Abstract
Objective
It has been suggested that fluid accumulation may delay recognition of acute kidney injury (AKI). We sought to determine the impact of fluid balance on the incidence of non-dialysis requiring AKI in patients with acute lung injury and to describe associated outcomes, including mortality.
Design
Analysis of the Fluid and Catheter Treatment Trial, a factorial randomized clinical trial of conservative versus liberal fluid management and of management guided by a central venous versus pulmonary artery catheter.
Setting and Patients
1000 patients at ARDS Network hospitals.
Measurements and Main Results
The incidence of AKI, defined as an absolute rise in creatinine of ≥ 0.3 mg/dL or a relative change of > 50% over 48 hours, was examined before and after adjustment of serum creatinine for fluid balance. The incidence of AKI before adjustment for fluid balance was greater in those managed with the conservative fluid protocol (57 versus 51%, p = 0.04). After adjustment for fluid balance, the incidence of AKI was greater in those managed with the liberal fluid protocol (66 versus 58%, p = 0.007). Patients who met AKI criteria after adjustment of creatinine for fluid balance (but not before) had a mortality rate that was significantly greater than those who did not meet AKI criteria both before and after adjustment for fluid balance (31 versus 12%, p < 0.001) and those who had AKI before but not after adjustment for fluid balance (31 versus 11%, p = 0.005). The mortality of those patients meeting AKI criteria after but not before adjustment for fluid balance was similar to patients with AKI both before and after adjustment for fluid balance (31% versus 38%, p = 0.18).
Conclusions
Fluid management influences serum creatinine and therefore the diagnosis of AKI using creatinine-based definitions. Patients with “unrecognized” AKI that is identified after adjusting for positive fluid balance have high mortality rates, and patients who have AKI before but not after adjusting for fluid balance have low mortality rates. Future studies of AKI should consider potential differences in serum creatinine caused by changes in fluid balance and the impact of these differences on diagnosis and prognosis.
Keywords: Acute kidney injury, acute lung injury, acute respiratory distress syndrome, fluid balance, creatinine, mortality
Introduction
Acute kidney injury (AKI) is a common complication in critically ill patients and is a major risk factor for death. For example, while acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) are associated with mortality rates between 25 and 40 percent (1–4), when patients with ALI/ARDS develop AKI, mortality rates increase substantially, to the range of 50–80% (5, 6).
For many years, differing definitions for AKI were used in the literature, making comparisons between studies difficult. Over the last decade, consensus definitions have advanced the field by allowing for improved comparisons between study populations (7, 8). More recently, it has been proposed that since creatinine distributes into both the intracellular and extracellular fluid compartments (9), fluid accumulation may delay recognition of AKI, as creatinine is diluted (10). Similarly, under conditions of negative fluid balance, creatinine may become relatively concentrated and patients may be more likely to meet criteria for AKI. Indeed, some have advocated that serum creatinine measurements should be adjusted for fluid balance and have demonstrated that AKI is recognized earlier if serum creatinine is adjusted for fluid balance in patients with AKI who underwent nephrology consultation (10).
To test the impact of fluid balance on AKI classification in a more generalized ICU setting, we used data from the Fluid and Catheter Treatment Trial (FACTT), a randomized factorialized clinical trial of a pulmonary artery catheter versus central venous catheter and a fluid-liberal versus fluid-conservative management strategy for patients with ALI (2). There was no difference in the incidence of dialysis or renal failure defined by the Brussels Organ Failure criteria (serum creatinine > 2 mg/dL) (11) between the fluid management arms. In this study, we have examined the impact of fluid balance on more sensitive definitions of AKI. We hypothesized that the incidence of AKI would increase in patients who were managed with a liberal fluid management protocol after adjusting for fluid balance. Furthermore, we hypothesized that patients who did not meet the definition of AKI using unadjusted serum creatinine values but who met criteria for AKI after adjusting creatinine for fluid balance would have poorer outcomes than patients who never met AKI criteria before or after adjustment for fluid balance.
Materials and Methods
We analyzed data from the ARDS Network Fluid and Catheter Treatment trial. This trial excluded patients in whom renal replacement therapy (for either acute or end stage renal disease) had been initiated or planned for at the time of screening (1, 2). The clinical data have been described previously and included chronic health conditions, laboratory data, ventilator parameters, PaO2/FiO2 ratio, and vasopressor use. Vasopressor use was defined as the use of dopamine at 6 mcg/kg/min or higher, or the use of other vasopressors including norepinephrine, vasopressin or epinephrine at any dose.
Definition of acute kidney injury
We used the Acute Kidney Injury Network (AKIN) consensus definition as our a priori definition of acute kidney injury (8). Because even small changes in serum creatinine are associated with increased mortality, our analysis focused primarily on AKIN stage 1 disease, defined as an increase in serum creatinine of 50% or an absolute increase or more than 0.3 mg/dL over a 48 hour window during study days 1–8. The initial baseline creatinine was obtained immediately pre-randomization; subsequently the new baseline creatinine for each 48 hour window was the lowest recorded study measurement on a given study day. Since patients were assigned to a fluid management strategy for 7 days, we examined the incidence of AKI over the first 8 days of the study. To calculate adjusted creatinine, we first estimated the volume of distribution for creatinine on the day of randomization, which is equal to total body water (TBW). We assumed that TBW was 60% of the patient's weight at the time of randomization (10). In the case of 76 patients where initial weight was not available, we used predicted body weight based on the patient's height and gender. In a sensitivity analysis, we excluded these 76 patients from the study population. For each study day, we calculated cumulative on-study fluid balance using the 24 hour fluid intake and output recorded in the case report forms for each study subject. Then, adjusted Cr = SCr*(1+[on study cumulative net fluid balance/TBW]) where SCr is measured serum creatinine, and TBW is 0.6 × patient weight at randomization. Patients were then classified as AKI/no AKI by creatinine before and after adjustment for fluid balance, yielding four groups of patients for analyses: those who did not have AKI before or after adjustment for fluid balance, those with AKI only after adjustment for fluid balance, those who had AKI before but not after adjustment for fluid balance, and those with AKI both before and after adjustment for fluid balance.
Statistical Analysis
We measured the proportion of AKIN stages 1–3 AKI in the fluid liberal and fluid conservative treatment arms using serum or adjusted creatinine. Proportions were calculated for the frequency of categorical variables in each subgroup. Differences in proportions by subgroup were assessed using the chi-square test. Means, standard deviations, medians, and inter-quartile ranges were calculated for continuous variables by subgroup. Differences in means by subgroup were assessed using single factor analysis of variance (ANOVA), and differences in medians were assessed using the Kruskal-Wallis test. No adjustments were made for multiple comparisons.
The statistical significance of the unadjusted association between acute kidney injury category (predictor) and death (outcome) was assessed using the chi-square test or Fisher's exact test, where appropriate. Multivariable logistic regression analysis was used to assess the adjusted odds of death associated with the four AKIN stage 1 AKI combinations of assessments before and after adjustment for fluid balance. Comparisons were made with reference to patients in the subgroup with no AKI before adjustment and no AKI after adjustment, controlling for patient age, race, severity of illness (APACHE III score), baseline vasopressor use, and baseline serum creatinine. The odds ratio for age was expressed per 10 year unit increases in age. Model fit (calibration) was assessed using the Hosmer-Lemeshow goodness-of-fit test, which compares model performance (observed vs. expected) across deciles of risk. A non-significant value for the Hosmer-Lemeshow chi-square test suggests there is no significant difference between observed and expected risk across deciles between groups. Similarly, among the subgroup of patients who met criteria for AKIN stage 1 AKI, we examined the unadjusted association between AKIN stage 2 category (predictor) and dialysis (outcome) was assessed using the chi-square test or Fisher's exact test, where appropriate. Data analysis was conducted using Stata 10.1 (StataCorp, College Station, TX). Differences for tests with p values < 0.05 were considered statistically significant.
Results
Baseline characteristics
Patient demographics and baseline hemodynamic and laboratory data have been previously reported (1, 2). At enrollment, study subjects had a median creatinine of 1.0 mg/dL, with a 25–75% interquartile range of 0.7–1.5 mg/dL; this was not different in the fluid liberal and fluid conservative treatment groups (p=0.39). The rate at which patients received renal replacement therapy through study day 60 was not different between groups (10% in the fluid conservative group, 14% in the fluid liberal group, p = 0.06).
Association of mild AKI with fluid management strategy without adjusting for differences in volume status
The incidence of AKI over the first eight days of the study stratified by treatment management protocols is shown in Table 1. There was an increased incidence of AKIN stage 1 AKI (defined as an increase in serum creatinine to greater than 1.5 fold the baseline value or 0.3 mg/dL absolute increase from baseline) in those managed with the fluid conservative protocol (57% in fluid conservative versus 51% in fluid liberal, p = 0.04). AKIN Stage 2 and 3 AKI were defined as an increase in serum creatinine to greater than 2 fold the baseline value and as an increase in creatinine to greater than 3 fold the baseline value, a 0.5 mg/dL absolute increase in baseline to a level of at least 4 mg/dL, or the need for dialysis. There was no difference in the proportion of patients with AKIN stages 2 or 3 AKI between those managed with the fluid conservative and fluid liberal treatment strategies (11 versus 14%, p = 0.17 and 15% versus 18%, p = 0.94, respectively). Thus, patients in the fluid conservative treatment arm more commonly had modest changes in serum creatinine than patients in the fluid liberal treatment arm, but there was no difference in the incidence of severe AKI or dialysis.
Table 1.
Development of AKI by treatment group before and after adjustment of serum creatinine for fluid balance
| Renal Outcomes | Liberal | Conservative | P-Value Liberal vs. Conservative | |||
|---|---|---|---|---|---|---|
| Not Adjusted | Adjusted | Not Adjusted | Adjusted | Not Adjusted | Adjusted | |
| AKIN Stage 1 n (%) | 253 (51%) | 328 (66%) | 288 (57%) | 290 (58%) | 0.04 | 0.007 |
| AKIN Stage 2 n (%) | 54 (11%) | 106 (21%) | 69 (14%) | 87 (17%) | 0.17 | 0.11 |
| AKIN Stage 3 n (%) | 75 (15%) | 89 (18%) | 75 (15%) | 83 (17%) | 0.94 | 0.56 |
*There was no difference in the incidence of AKI by PAC versus CVC management groups.
Differences in volume status between the fluid management arms may account for differences in the rates of AKI
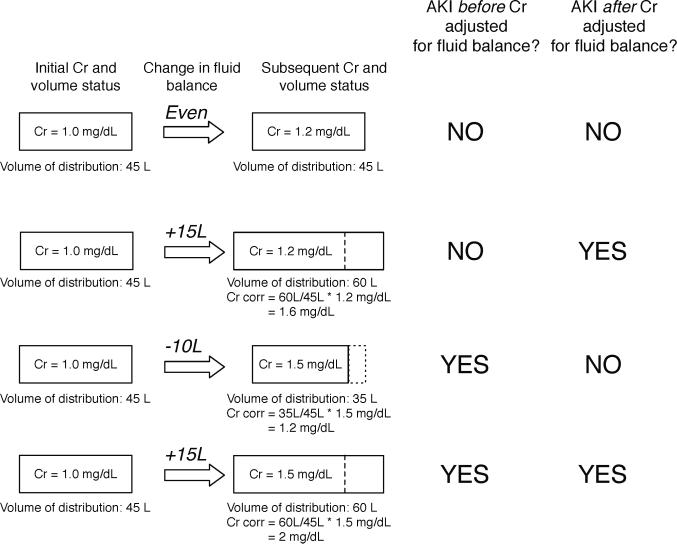
We next examined the incidence of AKI after adjusting for fluid balance, as has been done by Macedo et al (10). With this approach, the measured serum creatinine is adjusted upwards or downwards based on changes in fluid balance; changes in fluid balance impact total body water, which is the volume of distribution for creatinine (Figure 1). Patients in the fluid conservative treatment arm averaged a cumulative fluid balance over the 7 days of the protocol of – 200 ml. In contrast, patients in the fluid liberal arm had approximately 7 liters of positive fluid balance at day 7 of the protocol (2). Furthermore, the fluid balance in the liberal arm was similar to the “usual” management of patients enrolled in two prior ARDS Network clinical trials of a low tidal volume ventilation strategy and of a high positive end-expiratory pressure management strategy. After adjustment of creatinine for changes in fluid balance, the incidence of AKIN stage 1 AKI was lower in the fluid conservative than the fluid liberal group (66 versus 58%, p = 0.007). There was no difference in the incidence of AKIN stage 2 or 3 AKI between patients in the fluid liberal and fluid conservative arms (Table 1). Thus, when AKI is defined using modest changes in serum creatinine, prescribed fluid balance can affect disease ascertainment. There was little change in the incidence of AKIN stage 1 AKI in patients in the fluid conservative group before and after adjustment of creatinine for fluid balance (57 versus 58%). However, in patients who were managed with a more liberal fluid management strategy, the incidence of AKIN stage 1 AKI increased from 51 to 66% after adjustment for fluid balance.
Figure 1.
Impact of adjustment of serum creatinine for fluid balance on the ascertainment of AKI.
Mortality in those with “unrecognized” AKI is similar to that of those with clear-cut AKI
Patients were then categorized into four groups based on the presence or absence of AKIN stage I AKI before and after adjustment for fluid balance (Figure 1); for the sake of clarity we will refer to these groups as follows: Group A did not meet criteria before or after adjustment for fluid balance. In contrast, Group B met criteria for AKI only after adjustment of fluid balance. Group C met criteria for AKI before but not after adjustment for fluid balance. Finally, Group D met criteria for AKI both before and after adjustment for fluid balance. Group B comprised 131 patients (13.1%), who had a mortality rate similar to patients in Group D (31% vs. 38%, p = 0.18, Table 2) and significantly greater than those in Group A (31% versus 12%, p < 0.001). Thus, the mortality rate of patients who were classified as having AKI only after adjustment for fluid balance was similar to that of patients who had AKI both before and after adjustment for fluid balance, and was markedly higher than that of patients without AKI.
Table 2.
Patients with AKIN Stage 1 AKI after adjustment for fluid balance have similar mortality rates and ventilator free days to those with AKI regardless of fluid balance.
| AKIN Stage 1 AKI | Group | Survivors N (%) | Non-Survivors N (%) | Ventilator Free Days (VFDs) Median (25–75% IQR) | ||
|---|---|---|---|---|---|---|
| Before Adjustment for Fluid Balance | & | After Adjustment for Fluid Balance | ||||
| NO | & | NO | A* | 289 (88%) | 39 (12%) | 22 (16,25) |
| NO | & | YES | B** | 90 (69%) | 41 (31%) | 15 (0,21) |
| YES | & | NO | C*** | 48 (89%) | 6 (11%) | 22 (13,25) |
| YES | & | YES | D | 304 (62%) | 183 (38%) | 7 (0, 20) |
For mortality analysis: p < 0.001 for comparison to Groups B and D; p = 1.0 for comparison to Group C. For VFD analysis: p < 0.001 for comparison to Groups B and D; p = 0.71 for comparison to Group C.
For mortality analysis: p = 0.005 for comparison to Group C; p = 0.18 for comparison to Group D. For VFD analysis: p < 0.001 for comparison to Group C; p = 0.09 for comparison to Group D.
For mortality analysis: p < 0.001 for comparison to Group D. For VFD analysis: p < 0.001 for comparison to Group D.
In contrast, 54 patients (5.4%) met criteria for AKI before adjustment for fluid balance but not after adjustment (Group C). This group had a substantially lower mortality rate than patients in either Group B or D (11% vs. 31% and 38%, respectively, p < 0.001 for both comparisons). The mortality rate for patients in Group C was similar to those in Group A (11% vs 12%, p = 1.0). That is, the mortality rate of patients who had AKI before but not after adjustment for fluid balance was similar to that of patients who did not have AKI both before and after adjustment for fluid balance, and was lower than that of patients with AKI after adjustment for fluid balance.
These results were unchanged in sensitivity analyses where the 76 patients in whom actual body weight was missing (and predicted body weight was used instead) were excluded from the analysis and in an analysis where a correction factor for the predicted body weight was applied to these 76 participants based on the average difference in actual and predicted body weight in the other 924 participants. These participants made up 7% and 8.1% of the fluid liberal and fluid conservative groups, respectively (p=0.51). As another sensitivity analysis, we examined an alternate approach where changes in creatinine were applied as in the AKIN Stage 1 criteria, but where daily maximum creatinine (days 1–8) values were compared to a single baseline serum creatinine recorded pre-randomization. With this modification the incidence of AKI misclassification decreased (58 and 41 patients in groups B and C, respectively). However the findings with respect to mortality and VFDs were similar to our main results (Online Table 1).
In multivariable logistic regression, those in Group B (e.g, those who did not have AKI by serum creatinine but met criteria for AKI after adjustment of creatinine for fluid balance) had a 2.09-fold increase in the odds of death (95% CI 1.19–3.67, p =0.01, Table 3) compared to Group A (those who did not have AKI before and after adjustment for fluid balance), following adjustment for age, gender, race, severity of illness, baseline vasopressor use and baseline serum creatinine. In the same model, those in Group D (with AKI before and after adjustment of creatinine for fluid balance) had a 3.16 fold increase in the odds of death compared to Group A (95% CI 2.04–4.87, p < 0.001).
Table 3.
Multivariable clinical model for death. As described in the Methods, we controlled for age, sex, race, the trial interventions as well as other covariates that reflect the severity of acute illness, including baseline vasopressor use, the presence or absence of infection, baseline creatinine and the APACHE III score.
| Variable | Odds Ratio | 95% CI | P value |
|---|---|---|---|
| Age* | 1.35 | 1.22–1.50 | <0.001 |
| Male | 1.11 | 0.80–1.54 | 0.53 |
| Caucasian race | 0.57 | 0.41–0.80 | 0.001 |
| Fluid conservative strategy | 0.96 | 0.69–1.33 | 0.81 |
| Pulmonary artery catheter | 1.02 | 0.74–1.41 | 0.90 |
| Baseline vasopressor use | 1.06 | 0.74–1.51 | 0.76 |
| Baseline creatinine# | 0.98 | 0.81–1.17 | 0.81 |
| Acute lung injury secondary to infection | 1.24 | 0.85–1.83 | 0.27 |
| APACHE III score+ | 1.26 | 1.19–1.34 | <0.001 |
| No AKI before adjustment; AKI after adjustment for fluid balance (Group B referent to A) | 2.09 | 1.19–3.67 | 0.01 |
| AKI before adjustment; no AKI after adjustment for fluid balance (Group C referent to A) | 1.17 | 0.45–3.02 | 0.75 |
| AKI before AND after adjustment for fluid balance (Group D referent to A) | 3.16 | 2.04–4.87 | <0.001 |
Per 10 year increase in age
Per 1 mg/dL increase in creatinine
Per 10 point increase in APACHE III score
Hosmer-Lemeshow goodness of fit p = 0.90
Similarly, we tested the number of ventilator free days (VFDs) in those with and without AKI (Table 2). The number of ventilator free days (VFDs) in patients in Group B was significantly lower than the number of VFDs achieved by patients in Group A (median 15 days [25–75% IQR 0, 21] vs 22 [16, 25], p < 0.001). Patients in Groups B and D had similar number of ventilator free days (15 [0, 21] vs 7 [0, 20], p = 0.09). Additional data regarding mean arterial pressure, central venous pressure, vasopressor use, and fluid intake/output can be found in Table 4. To test the hypothesis that patients in Group B were more frequently oliguric than patients in other groups, we examined the proportion of individuals who had a fluid output of less than 0.5 cc/kg over a 24 hour period in each of the four study arms (eg, an extension of the AKIN Stage 1 urine output criteria to a 24 hour window since 24 hour fluid output but not urine output was available). The proportion of patients who had low fluid output in Groups A–D were 19%, 20%, 13%, and 39%; thus, a higher proportion of patients who had AKI before and after adjustment for fluid balance (Group D) experience low fluid output (and, along with this, possible oliguria) than patients in any of the other three groups (p<0.001 for all three comparisons). Results across categories were similar when we considered the impact of AKIN stage 2 disease recognized after adjustment for fluid balance (Online Table 2), with the exception that patients in Group B (those with AKI after adjustment for fluid balance but not before) had poorer outcomes than patients in Group D (those with AKI before or after adjustment for fluid balance). Our analysis of stage 3 disease was limited by the small number of patients where AKI classification changed after adjustment of creatinine for fluid balance.
Table 4.
Characteristics of patients by group
| AKIN Stage 1 AKI | Group A | Group B | Group C | Group D | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| *Before adjustment | After adjustment | Before adjustment | After adjustment | Before adjustment | After adjustment | Before adjustment | After adjustment | ||
| NO | NO | NO | YES | YES | NO | YES | YES | ||
| APACHE III | 81 ± 28 | 100 ± 30 | 81 ± 23 | 103 ± 31 | < 0.001 | ||||
| Fluid conservative | 169 (52%) | 46 (35%) | 44 (81%) | 244 (50%) | <0.001 | ||||
| PAC | 171 (52%) | 62 (47%) | 25 (46%) | 255 (52%) | 0.64 | ||||
| Baseline Cr (mg/dL) | 0.9 ± 0.5 | 1.4 ± 0.9 | 0.9 ± 0.4 | 1.5 ± 1.0 | <0.001 | ||||
| MAP (mm Hg) | 80 ± 14 | 75 ± 13 | 79 ± 14 | 76 ± 14 | <0.001 | ||||
| CVP (mm Hg)** | 12 ± 5 | 11 ± 5 | 12 ± 4 | 12 ± 5 | 0.61 | ||||
| Vasopressor use | 73 (22%) | 61 (47%) | 9 (17%) | 187 (38%) | < 0.001 | ||||
| Fluid intake (L/d) | 3.2 ± 1.6 L | 4.3 ± 2.4 L | 2.8 ± 1.3 L | 4.1 ± 2.6 L | < 0.001 | ||||
| Fluid output (L/d) | 3.5 ± 2 L | 3.1 ± 2 L | 3.8 ± 2.1 L | 3.1 ± 2.4 L | < 0.001 | ||||
For fluid balance
Prior to first study instruction
Incidence of dialysis in those with “unrecognized” severe AKI is similar to that of those with severe AKI
In a companion analysis, among patients who met criteria for AKIN stage 1 AKI, we examined the incidence of dialysis based on the presence or absence of severe AKI (defined as AKIN stage 2 disease) before and after adjustment of creatinine for fluid balance (Online Table 3). In this subgroup, the incidence of RRT in those who did not have severe AKI before adjustment for fluid balance, but did after adjustment (Group B) was similar to that of patients with who had AKI before and after adjustment of creatinine for fluid balance (Group D, 38% versus 37%, p = 0.84) and significantly greater than that of patients who did not have severe AKI by either definition (Group A; 38% versus 16%, p < 0.001). None of the patients who met criteria for severe AKI before adjustment for fluid balance but not after adjustment (Group C) required RRT.
Discussion
Using data from the FACTT trial, we found that the incidence of mild (AKIN stage 1) AKI increased after adjustment of serum creatinine for fluid balance among patients managed with a liberal fluid management strategy. In a significant fraction of patients (18%), adjustment of creatinine for cumulative fluid balance reclassified their AKI status. We found that patients who met the definition of AKI after adjustment of creatinine for fluid balance but not serum creatinine criteria (Group B) had mortality rates that were not different from patients with AKI before and after adjustment of creatinine for fluid balance (Group D). Furthermore, patients who met the definition of AKI by serum creatinine only before and not after adjustment for fluid balance (Group C) had a mortality rate that was lower than patients who met the definition of AKI only after adjustment of creatinine for fluid balance (Group B) and similar to patients who did not have AKI before or after adjustment of creatinine for fluid balance (Group A).
Our data have important implications for clinical practice. Patients who had AKI only after adjustment of creatinine for fluid balance had to, almost by definition, have positive cumulative fluid balance. Because of the protocolized nature of the fluid conservative management arm and the high compliance with instructions at the ARDS Network sites, net fluid gain was likely lower than it would be in usual clinical practice. Indeed, the fluid liberal management strategy was very similar to management of patients in prior ARDS Network clinical trials where no guidance was provided with regard to fluid management (2). Positive cumulative fluid balance has been associated with poor outcomes in critically ill patients (12–15). Whether this is due to direct negative consequences of net fluid gain on end organs including the heart, lung and kidneys as has been suggested by some (16, 17) or whether this is because patients who receive larger amounts of fluid have patient characteristics that are associated with worse outcomes, such as hypotension, is unclear. Along the same lines, based on our analysis, positive fluid accumulation may be associated with poor outcomes in part because it masks kidney injury. However, further work to directly test whether or not these patients sustain kidney injury using novel, sensitive markers of kidney injury is needed.
Our study also highlights the important interplay between fluid management and newer, more sensitive definitions of AKI. Specifically, when we examined the incidence of AKIN Stage 1 disease, where the AKI threshold is low, the incidence was significantly greater in patients managed with a fluid conservative strategy compared to a fluid liberal strategy. After we adjusted for changes in volume status over the first 7 days of the study protocol, the incidence of AKI was greater in patients in the fluid liberal group than the fluid conservative group. This demonstrates that clinical management may affect recognition of mild AKI. This finding has implications for future clinical studies and randomized clinical trials, where it may be of vital importance to the study or trial design to identify and enroll patients with similar disease severity; serum creatinine-based definitions of AKI may again be problematic in this context.
Limitations to our study include limited data on urine output; we had available data on fluid intake and overall fluid output including urine. Urine output was not recorded separately from overall fluid output. Thus, we were unable to test the implications of the fluid conservative management strategy on urine output-based definitions for AKI. Our data is also limited by missing baseline weights in 76 patients, yet excluding these patients from the analysis did not alter the results. Urine biosamples were not stored as part of the FACTT protocol, so we were unable to test whether patients with AKI after adjustment for fluid balance had detectable levels of novel and potentially more sensitive biomarkers of AKI such as neutrophil-gelatinase associated lipocalin and kidney injury molecule-1 (18, 19). Finally, this is a secondary analysis of a randomized clinical trial of acute lung injury, and it is unclear how generalizable the results will be to other critically ill patient populations. However, the trial involved 39 medical centers and included medical and surgical patients. While this data was taken from a large randomized control trial, there can be confounders between groups that remain despite our multivariate adjustment. Nonetheless, this is the largest study to date of protocolized fluid management in critically ill patients, and the data have important implications for AKI diagnosis, as well as for prediction of mortality in critically ill patients.
The current AKI classification systems, including the RIFLE criteria and AKIN modification of the RIFLE criteria, define AKI using relative or absolute changes in serum creatinine without accounting for changes in fluid balance, and it is clear that patients with even mild forms of AKI (AKIN Stage 1/RIFLE “Risk” disease) are at an increased risk of death (20, 21). Our data suggest that changes in renal function need to be interpreted in the context of fluid management; not adjusting for positive fluid balance may obscure changes in renal function that are associated with risk of death. Further studies are needed to confirm and extend our findings.
Conclusions
The current analysis demonstrates that adjusting for fluid balance may impact the incidence of AKI in critically ill patients, who often have significant fluid accumulation over the first several days of their ICU stay. Furthermore, patients who have AKI only after adjusting serum creatinine for fluid balance have mortality rates similar to patients with AKI before and after adjusting serum creatinine for fluid balance. In contrast, patients who meet creatinine criteria for AKI before adjusting for fluid balance but not after adjustment have a lower risk of death than patients who have AKI only after adjustment for fluid balance. In fact, the mortality of the former group is similar to that of patients who do not have AKI before or after adjustment for fluid balance. Further studies are needed to determine if similar results are obtained in other critically ill patient populations; if these results are confirmed, we may need to further consider the impact of fluid balance on AKI ascertainment using creatinine-based criteria to define AKI.
Supplementary Material
Acknowledgements
This study was funded, in part, by the National Institutes of Health. Dr. Thompson and Dr. Douglas received a grant from NHLBI for ARDS Network activities. Dr. Wright received honoraria/speaking fees from GlaxoSmithKline.
The following persons and institutions participated in the trials: Steering Committee Chair — G.R. Bernard; Clinical Coordinating Center — D.A. Schoenfeld, B.T. Thompson, N. Ringwood, C. Oldmixon, F. Molay, A. Korpak, R. Morse, D. Hayden, M. Ancukiewicz, A. Minihan;Protocol-Review Committee — J.G.N. Garcia, R. Balk, S. Emerson, M. Shasby, W. Sibbald; Data Safety and Monitoring Board — R. Spragg, G. Corbie-Smith, J. Kelley, K. Leeper, A.S. Slutsky, B. Turnbull, C. Vreim;National Heart, Lung, and Blood Institute — A.L. Harabin, D. Gail, P. Lew, M. Waclawiw ARDS Clinical Trials Network Consultant — P. Parsons; Clinical Centers — University of Washington, Harborview — L. Hudson, K. Steinberg, M. Neff, R. Maier, K. Sims, C. Cooper, T. Berry-Bell, G. Carter, L. Andersson; University of Michigan — G.B. Toews, R.H. Bartlett, C. Watts, R. Hyzy, D. Arnoldi, R. Dechert, M. Purple; University of Maryland— H. Silverman, C. Shanholtz, A. Moore, L. Heinrich, W. Corral; Johns Hopkins University — R. Brower, D. Thompson, H. Fessler, S. Murray, A. Sculley; Cleveland Clinic Foundation — H.P. Wiedemann, A.C. Arroliga, J. Komara, T. Isabella, M. Ferrari; University Hospitals of Cleveland — J. Kern, R. Hejal, D. Haney; MetroHealth Medical Center — A.F. Connors; University of Colorado Health Sciences Center — E. Abraham, R. McIntyre, F. Piedalue; Denver Veterans Affairs Medical Center — C. Welsh; Denver Health Medical Center — I. Douglas, R. Wolkin; St. Anthony Hospital — T. Bost, B. Sagel, A. Hawkes; Duke University — N. MacIntyre, J. Govert, W. Fulkerson, L. Mallatrat, L. Brown, S. Everett, E. VanDyne, N. Knudsen, M. Gentile; University of North Carolina — P. Rock, S. Carson, C. Schuler, L. Baker, V. Salo; Vanderbilt University — A.P. Wheeler, G. Bernard, T. Rice, B. Christman, S. Bozeman, T. Welch; University of Pennsylvania — P. Lanken, J. Christie, B. Fuchs, B Finkel, S. Kaplan, V. Gracias, C.W. Hanson, P. Reilly, M.B. Shapiro, R. Burke, E. O'Connor, D. Wolfe; Jefferson Medical College — J. Gottlieb, P. Park, D.M. Dillon, A. Girod, J. Furlong; LDS Hospital — A. Morris, C. Grissom, L. Weaver, J. Orme, T. Clemmer, R. Davis, J. Gleed, S. Pies, T. Graydon, S. Anderson, K. Bennion, P. Skinner; McKay-Dee Hospital — C. Lawton, J. d'Hulst, D. Hanselman; Utah Valley Regional Medical Center — K. Sundar, T. Hill, K. Ludwig, D. Nielson; University of California, San Francisco — M.A. Matthay, M. Eisner, B. Daniel, O. Garcia; San Francisco General — J. Luce, R. Kallet; University of California, San Francisco, Fresno — M. Peterson, J. Lanford; Baylor College of Medicine — K. Guntupalli, V. Bandi, C. Pope; Baystate Medical Center — J. Steingrub, M. Tidswell, L. Kozikowski; Louisiana State University Health Sciences Center — B. deBoisblanc, J. Hunt, C. Glynn, P. Lauto, G. Meyaski, C. Romaine; Louisiana State University Earl K. Long Center — S. Brierre, C. LeBlanc, K. Reed; Alton-Ochsner Clinic Foundation — D. Taylor, C. Thompson; Tulane University Medical Center — F. Simeone, M. Johnston, M. Wright; University of Chicago — G. Schmidt, J. Hall, S. Hemmann, B. Gehlbach, A. Vinayak, W. Schweickert; Northwestern University — J. Dematte D'Amico, H. Donnelly; University of Texas Health Sciences Center — A. Anzueto, J. McCarthy, S. Kucera, J. Peters, T. Houlihan, R. Steward, D. Vines; University of Virginia — J.D. Truwit, A.F. Connors, M.H. Marshall, W. Matsumura, R. Brett; University of Pittsburgh — M. Donahoe, P. Linden, J. Puyana, L. Lucht, A. Verno; Wake Forest University — R.D. Hite, P. Morris, A. Howard, A. Nesser, S. Perez; Moses Cone Memorial Hospital — P. Wright, C. Carter-Cole, J. McLean; St. Paul's Hospital, Vancouver — J. Russell, L. Lazowski, K. Foley; Vancouver General Hospital — D. Chittock, L. Grandolfo; Mayo Foundation — M. Murray.
We are also appreciative to George J. Stukenborg, Ph.D., M.A. from the University of Virginia School of Medicine Department of Public Health Sciences for providing statistical review.
Supported by contracts (NO1-HR-46046-64 and NO1-HR-16146-54) with the National Heart, Lung, and Blood Institute, National Institutes of Health. KDL was supported by NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130.
Dr. Thompson and Dr. Douglas received a grant from NHLBI for ARDS Network activities. Dr. Wright received honoraria/speaking fees from GlaxoSmithKline.
Footnotes
The remaining authors have not disclosed any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 2.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. Jama. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 5.Liu KD, Glidden DV, Eisner MD, et al. Predictive and Pathogenetic Value of Plasma Biomarkers for Acute Kidney Injury In Patients with Acute Lung Injury. Crit Care Med. 2007;35:2755–2761. [PMC free article] [PubMed] [Google Scholar]
- 6.Esteban A, Alia I, Gordo F, et al. Prospective randomized trial comparing pressure-controlled ventilation and volume-controlled ventilation in ARDS. For the Spanish Lung Failure Collaborative Group. Chest. 2000;117(6):1690–1696. doi: 10.1378/chest.117.6.1690. [DOI] [PubMed] [Google Scholar]
- 7.Bellomo R, Ronco C, Kellum J, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative Group. Crit Care. 2004;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornsson TD. Use of serum creatinine concentrations to determine renal function. Clin Pharmacokinet. 1979;4(3):200–222. doi: 10.2165/00003088-197904030-00003. [DOI] [PubMed] [Google Scholar]
- 10.Macedo E, Bouchard J, Soroko SH, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14(3):R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernard G. The Brussels Score. Sepsis. 1997;1:43–44. [Google Scholar]
- 12.Goldstein SL, Currier H, Graf C, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 13.Michael M, Kuehnle I, Goldstein SL. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol. 2004;19(1):91–95. doi: 10.1007/s00467-003-1313-z. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 16.Prowle JR, Echeverri JE, Ligabo EV, et al. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6(2):107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 17.Bouchard J, Mehta RL. Fluid accumulation and acute kidney injury: consequence or cause. Curr Opin Crit Care. 2009;15(6):509–513. doi: 10.1097/MCC.0b013e328332f653. [DOI] [PubMed] [Google Scholar]
- 18.Parikh CR, Devarajan P. New biomarkers of acute kidney injury. Crit Care Med. 2008;36(4 Suppl):S159–165. doi: 10.1097/CCM.0b013e318168c652. [DOI] [PubMed] [Google Scholar]
- 19.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 21.Newsome BB, Warnock DG, McClellan WM, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168(6):609–616. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.