Abstract
Environmental enrichment (EE) during a period of forced abstinence attenuates incentive motivational effects of cocaine-paired stimuli. Here we examined whether EE during forced abstinence from cocaine self-administration would prevent time-dependent increases in cue-elicited cocaine-seeking behavior (i.e., the incubation effect). Rats were trained to self-administer cocaine, which was paired with light/tone cues, for 15 days while living in isolated conditions (IC). Controls received yoked saline infusions. Subsequently, rats were assigned to live in either continued IC or EE for either 1 or 21 days of forced abstinence prior to a test for cocaine-seeking behavior. During testing, responding resulted only in presentation of the light/tone cues. Contrary to our prediction, cocaine-seeking behavior increased over time regardless of living condition during abstinence; however, EE attenuated cocaine-seeking behavior relative to IC regardless of length of abstinence. Brains were harvested and trunk blood was collected immediately after the 60-min test and later assayed. Results indicated that short-term EE elevated hippocampal brain-derived neurotrophic factor and reduced plasma corticosterone compared to IC. Furthermore, 21 days of EE during forced abstinence prevented increases in the cue-elicited amygdala phosphorylated extracellular signal-regulated kinase expression that was observed in IC rats. These findings suggest that EE attenuates incentive motivational effects of cocaine cues through a mechanism other than preventing the incubation effect, perhaps involving reduction of stress and neural activity in response to cocaine-paired cues during acute withdrawal.
Keywords: brain-derived neurotrophic factor (BDNF), craving, corticosterone, drug-seeking behavior, extracellular signal-regulated kinase (ERK), incentive motivation
1. Introduction
Recent studies have demonstrated that environmental enrichment (EE) introduced during forced abstinence from cocaine self-administration reduces cue-elicited cocaine-seeking behavior (Chauvet et al. 2009; Thiel et al. 2009). In rats, this behavior is associated with neural activation patterns similar to human cocaine addicts exposed to drug-related cues that elicit cocaine craving (Childress et al. 1999; Goldstein & Volkow 2002; Feltenstein & See 2008; Kufahl et al. 2009). A troubling characteristic of cue-elicited drug craving and drug seeking is that it increases over time, leaving an individual vulnerable to relapse even after prolonged abstinence (Gawin & Kleber 1986; Tran-Nguyen et al. 1998; Neisewander et al. 2000; Grimm et al. 2001). This phenomenon is referred to as the incubation effect, which has also been observed with cue-elicited sucrose-seeking behavior (Grimm et al. 2003).
Two mechanisms involved in the development and expression of incubation are brain derived neurotrophic factor (BDNF) and extracellular signal-regulated kinase (ERK; Bossert et al. 2005). Cocaine-seeking behavior during forced abstinence from self-administration correlates with time-dependent increases in BDNF protein throughout the ventral tegmental area (VTA), nucleus accumbens (NAc), and amygdala (Grimm et al. 2003). BDNF infusions into the VTA after a final cocaine self-administration session enhance cue-elicited cocaine-seeking behavior for up to 30 days of abstinence. These BDNF effects are reversed via blockade of its downstream activation of mitogen-activated protein kinase (MAPK)/ERK signaling (Lu et al. 2004a). BDNF infused into the NAc shell produces an upward shift in the cocaine self-administration dose-response curve, increases breakpoints, and facilitates cocaine-seeking behavior, whereas infusion of BDNF antibody produces opposite effects (Graham et al. 2007). Incubation of cocaine-seeking behavior following 30 days, relative to 1 day, of forced abstinence increases cue-elicited p-ERK within the central amygdala (Lu et al. 2005). Infusion of the p-ERK inhibitor U0126 into central amygdala blocks expression of incubation of cocaine-seeking behavior, whereas an NMDA receptor agonist activation of ERK after just 1 day of abstinence increases cocaine-seeking behavior (Lu et al. 2005). Taken together, incubation of cocaine-seeking behavior appears to involve increased BDNF throughout the mesolimbic system, as well as increased cue-elicited ERK activity within the amygdala.
Grimm et al. (2008) reported that EE introduced during forced abstinence prevents the incubation of cue-elicited sucrose-seeking behavior. Furthermore, EE decreases basal phospho-CREB and BDNF mRNA expression in mesolimbic dopamine terminals (Green et al. 2010), and promotes neurogenesis and BDNF protein levels in the hippocampus (Ickes et al. 2000; Russo-Neustadt et al. 2001; Solinas et al. 2010). Thiriet et al. (2008) demonstrated that EE increases neural expression of several upstream mediators of the MAP/ERK signaling pathway, which in turn suggests that EE can ultimately regulate p-ERK activation. In addition, EE attenuates basal HPA-axis activity and stress-related responses (Belz et al. 2003; Benaroya-Milshtein et al. 2004).
The present study explored whether EE introduced as an intervention strategy during forced abstinence would prevent the incubation effect and its associated neurochemical correlates. In regard to the latter, we measured abstinence-induced changes in mesolimbic BDNF, amygdala p-ERK, and plasma corticosterone (CORT) levels.
2. Method
Animals and surgery
Adult male Sprague-Dawley rats weighing 225–250 g upon arrival to Arizona State University were housed under standard isolated conditions of 1 rat/cage (21.6 × 45.7 × 17.8 cm). Food and water were available ad libitum in a colony room with a 12-h reverse light:dark cycle (lights off at 07:00 h). Care and housing were in adherence to the Guide for the Care and Use of Laboratory Animals (1996). Rats were acclimated to handling for 5 days prior to surgically implanting intravenous (IV) catheters under 2–3% isoflurane anesthesia using procedures described previously (Pentkowski et al. 2010). Catheters were flushed daily with 0.1 ml saline containing heparin sodium (70 U/ml; APP Pharmaceuticals, Schaumburg, IL) and Timentin (66.7 mg/ml; GlaxoSmithKline, Research Triangle Park, NC) to maintain patency. Patency was tested periodically by administering 0.05 ml methohexital sodium (16.7 mg/ml; JHP Pharmaceuticals, Rochester, MI), a dose that produces transient anesthetic effects when administered IV.
Self-administration
Prior to self-administration training, rats were randomly divided into two groups: Cocaine self-administration (n=40) or Saline-yoked control (n=40). All rats remained housed in isolated conditions throughout self-administration training. Cocaine rats underwent 15 sessions of cocaine self-administration training across consecutive days for 3 h/day during their dark cycle. Each Cocaine rat was assigned a Saline-yoked partner that was placed in an adjacent self-administration chamber. For Cocaine rats, sessions initially began on a Fixed Ratio 1 (FR1) schedule of reinforcement and progressed to a Variable Ratio 5 (VR5) schedule based on individual performance, with the latter in effect exclusively during the last 5–8 sessions. Schedule completions on an “active” lever resulted in simultaneous presentation of a tone (500 Hz, 10 db above background), cue light above the lever, and house light, which were followed one second later by a cocaine infusion (0.75 mg/kg/0.1 ml, IV). Cocaine (salt form, cocaine hydrochloride; RTI International Triangle Park, NC) was dissolved in saline and filtered through a 0.2 μm membrane prior to use. Upon completion of the 6-s infusion, the cue light and tone ceased, but the house light remained on for an additional 20-s time-out. Saline-yoked rats received yoked IV infusions of saline (0.1 ml/infusion) along with the cue light/tone/house light stimulus complex each time their Cocaine partner received a cocaine infusion; however, their lever responses produced no consequences. Responses on another lever (i.e., inactive lever) produced no consequences for either Cocaine or Saline-yoked rats. Rats were restricted to 16 g of food/day beginning 2 days before training to facilitate exploration. A Cocaine rat and his Saline-yoked partner remained food-restricted until a criterion of ≥21 infusions/3 h was achieved on 2 consecutive days, after which food was available ad libitum in the home cage throughout the remainder of the experiment. All Cocaine rats had reached this criterion by the 8th session. One Cocaine rat was omitted from the analyses due to catheter failure but was used as an enrichment partner during the abstinence phase; his Saline-yoked partner was yoked to another Cocaine rat.
Forced abstinence
Following the last self-administration training session, all rats were returned to their home cages under isolated conditions to allow 24 h for cocaine clearance. Cocaine and Saline groups were then divided into two Living Conditions: Environmental Enrichment (EE) or Isolated Condition (IC). Rats in the IC group remained living alone in their home cages. We used IC rats as our standard housing control because past studies examining the incubation effect have almost exclusively used isolated-housed rats. The EE group lived in large plastic tubs (74 × 91 × 36 cm) that housed 5 rats and contained bedding, nesting material, 3 PVC pipes, 2 running wheels, 2 water bottles, 2 food dishes, and 2 small plastic toys. Toys were continually changed 3 times/week to maintain novelty. Rats within the EE and IC conditions were then further assigned to one of two Abstinence Lengths while living in those conditions: Short (i.e., 1 day) or Long (i.e., 21 days). Thus, there were a total of 8 groups (n= 9–10/group) that consisted of different Drug Histories (Cocaine or Saline), Living Conditions (EE or IC), and Abstinence Lengths (Short or Long). Group assignment was counterbalanced to equate for total cocaine intake and active lever responding. Each Cocaine rat and his Saline-yoked partner were assigned to the same Living Condition/Abstinence Length. Each enriched environment contained rats from both the Cocaine and Saline-yoked conditions.
Test for cocaine-seeking behavior
After their assigned length of abstinence, rats were put back into the self-administration chambers for 60 min to test for cocaine-seeking behavior. All testing took place between 11:00 – 15:00 h. Group testing was counterbalanced such that equal numbers of rats from each condition were sacrificed throughout this testing time period in order to avoid potential circadian effects on the various neurochemical correlates. To begin the test, a non-contingent presentation of the cocaine-paired stimulus complex (i.e., cue light, tone, and house light) was delivered. Thereafter, FR 1 schedule completions by a Cocaine rat activated this stimulus complex for both himself and his Saline-yoked counterpart. Lever responding by Saline-yoked rats had no consequences. It is important to note that cocaine-seeking behavior in this type of paradigm was driven in part by the contextual stimuli as well as the discrete response-contingent cues. There is a high correlation between cocaine-seeking behavior exhibited in response to the self-administration chambers without cues (i.e., extinction responding within the context) and that exhibited with response-contingent cue presentations (i.e., cue reinstatement), and based on this finding Lu et al. (2004c) suggest that there is overlap in the motivational state produced by the context and discrete cues that incubates throughout abstinence. Therefore, in the present study, we measured cocaine-seeking behavior in rats re-exposed to a previous drug-associated context with the opportunity to obtain response-contingent presentations of the discrete light-tone stimulus complex with the rationale to maximize resulting brain activation.
Tissue preparation
All rats were decapitated within 1 min of completing their test for cocaine-seeking behavior. Brains were rapidly harvested, frozen in −50°C isopentane, and stored at −80°C. Coronal sections (1 mm) of the brains were cut inside of a freezer kept at −20 °C using a block that had slots 1 mm apart through which razor blades were lowered. Subsequently, bilateral tissue punches (diameter indicated in parentheses) of the nucleus accumbens (NAc; 2 mm), amygdala (1.5 mm), posterior dorsal subregion of the hippocampus (2 mm), and ventral hippocampus (2 mm) from each section were collected. Single (2 mm) tissue punches of the VTA were collected along the midline of the brain that contained the VTA from both hemispheres. The coronal sections were obtained at +1.8 (NAc), −2.56 (amygdala), and −5.6 (VTA and hippocampal regions) mm from bregma according to Paxinos & Watson (1998; Figure 1). The NAc tissue punches included both the shell and core subregions. Efforts were made to limit the amygdala tissue punches to the central nucleus, but some neighboring basolateral nuclei were also included. Tissue punches from the NAc, VTA, and hippocampus were used for BDNF ELISA; punches from the amygdala were used for p-ERK Western Blot analysis.
Figure 1.
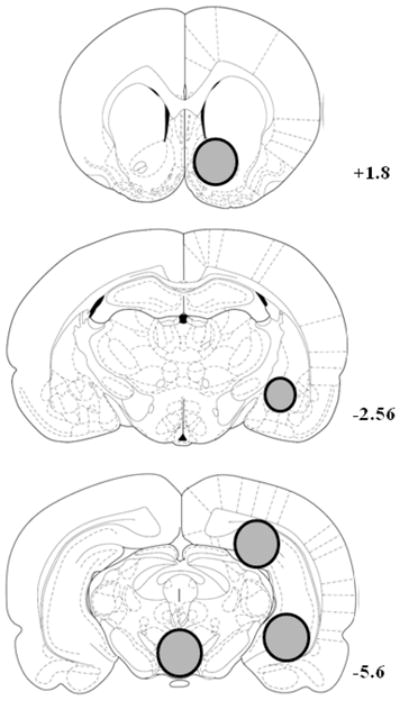
Schematic representation of coronal sections from which tissue punches were obtained at +1.8, −2.56, and −5.6 mm from bregma (Paxinos & Watson 1998). The shaded circles indicate the sampling area and are illustrated unilaterally in order to allow visibility of structures in the contralateral hemisphere. Bilateral 2 mm (diameter) tissue punches of the nucleus accumbens were obtained at +1.8 mm, bilateral 1.5 mm tissue punches of the amygdala (predominately central amygdala) were obtained at −2.56 mm, and bilateral 2 mm tissue punches of the posterior dorsal region of the hippocampus and the ventral hippocampus were obtained at −5.6 mm. Unilateral 2 mm tissue punches of the ventral tegmental area were also obtained along the midline of the brain at −5.6 mm.
CORT analysis
At the time of decapitation, trunk blood was collected from each rat into tubes coated with heparin and stored on ice. Subsequently, blood was centrifuged for 10 min at 5,000 rpm. Serum was removed and stored at −80°C. Samples were diluted 1:10 using assay buffer and then processed in duplicate. CORT concentrations were quantified using an enzyme immunoassay kit (ELISA; Assay Designs Inc., Ann Arbor, MI) according to the manufacturer’s protocol. Optical density values were measured at 405 nm using a microplate reader and CORT values were extrapolated from a standard curve. CORT levels are expressed as ng/ml.
BDNF protein levels
Tissue punches were incubated in 300 μl of cold lysis buffer consisting of 137 mM NaCl, 20 mM Tris, 10% glycerol, and a protease inhibitor cocktail set (Calbiochem, La Jolla, CA) for 30 min at 4°C. Supernatant was collected and stored overnight at −20°C to be used for the subsequent ELISA assay. Total protein concentration of the samples was determined using the Micro-BCA assay kit (Pierce, Rockford, IL) according to the manufacturer’s protocol. BDNF protein levels were quantified using the BDNF Emax ImmunoAssay System kit (Promega, Madison, WI) according to the manufacturer’s protocol. BDNF content was extrapolated from standard curves established for each plate (liner range of 7.8 – 500 pg/ml). For the assay kit used, cross reactivity with other trophic proteins is < 2 – 3%. BDNF protein levels were divided by total protein levels in each sample to determine pg of BDNF per mg of total protein.
p-ERK protein levels
Tissues were weighed and homogenized (1:10 sample to buffer ratio) in ice-cold nonyl phenoxylpolyethoxylethanol (NP-40) lysis buffer (20mM Tris-HCl, pH 8.0, with 137mM NaCl, 10% glycerol, 1% NP-40, 1 mM Na+ EDTA, 0.5mM Sodium Ortho-Vanadate), supplemented with Protease Inhibitors Cocktail (Sigma-Aldrich Corp., St. Louis, MO), 1mM phenylmethanesulfonylfluoride (PMSF; Sigma-Aldrich Corp., St. Louis, MO), and Phosphatase Inhibitor Cocktails 1 and 2 (Sigma-Aldrich Corp., St. Louis, MO) in dilution of 1:100. Samples were then vortexed for 1 min, followed by sonication with a 5 s pulse. The sample tubes were incubated on ice for 15 min, and then the vortex/sonication procedure was repeated.
Protein content within the homogenate was assessed by Bradford method using BSA as the standard. For electrophoresis, each sample was mixed with Laemly Loading buffer (Bio-Rad Laboratories, Hercules, CA) to a concentration of 2 μg/μL, and boiled for 3 minutes at 95°C, cooled on ice, and loaded onto 12% polyacramide bis-TRIS gels (Bio-Rad Laboratories, Hercules, CA), then ran at 175 V for 2.5 hours, and transferred to polyvinylidene fluoride (PVDF) membrane (0.45 μ Bio-Rad) for 2.5 hours at 325 mA constant. The membrane was blocked in 5% BSA in TBS with 0.5% Tween 20, pH 7.4, for 4 h at room temperature.
The blot was then incubated with rabbit anti-pERK [Cell Signaling Phospho-p44/42 MAPK (Erk1/2); Cell Signalling Technology, Danvers, MA] in 5% BSA in Tris-Buffered Saline (TBS) with 0.05% Tween 20, pH 7.4, overnight at 4°C. After 5 washes with Tris-Buffered Saline Tween-20 TBST, the blot was incubated in Santa Cruz Bio goat anti-mouse IgG-HRP (Santa Cruz Biotech, Santa Cruz, CA) at 1:100000 dilution in TBST, pH 7.4, with 5% nonfat milk for 2 h at RT. After 5 TBST washings, immunoreactive proteins were revealed with enhanced chemiluminescence (ECL; Pierce SuperSignal West Dura Extended Duration Substrate, Thermo Fisher Sci., Rockford, IL) on X-ray film (Thermo Fisher Sci., Rockford, IL), developed on an AFP “Mini-Medical” Series X-Ray Film Processors (AFP Imaging, Elmsford, NY), then their expression level was measured by densitometry. Blots were mildly striped with Restore Western Blot Stripping Buffer (Thermo Fisher Sci., Rockford, IL), and the process was repeated using rabbit anti-ERK [p44/42 MAPK (ERK1/2), - 1:1000 dilution], and again using Glyceraldehyde 3-phosphate dehyrdrogenase [GAPDH (IMGENEX, San Diego, CA), -1:2000 dilution] as a control protein.
The films were scanned on the Agfa Arcus II (Ridgefield Park, NJ), and the expression levels were calculated by densitometric analysis of the bands using QuantityOne software (Bio-Rad, Hercules, CA), with the results being presented as relative optical density adjusted for the GAPDH levels.
Data analysis
Cocaine-seeking behavior was operationally defined as active lever responses in the absence of cocaine reinforcement. Separate three-way ANOVAs using Drug (Cocaine vs. Saline-yoked), Abstinence Length (Short vs. Long), and Living Condition (IC vs. EE) were used to analyze active and inactive lever responses during the test for cocaine-seeking behavior as well as to analyze raw CORT, BDNF, and p-ERK1/2 levels. In addition, planned comparisons were made between Short and Long abstinence length groups for BDNF within the VTA and nucleus accumbens, and for p-ERK within the amygdala as increases in these measures have been reported previously in association with the incubation effect (Grimm et al. 2003; Lu et al. 2005). Significant effects were followed up with post hoc Newman-Keuls tests (p< 0.05). Due to varying degrees of protein expression between regions, the BDNF and ERK1/2 protein levels in each region were normalized by expressing the results as percent of controls.
3. Results
Cocaine intake
As noted above, group assignment was counterbalanced such that there were no differences in cocaine intake across groups. Self-administration results, including total infusions obtained, as well as infusions and active lever presses/session over the last 5 d of training are presented in Table 1.
Table 1.
Mean (± SEM) Reinforcement Rates and Active and Inactive Lever Presses During Self-Administration Training Prior to Examining Acute and Long-Term Effects of Enrichment
| Group (n) | Reinforcement rates
|
Lever press rates/session, last 5 sessions
|
||
|---|---|---|---|---|
| Total infusions | Infusions/session, last 5 sessions | Active lever | Inactive Lever | |
| Cocaine
|
||||
| IC/Short (10) | 479.5 ± 37.7 | 36.8 ± 1.8 | 211.3 ± 15.2 | 2.7 ± 1.6 |
| EE/Short (9) | 494.0 ± 37.1 | 40.7 ± 2.8 | 221.6 ± 19.8 | 5.5 ± 2.4 |
| IC/Long (10) | 489.4 ± 34.8 | 40.3 ± 2.2 | 218.0 ± 17.1 | 4.6 ± 3.8 |
| EE/Long (10) | 481.2 ± 40.3 | 37.8 ± 2.4 | 214.8 ± 15.7 | 6.5 ± 5.8 |
| Saline-Yoked
|
||||
| IC/Short (10) | N.A. | N.A. | 4.9 ± 1.8 | 6.2 ± 2.3 |
| EE/Short (10) | N.A. | N.A. | 3.6 ± 2.1 | 4.1 ± 1.9 |
| IC/Long (10) | N.A. | N.A. | 1.6 ± 0.9 | 5.6 ± 2.3 |
| EE/Long (10) | N.A. | N.A. | 2.4 ± 1.4 | 4.8 ± 1.7 |
Note. Group abbreviations refer to Isolated Condition (IC) or Environmental Enrichment (EE) for either a Short (i.e., 1 day) or Long (i.e., 21 days) period of forced abstinence.
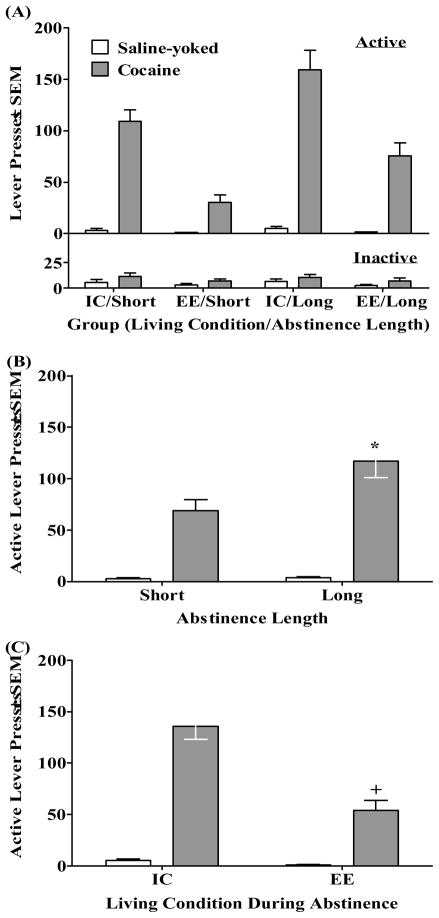
Cocaine-seeking behavior
Figure 2 illustrates cocaine-seeking behavior (i.e., active lever responding) and inactive lever responding on the test day for Cocaine and Saline-yoked rats after living in either IC or EE for either a Short or Long (i.e., 1 or 21 days) period of abstinence. There was no overall Drug × Living Condition × Abstinence Length interaction on active lever responding (see Figure 2A); however, there were significant Drug × Abstinence Length (F(1,71) = 11.08, p<0.001) and Drug × Living Condition (F(1,71) = 30.96, p<0.001) interactions. The Drug × Abstinence Length interaction is illustrated in Figure 2B where regardless of whether rats lived in IC or EE, cocaine-seeking behavior was higher in Cocaine rats that underwent Long abstinence than those that underwent Short abstinence (i.e., incubation effect, p<0.05, Newman-Keuls); however, there was no difference between Saline-yoked groups. The Drug × Living Condition interaction is illustrated in Figure 2C where regardless of whether rats underwent Short or Long abstinence, cocaine-seeking behavior was higher in rats that lived in IC relative to those living in EE (i.e., incubation effect, p<0.05, Newman-Keuls); however, there was no difference between Saline-yoked groups. Analysis of overall inactive lever responding revealed a main effect of Drug (F(1,71) = 7.44, p<0.01), indicating that Cocaine groups exhibited more inactive lever responding than Saline-yoked groups.
Figure 2.
(A) Cocaine-seeking behavior (i.e., active lever presses ± SEM; top panel) and inactive lever presses (bottom panel) across all groups during the 60-min test. Group designations indicate whether the animals were housed in an isolated condition (IC) or in an enriched environment (EE) during abstinence and whether the animals underwent a Short (i.e., 1 day) or Long (i.e., 21 days) abstinence length period while living in their assigned conditions prior to testing. Active lever responses by Cocaine rats resulted in presentation of the light/tone cue complex previously associated with cocaine infusions on an FR1 schedule for both the Cocaine rat and his Saline-yoked partner. Active lever presses for Saline-yoked rats produced no consequences. (B) Cocaine-seeking behavior (i.e., active lever presses) ± SEM during the 60-min test collapsed across Living Condition or (C) collapsed across Length of Abstinence. Asterisk (*) indicates greater active lever responding in the Long abstinence Cocaine rats relative to Short abstinence Cocaine rats (i.e., incubation effect, p<0.05, Newman-Keuls). Cross (+) indicates less active lever responding in the EE Cocaine rats relative to IC Cocaine rats (i.e., enrichment effect”, p<0.05, Newman-Keuls).
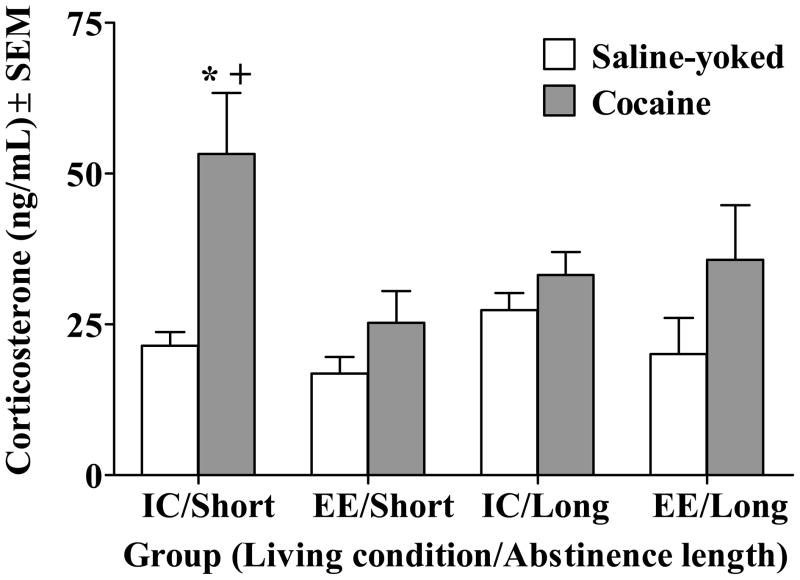
CORT
Figure 3 illustrates CORT levels across groups on the test day. Note that one sample from the IC/Short Cocaine group was lost during blood collection. The analysis revealed an overall Drug × Living Condition × Abstinence Length interaction (F(1,70) = 4.44, p<0.05). Cocaine rats in the IC/Short abstinence group demonstrated higher CORT levels relative to their Saline-yoked controls, as well as relative to Cocaine rats in the EE/Short abstinence group (p<0.05, Newman-Keuls). There were no significant differences among the other groups.
Figure 3.
Plasma corticosterone levels (ng/ml) for all groups presented as means ± SEM. Group designations are described in the caption of Figure 1. Asterisk (*) indicates difference from Saline-yoked control (p<0.05, Newman-Keuls). Cross (+) indicates difference from EE/Short Cocaine rats (p<0.05, Newman-Keuls).
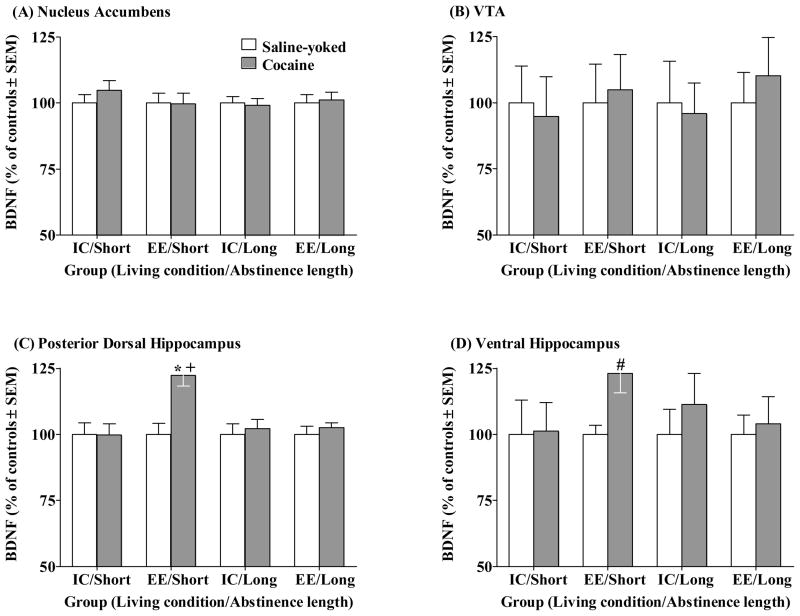
BDNF
Figure 4 illustrates BDNF protein levels (normalized to percent of Saline-yoked controls). Raw values of BDNF protein levels, expressed as pg BDNF/mg total protein, are presented in Table 2. Unexpectedly, there were no group differences in BDNF protein levels within either the NAc or VTA. Analysis of BDNF protein within the posterior dorsal hippocampus revealed an overall Drug × Living Condition × Abstinence Length interaction (F(1,71) = 4.44, p<0.05). Cocaine rats in the EE/Short abstinence group demonstrated greater BDNF relative to all other cocaine groups, as well as relative to their Saline-yoked controls (p<0.05, Newman-Keuls). Analysis of BDNF protein within the ventral hippocampus failed to reveal an overall Drug × Living Condition × Abstinence Length interaction, although there was a trend (F(1,71) = 2.49, p = 0.127). In light of increased BDNF within the posterior dorsal hippocampus in EE/Short Cocaine rats relative to IC/Short Cocaine rats, we examined whether the same trend would be observed in the ventral hippocampus. Thus, an ANOVA of ventral hippocampus BDNF in only the Cocaine groups revealed a Living Condition × Abstinence Length interaction (F(1,35) = 8.32, p<0.01). Post-hoc comparisons revealed that BDNF levels in the ventral hippocampus of Cocaine groups were higher in the EE/Short abstinence relative to IC/Short abstinence conditions (p<0.05, Newman-Keuls).
Figure 4.
BDNF protein levels ± SEM across all groups in the Nucleus Accumbens (A), VTA (B), Posterior Dorsal Hippocampus (C), and Ventral Hippocampus (D). Data are presented as percentage of saline controls. Group designations are described in the caption of Figure 1. Asterisk (*) indicates difference from Saline-yoked control (p<0.05, Newman-Keuls). Cross (+) indicates difference from all other Cocaine groups (p<0.05, Newman-Keuls). Pound (#) indicates difference from IC/Short abstinence, Cocaine groups (p<0.05).
Table 2.
Mean ± SEM levels of BDNF (pg/mg total protein) in the regions sampled
| Brain region | Cocaine rats
|
Saline-yoked rats
|
||||||
|---|---|---|---|---|---|---|---|---|
| IC/Short | EE/Short | IC/Long | EE/Long | IC/Short | EE/Short | IC/Long | EE/Long | |
| Nucleus acccumbens | 4.33 ± 0.15 | 4.21 ± 0.17 | 3.99 ± 0.10 | 4.38 ± 0.13 | 4.13 ± 0.13 | 4.22 ± 0.16 | 4.02 ± 0.10 | 4.33 ± 0.14 |
| Ventral tegmental area | 6.45 ± 1.02 | 5.90 ± 0.75 | 6.53 ± 0.78 | 5.86 ± 0.77 | 6.79 ± 0.95 | 5.62 ± 0.82 | 6.80 ± 1.07 | 5.32 ± 0.61 |
| Dorsal hippocampus | 2.40 ± 0.09 | 2.88 ± 0.09 | 2.53 ± 0.08 | 2.54 ± 0.05 | 2.40 ± 0.10 | 2.35 ± 0.10 | 2.47 ± 0.10 | 2.47 ± 0.08 |
| Ventral hippocampus | 1.32 ± 0.14 | 2.20 ± 0.13 | 1.76 ± 0.19 | 1.71 ± 0.17 | 1.31 ± 0.17 | 1.79 ± 0.06 | 1.58 ± 0.12 | 1.65 ± 0.16 |
Note. Group designation refers to subsequent assignment to isolate conditions (IC) or enriched environment (EE) during either a Short (i.e., 1 day) or Long (i.e., 21 days) period of forced abstinence.
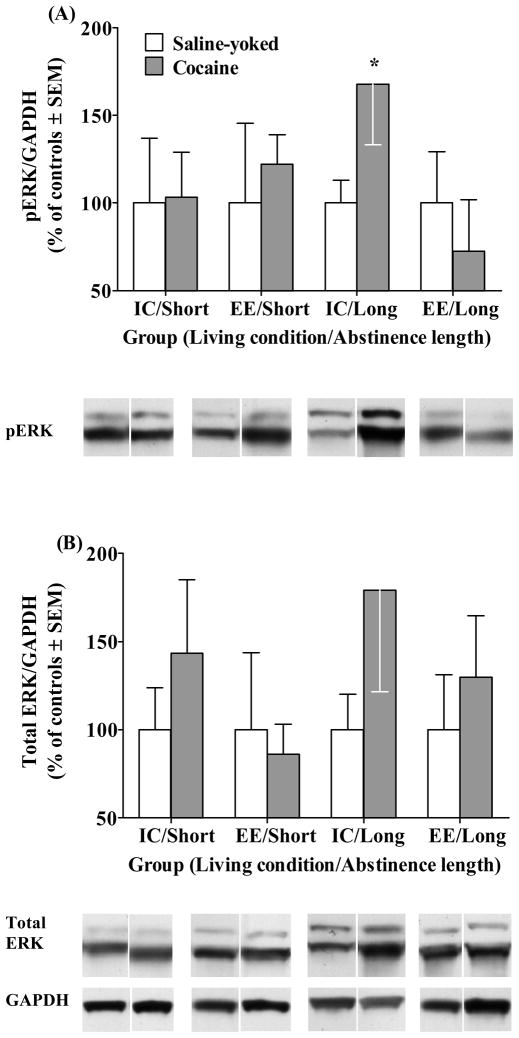
ERK
Figure 5 illustrates ERK1/2 protein levels (normalized to percent of Saline-yoked controls) within the amygdala. Note that one sample from the IC/Long Saline group was lost during tissue preparation. Although analysis of p-ERK1/2 within the amygdala failed to reveal an overall Drug × Living Condition × Abstinence Length interaction (F(1,70) = 1.20, p = 0.27), planned comparison t-tests between Cocaine and Saline-yoked rats within each Living Condition/Abstinence Length group revealed a marginally significant increase in p-ERK1/2 only in the IC/Long Cocaine rats relative to their Saline-yoked controls (t(17) = 1.78, p = 0.05, Figure 5A). Analysis of total ERK1/2 within the amygdala failed to reveal an overall Drug × Living Condition × Abstinence Length interaction (Figure 5B). In contrast to p-ERK1/2, t-tests between Cocaine and Saline-yoked rats within each Living Condition/Abstinence Length group failed to reveal a difference in total ERK1/2.
Figure 5.
p-ERK1/2 (A) and total ERK1/2 (B) protein levels ± SEM across all groups within the amygdala. Data are presented as percentage of saline controls. Representative bands, as determined by Western blot luminescence, are included for reference. Group designations are described in the caption of Figure 1. Asterisk (*) indicates a difference from Saline-yoked control (p=0.05).
4. Discussion
Contrary to our hypothesis, the incubation effect occurred despite an EE-induced attenuation of cocaine-seeking behavior. Incubation was evident as enhanced cocaine-seeking behavior with prolonged forced abstinence relative to brief abstinence, regardless of whether the rats lived in IC or EE during abstinence (Fig. 2). These results were unexpected given previous findings that EE blocks the incubation of sucrose seeking (Grimm et al. 2008). The decrease in cocaine-seeking behavior after only 1 day of EE is consistent with Chauvet and colleagues (2009) who demonstrated that 1 day of EE during forced abstinence attenuates cocaine-seeking behavior on the first day of extinction training relative to group-housed controls. One possible explanation for a short-term EE effect is that fatigue from undergoing a drastic change in living conditions may have reduced cocaine-seeking behavior. This explanation is mitigated by the finding that EE failed to alter inactive lever responding across groups. Furthermore, there is no relationship between the degree of wheel running exercise and subsequent responding for cocaine, which would be expected if fatigue influenced operant responding (Smith et al. 2008). Finally, stress associated with a change in living conditions per se is unlikely to explain the short-term enrichment effect, as there were no differences in plasma CORT between enriched and isolated saline-controls, and there was actually a decrease in plasma CORT in the enriched relative to isolated cocaine rats. We suggest that EE attenuation of cocaine-seeking behavior reflects a decrease in the incentive motivational effects of cocaine cues.
The incubation effect has been reported in rats undergoing forced drug abstinence while single-housed (e.g., Grimm et al. 2001; Lu et al. 2004b), suggesting that isolation stress could be a contributing factor. Indeed, social deprivation increases sensitivity to rewards and reward-related stimuli (Jones et al. 1990; van der Harst et al. 2003; Brenes & Fornaguera 2008). However, the present findings suggest that incubation of craving occurs independently of isolation stress, given that it was also observed in EE rats.
Contrary to previous studies suggesting that increased mesolimbic BDNF contributes to incubation of cue-elicited cocaine-seeking behavior (Grimm et al. 2003; Bossert et al. 2005; Lu et al. 2006), the incubation effect was observed in the present study without a concomitant increase in mesolimbic BDNF. Differences across studies include rat strains, schedules of reinforcement during cocaine training, cocaine dose, and length of access. Additionally, subtle differences in neural dissection technique and location may have contributed to the disparity. The previous studies used a longer period of access than the present study which may account for the discrepancy given that neuroadaptations vary depending on access length (Mantsch et al. 2003, 2004; Koob et al. 2004).
The reversal of the withdrawal-induced increase in CORT (Fig. 3) and the increase in hippocampal BDNF (Fig. 4) observed with 1 day of EE likely contribute to EE attenuation of cocaine-seeking behavior. The former effect of EE is consistent with previous research demonstrating that EE attenuates responses to stress, including stress-induced reinstatement of cocaine-seeking behavior (Adlard & Cotman 2004; Benaroya-Milshtein et al. 2004; Del Arco et al. 2007; Laviola et al. 2008; Segovia et al. 2008; Chauvet et al. 2009). Furthermore, the EE reversal of withdrawal-induced CORT is consistent with the increase in hippocampal BDNF as there is an established inverse relationship between CORT and BDNF protein levels throughout the entire hippocampus (Schaaf et al. 1998, 2000; Jacobsen & Mork 2006). Increases in hippocampal BDNF are associated with neurogenesis and antidepressant effects (van Praag et al. 1999; Malberg et al. 2000; Paizanis et al. 2007; Perera et al. 2007; Duman et al. 2008), and both hippocampal neurogenesis and antidepressants attenuate cocaine-seeking behavior (Fuchs et al. 1998; Baker et al. 2001; Burmeister et al. 2004; Noonan et al. 2010). Rogers & See (2007) suggest that the ventral hippocampus interacts with other limbic structures, such as the amygdala, to regulate cue-elicited cocaine-seeking behavior. Brief alterations in hippocampal neurochemistry, such as elevated BDNF, might therefore contribute to greater behavioral control in the presence of drug cues. Taken together, the ability of EE to decrease cocaine-seeking behavior within just 1 day may be due to reversal of withdrawal-induced stress and depression as suggested by the pattern of CORT and hippocampal BDNF changes observed.
It is possible that the CORT changes observed in the present study were due to a transient conditioned-CORT response associated with extinction as reported previously (De Vries et al. 1998; Peltier et al. 2001). However, there are some factors that mitigate this explanation. For one, peak increases in CORT during extinction and cue reinstatement tests are found within 15 min (Clampitt & Goeders 2002) and decline to baseline levels by the end of 2-h sessions (Clampitt & Goeders 2002; Feltenstein & See 2006). Therefore, the CORT levels observed at the end of the 60-min session in the present study are unlikely due to a conditioned-CORT response. Secondly, if the CORT increase observed in the IC/Short rats in the present study was activational, then we would have expected to observe cue-induced CORT elevations in the IC/Long rats as well. During the course of withdrawal from chronic cocaine exposure, CORT levels are initially high and then fall to control levels over time (Zhou et al. 2003a,b). Thus, it is most likely that EE reduced elevated plasma CORT associated with acute withdrawal, but that plasma CORT levels normalize with prolonged withdrawal, such that differences were not observed between IC and EE Cocaine rats after long-term abstinence.
Incubation of cocaine-seeking behavior is thought to be critically mediated by elevated p-ERK induction in the central amygdala (Bossert et al. 2005; Lu et al. 2006). In the present study, the only group to demonstrate increased p-ERK in the amygdala relative to controls was the IC cocaine rats that had undergone prolonged forced abstinence (Fig. 5). It is possible that higher p-ERK in the IC/Long Cocaine rats was related to their high total ERK levels; however, it should be noted that in contrast to the IC/Long Cocaine rats that had high levels of both total and p-ERK relative to controls, the EE/Long Cocaine rats demonstrated higher total ERK levels, yet lower p-ERK levels, compared to their controls. Thus, the pattern of p-ERK across all groups in the present study, together with the previous finding that incubation of cocaine-seeking is associated with cue-evoked increases in p-ERK in the central amygdala, suggests that lack of an increase in p-ERK in EE/Long Cocaine rats is likely due to EE-attenuated amygdala activation that occurs in response to cocaine cues after an incubation period. We further suggest that dampening central amygdala activation may represent a neurobiological mechanism of the long-term protective effects of EE against cocaine-seeking behavior. Consistent with this finding, we have recently demonstrated blunted activation of the amygdala in response to cocaine-associated cues in EE rats through the use of Fos protein expression (Thiel et al. 2010).
Induction of p- ERK within the amygdala in response to cues is thought to be glutamate-mediated as infusion of glutamatergic antagonists into the central amygdala blocks p-ERK expression as well as incubation, whereas NMDA receptor stimulation of this region after acute withdrawal stimulates p-ERK and robust cocaine-seeking behavior (Lu et al. 2005). Importantly, EE leads to alterations in glutamatergic neurotransmission throughout brain regions which have been implicated in craving and drug-seeking, including improved metabotropic glutamate receptor functioning in the prefrontal cortex (Melendez et al. 2004), increased GluR2-containing AMPA receptor subunit expression in the hippocampus (Naka et al. 2005), and reduced NMDA receptor expression throughout the limbic system (Wood et al. 2005). These findings provide insight into a possible mechanism through which EE may reverse drug-induced neural alterations in glutamate receptor expression and functioning, which in turn may blunt p-ERK activation and thus reduce responsiveness to drug cues.
The dissociation between the incubation effect and increased amygdala p-ERK expression is a novel, important finding that suggests that ERK activation in this region may not play a necessary role in incubation. This finding is surprising in light of Lynch et al. (2010) who recently reported that a two-week exercise intervention introduced after cocaine self-administration attenuates cue-elicited p-ERK expression within the prefrontal cortex, a finding that has also been linked to incubation (Koya et al. 2009). Although this finding supports p-ERK as a target by which environmental stimulation protects against cocaine-seeking behavior, it does not explain how incubation continues to develop within EE rats. It is possible that elevated p-ERK observed in IC rats in this study and others is, at least in part, a consequence of isolation stress, as isolated housing in adult rats leads to augmented glutamaterigic and dopaminergic receptor functioning (Hall 1998), which in turn could alter p-ERK expression. Potential isolation stress-induced alterations in p-ERK may interact with other incubation mechanisms that have yet to be identified, but which remain present in EE rats. Further research will be required to pinpoint these neuroadaptations.
In summary, EE attenuated cue-elicited cocaine-seeking behavior regardless of whether exposure was for 1 or 21 days, suggesting that EE does not prevent the incubation effect. It is possible that different mechanisms underlie EE’s protective effects at different time points. The neurochemical results suggest that the primary mechanism by which EE attenuates incentive motivation during acute abstinence may involve elevation of BDNF protein levels in the hippocampus, which in turn corresponds with reduced stress hormone output during acute cocaine withdrawal. On the other hand, EE’s protective effects following a protracted period of abstinence were associated with blunted amygdala p-ERK expression in response to cocaine-associated cues. Thus, it may be that early on during abstinence EE reduces withdrawal-related stress, which may contribute to its protective effects at that time. Once the withdrawal effects subside (i.e., at more protracted time points), it may be that the history of cognitive and physical stimulation within the enriched environment has served as a type of alternative reinforcement which reduces incentive motivation elicited by drug-paired stimuli. To this end, long-term placement into EE may dampen neural activity associated with sensitized incentive motivation; however, it is unlikely that EE counters all neuroadaptations that contribute to the incubation effect. Elucidating the behavioral and neurobiological consequences of EE as a cocaine intervention is a necessary step toward advancing this paradigm as a viable treatment for cocaine addiction.
Acknowledgments
This work was supported by grants R01DA11064, R21DA023123, F31DA023746, and DA027683 from the National Institute on Drug Abuse (NIDA). The content is solely the responsibility of the authors and does not necessarily represent the official view of NIDA or the National Institutes of Health. The authors thank Natalie Peartree and Lara Pockros for their expert technical assistance with surgical procedures, and Joshua Talboom and Sanya Fanous for technical assistance with ELISA assays. The authors also thank Drs. Heather Bimonte-Nelson, Cheryl Conrad, and Ronald Hammer, Jr. for helpful comments on the manuscript and use of equipment.
Footnotes
Author Contributions
KJT, MRP, NSP, and JLN were responsible for the study concept and design. KJT, MRP, and NSP contributed to collecting all of the behavioral data. KJT and MRP performed the ELISA analyses. DM and CAC were responsible for the Western Blot analysis. KJT and JLN drafted the manuscript. All authors assisted with interpretation of the findings and provided critical revision of the manuscript for important intellectual content. In addition, all authors critically reviewed the content and approved the final version for publication.
References
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–992. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2001;155:18–26. doi: 10.1007/s002130000676. [DOI] [PubMed] [Google Scholar]
- Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–486. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick CG. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur J Neurosci. 2004;20:1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Fornaguera J. Effects of environmental enrichment and social isolation on sucrose consumption and preference: associations with depressive-like behavior and ventral striatum dopamine. Neurosci Lett. 2008;436:278–282. doi: 10.1016/j.neulet.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2004;29:660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Garrido P, de Blas M, Mora F. Stress, prefrontal cortex and environmental enrichment: studies on dopamine and acetylcholine release and working memory performance in rats. Behav Brain Res. 2007;176:267–273. doi: 10.1016/j.bbr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Taymans SE, Sundstrom JM, Pert A. Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releaseing factor. Brain Res. 1998;786:39–46. doi: 10.1016/s0006-8993(97)01328-0. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology (Berl) 1998;135:151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatment of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry. 2010;67:28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, Harkness JH. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol. 2008;19:777–785. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Jacobsen JP, Mork A. Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res. 2006;1110:221–225. doi: 10.1016/j.brainres.2006.06.077. [DOI] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, Robbins TW. Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms of the nucleus accumbens. Psychopharmacology (Berl) 1990;102:364–372. doi: 10.1007/BF02244105. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL. c-Fos Expression Associated With Reinstatement of Cocaine-Seeking Behavior by Response-Contingent Conditioned Cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Hannan AJ, Macri S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis. 2008;31:159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004a;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004b;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004c;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010;68:774–777. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology. 2003;28:836–862. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology. 2004;29:1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- Naka F, Narita N, Okado N, Narita M. Modification of AMPA receptor properties following environmental enrichment. Brain Dev. 2005;27:275–278. doi: 10.1016/j.braindev.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paizanis E, Kelai S, Renoir T, Hamon M, Lanfumey L. Life-long hippocampal neurogenesis: environmental, pharmacological and neurochemical modulations. Neurochem Res. 2007;32:1762–1771. doi: 10.1007/s11064-007-9330-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Peltier Rl, Guerin GF, Dorairaj N, Goeders NE. Effects of saline substitution on responding and plama corticosterone in rats trained to self-administer different doses of cocaine. J Pharmacol Exp Ther. 2001;299:114–120. [PubMed] [Google Scholar]
- Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, Thiel KJ, Neisewander JL. Stimulation of medial prefrontal cortex serotonin 2C (5-HT(2C)) receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology. 2010;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:699–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res. 2001;120:87–95. doi: 10.1016/s0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, Garrido P, de Blas M, Mora F. Environmental enrichment reduces the response to stress of the cholinergic system in the prefrontal cortex during aging. Neurochem Int. 2008;52:1198–1203. doi: 10.1016/j.neuint.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL. Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression. Neuroscience. 2010;171:1187–1196. doi: 10.1016/j.neuroscience.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychoph. 2009;12:1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriet N, Amar L, Toussay X, Lardeux V, Ladenheim B, Becker KG, Cadet JL, Solinas M, Jaber M. Environmental enrichment during adolescence regulates gene expression in the striatum of mice. Brain Res. 2008;1222:31–41. doi: 10.1016/j.brainres.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- van der Harst JE, Baars AM, Spruijt BM. Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav Brain Res. 2003;142:151–156. doi: 10.1016/s0166-4328(02)00403-5. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DA, Buse JE, Wellman CL, Rebec GV. Differential environmental exposure alters NMDA but not AMPA receptor subunit expression in nucleus accumbens core and shell. Brain Res. 2005;1042:176–183. doi: 10.1016/j.brainres.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Ho A, Kreek MJ. Increased CRH mRNA levels in the rat amygdala during short-term withdrawal from chronic ‘binge’ cocaine. Brain Res Mol Brain Res. 2003a;114:73–79. doi: 10.1016/s0169-328x(03)00139-6. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res. 2003b;964:187–199. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]