Abstract
Objective
The purpose of this study was to determine whether myeloid differentiation factor 88 (MyD88) and its related Toll-like receptors (TLRs) 2 and 4 contributed to the development of angiotensin II (AngII)-induced abdominal aortic aneurysms (AAAs) and atherosclerosis.
Methods and Results
AngII was infused into either apoE−/− or LDL receptor (LDLR)−/− male mice that were either MyD88+/+ or −/−. MyD88 deficiency profoundly reduced AngII-induced AAAs and atherosclerosis in both strains. To define whether deficiency of specific TLRs had similar effects, AngII was infused into LDLR−/− mice that were also deficient in either TLR2 or TLR4. TLR2 deficiency had no effect on AAA development, but inhibited atherosclerosis. In contrast, TLR4 deficiency attenuated both AAAs and atherosclerosis. To resolve whether MyD88 and TLR4 exerted their effects through cells of hematopoietic lineage, LDLR−/− mice were lethally irradiated and repopulated with bone marrow-derived cells from either MyD88 or TLR4 strains. MyD88 deficiency in bone marrow-derived cells profoundly reduced both AngII-induced AAAs and atherosclerosis. However, TLR4 deficiency in bone marrow-derived cells had no effect on either pathology.
Conclusions
These studies demonstrate that MyD88 deficiency in leukocytes profoundly reduces AngII-induced AAAs and atherosclerosis via mechanisms independent of either TLR2 or 4.
Keywords: aneurysms, atherosclerosis, angiotensin II, myeloid differentiation factor 88, toll-like receptors
Introduction
Abdominal aortic aneurysms (AAAs) in humans are characterized by permanent dilations that have an increasing propensity to rupture with expansion. Despite the devastating impact of the disease in an increasingly larger number of people, the mechanistic basis for the initiation, progression, and rupture of AAAs has not been defined. Aneurysmal tissues are highly heterogeneous that is manifested as accumulation of several types of leukocytes, substantial fragmentation of extracellular matrix, and atherosclerosis.1 Notably, cells of the innate and adaptive immune systems accumulate in human AAA tissue and have been linked to the development of the disease.2
Several animal models of AAAs have become widely used to gain insight into mechanisms of the human disease.3 One of the most commonly used AAA models generates the disease by chronic subcutaneous infusion of AngII into mice.4 AngII infusion promotes aneurysmal disease in both normo- and hypercholesterolemic mice, although AAAs occur more frequently in hypercholesterolemic mice.5,6 In addition to AAAs, infusion of AngII to hypercholesterolemic mice also augments atherosclerosis.7,8 Like the human disease, AAAs developed during AngII infusion are characterized by progressive leukocyte accumulation, extracellular matrix degradation, lumen expansion, thrombus, and atherosclerosis.4
Both innate and adaptive immunities have proposed effects on AAA formation,9 although many facets are unknown regarding the relative contribution and mechanism. Myeloid differentiation factor 88 (MyD88) is a mediator of signaling cascades that directly influences leukocytes involved in innate immunity and has indirect effects on adaptive immunity.10 MyD88 was initially identified as a myeloid differentiation marker and was subsequently defined as an adaptor protein for signaling.11 It was first demonstrated to act as an adaptor protein in signaling mechanisms that follow the engagement of interleukin-1 (IL-1) with IL-1 receptors.12 Later, MyD88 was identified as a critical adaptor protein engaged by the majority of the Toll-like receptors (TLRs). MyD88 deficiency abolishes signaling of most TLRs, with the exception of the TLR3 and TLR4 MyD88-independent signaling pathways.13 Stimulation of MyD88 activates several signaling pathways including nuclear factor κB and mitogen-activated protein kinase that trigger transcription of numerous inflammatory cytokines to promote leukocyte recruitment.10
MyD88 and its association with TLRs have been studied in hypercholesterolemia-induced atherosclerosis.14–18 However, a role for MyD88 in development of AAAs is currently unknown. Therefore, we determined the role of MyD88 in AngII-induced AAAs and atherosclerosis in both apolipoprotein E deficient (apoE−/−) and low density lipoprotein receptor deficient (LDLR−/−) mice. MyD88 deficiency led to profound reductions on AngII-induced AAAs and atherosclerosis in both strains. In addition, the effects of MyD88 on these two vascular pathologies were predominantly mediated by cells of the hematopoietic lineage. Although TLR4 also contributed to AngII-induced AAA formation and atherosclerosis, this TLR was not responsible for the MyD88-dependent signaling in hematopoietic cells that promoted these two vascular pathologies.
Methods
A detailed description of all methods is presented in the Supplemental Materials.
Experimental Animal Models
MyD88−/−,14 TLR2−/−,16 or TLR4−/−19 mice were bred to either apoE−/− or LDLR−/− backgrounds as described in detail in the Supplemental Materials. AngII (1,000 ng/kg/min) was infused into male mice to develop AAAs and atherosclerosis as described previously.4 Bone marrow transplantations were performed as described previously.20 All studies were performed with the approval of the University of Kentucky Institutional Animal Care and Use Committee.
Genotyping by Polymerase Chain Reaction
All mice were genotyped by polymerase chain reaction (PCR) as described in detail in the Supplemental Materials (Supplemental Table I).
Measurements of Vascular Pathologies
AAAs and atherosclerosis were quantified as described previously.21–23
Statistics
All statistical analyses were performed using either version 3.5 of SigmaStat or version 8.2 of SAS. All measurements are represented as the mean±SEM. Analyses were performed using tests that were appropriate for the number of groups and the parametric versus non parametric nature of the data.
Results
Deficiency of MyD88 Reduced AngII-induced AAAs and Atherosclerosis
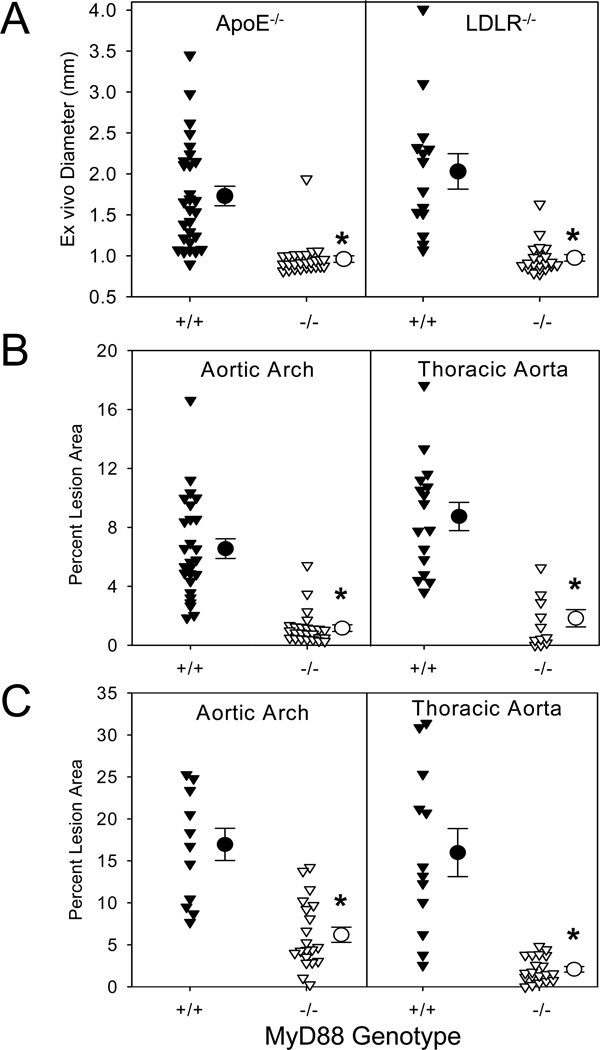
To determine the contribution of MyD88 to AngII-induced AAAs and atherosclerosis, initial studies were performed in male apoE−/− mice that were either MyD88+/+ or −/−. These mice were infused with AngII (1,000 ng/kg/min) for 28 days. The absence of MyD88 profoundly reduced AAA development, with external aortic diameters of 2.0 ± 0.2 mm versus 0.97 ± 0.08 mm in +/+ and −/− mice, respectively (P<0.001; Figure 1A). MyD88 deficiency also led to significant reductions in death due to aorta rupture (P<0.01; Supplemental Table II). The absence of MyD88 markedly reduced AngII-induced atherosclerosis in both aortic arches and thoracic aortas (82% and 79% reductions, respectively, P<0.001; Figure 1B). These reductions in AngII-induced AAAs and atherosclerosis occurred in the absence of MyD88 exerting any effects on plasma cholesterol concentrations, lipoprotein-cholesterol concentrations, or systolic blood pressure (Supplemental Table III and Supplemental Figure I).
Figure 1. MyD88 deficiency attenuated AngII-induced AAAs and atherosclerosis in both apoE−/− and LDLR−/− mice.
(A) Maximal external width of abdominal aortas was measured in apoE−/− and LDLR−/− mice that were either MyD88+/+ or −/−. Percent atherosclerotic lesion area was determined on aortic arches and thoracic aortas in apoE−/− (B) and LDLR−/− (C) mice that were either MyD88+/+ or −/−. Triangles represent individual mice. Circles represent means, and bars are SEM. * denotes P<0.001 by Mann-Whitney Rank Sum analyses.
Since apoE has many effects on immunity,24–26 we also examined the effect of MyD88 deficiency on AngII-induced AAAs and atherosclerosis in mice with LDLR deficiency. To induce hypercholesterolemia, these mice were fed a diet enriched in saturated fat. Comparable to apoE−/− mice, MyD88 deficiency in LDLR−/− mice greatly reduced AAA development (P<0.001; Figure 1A and Supplemental Figure II) and death due to aortic ruptures (P<0.01; Supplemental Table II). Furthermore, deficiency of MyD88 in male LDLR−/− mice resulted in profound reductions of atherosclerosis in both arch and thoracic regions (64% and 87%, respectively, P<0.001; Figure 1C). Again, reductions of these two vascular pathologies in MyD88 deficient mice occurred in the absence of any discernable effects on plasma cholesterol concentrations, lipoprotein-cholesterol concentrations, or systolic blood pressure (Supplemental Table III and Supplemental Figure I). All subsequent studies were performed using male LDLR −/− mice fed the saturated fat-enriched diet.
TLR2 and TLR4 Deficiencies Exerted Differential Effects on AngII-induced AAAs and Atherosclerosis
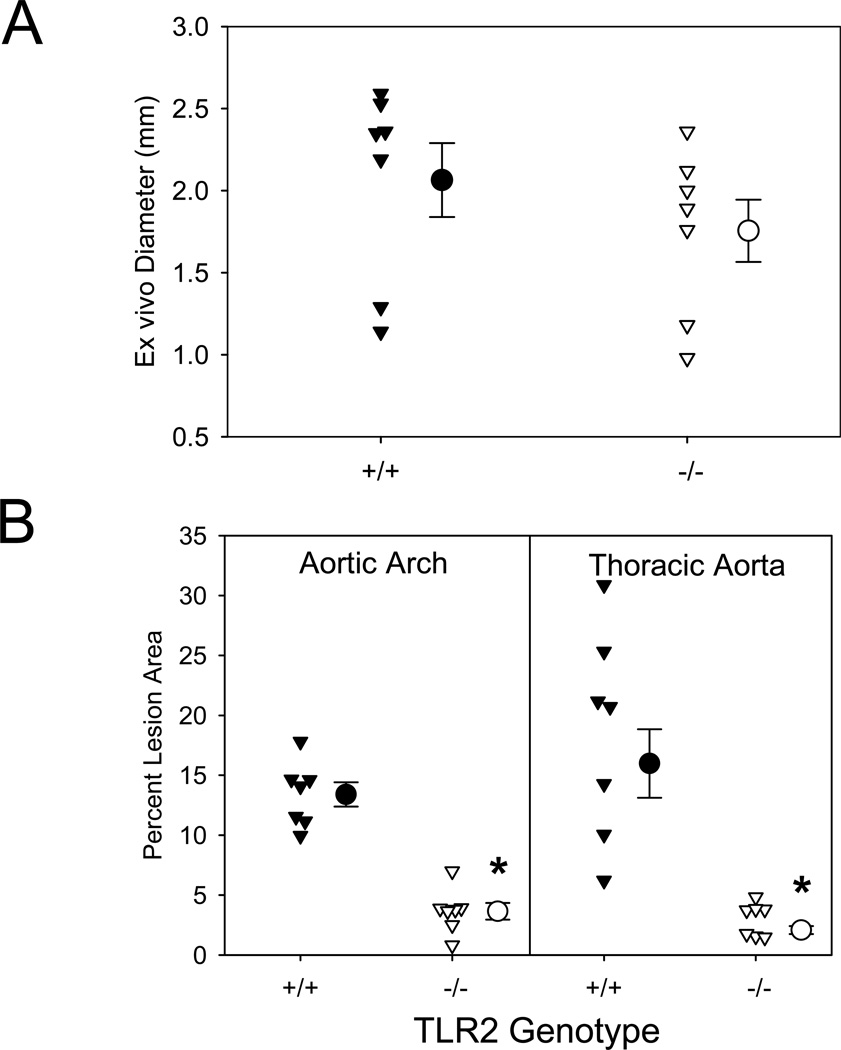
TLR2 signaling occurs via a MyD88-dependent signaling pathway. Therefore, to determine whether TLR2 deficiency mimicked the effects of MyD88 deficiency, male LDLR−/− mice that were either TLR2+/+ or −/− were fed the saturated fat-enriched diet for 5 weeks and also infused with AngII (1,000 ng/kg/min) during the final 4 weeks. Surprisingly, TLR2 deficiency exerted no significant effect on AngII-induced expansion of suprarenal aortic diameter, AAA incidence, or death due to aortic rupture (Figure 2A and Supplemental Table II). Similar to reported effects in hypercholesterolemic mice,16–18 TLR2 deficiency reduced AngII-induced atherosclerosis in both aortic arches and thoracic aortas to a similar extent as observed in MyD88 deficient mice (73% and 87%, respectively, P<0.001; Figure 2B). TLR2 deficiency had no effect on plasma cholesterol concentrations, systolic blood pressure, or body weight (Supplemental Table III). These data demonstrate that reductions in AAA formation in MyD88 deficient mice are independent of TLR2-MyD88 signaling.
Figure 2. TLR2 deficiency did not change AAAs, but reduced AngII-induced atherosclerosis in LDLR−/− mice.
(A) AAAs were defined by measuring maximal external width of abdominal aortas. (B) Percent atherosclerotic lesion area was determined on aortic arches and thoracic aortas. Triangles represent individual mice. Circles represent means, and bars are SEM. * denotes P<0.001 by Mann-Whitney Rank Sum analysis.
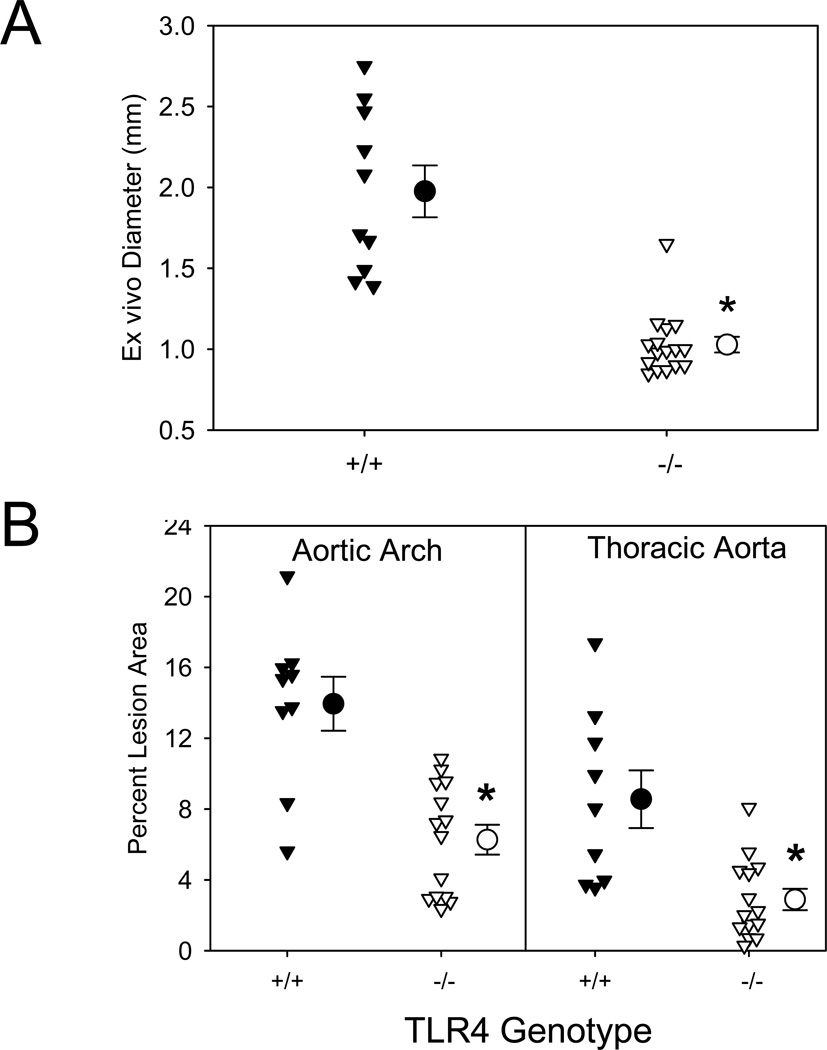
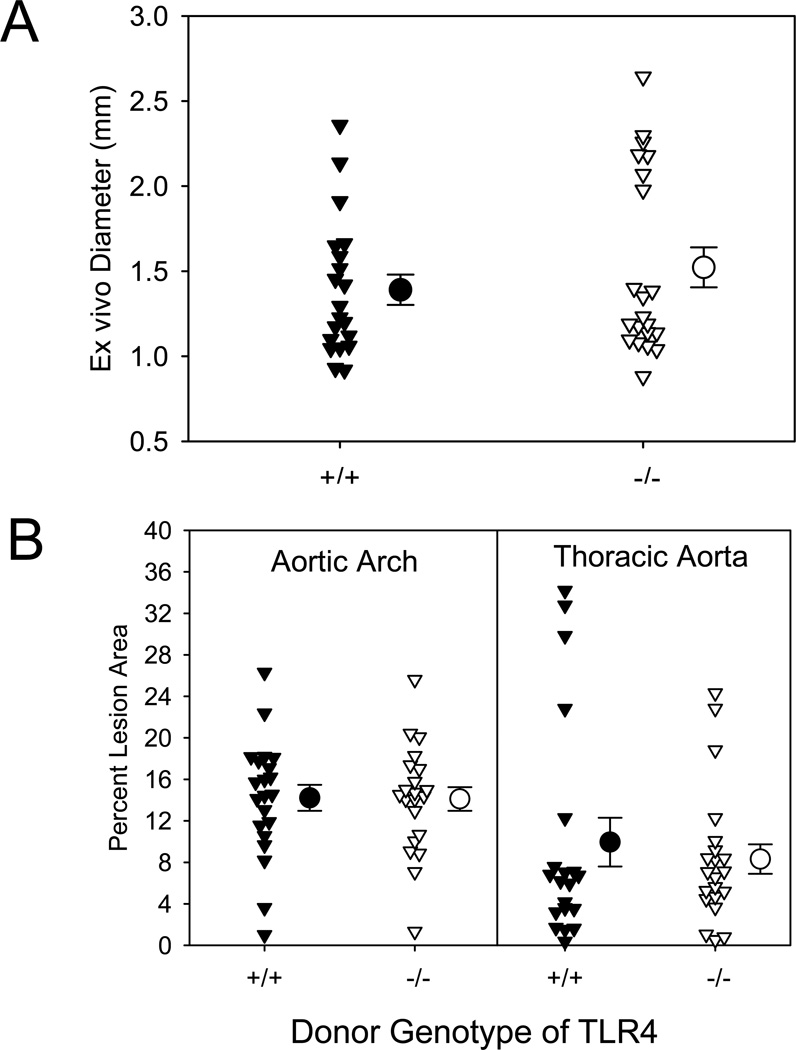
TLR4 signaling is mediated through either MyD88 dependent or independent mechanisms. We determined whether TLR4 deficiency mimicked the effects of MyD88 deficiency on the AngII-induced vascular pathologies. Unlike TLR2 deficiency, but comparable to MyD88 deficiency, TLR4 deficiency nearly ablated AngII-induced increases in external diameters of suprarenal aortas and AAA incidence (P<0.001; Figure 3A and Supplemental Table II). Deficiency of TLR4 in male LDLR−/− mice also resulted in reductions of atherosclerotic lesion size in both aortic arches and thoracic aortas (55% and 66%, respectively, P<0.001; Figure 3B). TLR4 deficiency had no effect on plasma cholesterol concentrations (Supplemental Table III), but a modest effect in reducing systolic blood pressure. Since AngII-induced increases in systolic blood pressure have minimal impact on AAAs and atherosclerosis, these changes in systolic blood pressure are not likely to have contributed to reduced vascular pathologies in TLR4 deficient mice.8,27 These data were generally consistent with TLR4 being the major receptor for MyD88 in the regulation of AngII-induced vascular pathologies.
Figure 3. TLR4 deficiency attenuated AngII-induced vascular pathologies in LDLR−/− mice.
(A) AAAs were defined by measuring maximal external width of abdominal aortas. (B) Percent atherosclerotic lesion area was determined on aortic arches and thoracic aortas. Triangles represent individual mice. Circles represent means, and bars are SEM. * denotes P<0.001 by Mann-Whitney Rank Sum analysis.
MyD88 Expression in Bone Marrow-derived Cells Determined AngII-induced AAAs and Atherosclerosis Independent of TLR4 in Hematopoietic Cells
Given the critical roles of MyD88 and TLR4 in the development of AngII-induced vascular pathologies, we used bone marrow transplantation to define whether the effect of MyD88 or TLR4 deficiency on AngII-induced AAAs and atherosclerosis was mediated by cells of the hematopoietic lineage. Recipients with MyD88+/+ or −/− donor cells had no change in plasma cholesterol concentrations, systolic blood pressure, body wieght, and white blood cells (Supplemental Tables IV and V). At the termination of each experiment, bone marrow was harvested and genotyping was performed to confirm chimerism.
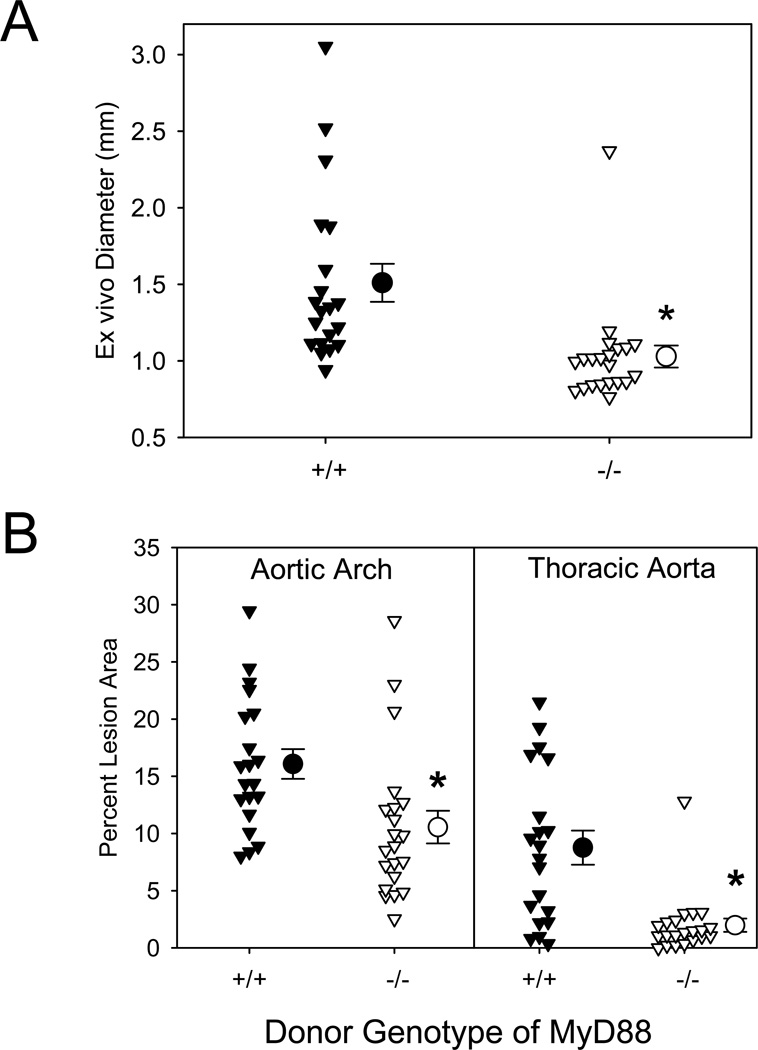
Similar to the results of whole body deletion, deficiency of MyD88 in bone marrow-derived cells significantly attenuated suprarenal aortic width (Figure 4A) and atherosclerosis (Figure 4B). AngII infusion into LDLR−/− mice repopulated with MyD88+/+ bone marrow-derived cells resulted in a 68% incidence of AAAs versus only 9% (P<0.001) in mice repopulated with MyD88−/− bone marrow-derived cells. Furthermore, atherosclerotic lesions were significantly attenuated in both aortic arches and thoracic aortas (P<0.006; Figure 4B) of mice repopulated with MyD88−/− bone marrow-derived cells, compared to those repopulated with MyD88+/+ donor cells.
Figure 4. AngII-induced AAAs and atherosclerosis were attenuated by MyD88 deficiency in bone marrow-derived cells.
(A) AAAs were defined by measuring maximal external width of abdominal aortas. (B) Percent atherosclerotic lesion area was determined on aortic arches and thoracic aortas. Triangles represent individual mice. Circles represent means, and bars are SEM. * denotes P<0.006 by Mann-Whitney Rank Sum analyses.
Chimerism of LDLR−/− mice repopulated with TLR4+/+ or −/− donor cells was confirmed by genotyping of bone marrow DNA at the end of the experiment and no effect was found on plasma cholesterol concentrations, systolic blood pressure, and body weight (Supplemental Table IV). Although the overall numbers of WBCs were not changed, there was a small reduction of platelets in mice repopulated with TLR4−/− donor cells (P=0.04; Supplemental Table V). Surprisingly, TLR4 deficiency in bone marrow-derived cells had no effect on either the expansion of suprarenal aortas (Figure 5A) or the development of atherosclerosis in aortic arches and thoracic aortas (Figure 5B) of LDLR−/− recipient mice infused with AngII. These data imply that unlike MyD88, the effects of TLR4 on AAAs and atherosclerosis are mediated by non-hematopoietic cells.
Figure 5. AngII-induced AAAs and atherosclerosis were not changed by TLR4 deficiency in bone marrow-derived cells.
(A) AAAs were defined by measuring maximal external width of abdominal aortas. (B) Percent atherosclerotic lesion area was determined on aortic arches and thoracic aortas. Triangles represent individual mice. Circles represent means, and bars are SEM.
MyD88 Deficiency Attenuated AngII-induced Monocytosis and Redistribution of Ly-6C Populations
Chronic AngII infusion for 28 days significantly increased leukocytes, neutrophils, and monocytes (P<0.001) in peripheral blood of LDLR−/− mice. While MyD88+/+ and −/− mice had no differences on leukocyte numbers prior to AngII infusion, MyD88 deficiency completely attenuated the AngII-induced increases of leukocyte numbers, with equivalent effects on neutrophils and monocytes (Supplemental Figure III). However, TLR4 deficiency did not change leukocyte and its subtype numbers.
Redistribution of Ly-6C populations has been detected in AngII-induced AAAs and implicated in the progression of atherosclerosis in apoE−/− mice.28,29 Therefore, we investigated the effects of AngII on the Ly-6C monocytes in peripheral blood that were categorized into 3 populations of Ly-6C (Ly-6Clow, Ly-6Cint, and Ly-6Chi).30 MyD88 deficiency ablated AngII-induced increases of Ly-6Chi and reductions of Ly-6Clow monocyte numbers (P<0.007; Supplemental Figure IV). However, TLR4 deficiency did not change AngII-induced redistribution of Ly-6C populations.
There is evidence that spleen acts as a major reservoir for monocytes in peripheral blood.29 However, there was no overt difference in spleens of MyD88+/+ versus −/− mice in regard to CD68 immunoreactivity when infused with AngII. Consistent with the changes of Ly-6C monocyte populations in peripheral blood, MyD88 deficiency abolished AngII-induced increases of Ly-6Chi and reductions of Ly-6Clow monocyte numbers in spleens (P<0.001; Supplemental Figure V).
To determine whether MyD88−/− mice had impaired response to macrophage elicitation, thioglycollate was injected into mouse peritoneal cavities. MyD88 deficiency significantly reduced the numbers of macrophages elicited to peritoneal cavities (P<0.001; Supplemental Figure VI), whereas TLR4 deficiency showed no significant effect on macrophage elicitation to peritoneal cavities. All of these findings infer differential effects of MyD88 and TLR4 on monocyte/macrophage behaviors.
We also examined whether AngII had differential effects on MyD88 and TLR4 in smooth muscle cells isolated from the aneurysmal prone suprarenal aortic region. While AngII had no effect on the expression of MyD88 in smooth muscle cells, it greatly increased the abundance of TLR4 mRNA in these cells that was comparable to the TLR4 agonist, lipopolysaccharide (P<0.001; Supplemental Figure VII).
Discussion
AngII infusion into hypercholesterolemic mice has been used in many studies to induce AAAs and augment the development of atherosclerosis.4,8 These diseases are frequently co-morbid, and therefore the model permits simultaneous evaluation of both vascular pathologies. Previous studies have demonstrated distinctions between mechanisms that influence the processes of AAAs versus atherosclerosis.31–34 In the present study, MyD88 and TLR4 deficiencies had equivalent effects on AngII-induced AAAs and atherosclerosis. However, while TLR2 deficiency reduced AngII-induced atherosclerosis, we were not able to discern any effect on the development of AAAs.
Deficiency of MyD88 was studied on the effects of AngII-induced AAAs in both apoE−/− mice fed a normal laboratory diet and LDLR−/− mice fed a saturated fat-enriched diet. There are marked differences in plasma cholesterol concentrations and lipoprotein characteristics between the two strains. There is also evidence that apoE has direct effects on innate and adaptive immune responses independent of lipoproteins, which has the potential to lead to different responses between apoE−/− and LDLR−/− mice.24–26 Despite these differences, aneurysms that form during AngII infusion in the two strains are similar in size and characteristics. Consistently as demonstrated in this study, deficiency of MyD88 had a similar effect in attenuating AngII-induced AAAs in both strains.
Infusion of AngII leads to medial accumulation of macrophages in regions that are prone to aneurysmal formation.35 Following luminal dilation, there is enhanced macrophage accumulation and the presence of other leukocyte types, such as lymphocytes.35 Macrophages are the most abundant leukocyte type that infiltrates aortic tissue at all stages of aneurysmal formation.35,36 Despite this abundance, the function of macrophages in aneurysmal pathology has not been determined. Our previous study using osteopetrotic mice that have marked reductions in circulating monocytes37 was confounded by several defects in this strain and a low incidence of AngII-induced AAAs in the genetic background-matched wild type mice. A later study demonstrated that substantial reductions of circulating monocytes by clodronate-lipoproteins decreased severity of AngII-induced AAAs.6 In the present study, we found that the striking effect of MyD88 on AAA formation was attenuated by selective deletion of MyD88 in hematopoietic cells, strongly suggesting a role for MyD88 signaling in leukocytes in promoting AAAs. Interestingly, the same effect did not occur for TLR4 deficiency in hematopoietic cells, despite the pronounced attenuation of AAA formation in mice with whole body TLR4 deficiency.
Ly-6Chi monocytes are the predominant population of macrophages accumulating in AngII-induced AAAs.28 We demonstrated that MyD88 deficiency ablated AngII-induced Ly-6Clow to Ly-6Chi monocyte switching in both peripheral blood and spleens. These results are consistent with the previous report that AngII mediates monocyte recruitment from the spleen to peripheral tissues after injury.38 It is possible that MyD88 deficiency blunts macrophage infiltration into the aortic wall via abolishing the ability of AngII-induced Ly-6C monocyte switching.
AngII exerts its bioactive effects predominantly through binding AT1a receptors. AT1a receptors are ubiquitously present on many cell types including macrophages and resident cell types of the aorta. Comparable to whole body deficiency of AT1a receptors,39 whole body deficiency of MyD88 strikingly reduced AngII-induced AAAs. However, while MyD88 deficiency in bone marrow-derived cells also profoundly reduced AngII-induced AAAs, AT1a receptor deficiency on bone marrow-derived cells failed to influence AngII-induced AAAs.39 These findings infer that AngII induces changes in cells of non-hematopioetic origins that subsequently promote AAAs through a mechanism based on MyD88 of hematopoietic origin.
The role of MyD88 and its link to TLRs in the development of atherosclerosis have been previously studied in apoE−/− mice.14,15,17 Consistent with the previous studies, we demonstrated that deficiency of MyD88 also decreased atherosclerotic lesions that were augmented by AngII infusion in apoE−/− mice. We also demonstrated that deficiency of MyD88 had a comparable effect on the reduction of atherosclerosis in LDLR−/− mice. The effect of MyD88 deficiency on AngII-induced atherosclerosis occurred in both mouse strains, despite profound differences in both concentrations and characteristics of plasma lipoprotein cholesterol distributions. Our study advances the previous understanding of the role of MyD88 in atherosclerosis by demonstrating that MyD88 in bone marrow-derived cells accounts for the predominant effect of this adaptor protein in the development of atherosclerosis. Deficiency of TLR2 or TLR4 decreases atherosclerosis in both apoE−/− and LDLR−/− mice.15–18 The present study also demonstrated that deficiency of these TLRs decreased atherosclerosis in AngII-infused LDLR−/− mice. There is compelling evidence for the similarities of the effects of MyD88, TLR2, and TLR4 in the development of atherosclerosis. However, it was unclear whether MyD88 served as a required adaptor signaling molecule for TLR2 or TLR4 to promote atherosclerosis. Our findings using bone marrow transplantation in the present study clearly demonstrate that reductions in atherosclerosis in mice with complete deficiency of TLR4 were attributable to MyD88-independent mechanisms. This conclusion is consistent with the recent demonstration that TRIF deficiency reduced atherosclerosis by a mechanism not involving hematopoietic cells.40
In conclusion, this study demonstrates that MyD88 deficiency in hematopoietic cells has a profound effect in reducing AngII-induced AAAs and atherosclerosis. While whole body deficiency of TLR4 had a similar ability to reduce AngII-induced vascular pathologies, the use of bone marrow transplantation clearly demonstrated that these effects were unrelated to MyD88 mediated signaling in hematopoietic cells. Future studies will systemically evaluate other MyD88-linked receptors, such as IL-1β and IL-18, in AngII-induced AAA formation and atherosclerosis.41–44
Supplementary Material
Acknowledgments
We acknowledge the assistance of Jessica J. Moorleghen and Congqing Wu.
Sources of Funding
These studies were supported by funding from the NIH (HL08100 and AG20255). A. Phillip Owens III was supported by a Predoctoral Fellowship from the Ohio Valley Affiliate of the American Heart Association (0615222B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
REFERENCES
- 1.Wang YX, Martin-McNulty B, Freay AD, Sukovich DA, Halks-Miller M, Li WW, Vergona R, Sullivan ME, Morser J, Dole WP, Deng GG. Angiotensin II increases urokinase-type plasminogen activator expression and induces aneurysm in the abdominal aorta of apolipoprotein E-deficient mice. Am J Pathol. 2001;159:1455–1464. doi: 10.1016/S0002-9440(10)62532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizas KD, Ippagunta N, Tilson MD., 3rd Immune cells and molecular mediators in the pathogenesis of the abdominal aortic aneurysm. Cardiol Rev. 2009;17:201–210. doi: 10.1097/CRD.0b013e3181b04698. [DOI] [PubMed] [Google Scholar]
- 3.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 4.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, Loskutoff DJ, Carmeliet P, Dole WP, Wang YX. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest. 2010;120:422–432. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann NY Acad Sci. 1999;892:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- 8.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 10.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 11.Lord KA, Hoffman-Liebermann B, Liebermann DA. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene. 1990;5:1095–1097. [PubMed] [Google Scholar]
- 12.Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer JL, Di Marco F, French L, Tschopp J. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 13.Watters TM, Kenny EF, O'Neill LA. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol. 2007;85:411–419. doi: 10.1038/sj.icb.7100095. [DOI] [PubMed] [Google Scholar]
- 14.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 15.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schroder NW, Crother TR, Bulut Y, Doherty TM, Bradley M, Shaposhnik Z, Peterson EM, Tontonoz P, Shah PK, Arditi M. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008;181:7176–7185. doi: 10.4049/jimmunol.181.10.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC, 3rd, Genco CA. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2008;196:146–154. doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 20.Lu H, Rateri DL, Feldman DL, Charnigo RJ, Jr, Fukamizu A, Ishida J, Oesterling EG, Cassis LA, Daugherty A. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. doi: 10.1172/JCI32970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]
- 22.Wang YX, Cassis LA, Daugherty A. Angiotensin II-induced abdominal aortic aneurysms. In: Xu Q, editor. A Handbook of Mouse Models for Cardiovascular Disease. John Wiley & Sons; 2006. pp. 125–136. [Google Scholar]
- 23.Daugherty A, Lu H, Howatt DA, Rateri DL. Modes of defining atherosclerosis in mouse models: relative merits and evolving standards. Methods Mol Biol. 2009;573:1–15. doi: 10.1007/978-1-60761-247-6_1. [DOI] [PubMed] [Google Scholar]
- 24.Pepe MG, Curtiss LK. Apolipoprotein E is a biologically active constituent of the normal immunoregulatory lipoprotein LDL-In. J. Immunol. 1986;136:3716–3723. [PubMed] [Google Scholar]
- 25.Kelly ME, Clay MA, Mistry MJ, Hsieh-Li H-M, Harmony JAK. Apolipoprotein E inhibition of proliferation of mitogen-activated T lymphocytes: Production of interleukin 2 with reduced biological activity. Cell Immunol. 1994;159:124–139. doi: 10.1006/cimm.1994.1302. [DOI] [PubMed] [Google Scholar]
- 26.Roselaar SE, Daugherty A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res. 1998;39:1740–1743. [PubMed] [Google Scholar]
- 27.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. Angiotensin II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–H1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nahrendorf M, Keliher E, Marinelli B, Leuschner F, Robbins CS, Gerszten RE, Pittet MJ, Swirski FK, Weissleder R. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol. 2011;31:750–757. doi: 10.1161/ATVBAHA.110.221499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 32.Henriques TA, Huang J, D'Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology. 2004;145:3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 33.Uchida HA, Kristo F, Rateri DL, Lu H, Charnigo R, Cassis LA, Daugherty A. Total lymphocyte deficiency attenuates AngII-induced atherosclerosis in males but not abdominal aortic aneurysms in apoE deficient mice. Atherosclerosis. 2010;211:399–403. doi: 10.1016/j.atherosclerosis.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchida HA, Poduri A, Subramanian V, Cassis LA, Daugherty A. Urokinase-Type Plasminogen activator deficiency in bone marrow-derived cells augments rupture of angiotensin II-induced abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.234997. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 36.Rateri DL, Howatt DA, Moorleghen JJ, Charnigo R, Cassis LA, Daugherty A. Prolonged infusion of angiotensin II in apoE−/− mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysms. Am J Pathol. 2011;179:1542–1548. doi: 10.1016/j.ajpath.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babamusta F, Rateri DL, Moorleghen JJ, Howatt DA, Li XA, Daugherty A. Angiotensin II infusion induces site-specific intra-laminar hemorrhage in macrophage colony-stimulating factor-deficient mice. Atherosclerosis. 2005;186:282–290. doi: 10.1016/j.atherosclerosis.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 40.Richards MR, Black AS, Bonnet DJ, McKeon KK, Barish GD, Hoebe KH, Tobias PT, Curtiss LK. TRIF deficiency reduces atherosclerosis in hyperlipidemic LDL-receptor knockout mice (Abstract) Arterioscler Thromb Vasc Biol. 2010;30:e295. [Google Scholar]
- 41.Boraschi D, Tagliabue A. The interleukin-1 receptor family. Vitam Horm. 2006;74:229–254. doi: 10.1016/S0083-6729(06)74009-2. [DOI] [PubMed] [Google Scholar]
- 42.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1 beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arteriosclero Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 43.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 44.Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res. 2001;89:E41–E45. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.