Abstract
Aims
We have previously demonstrated that pretreatment with (+)-morphine given intrathecally attenuates the intrathecal (−)-morphine-produced tail-flick inhibition. The phenomenon has been defined as antianalgesia against (−)-morphine-produced analgesia. Present experiments were then undertaken to determine if the antianalgesic effect induced by (+)-morphine given spinally is mediated by the stimulation of the sigma-1 receptor in the mouse spinal cord.
Main methods
Sigma-1 receptor ligands, N-[2-(3,4-Dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide (BD1047) and (+)-pentazocine were used to determine if (+)-morphine-induced antianalgesia is mediated by the stimulation of sigma-1 receptors in the mouse spinal cord. Tail-flick test was employed to measure the nociceptive response. All compounds were given intrathecally.
Key findings
Pretreatment with BD1047 (1–10 μg) or (+)-pentazocine (0.1–10 μg) dose-dependently reversed the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine (10 pg). BD1047 and (+)-pentazocine injected alone did not affect (−)-morphine-produced tail-flick inhibition.
Significance
The finding indicates that (+)-morphine attenuates the (−)-morphine-produced tail-flick inhibition via the activation of the sigma-1 receptors in the mouse spinal cord. Sigma-1 receptors may play an important role in opioid analgesia in the mouse spinal cord.
Keywords: sigma-1 receptors, opioid, analgesia, spinal cord
Introduction
Unlike levorotatory (−)-morphine, which interacts with μ-opioid receptors to produce potent analgesic and other pharmacologic effects, the dextrorotatory (+)-morphine is inert to produce any analgesic and other μ-opioid receptor-mediated pharmacological effects (Jacquet et al. 1977). However, we have previously demonstrated that (+)-morphine attenuates the tail-flick inhibition produced by (−)-morphine when both are given either systemically or intrathecally in the mouse and intracerebrally in the rat (Wu et al. 2005, 2007; Terashvili et al. 2007). We also found that the antinociception produced by (−)morphine is reversed by the sigma-1 receptor antagonist N-[2-(3,4-Dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide (BD1047). The findings suggest that attenuation of the (−)-morphine-produced antinociception induced by (+)-morphine given systemically or intracerebrally is mediated by the stimulation of sigma-1-receptors (Wu et al. 2007; Terashvili et al. 2007).
Sigma-1 receptors are expressed in the dorsal horn of the spinal cord where opioid receptors are enriched (Alonso et al. 2000; Hayashi and Su 2005). Indeed, sigma receptor has been demonstrated to modulate opioid antinociceptive effects. Pretreatment with (+)-Pentazocine, a highly selective ligand for sigma-1 receptors (Bowen et al. 1990; Quirion et al. 1992), blocks (−)-morphine-produced antinociception, suggesting the involvement of sigma-1 receptors in modulating the (−)-morphine produced antinociception (Wu et al. 2007; Chien and Pasternak 1994; Mei and Pasternak 2002, 2007).
In present study, BD1047 and (+)-pentazocine were then used as pharmacological tools to determine if the attenuation of the (−)-morphine-produced antinociception by (+)morphine is mediated through activation of sigma-1 receptors in the mouse spinal cord.
Materials and methods
Animals
Male CD-1 mice weighing 25–30 g (Charles River Breeding Laboratory, Wilmington, MA) were used. Animals were housed five per cage in a room maintained at 22 ± 0.5 °C with an alternating 12-h light-dark cycle. Food and water were available ad libitum. Each animal was used only once. All experiments were approved by and conformed to the guidelines of the Animal Care Committee of the Medical College of Wisconsin.
Assessment of Antinociception
Nociceptive responses were measured with the tail-flick test (D’Amour and Smith, 1941). Mice were gently held with the tail placed on the apparatus to measure the latency of the tail-flick response (Model TF6, EMDIE Instrument Co., Maidens, VA) elicited by applying radiant heat to the dorsal surface of the tail. The heat stimulus was set to provide a pre-drug tail-flick response time of 3 to 4 s. The cutoff time for the heat stimulus was set at 10 s to avoid tissue damage (Wu et al. 2007).
Experimental Protocols
The following experiments were performed; 1) the effects of sigma receptor antagonist BD1047 on the attenuation of (−)-morphine-produced tail-flick inhibition induced by (+)--morphine; 2) the effects of sigma receptor ligand (+)-pentazocine on the attenuation of (−)-morphine-produced tail-flick inhibition induced by (+)-morphine.
Drugs and drug administration
(−)-Morphine sulfate, (+)-pentazocine and (+)-morphine were obtained from the National Institute on Drug Abuse (Baltimore, MD, USA). N-[2-(3,4-Dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide (BD1047) was purchased from Tocris (Ellisville, MO). (−)-Morphine, BD1047 and (+)-pentazocine were dissolved in 0.9% saline for intrathecal injection. The (+)-morphine was dissolved in 10 N hydrochloric acid and then titrated with 1 N sodium hydroxide to pH 6–7, which was then diluted to the intended dose in 0.9% saline. Intrathecal injection was performed according to the procedure described in a previous publication (Wu et al. 2005), using a 25-μl Hamilton syringe with a 30-gauge needle. The volume of injected drug solution was 5 μl.
Statistical analysis
The tail-flick latencies in seconds are presented as mean±SEM. One-way analysis of variance (ANOVA) followed by Dunnett’s post-test or Two-way ANOVA followed by Bonferroni post-test was used to test the differences between groups. Each group has 8 to 9 mice. GraphPad Prism software was used to perform the statistics (version 4.1; GraphPad Software, Inc., San Diego, CA).
Results
Effect of BD1047 or (+)-pentazocine pretreatment on the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine
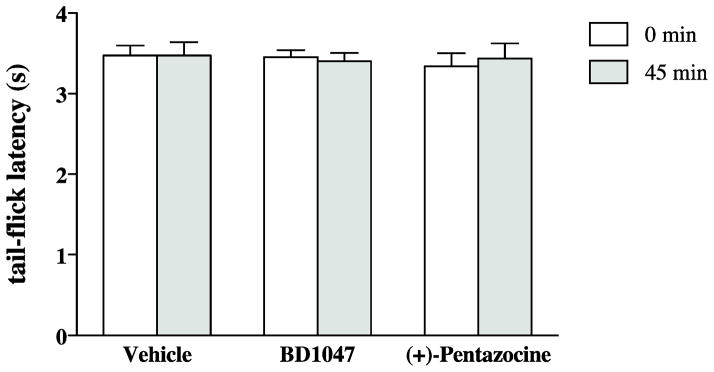
The sigma-1 receptor ligands BD1047 and (+)-pentazocine were used to determine if the sigma-1 receptors are involved in mediating the attenuation of the (−)-morphine-produced tail-flick inhibition by (+)-morphine in the mouse spinal cord. Intrathecal injection of BD1047 or (+)-pentazocine at a dose of 10 μg each did not affect the tail-flick latency observed at 45 min after intrathecal injection compared with the latency before the injection (Fig 1).
Fig. 1.
BD1047 or (+)-pentazocine given intrathecally did not affect tail-flick latency. Groups of mice were injected intrathecally with BD1047 (10 μg), (+)-pentazocine (10 μg) or vehicle and the tail-flick responses were measured before and 45 min after injection. Each column represents the mean and the vertical bar represents the SEM with 8 to 9 mice in each group. Two-way ANOVA followed by Bonferroni post-test was used to test the difference between groups;
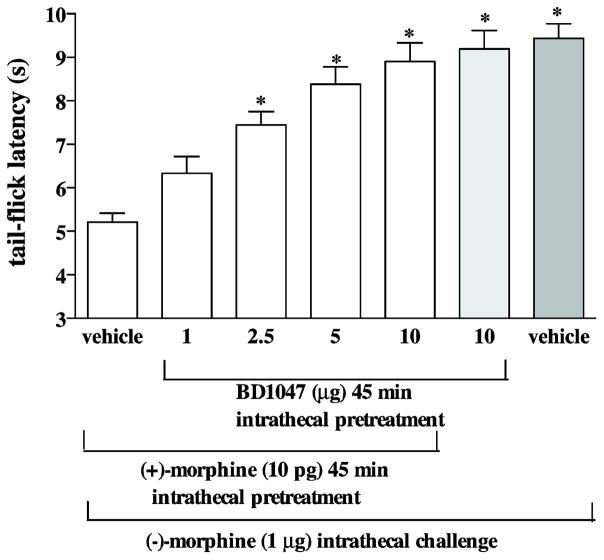
Different groups of mice were pretreated intrathecally with (+)-morphine (10 pg) combined with various doses of BD1047 (1–10 μg) for 45 min before the challenge dose of intrathecal (−)-morphine (1 μg), and the tail-flick response was measured 15 min later. Intrathecal pretreatment with (+)-morphine (10 pg) attenuated the (−)-morphine-produced tail-flick inhibition. Attenuation of the (−)-morphine-produced tail-flick inhibition by (+)morphine was reversed by BD1047 (Fig. 2) in a dose-dependent manner.
Fig. 2.
Pretreatment with BD1047 reverses the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine. Groups of mice were co-administered with different doses (1–10 μg) of BD1047 and (+)-morphine (10 pg) 45 min before (−)morphine (1 μg) given intrathecally. The tail-flick responses were measured 15 min after last injection. Each column represents the mean and the vertical bar represents the SEM with 8 to 9 mice in each group. One-way ANOVA followed by Dunnett’s post-test was used to test the difference between groups; * P < 0.001 compared with the vehicle injected group (the first column from the left).
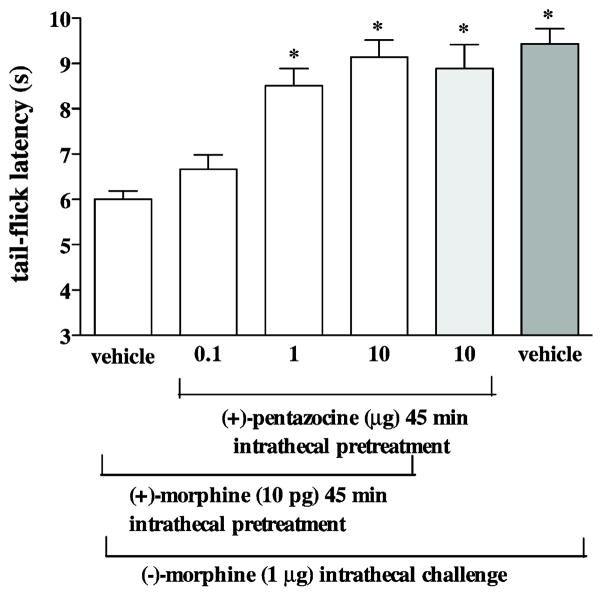
In the other experiment, different groups of mice were pretreated intrathecally with (+)morphine (10 pg) combined with various doses of (+)-pentazocine (0.1–10 μg) for 45 min before the challenge dose of intrathecal (−)-morphine (1 μg), and the tail-flick response was measured 15 min later. Intrathecal pretreatment with (+)-morphine (10 pg) attenuated the (−)-morphine-produced tail-flick inhibition. Attenuation of the (−)-morphine-produced tail-flick inhibition by (+)-morphine was dose-dependently reversed by (+)-pentazocine pretreatment (Fig 3), similar to the effect of BD1047. Intrathecal pretreatment with BD1047 (10 μg) or (+)-pentazocine (10 μg) given alone 45 min prior to intrathecal injection of (−)-morphine (1 μg) did not affect the (−)-morphine-produced tail-flick inhibition (Fig. 2 and 3).
Fig. 3.
Pretreatment with (+)-pentazocine reverses the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine. Groups of mice were co-administered with different doses (0.1–10 μg) of (+)-pentazocine and (+)-morphine (10 pg) 45 min before (−)-morphine (1 μg) given intrathecally. The tail-flick responses were measured 15 min after last injection. Each column represents the mean and the vertical bar represents the SEM with 8 to 9 mice in each group. One-way ANOVA followed by Dunnett’s post-test was used to test the difference between groups; * P < 0.001 compared with the vehicle injected group (the first column from the left).
Discussions
The present study demonstrates that blockade of the sigma-1 receptor in the spinal cord by pretreatment with the sigma-1 receptor antagonist BD1047 reverses the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine given intrathecally. The result supports the contention that (+)-morphine acts as a sigma-receptor agonist and attenuates physiologically the antinociception produced by (−)morphine in the spinal cord. This result is in line with our previous finding that BD1047 antagonized (+)-morphine-induced antianalgesia against (−)-morphine-produced antinociception (Wu et al. 2007; Terashvili et al. 2007). Our findings clearly indicate that the sigma-1 receptor may play an important role in modulating the analgesia produced by (−)-morphine both at spinal and supraspinal sites.
We found in the present study that, similar to BD1047, (+)-pentazocine pretreated intrathecally also reverses the attenuation of the (−)-morphine produced tail-flick inhibition induced by (+)-morphine. This finding indicates that (+)-pentazocine elicits antagonistic effect on the sigma-1 receptors when given spinally to block the antianalgesia induced by (+)-morphine in the spinal cord. These observations provide additional evidence that (+)-morphine attenuates the (−)-morphine-produced antinociception via activation of sigma-1 receptors in the mouse spinal cord. It has been demonstrated previously that (+)-pentazocine attenuates the antinociception produced by (−)-morphine when both are given systemically (Wu et al. 2007) or supraspinally (Mei and Pasternak 2002, 2007), which is prevented by systemic administration of sigma-1 receptor blocker BD1047 or by supraspinal administration of haloperidol. However, (+)-pentazocine given intrathecally does not attenuate the intrathecal (−)-morphine-produced tail-flick inhibition (Mei and Pasternak 2002). Mei and Pasternak (2002) conclude that supraspinal site is the active site of (+)-pentazocine-induced anti-opioid effect based on the findings that (+)-pentazocine is without effect against (−)-morphine when both are given spinally in mice. Thus, (+)-pentazocine may acts as a partial agonist with higher intrinsic activity at the supraspinal sites and very low intrinsic activity at the spinal sites for sigma-1 receptors.
The sigma-1 receptors, an inter-organelle signaling modulator, have been shown to modulate a number of physiological and pharmacological responses (review see Su et al. 2010). Although there is no evidence that sigma-1 receptor ligands bind with opioid receptors (Chien and Pasternak, 1995; Kim et al. 2010), it has been shown that there is an antagonistic physiological interaction between the sigma-1 receptors and μ-opioid receptors (Kim et al. 2010). Since (+)-morphine does not have any affinity for μ-opioid receptors (Jacquet et al. 1977), it is highly unlikely that (+)-morphine acts directly on μ-opioid receptors to attenuate the (−)-morphine-produced antinociception. We propose that (+)-morphine activates the sigma-1 receptors to attenuate physiologically, if not pharmacologically, the antinociception produced by (−)-morphine, which stimulates the -opioid receptors in the dorsal horn of the spinal cord.
Conclusions
It is concluded that pretreatment with sigma-1 ligands, BD1047 or (+)-pentazocine, dose-dependently reversed the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine. The finding indicates that (+)-morphine attenuates the (−)morphine-produced tail-flick inhibition via the activation of the sigma-1 receptors in the mouse spinal cord. Sigma-1 receptors may play an important role in modulating the opioid analgesia in the mouse spinal cord.
Acknowledgments
This work was supported by grant K01DA024751 (PI:HEW) and DA12588 (PI:LFT) from the National Institute of Health, National Institute on Drug Abuse.
Footnotes
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso G, Phan VL, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma1 receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Bowen WD, de Costa BR, Hellewell SB, Thurkauf A, Walker JM, Rice KC. Characterization of [3H](+)-pentazocine, a highly selective sigma ligand. Prog Clin Biol Res. 1990;328:117–120. [PubMed] [Google Scholar]
- Chien CC, Pasternak GW. Selective antagonism of opioid analgesia by a sigma system. J Pharmacol Exp Ther. 1994;271:1583–1884. [PubMed] [Google Scholar]
- Chien CC, Pasternak GW. (−)-Pentazocine analgesia in mice: interaction with a σ receptor. Eur J Pharmacol. 1995;294:303–308. doi: 10.1016/0014-2999(95)00552-8. [DOI] [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Hayashi T, Su TP. The potential role of sigma-1 receptors in lipid transport and lipid raft reconstitution in the brain: implication for drug abuse. Life Sci. 2005;77:1612–1624. doi: 10.1016/j.lfs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Klee WA, Rice KC, Iijima I, Minamikawa J. Stereospecific and nonstereospecific effects of (+)- and (−)-morphine: evidence for a new class of receptors. Science. 1977;198:842–845. doi: 10.1126/science.199942. [DOI] [PubMed] [Google Scholar]
- Kim FJ, Kovalyshyn I, Burgman M, Neilan C, Chien C-C, Pasternak GW. σ1 Receptor modulation of G-protein-coupled receptor signaling: potentiation of opioid transduction independent from receptor binding. Mol Phamracol. 2010;77:695–703. doi: 10.1124/mol.109.057083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J, Pasternak GW. σ1 Receptor modulation of opioid analgesia in the mouse. J Pharamcol Exp Ther. 2002;300:1070–1074. doi: 10.1124/jpet.300.3.1070. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak GW. Modulation of brainstem opiate analgesia in the rat by sigma1 receptors: A microinjection study. J Pharmacol Exp Ther. 2007;322:1278–1285. doi: 10.1124/jpet.107.121137. [DOI] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Su T-P, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacolo Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashvili M, Wu H-E, Moore RM, Harder DR, Tseng LF. (+)-Morphine and (−)morphine stereoselectively attenuate the (−)-morphine-produced tail-flick inhibition via the naloxone-sensitive sigma receptor in the ventral periaqueductal gray of the rat. Eur J Pharmacol. 2007;571:1–7. doi: 10.1016/j.ejphar.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-E, Thompson J, Sun HS, Terashvili M, Tseng LF. Antianalgesia: stereoselective action of dextro-morphine over levo-morphine on glia in the mouse spinal cord. J Pharmacol Exp Ther. 2005;314:1101–1108. doi: 10.1124/jpet.105.087130. [DOI] [PubMed] [Google Scholar]
- Wu H-E, Hong JS, Tseng LF. Stereoselective action of (+)-morphine over (−)-morphine in attenuating the (−)-morphine-produced antinociception via the naloxone-sensitive sigma receptor in the mouse. Eur J Pharamcol. 2007;571:145–151. doi: 10.1016/j.ejphar.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]