Abstract
AIM
The aim of this study was to compare total and regional cerebral volumes in children with isolated cerebellar malformations (CBMs) with those in typically developing children, and to examine the extent to which cerebellar volumetric reductions are associated with total and regional cerebral volumes.
METHOD
This is a case–control study of children diagnosed with isolated CBMs. Each child was matched on age and sex to two typically developing children. Using advanced three-dimensional volumetric magnetic resonance imaging, the cerebrum was segmented into tissue classes and partitioned into eight regions. Analysis of variance was used to compare cerebral volumes between children with CBMs and comparison children, and linear regressions to examine the impact of cerebellar volume reduction on cerebral volumes.
RESULTS
Magnetic resonance imaging was performed at a mean age of 27 months in 20 children (10 males, 10 females) with CBMs and 40 typically developing children. Children with CBMs showed significantly smaller deep grey matter nuclei (p<0.001), subgenual white matter (p=0.03), midtemporal white matter (p=0.02), and inferior occipital grey matter (p=0.03) volumes than typically developing children. Greater cerebellar volumetric reduction in children with CBMs was associated with decreased total cerebral volume and deep grey matter nuclei (p=0.02), subgenual white/grey matter (p=0.001), midtemporal white (p=0.02) and grey matter (p=0.01), and parieto-occipital grey matter (p=0.004).
INTERPRETATION
CBMs are associated with impaired regional cerebral growth, suggesting deactivation of principal cerebello-cerebral pathways.
Converging evidence from clinical and neuroimaging studies in adults with cerebellar lesions increasingly supports the involvement of the cerebellum in high-order cognitive skills, behavior, and affect.1 These new insights into the expanded functional role of the cerebellum have led to a more comprehensive understanding of the impact of cerebellar malformations (CBMs) on subsequent cerebral development.
Normal brain development is dependent on appropriate neuronal activation through developing neural circuits. The cerebellar output projects from the deep cerebellar nuclei through the red nucleus to the thalamus. From there it proceeds to the motor cortex and the supplementary motor area, the posterior and inferior parietal cortex, the superior temporal cortex, the prefrontal cortex, as well as the cingulate gyrus and the parahippocampal gyrus.2– 4 The presence of these strong efferent connections between the cerebellum and the cerebrum raises the question of whether trophic deactivation of the cerebello-cerebral pathways caused by impaired cerebellar development could result secondarily in altered cerebral development.
Cerebello-cerebral diaschisis, defined as a loss of function in the cerebral cortex after a cerebellar lesion, has been previously reported in adults and in older children after cerebellar tumor resection.5–8 Decreased perfusion, metabolism, and oxygen consumption are characteristics of this phenomenon that is presumed to be present in up to 77% of patients after posterior fossa injury.5–8 Using single-photon emission computed tomography and dynamic susceptibility contrast magnetic resonance imaging (MRI), cerebello-cerebral diaschisis has been reported in the basal ganglia, thalamus, striatum, prefrontal, frontal, parietal, and temporal regions and has been attributed to changes in the cerebello-cortical pathways.5–8 However, these studies examined the effects of acquired injury after tumor resection. Consequently, very little is known about the impact of congenital cerebellar anomalies on early cerebral development.
It is increasingly appreciated that CBMs are associated with global and pervasive developmental and functional deficits.9,10 The potential contribution of secondary cerebral growth impairment in the high prevalence of neurodevelopmental impairment in children with CBMs remains unclear. The aim of this study was to compare total and regional cerebral volumes in children with CBMs and typically developing age- and sex-matched children, using advanced three-dimensional volumetric MRI and parcellation techniques. We also examined the extent to which cerebellar volumetric reductions are associated with total and regional cerebral volumes in children with CBMs. We hypothesized that children with isolated CBMs would show decreased regional cerebral volume in known projection areas of the cerebellum compared with healthy age-matched controls. We also postulated that greater reductions in cerebellar volumes would be associated with volumetric reductions in specific regions of the cerebrum.
METHOD
Procedures
Children born between 2003 and 2008 with a prenatal or postnatal diagnosis of isolated CBMs (i.e. absence of supratentorial lesions, as well as the absence of chromosomal abnormalities and syndromic diagnoses) were identified though a systematic electronic search of the MRI database at The Children’s Hospital Boston for this case–control study. Each case was matched to two typically developing children according to their age and sex for MRI comparisons, to increase the efficiency of our study. The comparison group were selected on the basis of their age and sex from the database of the MRI Study of Normal Brain Development funded by the US National Institutes of Health.11,12 Term infants (≥37 gestational wks) who were diagnosed with Dandy–Walker malformation, an inferior vermis hypoplasia, a cerebellar and/or vermis hypoplasia, or a rhombencephalosynapsis were accrued for this study. Diagnostic criteria were detailed in a previous paper.13 Children with fetal or neonatal central nervous system infection, major intracranial birth trauma, an inherited metabolic disease, or major pre- or postnatal cerebral ischemias were excluded. Children with concomitant supratentorial lesions or chromosomal abnormalities were also excluded. Additionally, children with ventricular peritoneal shunts were excluded because reliable cerebral volumes could not be obtained owing to the presence of metal artifacts on the MRIs. The clinical outcome of this cohort of children has been examined, by our group, in the context of another paper.13 The study was approved by The Children’s Hospital Boston’s Committee on Clinical Investigation. Informed written consent was obtained from all parents or legal guardians.
MRI acquisition
MRI was performed for children with CBMs at The Children’s Hospital Boston using a standardized protocol. A 1.5T General Electric System (GE-Medical Systems, Milwaukee, WI, USA) was used to image all children using a quadrature or an eight-channel phased array head coil. Detailed information of the MRI acquisition protocol used in the study is provided in Appendix S1 (supporting information published online).
MRI analysis
Conventional MRI
An experienced pediatric neuroradiologist (RLR), blinded to the medical history and developmental outcome of the child, reviewed the conventional T1- and T2-weighted images to categorize the CBMs. Multiplanar images (i.e. axial, coronal, and sagittal) were used by the pediatric neuroradiologist to confirm the diagnosis of CBMs. Our cohort had serial MRI studies in the fetal and early postnatal period for clinical purposes in addition to the follow-up MRI study that is presented here. The pediatric neuroradiologist reviewed all the MRI studies and distinguished between cerebellar dysplasia (i.e. abnormality of the cerebellar cortex or pattern of the cerebellar folia) and atrophy. Cerebellar atrophy is marked by thin folia and widened sulci. Based on these two observations, none of the participants showed evidence of atrophy on any of the MRI studies.
Quantitative MRI
Brain extraction, cerebral and cerebellar tissue classification, and cerebellar extraction were performed. Detailed information on the extraction and tissue classification methods is provided in Appendix S1. Volumetric measurements in cubic centimeters (cm3) were obtained for cerebellar volume, total cerebral volume, cerebral white matter and cortical grey matter, and deep grey matter nuclei (Fig. 1). The cerebrum was then partitioned into eight regions using the parcellation scheme previously described and validated in children by a number investigators,14 including our group15 (Fig. 2). Detailed information on the cerebral parcellation is provided in Appendix S1.
Figure 1.
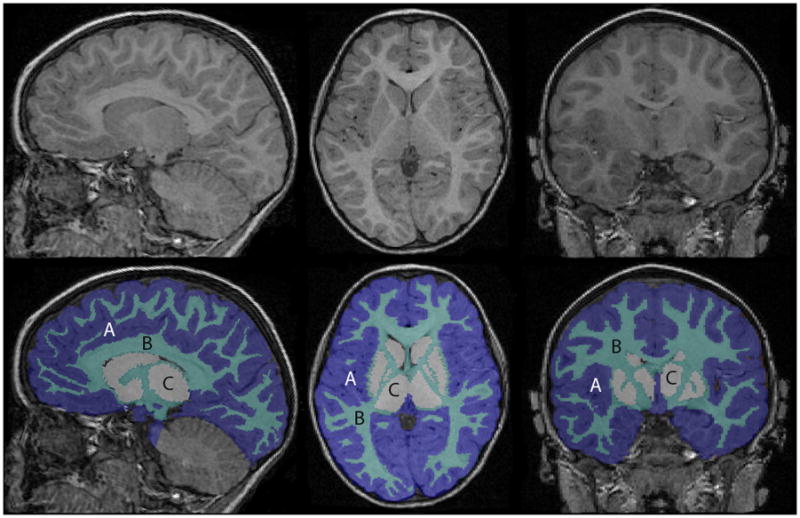
Brain segmentation into tissue classes: A, grey matter; B, white matter; C, deep grey matter nuclei
[Typesetter: Change A B C labels to lower case. Authors are not paying for colour printing.]
Figure 2.
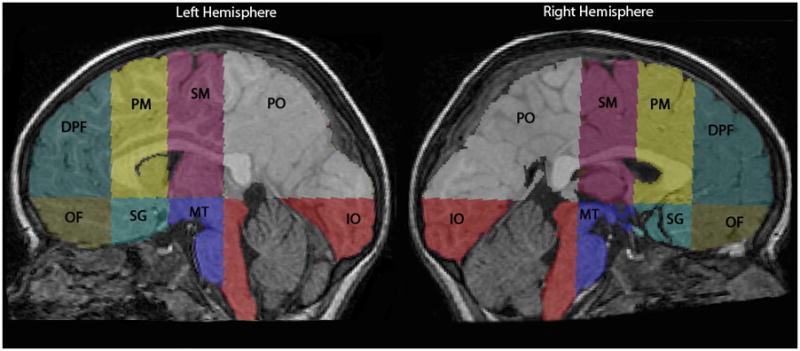
Cerebral parcellation into eight regions: dorsolateral prefrontal (DPF), orbitofrontal (OF), premotor (PM), subgenual (SG), sensorimotor (SM), midtemporal (MT), parieto-occipital (PO), and inferior occipital (IO).
Because most of the children in our cohort with CBMs were affected in both cerebellar hemispheres or had midline anomalies involving the vermis (85%), we did not analyze the right and left cerebral hemispheres separately.
Statistical analysis
For the analyses, each child with CBMs was matched to two typically developing children according to their age and sex. Clinical characteristics of all the children were summarized by means and standard deviations for continuous data. Analysis of variance (ANOVA) was performed using a mixed-effects (both fixed and random effects) model to compare total cerebral volume, total white matter, total cortical grey matter, deep grey matter nuclei, and 16 regional cerebral volumes between children with CBMs and healthy age- and sex-matched comparison children (fixed effect), and to take into account the dependency between children with CBMs and corresponding matched comparisons (random effect). In addition, linear regressions were used to examine if cerebellar volume reductions (determined by subtracting the total cerebellar volume of each child with CBMs from the average total cerebellar volume of the two age- and sex-matched children) were associated with total cerebral volume, total white matter, total cortical grey matter, deep grey matter nuclei, and the 16 regional cerebral volumes in children with CBMs. Estimates of effect and confidence intervals are reported. All assumptions were verified for ANOVA and linear regression models. We also performed a subgroup analysis (t-tests) to compare children with inferior vermian hypoplasia and the rest of the cohort. A significance level was defined at p<0.05. Statistical analyses used SPSS version 17.0 (SPSS Inc, Chicago, IL, USA) and SAS version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
Characteristics of the cohort
Thirty-eight children with isolated CBMs met our inclusion criteria. Among these children, four died in infancy and three were lost to follow-up. Twenty-seven (87%) of the 31 families consented to the MRI; however, imaging could not be completed in seven children. The remaining 20 children who composed the sample in this study were then matched to 40 healthy age- and sex-matched comparison children. There was no significant difference between the 18 children who did not participate in the study and those who did in baseline characteristics, including mean age at enrollment (27.5mo [SD 19.3] vs 23.9mo [SD 13.9] respectively) and sex (10 males, 10 females vs 10 males, 6 females respectively).
The clinical diagnoses of the children with CBMs included inferior vermis hypoplasia (n=9), cerebellar hypoplasia (n=4), unilateral cerebellar hypoplasia (n=3), vermis hypoplasia (n=2), rhombencephalosynapsis (n=1), and Dandy–Walker malformation (n=1). Our sample was composed of 10 males (50%) and 10 females (50%) with CBMs who were matched to 20 typically developing males (50%) and 20 typically developing females (50%). All children with CBMs were successfully matched to two same-sex comparisons. The age difference between the cases and comparison children was at not more than 2 months for 73% of the pairs and not more than 6 months for 85% of the pairs. MRI was performed at a mean age of 27.1 (SD 16.1) and 27.0 (SD 17.1) months in the children with CBMs and healthy comparison children respectively (p=0.99).
Reliability of the cerebral parcellation scheme and cerebellar volume measurements
To ensure consistency in our volume measurements, the same cerebral parcellation method was completed a second time by the same investigator (M-EB) on 10 different scans selected across ages, of which 85% of regions parcellated twice were within 10.43cm3, and the volume difference averaged 5.29cm3. Finally, five cerebella were manually outlined a second time by the same investigator (M-EB) to estimate the intrarater reliability of cerebellar volumes. Volumes for total cerebellar volume were all within 2.75cm3, with an average difference of 0.79cm3.
Comparison of cerebellar, total cerebral, and tissue class volumes
Children with CBMs showed a statistically significant decrease in total cerebellar volume (p=0.002) and in the deep grey matter nuclei (basal ganglia and thalamus; p<0.001) compared with typically developing children. Total cerebral volume and total cortical grey and white matter volumes, although smaller in the children with CBMs, were not significantly different between the two groups. The results are presented in Tables I and II. In a subgroup analysis comparing children with inferior vermis hypoplasia to the rest of our cohort, there was no significant difference in total brain volume, total grey matter, or total white matter volumes (p>0.05).
Table I.
Cerebellar volume, total cerebral volume, tissue classes, and regional cerebral volumes in children with cerebellar malformations (CBMs) and typically developing children (TDC)
| Regional cerebellar and cerebral volumes (cm3) | CBMs group, (cm3); n=20 | TDC group (cm3); n=40 |
|---|---|---|
| Total cerebellar | 93.1 (38.8) | 115.1 (13.39) |
| Total cerebral | 899.2 (146.9) | 922.2 (126.4) |
| Total cerebral grey matter | 601.4 (85.4) | 609.3 (71.2) |
| Total cerebral white matter | 270.0 (66.1) | 280.3 (72.2) |
| Deep grey matter nuclei | 27.9 (4.5) | 33.2 (4.8) |
| Dorsolateral prefrontal white matter | 25.2 (9.4) | 27.2 (8.4) |
| Dorsolateral prefrontal grey matter | 64.7 (13.7) | 64.3 (11.1) |
| Orbitofrontal white matter | 6.5 (3.9) | 7.0 (5.3) |
| Orbitofrontal grey matter | 25.2 (7.3) | 25.2 (7.8) |
| Premotor white matter | 38.8 (10.8) | 37.8 (12.0) |
| Premotor grey matter | 69.6 (12.6) | 67.6 (13.1) |
| Subgenual white matter | 18.9 (4.9) | 22.5 (6.0) |
| Subgenual grey matter | 45.2 (5.6) | 45.8 (6.0) |
| Sensorimotor white matter | 48.9 (12.6) | 48.1 (12.7) |
| Sensorimotor grey matter | 73.3 (12.1) | 75.8 (8.8) |
| Midtemporal white matter | 6.9 (2.2) | 9.1 (3.7) |
| Midtemporal grey matter | 40.4 (7.8) | 40.3 (8.7) |
| Parieto-occipital white matter | 94.7 (23.7) | 96.3 (24.6) |
| Parieto-occipital grey matter | 202.4 (30.8) | 198.9 (30.0 |
| Inferior occipital white matter | 30.1 (9.8) | 32.3 (9.6) |
| Inferior occipital grey matter | 80.8 (19.9) | 91.4 (16.1) |
Values are mean (SD).
Table II.
Differences in cerebellar volume, total cerebral volume, tissue classes, and regional cerebral volumes in children with cerebellar malformations compared with typically developing children
| Regional cerebellar and cerebral volumes (cm3) | Mean difference (cm3) | 95% CI | p value |
|---|---|---|---|
| Total cerebellar | −22.0 | −35.7 to 8.2 | 0.002 |
| Total cerebral | −23.0 | −97.2 to 49.9 | 0.52 |
| Total cerebral grey matter | −7.9 | −49.9 to 34.0 | 0.71 |
| Total cerebral white matter | −10.3 | −49.0 to 28.3 | 0.60 |
| Deep grey matter nuclei | −5.3 | −7.9 to 2.8 | <0.001 |
| Dorsolateral prefrontal white matter | −2.0 | −6.8 to 2.8 | 0.40 |
| Dorsolateral prefrontal grey matter | 0.4 | −6.3 to 7.0 | 0.91 |
| Orbitofrontal white matter | −0.5 | −3.2 to 2.1 | 0.70 |
| Orbitofrontal grey matter | 0.0 | −4.2 to 4.2 | 1.00 |
| Premotor white matter | 1.0 | −5.4 to 7.4 | 0.76 |
| Premotor grey matter | 2.0 | −5.1 to 9.1 | 0.57 |
| Subgenual white matter | −3.6 | −6.7 to 0.5 | 0.03 |
| Subgenual grey matter | −0.6 | −3.9 to 2.6 | 0.69 |
| Sensorimotor white matter | 0.8 | −6.1 to 7.8 | 0.82 |
| Sensorimotor grey matter | −2.5 | −8.1 to 3.0 | 0.36 |
| Midtemporal white matter | −2.2 | −4.0 to 0.4 | 0.02 |
| Midtemporal grey matter | 0.1 | −4.6 to 4.6 | 1.0 |
| Parieto-occipital white matter | −1.6 | −15.0 to 11.8 | 0.81 |
| Parieto-occipital grey matter | 3.5 | −13.2 to 20.1 | 0.68 |
| Inferior occipital white matter | −2.2 | −7.5 to 3.1 | 0.40 |
| Inferior occipital grey matter | −10.6 | −20.2 to 1.0 | 0.03 |
CI, confidence interval.
Comparison of regional cerebral volumes
The ANOVA models demonstrated statistically significant reductions in regional cerebral volumes between children with CBMs and age- and sex-matched comparison children in the cortical grey matter of the inferior occipital region (p=0.03). Significant decreases in white matter volume were evident in the subgenual (p=0.03) and midtemporal regions (p=0.02). In addition, grey matter volumes were smaller in the subgenual and the sensorimotor regions, although the differences were not statistically significant. In children with CBMs, white matter volumes were also smaller in the dorsolateral prefrontal, orbitofrontal, parieto-occipital, and inferior occipital regions but the differences were not statistically significant. The results are presented in Tables I and II. There were no statistical differences in regional volumes between children with inferior vermis hypoplasia and those with other CBMs in our cohort (p>0.05).
Association between cerebellar volume and cerebral development
Linear regression analyses showed that cerebellar volume reductions in children with CBMs were significantly associated with smaller total cerebral volume by 1.28 (p=0.02), total grey matter by 0.82 (p=0.01), and deep grey matter nuclei volumes by 0.04 (p=0.02). In addition, cerebellar volume reduction was associated with lower volumes of subgenual white and grey matter by 0.06 (p=0.001) and 0.07 (p=0.001) respectively, midtemporal white by 0.20 (p=0.02), and grey matter by 0.08 (p=0.01), as well as parieto-occipital grey matter by 0.34 (p=0.004). Results are presented in Table SI (supporting information published online). Finally, there was a borderline association between the cerebellar volume reduction and the dorsolateral prefrontal grey matter by 0.10 (p=0.06), premotor grey matter by 0.09 (p=0.07), and parieto-occipital white matter by 0.17 (p=0.06).
DISCUSSION
In the present study we used quantitative MRI techniques to demonstrate that children with congenital CBMs have significantly reduced volumes in specific regions of the cerebral hemispheres compared with healthy age and sex-matched typically developing children at long-term follow-up. These regions include the deep grey matter nuclei (basal ganglia and thalamus) and inferior occipital grey matter, and the subgenual and midtemporal white matter. We also show that greater volumetric reduction of a malformed cerebellum was associated with decreased total cerebral volume, cerebral grey matter, and deep grey matter nuclei volumes, as well as regional volume reductions in the subgenual white and grey matter, midtemporal white and grey matter, and parieto-occipital grey matter. A borderline association between cerebellar volume reduction and the dorsolateral prefrontal, premotor grey matter, and parieto-occipital region white matter was also present. To our knowledge, this is the first three-dimensional volumetric MRI study to demonstrate total and regional growth impairment of the cerebral hemispheres in children with CBMs.
Crossed cerebello-cerebral diaschisis is described as a decrease in neuronal activation and blood flow in uninjured regions of the cerebrum remote from, but neurally connected to, an area of acute cerebellar injury.8 In some descriptions, this regional decrease in cerebral blood flow is followed by cerebral structural changes in the same area over time.16 Evidence of the presence of crossed cerebello-cerebral diaschisis in adult patients with stroke and in children after tumor resection is accruing.5–8 Several scintigraphic single-photon emission computed tomography studies and one dynamic susceptibility contrast MRI report have described hypoperfusion in specific cerebral regions (predominantly in the contralateral cerebral hemisphere), including the prefrontal cortex, basal ganglia, thalamus, and striatum, as well as the frontal, parietal, and temporal lobes.5–8 The presence of decreased blood flow in these regions has been attributed to a deactivation of the cerebello-ponto-thalamo-cerebral pathways.5 Finally, there has been a report of decreased blood flow in the occipital lobe in two children following posterior fossa tumor resection.7
Two studies by our group have described volumetric loss in the cerebrum following early-life cerebellar hemorrhagic injury in survivors of preterm birth.15,17 In these studies we reported decreased total contralateral cerebral growth in preterm infants with unilateral cerebellar hemorrhagic injury as early as term equivalent.17 Our group also recently described regional reductions in grey and white matter volumes in the contralateral dorsolateral prefrontal, premotor, sensorimotor, midtemporal, and deep grey matter nuclei in children with unilateral cerebellar injury.15
Noteworthy is the fact that very few studies have examined the impact of CBMs on cerebral development. One study reported normal perfusion in the frontal region in an adult patient with cerebellar hypoplasia.6 Interestingly, all four individuals with acquired cerebellar damage showed decreased frontal-lobe neuronal activity in the same study.6 The authors speculate that developmental remodeling could be the basis of this disparity.
Our results are in agreement with previous studies suggesting that lesions to the cerebellum result in decreased neuronal activity in the deep grey matter nuclei (basal ganglia/thalamus) and the temporal lobe,5,7,8,15 which are known projection areas for efferent cerebellar pathways. We also show that the extent of a CBM is associated with greater volumetric loss in these regions. Reduced volumes of deep grey matter nuclei have been shown to relate to motor, cognitive, behavioral, and emotional function,10,18 which are developmental domains commonly impaired in children with CBMs.13 Moreover, greater cerebellar volume loss was also associated with smaller volumes in the parieto-occipital grey matter. Interestingly, the parietal lobe is also a projection area for the cerebellar efferent connections.4 Although no study so far has described an association between CBMs and volumetric reductions in the subgenual white and grey matter regions of the cerebrum, hypoperfusion has been described in frontal and temporal regions in several studies, an area where the subgenual region is located.5–7 From a functional perspective, the subgenual region is known to be involved in emotion modulation, language function, and behavior,19 which are functional domains that are commonly affected in children with CBMs.9
Finally, our data show that in comparison with typically developing children, children with CBMs have volumetric reductions in the inferior occipital grey matter. These data are in line with a previous study by De Smet et al.7 that described changes in perfusion of the occipital lobe in patients with cerebellar injury.7 Although there are no known anatomical efferent pathways that connect the cerebellum to the occipital regions, recent functional imaging studies support the presence of connections between the cerebellum and the occipital lobe through the red nucleus.2,20 Given the numerous regions that connect the cerebellum and cerebrum, ‘global’ cerebral disruption has been postulated following an insult to the cerebellar-cerebral circuitry.8 Nevertheless, the exact mechanisms underlying regional reductions in occipital volume remain unclear. Interestingly, in a previous study by our group, abnormal eye movements, a function that may be related to the occipital lobe,21–23 were reported in 37% of the children with CBMs.13
Unlike our previous study in ex-preterm infants with cerebellar injury15 the current results do not show a significant volumetric difference in prefrontal and frontal regions in children with CBMs compared with healthy controls. However, our data revealed a borderline significant association between cerebellar volumetric differences in cases and comparison children in the dorsolateral prefrontal and premotor grey matter regions. The presence of key output connections from the cerebellum to these regions has been previously demonstrated and the effect of acquired cerebellar injury on the prefrontal and frontal regions has been reported.15 We hypothesize that the lack of a significant relationship in these regions can be partly explained by our small sample size combined with high variability in regional cerebral volumes in our cohort. Conversely, the possibility that CBMs have an impact on cerebral development in a different manner than acquired cerebellar injury also needs to be considered and warrants further study.
Children with CBMs experience a wide spectrum of developmental and functional disabilities that affect global development, cognition, language, motor skills, and behavior.9 Although the functional impact of these regional volumetric reductions in the cerebrum is currently unknown, our data suggest that the extent of cerebellar volumetric impairments is associated with altered growth in specific regions of the cerebrum, which could, in turn, contribute to the presence of functional disabilities. Studies examining the association between regional cerebral volumes and functional outcome in children with CBMs are warranted.
Several potential limitations of this study are worthy of note. Owing to the size of our sample and the amount of variability in the volume of the different regions of the cerebrum, it is likely that our study was underpowered to detect all statistically significant region-specific volumetric differences in the cerebrum in children with CBMs. In addition, despite the fact that there were no significant differences in our matching between children with CBMs and typically developing children, potential small differences in age matching could have confounded our volumetric comparisons. However, for all matches with an age difference greater than 6 months, the typically developing children were older than those with CBMs. Consequently, we could have underestimated the true difference in cerebral brain volumes between the two groups. A substantial proportion of children in our study had isolated inferior vermis hypoplasia, a less severe form of cerebellar dysgenesis, which has been shown to be associated with a favorable outcome.9 Therefore, cerebral development may not be significantly altered in this subgroup of children. However, cerebral volumes were not significantly different between those two groups. Additionally, because of the heterogeneity of our sample, specific regional volumetric impairments may have been difficult to detect. Finally, the causal relationship between CBMs and impaired cerebellar growth cannot be formally established owing to the nature of our study design and the developmental nature of the CBMs; however, the sample of children was carefully selected to exclude the presence of associated supratentorial or chromosome anomalies.
In summary, this is the first study, to our knowledge, to show that CBMs are associated with impaired regional cerebral growth. We speculate that these findings originate from disturbed development of the major cerebello-cerebral pathways decreasing the normal trophic activation of these projection areas during critical phases of cerebral development. Our results now justify the need for larger multicenter studies on the effect of CBMs on cerebral development. Longitudinal structure–function studies are also needed to delineate better the impact of CBMs on cerebral development and child function. Future voxel-based morphometry studies may help to elucidate better the local differences in cerebral development in children with CBMs compared with their typically developing aged-matched peers. Additionally, elucidating the effects of regional cerebellar volume (e.g. vermis volume, dentate nucleus volume) and brainstem volume on supratentorial growth awaits further study. Finally, studies examining the impact of disturbed cerebral growth on defined functional skills are needed to understand better the contribution of cerebral volumetric loss on child function.
Supplementary Material
What this paper adds
Isolated CBMs are associated with regional reductions in cerebral volumes.
CBMs are associated with deactivation of principal cerebello-cerebral pathways.
This study begins to define possible mechanisms underlying developmental disabilities in children with CBMs.
Acknowledgments
We thank Dr Nicol Korner-Bitensky for her methodological expertise in the design of the study. We acknowledge the financial support of the Lifebridge Fund, the Caroline Levine Foundation, and the Trust Family Foundation for this study. M-EB received a studentship from the McGill University Health Centre Research Institute Scholarship. The Canada Research Chairs Program supports CL; AJDP is supported by National Institutes of Health grant 1K24NS057568-01. We also thank all the children and their families who were part of this study.
Footnotes
ONLINE MATERIAL/SUPPORTING INFORMATION
Additional material and supporting information may be found in the online version of this article.
References
- 1.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain Dev. 1998;121:561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 2.Allen G, McColl R, Barnard H, Ringe WK, Fleckenstein J, Cullum CM. Magnetic resonance imaging of the cerebellar-prefrontal and cerebellar-parietal functional connectivity. NeuroImage. 2005;28:39–48. doi: 10.1016/j.neuroimage.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–12. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 5.Attig E, Botez MI, Hublet C, Vervonck C, Jacquy J, Capon A. Cerebral crossed diaschisis caused by cerebellar lesion: role of the cerebellum in mental functions. Rev Neurol (Paris) 1991;147:200–7. (In French) [PubMed] [Google Scholar]
- 6.Boni S, Valle G, Cioffi RP, et al. Crossed cerebello-cerebral diaschisis: a SPECT study. Nucl Med Commun. 1992;13:824–31. doi: 10.1097/00006231-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 7.De Smet HJ, Baillieux H, Wackenier P, De Praeter M, Engelborghs S, Paquier PF, et al. Long-term cognitive deficits following posterior fossa tumor resection: a neuropsychological and functional neuroimaging follow-up study. Neuropsychology. 2009;23:694–704. doi: 10.1037/a0016106. [DOI] [PubMed] [Google Scholar]
- 8.Miller NG, Reddick WE, Kocak M, et al. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. Am J Neuroradiol. 2009;31:288–294. doi: 10.3174/ajnr.A1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolduc ME, Limperopoulos C. Neurodevelopmental outcomes in children with cerebellar malformations: a systematic review. Dev Med Child Neurol. 2009;51:256–67. doi: 10.1111/j.1469-8749.2008.03224.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmahmann JD. In: The Cerebellum and Cognition. Bradley RJ, Harris RA, Jenner P, editors. San Diego: Academic Press; 1997. p. 665. [Google Scholar]
- 11.Almli CR, Rivkin MJ, McKinstryc RC, Group BDC. The NIH MRI study of normal brain development (Objective-2): newborns, infants, toddlers, and preschoolers. NeuroImage. 2007;35:308–25. doi: 10.1016/j.neuroimage.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 12.Evans AC Brain Development Cooperative Group. The NIH MRI study of normal brain development. NeuroImage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 13.Bolduc ME, Du Plessis AJ, Sullivan N, et al. Spectrum of neurodisabilities in children with cerebellar malformations. Dev Med Child Neurol. 2011;53:409–16. doi: 10.1111/j.1469-8749.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 14.Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–48. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 15.Limperopoulos C, Chilingaryan G, Guizard N, Robertson RL, du Plessis AJ. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr Res. 2010;68:145–50. doi: 10.1203/PDR.0b013e3181e1d032. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarty A. Crossed cerebral-cerebellar diaschisis: MRI evaluation. Neurol India. 2002;50:322–5. [PubMed] [Google Scholar]
- 17.Limperopoulos C, Soul JS, Haidar H, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116:844–50. doi: 10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- 18.Leiner HC, Leiner AL, Dow RS. The human cerebro-cerebellar system: its computing, cognitive and language skills. Behav Brain Res. 1991;44:113–28. doi: 10.1016/s0166-4328(05)80016-6. [DOI] [PubMed] [Google Scholar]
- 19.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4. McGraw-Hill; 2000. p. 1414. [Google Scholar]
- 20.Nioche C, Cabanis EA, Habas C. Functional connectivity of the human red nucleus in the brain resting state at 3T. Am J Neuroradiol. 2009;30:396–403. doi: 10.3174/ajnr.A1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salanova V, Andermann F, Olivier A, Rasmussen T, Quesney LF. Occipital lobe epilepsy: electroclinical manifestations, electrocorticography, cortical stimulation and outcome in 42 patients treated between 1930 and 1991. Surgery of occipital lobe epilepsy. Brain. 1992;115:1655–80. doi: 10.1093/brain/115.6.1655. [DOI] [PubMed] [Google Scholar]
- 22.Stolz S, Chatrian G-E, Spence AM. Epileptic nystagmus. Epilepsia. 1991;32:910–8. doi: 10.1111/j.1528-1157.1991.tb05550.x. [DOI] [PubMed] [Google Scholar]
- 23.Zee DS, Tusa RJ, Herdman SJ, Butler PH, Gücer G. Effects of occipital lobectomy upon eye movements in primates. J Neurophysiol. 1987;58:883–907. doi: 10.1152/jn.1987.58.4.883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


