar]
- 135.Signorello LB, Cohen SS, Bosetti C, Stovall M, Kasper CE, Weathers RE, et al. Female survivors of childhood cancer: preterm birth and low birth weight among their children. J Natl Cancer Inst. 2006;98(20):1453–61. doi: 10.1093/jnci/djj394. Epub 2006/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Green DM, Whitton JA, Stovall M, Mertens AC, Donaldson SS, Ruymann FB, et al. Pregnancy outcome of partners of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(4):716–21. doi: 10.1200/JCO.2003.04.085. Epub 2003/02/15. [DOI] [PubMed] [Google Scholar]


