Abstract
Inflammatory responses and associated products have been implicated in cancer metastasis. However, the relationship between these two processes is uncertain due to the lack of a suitable model. Taking advantage of localized and controllable inflammatory responses induced by biomaterial implantation and the capability of tissue scaffolds to release a wide variety of chemokines, we report a novel system for studying the molecular mechanisms of inflammation- mediated cancer metastasis. The animal model is comprised of an initial subcutaneous implantation of biomaterial microspheres which prompt localized inflammatory responses, followed by the transplantation of metastatic cancer cells into the peritoneal cavity or blood circulation. Histological results demonstrated that substantial numbers of B16F10 cells were recruited to the site nearby biomaterial implants. There was a strong correlation between the degree of biomaterial-mediated inflammatory responses and number of recruited cancer cells. Inflammation-mediated cancer cell migration was inhibited by small molecule inhibitors of CXCR4 but not by neutralizing antibody against CCL21. Using chemokine-releasing scaffolds, further studies were carried out to explore the possibility of enhancing cancer cell recruitment. Interestingly, erythropoietin (EPO) releasing scaffolds, but not stromal derived growth factors-1α-releasing scaffolds, were found to accumulate substantially more melanoma cells than controls. Rather unexpectedly, perhaps by indirectly reducing circulating cancer cells, mice implanted with EPO releasing scaffolds had ~30% longer lifespan than other groups. These results suggest that chemokine-releasing scaffolds may potentially function as implantable cancer traps and serve as powerful tools for studying cancer distraction and even selective annihilation of circulating metastatic cancer cells.
Keywords: Cancer metastasis, inflammation, foreign body reactions, B16F10 melanoma cells, mice, animal model
1. Introduction
Long term exposure to damaging UV radiation from the sun increases the risk for melanoma - the most serious form of skin cancer. Metastasis to different organs is a common complication and the leading cause of fatality in patients with cancer. Cancer metastasis is thought to involve a series of steps including invasion of extracellular matrix, intravasation into lymphatic or blood vessels, survival in the circulation, extravasation into distant sites, and progressive growth at those sites.[1, 2] Despite intensive research efforts, detailed mechanisms of cancer metastasis are not entirely understood. The lack of an animal model, which can be used to quantify the extent of cancer metastasis in a controllable manner is, at least partially, responsible for this deficiency. Several in vitro and in vivo models have been used in the past to assess cancer metastasis. Most studies of metastasis have been carried out on rodents with tumor xenografts. [3-5] In assays of spontaneous metastasis, tumor cells are injected into a site, preferably an orthotopic location. The primary tumor forms and metastases develop which are then monitored through time. Although this assay measures the complete metastatic process, this method is usually qualitative and time consuming.[6, 7] Metastasis evaluation has also been carried out by quantifying tumor growth in vital organs following by injection of tumor cells into the bloodstream. This method can only provide information about the post-intravasation stage of metastasis. It should also be noted that several transgenic mouse strains have been used to study primary tumorigenesis and spontaneous metastases.[7-10] A significant disadvantage of these systems however is the expense, unpredictability, and lack of versatility.
Numerous recent reports have implicated inflammation as an important process in the uncontrolled growth of cancer cells. Inflammatory signals have also been shown to facilitate the escape and spread of metastatic cells from the original tumor to new sites. [11-13] This is supported by a recent observation that tumor infiltrating macrophages promote both the development of breast cancers and their eventual spread to other sites in the body.[14] Furthermore, an increasing body of evidence suggests that inflammatory responses play an important role in tumor development and progression.[12, 13, 15-17] For example, inflammatory chemokines, such as CXCL12, CCL21, MIP-1α/CCL3, IL-8/CXCL8 and RANTES/CCL5, have been associated with metastasis of breast cancer, melanoma, myeloma, colorectal carcinoma, ovarian carcinoma and non-small cell lung cancer.[18-22] Human and murine tumors are also found to secrete various inflammatory cytokines, CXC chemokines and express their receptors.[18, 23, 24] Inflammatory chemokine receptors such as CXCR4 and CCR7 are commonly found to be expressed in human breast cancer.[25] CCR7 has also been found to dramatically increase metastasis of B16 murine melanoma to regional lymph nodes.[26] Colorectal cancer cells also express chemokine receptor/ligand such as CCR6/CCL20 and respond to chemokine gradients as do inflammatory cells.[27, 28] Interestingly, many chemokine ligands, such as CXCL12 (SDF-1) and CCL21 for SLC (lymphoid-tissue chemokine), are highly expressed in the target organs of breast cancer metastasis.[20, 25, 29]
The present investigations were aimed at the development of a reproducible animal model to investigate the processes governing inflammation-mediated cancer metastasis. We have created a two-step in vivo model to study cancer metastasis. First, subcutaneous implantation of biomaterial microspheres was used to create localized inflammatory responses. This maneuver is based on extensive studies showing that the implantation of biomaterials will prompt varying levels of inflammatory responses.[30-32] Second, metastatic cancer cells were then injected into the peritoneal cavity, which has widely been used to study cancer migration via lymph nodes and circulation.[33-35] After cancer cell implantation for different periods of time, lymph nodes, subcutaneous microsphere implants, and surrounding tissues were recovered for histological analyses. The numbers of cancer cells can be quantified in both lymph nodes and implantation site tissues to reflect the extent of cancer metastasis. Finally, chemokine-releasing scaffolds were fabricated to test the influence of various chemokines on promoting melanoma recruitment to scaffold implants in vivo.
2. Materials & Methods
2.1 Cancer cell culture
B16F10 melanoma cells, Lewis Lung carcinoma (LLC) cells, rat prostate cancer cell line (JHU-31), human prostate adenocarcinoma (PC-3), and human breast cancer cell line (MDA- MB-231) used in this investigation were purchased from American Type Culture Collection (ATCC) (Manassas, Virginia, USA). B16F10 melanoma cells are skin melanoma cell line isolated from C57BL/6J mice. LLC cells isolated from C57BL/6J mice are widely used as a model for cancer metastasis. JHU-31 cells are derived from rat and exhibit a high rate of metastasis to the lung and lymph nodes (>75%). PC-3 cells originate from a 62-year-old male Caucasian with bone metastatic prostate adenocarcinoma. MDA-MB231 cells are derived from breast adenocarcinoma metastasized pleural effusion. All cells were maintained in DMEM supplemented with 10% heat inactivated fetal bovine serum at 37°C, 5% CO2 humidified environment. For in vitro tracking, some of the cancer cells were labeled with Kodak X-Sight 761 Nanospheres (Carestream Health Inc, New Haven, CT, USA) according to the user manual and our previous study.[36, 37]
2.2 Microsphere preparation
To prompt various degrees of foreign body reactions, microspheres made of different materials, including poly-L-lactic acid (PLA), aluminum hydroxide (Alhydrogel 85), glass (Glasperlen®), were used in this investigation. PLA microspheres were synthesized according to a modified precipitation method.[32, 38] The average sizes of the microspheres were 8.23 ± 2.12, 10, and 450-500 μm in diameter, respectively. All microspheres were sterilized with 70% ethanol and then transferred to phosphate buffered saline (PBS, 100 mM, pH 7.2) prior to experiments.
2.3 Chemokine releasing scaffold fabrication
Chemokines SDF-1α (Prospec-Tany TechnoGene, Rehovot, Israel) and EPO (Cell Sciences, Canton, MA) releasing PLGA scaffolds were fabricated using our previously published method.[39] Briefly, albumin microbubbles made by sonicating 10% w/v bovine serum albumin under nitrogen gas bubbling, loaded with SDF-1α (1 μg/ml) or EPO (100 I.U.) were added to 10% w/v PLGA solution in 1,4-dioxane. Such mixtures were frozen in liquid nitrogen and lyophilized for at least 72 hours to result in the formation of 3-D degradable cancer traps loaded with either SDF-1α or EPO.
2.4 Cancer metastasis animal model
The animal experiments were carried out using C57BL/6 mice (6-10 week old) from Jackson Laboratory (Bar Harbor, ME, USA). This murine cancer metastasis model comprised of two consecutive steps. First, biomaterial microspheres (75 mg/0.5 ml saline/ mouse) were implanted in the dorsal subcutaneous space of mice to elicit localized subcutaneous inflammation. Second, after microsphere implantation for different periods of time (6 hours-14 days), cancer cells (5 × 106 cells/0.2 ml/mouse) were transplanted in the peritoneal cavity. Twenty four hours after tumor cell transplantation the animals were sacrificed. The vital organs, lymph nodes, the microsphere implants and surrounding tissues were then recovered and frozen in OCT embedding media (Polysciences Inc., Warrington, PA, USA) at -800C. The peripheral blood was also collected for further analysis. Eight μm thick sections were sliced using a Leica Cryostat (CM1850) and placed on poly-L-lysine coated slides for histological and immunohistochemical analyses. To reduce the extent of foreign body responses, some PLA microspheres, prior to administration, were soaked with anti-inflammatory agent, dexamethasone (0.1 mg drug/0.5 ml microsphere suspension). For whole body imaging of cancer cell migration, parallel studies were carried out to monitor the migration of X-Sight 761 Nanosphere-labeled B16F10 cells in C57BL/B6 mice. The animals were then imaged using Kodak In-Vivo Imaging System FX Pro (Carestream Health Inc, New Haven, CT).
2.5 Biodistribution analysis
To track the cell migration in animals, B16F10 cells were transduced by Ad5 virus bearing green fluorescent protein (pEGFP-N1, Clontech Laboratories Inc., Mountain View, CA) at MOI of 50 for 24 hours before injection to the peritoneal cavity. The GFP Ad5 infectivity to B16F10 was assessed by GFP expression visualized by fluorescent microscopy prior to experiments. For biodistribution analyses, GFP-B16F10 (1.0 × 107) suspended in the culture medium (0.2 ml) were injected into the peritonea of C57BL/6 mice as described before. After sacrificing the animals, the tissue sections were analyzed under fluorescent microscope. Cancer cell densities in different tissues were quantified to reflect the degree of cancer metastasis.
2,6 Influence of chemokine inhibitors and neutralizing antibodies in cancer cell migration
To determine the role of CXCR4/SDF-1α and CCR7/CCL21 pathways in cancer metastasis, AMD3100 and CCL21 neutralizing antibodies were used to block CXCR4/SDF-1α and CCR7/CCL21 pathways, respectively. Specifically, microsphere-implanted animals were injected intraperitoneally with either AMD3100 (250μg/0.1 ml/mouse, Sigma-Aldrich Inc., St. Louis, MO, USA) or CCL21 neutralizing antibody (1 mg/0.1 ml/mouse; R&D Systems Inc, Minneapolis, MN, USA) 1 hour prior and 12 hours post tumor injection.
2.7 Histological quantification of inflammatory responses and cancer cell migration
Immunohistological analyses for CD11b+ inflammatory cells and HMB45+ melanoma cells were carried out to assess the degree of implant-mediated inflammatory responses and melanoma cancer cell migration, respectively. Briefly, tissue sections were incubated with the primary anti-melanoma antibody (HMB45, 1:50 dilution, Abcam, Cambridge, MA, USA) or anti-mouse CD11b antibody (1:20 dilution, Serotec Inc., Raleigh, NC, USA) for 1 hr at 37°C. After washing thrice with PBS, the slides were then incubated with either HRP-conjugated or FITC-conjugated secondary antibody (1:500 dilution, Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania, USA) for 1 hr at 37°C. FITC-conjugated antibody incubated tissue section were ready for image analysis. HRP-conjugated antibody incubated sections were developed with a DAB liquid Substrate System for 15 minutes. All tissue section images were taken using a Leica fluorescence microscope (Leica Microsystems, Wetzlar, GmbH) equipped with a Retiga-EXi CCD camera (QImaging, Surrey, BC, Canada) as described earlier.[36, 37, 39]
2.8 Statistical analyses
Statistical comparison between different groups was carried out using Student t- test or one-way ANOVA. Differences were considered statistically significant when p < 0.05.
3.Results
3.1 Recruitment of cancer cells toward biomaterial implants
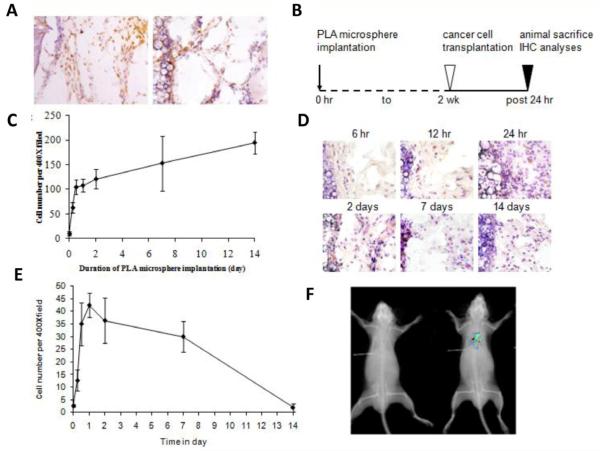
Biomaterial-mediated inflammatory responses involve a series of processes with the participation of various immune cells and inflammatory cytokines/chemokines. We found that 1- day old subcutaneous implants attract the infiltration of inflammatory cells (CD11b+) and intraperitoneally transplanted B16F10 melanoma cells (HMB45+) (Figure 1A). The immigration of melanoma cells into distant inflammatory sites suggests that inflammatory signals may serve as chemoattractants for melanoma cells. To test this, we analyzed the influence of varying degrees of inflammatory responses on melanoma cell migration. To create a localized environment with varying inflammatory intensities, poly-L-lactic acid (PLA) microspheres were implanted in the subcutaneous space for different periods of time (6 hours, 12 hours, 24 hours, 2 days, 7 days and 14 days) (Figure 1B). As expected, most of the inflammatory cell (CD11b+) recruitment occurs within 12 hours following microsphere implantation with insignificant increase after 24 hours (Figure 1C). To determine the importance of stage and intensity of the inflammatory processes in cancer cell migration, B16F10 melanoma cells were transplanted in the peritonea of mice bearing subcutaneous microsphere implants for different periods of time. At various time points following the initiation of inflammation, the numbers of melanoma cells immigrating into subcutaneous microsphere implantation sites were analyzed (Figure 1D&E). Interestingly, we found that the numbers of recruited melanoma cells varied greatly in mice bearing implants for different periods of time (Figure 1D&E). The accumulation of melanoma cells appears to be responding to acute inflammatory responses triggered by microsphere implantation for 12 hours up to 7 days. However, only a few melanoma cells were recruited to the sites with microspheres implanted for less than 6 hours and longer than 2 weeks (Figure 1 D). The specificity of the inflammatory response-mediated cancer metastasis could also be demonstrated by an optical imaging method using B16F10 melanoma cells labeled with near- infrared Kodak X-Sight 761 nanospheres. The in vivo image shows that transplanted melanoma cells were only recruited to the dorsal skin site with microsphere implants (Figure 1F).
Figure 1.
Foreign body reactions trigger tumor cell migration. Pre-existing 1-day old subcutaneous implants were found to attract the immigration of CD11b+ inflammatory cells (A, left) and intraperitoneally transplanted B16F10 melanoma cells (A, right). To determine the influence of inflammatory signals in cancer cell migration, varying degrees of inflammatory stimuli intensities were stimulated from 6 hours to 2 weeks according to the experimental time table (B). We found that large numbers of CD11b+ inflammatory cells were recruited to the implantation sites in 12 hours and the influx of inflammatory cells was slowed down after that. These results depict different stages of biomaterial-mediated inflammatory responses (C). The stages of inflammatory responses also affect the extent of melanoma cell recruitment (D).Melanoma cell accumulation in the implant area reached a peak around 24 hours post microsphere implantation (E). Inflammation-induced cancer metastasis is also detected in optical imaging method by labeling melanoma cells with Kodak X-Sight 761 near infrared nanospheres (F).
3.2 Effect of inflammation-suppression on cancer cell immigration
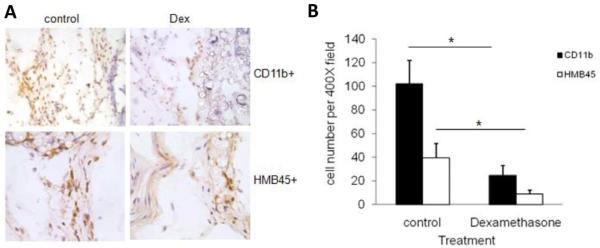
To verify the importance of inflammatory reactions in triggering melanoma cell immigration, similar subcutaneous PLA microsphere implantations were carried out in the presence or absence of the anti-inflammatory agent, dexamethasone. As expected, dexamethasone-treated microspheres prompted substantially less inflammatory cell (CD11b+) recruitment than saline-incubated microsphere controls (Figure 2A, panel of photos: CD11b+ vs. HMB45+, dexamethasone-treated vs. controls). Coincidentally, the recruitment of melanoma cells (HMB45+) was also diminished by localized release of dexamethasone (Figure 2A). The effects of locally released dexamethasone on the reduction of inflammatory cell and melanoma cells are statistically significant (Figure 2B). These results provide strong support to the idea that inflammatory reactions are essential for the initiation of cancer cell migration from the peritoneal cavity to subcutaneous microsphere implantation sites.
Figure 2.
Immunohistochemical staining of subcutaneous tissues surrounding the PLA microspheres with or without the treatment of dexamethasone (Dex). The accumulation of inflammatory cell (CD11b+) in tissue implanted with PLA microspheres (A, top left) or PLA microspheres soaked with dexamethansone (A, top right) can be observed (200X). The recruitment of melanoma cells (HMB45+) was also observed in tissues implantedwith PLA microspheres (A, bottom left) or dexamethansone embedded PLA microspheres (A, bottom right) (400X). Quantification of the numbers of inflammatory cells and melanoma cells in the subcutaneous tissues with both treatments were graphed and statistically analyzed (B). Data are mean ± SD (n = 6 per group). *P< 0.05, t-test.
3.3 Effect of biomaterial properties on cancer cell recruitment
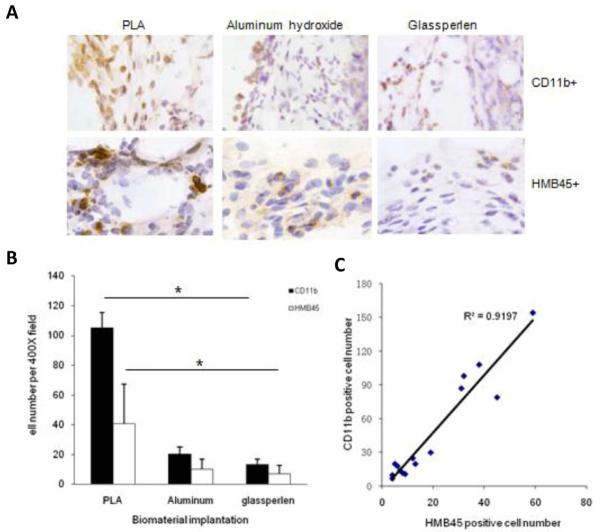
It is well established that different materials trigger varying degrees of inflammatory responses. If the extent of biomaterial-mediated inflammatory responses affects the degree of cancer cell immigration, materials with different tissue compatibility might differentially influence melanoma cell recruitment. To test this hypothesis, microspheres made of PLA, aluminum hydroxide, and glass were tested using the same animal model. As expected, these implanted microspheres triggered different extent of inflammatory responses and melanoma cell immigration as shown by immunohistochemical analysis (Figure 3A). PLA microspheres were found to prompt more inflammatory cell (CD11b+) and melanoma cell (HMB45+) recruitment than microspheres made of aluminum hydroxide and glass (Figure 3B). By comparing the numbers of both cell types, our results showed that there is an excellent correlation (R2 = 0.92) between the extent of inflammatory reactions and melanoma cell recruitment (Figure 3C). These results lend strong support to our hypothesis that inflammatory responses influence the migration and, perhaps, metastatic behavior of melanoma cells.
Figure 3.
Extent of foreign body responses and melanoma cell recruitment to different biomaterial implants. Immunohistochemistry staining of the tissue was carried out to assess the degree of foreign body reactions and quantify the accumulation of CD11b+ inflammatory cells and HMB45+ melanoma cells surround the implants, including PLA, aluminum hydroxide and Glasperlen (A). The quantification analysis of cell recruitment was graphed (B) and the correlation between the melanoma cell numbers and inflammatory cell numbers in surrounding tissue of implanted microspheres statistically analyzed (C). Data are mean ± SD (n = 5 per group). *P< 0.05, ANOVA.
3.4 Assessment of cancer cell biodistribution
Although our histological results support the idea that localized inflammatory responses attract melanoma cell immigration from the peritoneal cavity to the subcutaneous implantation site, it is not clear whether the inflamed tissue/microsphere implantation site is the only target for the migrating melanoma cells. We therefore assessed the overall distribution of GFP-expressing B16F10 melanoma cells in major organs (including lung, liver, kidney, spleen, and lymph nodes) using histological analyses. In addition to the subcutaneous implantation site, high numbers of cancer cells were found in the lymph nodes and spleen. However, relatively low densities of cancer cells were found in skin, lung, liver, and kidney (Figure 4). The accumulation of melanoma cells in the spleen may be associated with its blood filter activities. The accumulation of large numbers of cancer cells in the lymph nodes suggests that melanoma cell migration from peritoneum to the blood might involve passage through the lymphatic system.
Figure 4.
Biodistribution evaluation of B16F10 cell recruitment to the microsphere implant area based on immunohistological analyses. To observe the biodistribution, GFP expressing B16F10 cells were administered intraperitoneally 24 hours following PLA microsphere implantation. High densities of cancer cells were found in the lymph nodes, spleen and implantation area. However, relatively low densities of cancer cells were found in skin, lung, liver, and kidney.
Inflammatory responses have been implicated in the process of metastasis of various cancers.[40, 41] Although our results so far support the hypothesis that inflammation will cause B16F10 melanoma cells to accumulate in the inflamed area, it was not clear whether other types of cancer cells might respond similarly. By labeling several cancer cells (Lewis lung cancer, human MDA-MB231 breast cancer, human PC-3 prostate cancer, rat JHU-31 prostate cancer), originating from different sources, with a NIR probe, the same animal model was tested. Interestingly, we found that all cancer cells tested here migrated to the subcutaneous implantation sites, although the extent of cancer cell migration varied between the cell types (Figure 5).
Figure 5.
Cancer cell recruitment in response to inflammatory stimulus is universal in different cancer cell types, including Lewis lung cancer, human MDA-MB231 breast cancer, human PC-3 prostate cancer, rat and JHU-31 prostate cancer. Animal bearing PLA implant transplanted with non-labeled cancer cells served as control.
3.5 Molecular pathway associated with inflammation-mediated cancer migration
Despite of the above observations on the recruitment of cancer cells to microsphere implantation sites, it was still not clear whether this animal model might reflect cellular and physiological responses resembling other earlier cancer metastasis models. To test the relevance of this model, we first identified the molecular processes governing the foreign body reaction-mediated cancer migration. Since both CXCR4/CXCL12 and CCR7/CCL21 pathways have been shown to play an important role in melanoma cancer metastasis, the potential role of both pathways in foreign body reaction-mediated cancer migration was assessed.
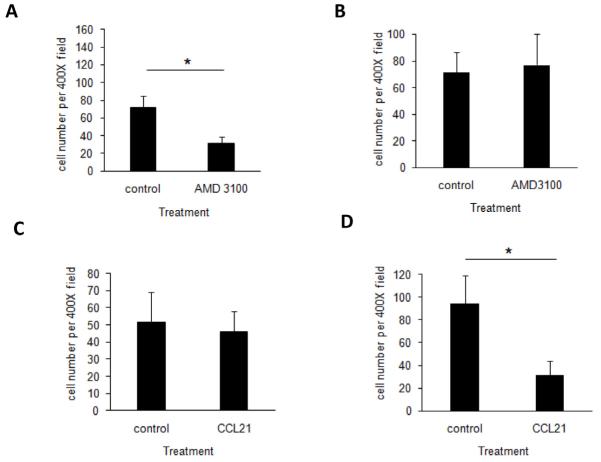
We first tested whether CXCR4/CXCL12 pathway was involved in our animal model. Indeed, treatment with AMD3100, an antagonist of the SDF-1α receptor - CXCR4, drastically reduced the recruitment of both melanoma cells and inflammatory cells to the subcutaneous microsphere implantation sites (Figure 6A). On the other hand, AMD3100 treatment had no effect on the accumulation of melanoma cells in lymph nodes (Figure 6B). To test the importance of CCR7/CCL21 pathway in B16F10 melanoma cell accumulation in the inflamed sites, microsphere-bearing mice were treated with either CCL21 neutralizing antibody or saline prior to melanoma cell transplantation. We observed that the number of tumor cells migrating to the microsphere implantation site was not affected by the treatment with CCL21 neutralizing antibody (Figure 6C). On the other hand, CCL21 neutralizing antibody treatment dramatically diminished the presence of B16F10 melanoma cells in the lymph nodes (Figure 6D). These results show that CCR7/CCL21 pathway, but not CXCR4/CXCL12 pathway, is critical to melanoma migration through lymphatic system. On the other hand, CXCR4/CXCL12 pathway, but not CCR7/CCL21 pathway, is essential to cell immigration into the subcutaneous implantation site.
Figure 6.
AMD3100 treatment inhibited the cell recruitment of B16F10 melanoma to the implant site (A). However, AMD 3100 blockage exerted no effect on the accumulation of melanoma cells in lymph node (B). On the other hand, CCR7/CCL21 pathway in B16F10 melanoma cell accumulation in the inflamed sites was also examined by CCL21 neutralizing antibody treatments. In contrast, the number of tumor cells migration to microsphere implantation site was not affected (C). However, the presence of B16F10 melanoma cells in the lymph node drastically diminished (D). *P< 0.05, t-test.
3.6 Application of chemokine-releasing scaffolds to enhance cancer cell recruitment
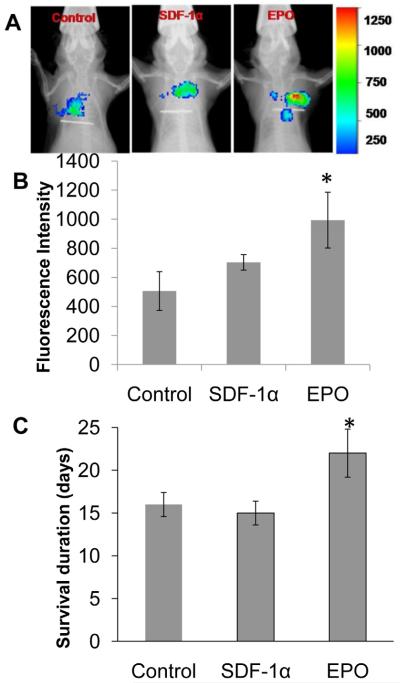
Previous results support that implant-associated inflammatory products actively recruit circulating cancer cells. The next question is whether cancer cell recruitment can be enhanced by cancer cell migration-specific chemokines. To find the answer, we were interested in SDF-1α and EPO, since both of these chemokines have been shown to enhance cancer cell migration and are also upregulated on metastatic cancer cells. [19, 42] To test this hypothesis, SDF-1-releasing scaffolds and EPO-releasing scaffolds were fabricated. Our results have shown that these scaffolds are capable of releasing 10% of the loaded drug for a duration of approximately 10 days. When implanted subcutaneously in mice and followed with NIR-labeled B16F10 melanoma cell transplantation, we found that the localized release of EPO prompted the highest cancer cell recruitment, as compared to SDF-1α which was not significantly different from the control (Figure 7A&B). The survival duration of scaffold-bearing animals was also evaluated after completion of the in vivo imaging detection. Very interestingly, we found that the release of EPO significantly prolonged the survival (>30%) of the cancer-bearing mice as compared to SDF-1α loaded scaffolds (Figure 7C).
Figure 7.
EPO and SDF-1α loaded tissue scaffold along with control scaffolds were tested for their melanoma recruitment ability using our murine melanoma metastasis model. Real time in vivo imaging showed accumulation of labeled B16F10 melanoma cells around the tissue scaffolds (A). EPO-releasing tissue scaffolds showed enhanced >1 fold accumulation of melanoma cells detected using Kodak imaging system (B). EPO-releasing scaffolds significantly enhanced the life span of cancer bearing animals (C). *P< 0.05, t-test
4. Discussion
Cancer metastasis, a lethal complication of many cancers, consists of a series of discrete biological processes including tumor cell intravasation, survival in circulation, extravasation into a distant organ, angiogenesis and uninhibited growth.[1, 43, 44] Despite intense research efforts, the study of cancer metastasis has been difficult due to the lack of a suitable animal model which can target, recruit and quantify the numbers of immigrated metastatic cells. Many recent studies indicate that inflammatory products, such as CSF-1, TNF-α, and MCP-1 promote the migration of several human metastatic cell lines such as colon cancer, breast cancer, and malignant melanoma.[40, 45-48] Also, inflammatory chemokines, such as CXCL12, CCL21, MIP- 1α/CCL3, IL-8/CXCL8 and RANTES/CCL5, have also been associated with metastasis of breast cancer, melanoma, myeloma, colorectal carcinoma, ovarian carcinoma and non-small cell lung cancer.[18, 19, 21, 22] It is likely that the signals released during inflammatory reactions may serve as chemoattractant for metastatic cancer cells, since many types of cancer cells share similar chemokine receptors with inflammatory cells. Based on this assumption, we have developed a biomaterial implant-mediated cancer metastasis animal model to specifically recruit distally transplanted metastatic cancer cells (B16F10 melanoma cells, LLC carcinoma cells, JHU-31, PC-3 prostate cancer cells and MDA-MB231 breast cancer cells) to sites of inflammation. In this animal model, localized inflammatory responses were created by subcutaneous implantation of microspheres. We used this biomaterial-based model because of numerous earlier observations. First, almost all implanted biomaterials trigger varying extents of foreign body reaction accompanied by an accumulation of macrophages and neutrophils on and adjacent to the implant surface.[49-51] Second, biomaterial implants prompt the production of a variety of pro-inflammatory cytokines and chemokines, including TNF-α, IL-1β, MCP-1 and MIP-1, which are similar to other inflammatory or wound healing reactions.[52, 53] Third, the inflammatory response could be affected by the chemical composition of the microsphere surface.[30, 31] Finally, differing from many other inflammatory models, biomaterial implants elicit localized inflammatory responses and rarely prompt systemic immune reactions.[30, 53, 54] The subcutaneously induced inflammatory responses were tested for their ability to attract cancer cells transplanted in a distal site - the peritoneal space. It should be noted that several recent studies have used tissue engineering scaffolds to generate bone environment to study breast cancer bone metastasis.[55, 56] However, the influence of inflammatory responses and associated products on inflammation-associated cancer metastasis was not the focus of these works.
Our results support the hypothesis that microsphere-mediated inflammatory responses are sufficient to trigger the migration of melanoma cells from the peritoneum to the subcutaneous implant. The recruitment of cancer cells to the microsphere implantation sites was substantially reduced by dexamethasone which is commonly used to diminish the degree of biomaterial- mediated inflammatory responses.[36, 57-59] The extent of cancer cell recruitment could also be varied with the implantation of microspheres triggering different levels of inflammation. Our results indicate that the extent of cancer cell recruitment by subcutaneous implants is positively correlated with the degree of biomaterial-mediated inflammatory responses.
To determine whether cancer cells are preferentially recruited to sites of inflammation, we examined the cancer cell distribution in most vital organs and tissues using GFP-expressing B16F10 cancer cells. In addition to the microsphere implantation sites, cancer cells were found in lymph nodes and spleen. These results are consistent with early findings that lymph nodes often serve as a passage for cancer metastasis, [60, 61] and the spleen functions as a filter to remove migrating cancer cells from circulation.[46] Both histological evaluation and in vivo imaging results suggested that substantial numbers of the transplanted cancer cells were recruited to the subcutaneous microsphere implantation site. Though the events involved in cell migration from the primary site to the inflamed tissue, probably involving lymphatic and blood circulation are yet to be determined, we believe that this newly developed animal model may serve as a powerful tool for dissecting possible “cause and effect” processes involved in cancer metastasis.
Based on the results from others,[62] and from our own laboratory, the migration of cancer cells from peritoneal transplantation sites to the subcutaneous microsphere implantation sites can be divided into at least three consecutive steps: cancer cell invasion into capillaries possibly via the lymphatics, cancer cell circulation, and extravasation of cancer cells into the subcutaneous implantation sites.[61] Our results have shown that CCR7 expression is essential to melanoma cell accumulation in lymph nodes and neutralization of its ligand CCL21, reduced the accumulation of melanoma cells in lymph nodes. These findings are in agreement with many recent observations that the CCR7/CCL21 pathway is important for cancer cell trafficking through lymph nodes. First, previous studies have demonstrated that lymph nodes play an important role in metastasis of many tumor cells, including melanoma, breast cancer, prostate, colon, and gastric cancer.[62] Second, lymph node-mediated metastasis is strongly associated clinically with poor prognosis in many tumors, including melanoma, breast cancer and colon cancer.[60, 63-66] Third, enhanced CCR7 expression may promote B16F10 melanoma cell metastasis to draining lymph nodes.[67, 68] Finally, CCL21 induces CCR7 expression and associated Ca2+ flux in lymphatic endothelial cells.[68] However, the reduction of B16F10 accumulation in lymph nodes has little effect on cancer cell accumulation at subcutaneous sites of inflammation. These results suggest that, during cancer metastasis, cancer cells may enter the blood circulation either through direct invasion of capillaries or via primary spread from lymphatics to capillaries as suggested earlier.[33-35, 62]
Since cancer cells, including breast cancer, ovarian cancer, melanoma and prostate cancer, express high levels of CXCR4, and cancer metastatic organs (lymph nodes, lungs, liver and bones) release high concentration of SDF-1α, the SDF-1α/CXCR4 pathway is believed to participate in cancer metastasis.[19, 42] Indeed, treatment with the CXCR4 antagonist - AMD3100 - substantially reduced the accumulation of cancer cells in the subcutaneous microsphere implantation sites. Our results suggest that CXCR4/CXCL12 (SDF-1α) axis is involved in melanoma migration from the peritoneal cavity to the microsphere implant site.
Many chemokines have been shown to affect cancer metastasis. It is thus likely that material implants can be made to recruit large number of cancer cells by releasing cancer- specific chemokines. This hypothesis was tested using scaffolds fabricated to release either SDF- 1α or EPO. SDF-1α was found to participate in cancer metastasis via the SDF-1α/CXCR4 pathway.[19, 42] On the other hand, EPO receptor is highly expressed on malignant melanoma and EPO has been shown to induce lymph node tumor metastasis.[69, 70] Rather unexpectedly, SDF-1α-releasing scaffold implant did not exert a very significant influence on cancer cell migration as compared to the control. On the other hand, EPO-releasing scaffolds prompted more melanoma cell migration than both SDF-1α and control scaffolds. Although the cause for the different cancer cell recruitment responses between localized release of SDF-1α and EPO are yet to be determined, it is possible that the localized release of EPO by scaffolds may alter melanoma cancer cell responses. Indeed, animals that received EPO scaffolds had longer (>30%) survival while mice implanted with SDF-1α scaffolds had a shorter period of survival. A possible reason for the increased cancer cell recruitment could be found in the observations of recent studies that have demonstrated high levels of EPO and EPO receptor expression in many different cell types, including cancer cells such as breast, head-and-neck tumors, colon, lung, prostate and melanoma.[69, 71, 72] EPO/EPO receptors are known to induce proliferation, chemotaxis, and angiogenesis, and inhibit apoptosis.[73, 74] It has also been demonstrated in murine model that EPO mediates an augmented B-cell response,[75] and may thus play a role in immunomodulation.[76]
This study demonstrates the amalgamation of a biomaterial scaffold fabrication technology and the biological phenomenon of cancer cell migration in response to inflammatory chemokines to develop a cancer metastasis model for uncovering the processes governing inflammation-mediated cancer metastasis in vivo. Our studies support, for the first time, that chemokine-releasing scaffold can be made to lure circulating cancer cells and additionally increase the survival rate of the animals. A more comprehensive understanding of the ensuing sequence of events may lead to the development and discovery of new cancer therapies for drastically reducing cancer metastasis.
5. Conclusions
Inflammatory responses have been shown to influence cancer metastasis. However, due to the lack of proper animal model, the mechanism of inflammation-mediated cancer metastasis is not totally understood. To address the gap in knowledge, we have created a cancer metastasis model in which biomaterial implants were employed to prompt controllable localized inflammatory responses. As anticipated, we found a very good relationship between the degree of implant-associated inflammatory responses and cancer cell recruitment. Furthermore, implant- associated cancer metastasis was substantially suppressed by reduced foreign body reactions with localized release of anti-inflammatory agent - dexamethasone. Subsequently, we explored the idea whether cancer cell recruitment can be significantly enhanced with localized release of cancer chemokines, such as EPO and SDF-1α. Very interestingly, we found that EPO releasing but not SDF-1α releasing scaffolds were capable of significantly improving the recruitment of circulating cancer cells in mice by compared with scaffold controls. Rather unexpectedly, mice with such EPO scaffolds showed an improved survival rate as compared to SDF-1α and untreated controls. Overall, our findings suggest that biomaterial implant based animal model of cancer metastasis can be used for extensive studies aimed at elucidating the molecular mechanisms of cancer metastasis. In addition and rather serendipitously, we found that specific chemokine loaded tissue engineering scaffolds could be used as “cancer traps” for distracting metastatic cancer cells and perhaps improve the survival rate of cancer afflicted individuals.
Acknowledgements
This work was supported by NIH grant RO1 EB007271. The authors would like to acknowledge Professor John W. Eaton for his critical review of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial support: NIH grant RO1 EB007271
References
- [1].Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- [2].Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- [3].Welch DR. Technical considerations for studying cancer metastasis in vivo. Clin Exp Metastasis. 1997;15:272–306. doi: 10.1023/a:1018477516367. [DOI] [PubMed] [Google Scholar]
- [4].Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K, et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci U S A. 2007;104:19506–19511. doi: 10.1073/pnas.0709185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yamamoto M, Kikuchi H, Ohta M, Kawabata T, Hiramatsu Y, Kondo K, et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 2008;68:9754–9762. doi: 10.1158/0008-5472.CAN-08-1748. [DOI] [PubMed] [Google Scholar]
- [6].Cespedes MV, Casanova I, Parreno M, Mangues R. Mouse models in oncogenesis and cancer therapy. Clin Transl Oncol. 2006;8:318–329. doi: 10.1007/s12094-006-0177-7. [DOI] [PubMed] [Google Scholar]
- [7].Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khanna C, Hunter K. Modeling metastasis in vivo. Carcinogenesis. 2005;26:513–523. doi: 10.1093/carcin/bgh261. [DOI] [PubMed] [Google Scholar]
- [9].Schwertfeger KL, Xian W, Kaplan AM, Burnett SH, Cohen DA, Rosen JM. A critical role for the inflammatory response in a mouse model of preneoplastic progression. Cancer Res. 2006;66:5676–5685. doi: 10.1158/0008-5472.CAN-05-3781. [DOI] [PubMed] [Google Scholar]
- [10].Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology. 2009;136:780–798. doi: 10.1053/j.gastro.2008.12.049. [DOI] [PubMed] [Google Scholar]
- [11].Marx J. Cancer research. Inflammation and cancer: the link grows stronger. Science. 2004;306:966–968. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- [12].Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- [13].Lorusso G, Ruegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol. 2008;130:1091–1103. doi: 10.1007/s00418-008-0530-8. [DOI] [PubMed] [Google Scholar]
- [14].Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- [15].Melnikova VO, Bar-Eli M. Inflammation and melanoma metastasis. Pigment Cell Melanoma Res. 2009;22:257–267. doi: 10.1111/j.1755-148X.2009.00570.x. [DOI] [PubMed] [Google Scholar]
- [16].Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- [17].Arias JI, Aller MA, Arias J. Cancer cell: using inflammation to invade the host. Mol Cancer. 2007;6:29. doi: 10.1186/1476-4598-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006;25:357–371. doi: 10.1007/s10555-006-9003-5. [DOI] [PubMed] [Google Scholar]
- [19].Gomperts BN, Strieter RM. Chemokine-directed metastasis. Contrib Microbiol. 2006;13:170–190. doi: 10.1159/000092972. [DOI] [PubMed] [Google Scholar]
- [20].Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79:639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- [21].Shields JD, Emmett MS, Dunn DB, Joory KD, Sage LM, Rigby H, et al. Chemokine- mediated migration of melanoma cells towards lymphatics--a mechanism contributing to metastasis. Oncogene. 2007;26:2997–3005. doi: 10.1038/sj.onc.1210114. [DOI] [PubMed] [Google Scholar]
- [22].Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- [23].Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374–379. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- [24].Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, et al. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59:4681–4687. [PubMed] [Google Scholar]
- [25].Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- [26].Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- [27].Ghadjar P, Coupland SE, Na IK, Noutsias M, Letsch A, Stroux A, et al. Chemokine receptor CCR6 expression level and liver metastases in colorectal cancer. J Clin Oncol. 2006;24:1910–1916. doi: 10.1200/JCO.2005.04.1822. [DOI] [PubMed] [Google Scholar]
- [28].Ghadjar P, Rubie C, Aebersold DM, Keilholz U. The chemokine CCL20 and its receptor CCR6 in human malignancy with focus on colorectal cancer. Int J Cancer. 2009;125:741–745. doi: 10.1002/ijc.24468. [DOI] [PubMed] [Google Scholar]
- [29].Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- [30].Kamath S, Bhattacharyya D, Padukudru C, Timmons RB, Tang L. Surface chemistry influences implant-mediated host tissue responses. J Biomed Mater Res A. 2008;86:617–626. doi: 10.1002/jbm.a.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nair A, Zou L, Bhattacharyya D, Timmons RB, Tang L. Species and density of implant surface chemistry affect the extent of foreign body reactions. Langmuir. 2008;24:2015–2024. doi: 10.1021/la7025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weng H, Zhou J, Tang L, Hu Z. Tissue responses to thermally-responsive hydrogel nanoparticles. J Biomater Sci Polym Ed. 2004;15:1167–1180. doi: 10.1163/1568562041753106. [DOI] [PubMed] [Google Scholar]
- [33].Carvalho MA, Zecchin KG, Seguin F, Bastos DC, Agostini M, Rangel AL, et al. Fatty acid synthase inhibition with Orlistat promotes apoptosis and reduces cell growth and lymph node metastasis in a mouse melanoma model. Int J Cancer. 2008;123:2557–2565. doi: 10.1002/ijc.23835. [DOI] [PubMed] [Google Scholar]
- [34].Gerber SA, Rybalko VY, Bigelow CE, Lugade AA, Foster TH, Frelinger JG, et al. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol. 2006;169:1739–1752. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K, et al. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001;61:889–895. [PubMed] [Google Scholar]
- [36].Nair A, Shen J, Lotfi P, Ko CY, Zhang CC, Tang L. Biomaterial implants mediate autologous stem cell recruitment in mice. Acta Biomater. 2011 doi: 10.1016/j.actbio.2011.06.050. Available from URL: http://www.ncbi.nlm.nih.gov/pubmed/21784181 (DOI: doi:10.1016/j.actbio.2011.06.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thevenot PT, Nair AM, Shen J, Lotfi P, Ko CY, Tang L. The effect of incorporation of SDF-1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 2010;31:3997–4008. doi: 10.1016/j.biomaterials.2010.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsules formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55:R1–R4. [Google Scholar]
- [39].Nair A, Thevenot P, Dey J, Shen J, Sun MW, Yang J, et al. Novel polymeric scaffolds using protein microbubbles as porogen and growth factor carriers. Tissue Eng Part C Methods. 2010;16:23–32. doi: 10.1089/ten.tec.2009.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ikebe M, Kitaura Y, Nakamura M, Tanaka H, Yamasaki A, Nagai S, et al. Lipopolysaccharide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathway. J Surg Oncol. 2009;100:725–731. doi: 10.1002/jso.21392. [DOI] [PubMed] [Google Scholar]
- [41].Koller FL, Hwang DG, Dozier EA, Fingleton B. Epithelial interleukin-4 receptor expression promotes colon tumor growth. Carcinogenesis. 2010;31:1010–1017. doi: 10.1093/carcin/bgq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Longo-Imedio MI, Longo N, Trevino I, Lazaro P, Sanchez-Mateos P. Clinical significance of CXCR3 and CXCR4 expression in primary melanoma. Int J Cancer. 2005;117:861–865. doi: 10.1002/ijc.21269. [DOI] [PubMed] [Google Scholar]
- [43].Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- [44].Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- [45].Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev. 2006;25:611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- [46].Lee SS, Morgenstern L, Phillips EH, Hiatt JR, Margulies DR. Splenectomy for splenic metastases: a changing clinical spectrum. Am Surg. 2000;66:837–840. [PubMed] [Google Scholar]
- [47].Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mochizuki Y, Nakanishi H, Kodera Y, Ito S, Yamamura Y, Kato T, et al. TNF-alpha promotes progression of peritoneal metastasis as demonstrated using a green fluorescence protein (GFP)-tagged human gastric cancer cell line. Clin Exp Metastasis. 2004;21:39–47. doi: 10.1023/b:clin.0000017181.01474.35. [DOI] [PubMed] [Google Scholar]
- [49].Tang L, Jennings TA, Eaton JW. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc Natl Acad Sci U S A. 1998;95:8841–8846. doi: 10.1073/pnas.95.15.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tang L, Lucas AH, Eaton JW. Inflammatory responses to implanted polymeric biomaterials: role of surface-adsorbed immunoglobulin G. J Lab Clin Med. 1993;122:292–300. [PubMed] [Google Scholar]
- [51].Zdolsek J, Eaton JW, Tang L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J Transl Med. 2007;5:31. doi: 10.1186/1479-5876-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tang L, Eaton JW. Inflammatory responses to biomaterials. Am J Clin Pathol. 1995;103:466–471. doi: 10.1093/ajcp/103.4.466. [DOI] [PubMed] [Google Scholar]
- [53].Tang L, Eaton JW. Natural responses to unnatural materials: A molecular mechanism for foreign body reactions. Mol Med. 1999;5:351–358. [PMC free article] [PubMed] [Google Scholar]
- [54].Tang L, Hu W. Molecular determinants of biocompatibility. Expert Rev Med Devices. 2005;2:493–500. doi: 10.1586/17434440.2.4.493. [DOI] [PubMed] [Google Scholar]
- [55].Pathi SP, Lin DD, Dorvee JR, Estroff LA, Fischbach C. Hydroxyapatite nanoparticle- containing scaffolds for the study of breast cancer bone metastasis. Biomaterials. 2011;32:5112–5122. doi: 10.1016/j.biomaterials.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Moreau JE, Anderson K, Mauney JR, Nguyen T, Kaplan DL, Rosenblatt M. Tissue- engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res. 2007;67:10304–10308. doi: 10.1158/0008-5472.CAN-07-2483. [DOI] [PubMed] [Google Scholar]
- [57].Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling acute inflammation with fast releasing dexamethasone-PLGA microsphere/pva hydrogel composites for implantable devices. J Diabetes Sci Technol. 2007;1:8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117:68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- [59].Zolnik BS, Burgess DJ. Evaluation of in vivo-in vitro release of dexamethasone from PLGA microspheres. J Control Release. 2008;127:137–145. doi: 10.1016/j.jconrel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- [60].Pugliese MS, Beatty JD, Tickman RJ, Allison KH, Atwood MK, Szymonifka J, et al. Impact and outcomes of routine microstaging of sentinel lymph nodes in breast cancer: significance of the pN0(i+) and pN1mi categories. Ann Surg Oncol. 2009;16:113–120. doi: 10.1245/s10434-008-0121-x. [DOI] [PubMed] [Google Scholar]
- [61].Tachibana T, Yoshida K. Role of the regional lymph node in cancer metastasis. Cancer Metastasis Rev. 1986;5:55–66. doi: 10.1007/BF00046422. [DOI] [PubMed] [Google Scholar]
- [62].Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- [63].Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- [64].Darling G. The role of lymphadenectomy in esophageal cancer. J Surg Oncol. 2009;99:189–193. doi: 10.1002/jso.21209. [DOI] [PubMed] [Google Scholar]
- [65].Das S, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci. 2008;1131:235–241. doi: 10.1196/annals.1413.021. [DOI] [PubMed] [Google Scholar]
- [66].Uharcek P. Prognostic factors in endometrial carcinoma. J Obstet Gynaecol Res. 2008;34:776–783. doi: 10.1111/j.1447-0756.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- [67].Maekawa S, Iwasaki A, Shirakusa T, Kawakami T, Yanagisawa J, Tanaka T, et al. Association between the expression of chemokine receptors CCR7 and CXCR3, and lymph node metastatic potential in lung adenocarcinoma. Oncol Rep. 2008;19:1461–1468. [PubMed] [Google Scholar]
- [68].Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- [69].Mirmohammadsadegh A, Marini A, Gustrau A, Delia D, Nambiar S, Hassan M, et al. Role of erythropoietin receptor expression in malignant melanoma. J Invest Dermatol. 2010;130:201–210. doi: 10.1038/jid.2009.162. [DOI] [PubMed] [Google Scholar]
- [70].Lee AS, Kim DH, Lee JE, Jung YJ, Kang KP, Lee S, et al. Erythropoietin induces lymph node lymphangiogenesis and lymph node tumor metastasis. Cancer Res. 2011;71:4506–4517. doi: 10.1158/0008-5472.CAN-10-3787. [DOI] [PubMed] [Google Scholar]
- [71].Arcasoy MO, Jiang X, Haroon ZA. Expression of erythropoietin receptor splice variants in human cancer. Biochem Biophys Res Commun. 2003;307:999–1007. doi: 10.1016/s0006-291x(03)01303-2. [DOI] [PubMed] [Google Scholar]
- [72].Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double- blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- [73].Lappin TR, Maxwell AP, Johnston PG. EPO’s alter ego: erythropoietin has multiple actions. Stem Cells. 2002;20:485–492. doi: 10.1634/stemcells.20-6-485. [DOI] [PubMed] [Google Scholar]
- [74].Weiss MJ. New insights into erythropoietin and epoetin alfa: mechanisms of action, target tissues, and clinical applications. Oncologist. 2003;8(Suppl 3):18–29. doi: 10.1634/theoncologist.8-suppl_3-18. [DOI] [PubMed] [Google Scholar]
- [75].Katz O, Gil L, Lifshitz L, Prutchi-Sagiv S, Gassmann M, Mittelman M, et al. Erythropoietin enhances immune responses in mice. Eur J Immunol. 2007;37:1584–1593. doi: 10.1002/eji.200637025. [DOI] [PubMed] [Google Scholar]
- [76].Yuan R, Maeda Y, Li W, Lu W, Cook S, Dowling P. Erythropoietin: a potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE. PLoS One. 2008;3:e1924. doi: 10.1371/journal.pone.0001924. [DOI] [PMC free article] [PubMed] [Google Scholar]