Mitral valve prolapse (MVP) often leads to important mitral regurgitation (MR), particularly after chordal rupture, but its mechanisms remain elusive because of the lack of an appropriate model. This study aimed to create such a model by implanting a pericardial patch within the anterior mitral leaflet (AML) in large animals (sheep, pig) allowing acute primary biomechanical consequences of MVP on chordae tendineae force (CTF) to be evaluated.
METHODS
Surgical procedure
Ex vivo studies in 3 pig and 6 sheep hearts showed that the use of a large autologous oval pericardial patch (area at least equal to and not more than twice that of the AML) sutured along the base of the AML (after an incision along the annulus) was the most appropriate procedure to create MVP without MR, as demonstrated by echography performed during heart immersion in a saline solution while pressurized water was injected into the left ventricle (LV) through the aortic root (fig. 1).
Figure 1. Techniques used in ex vivo (A–E) and in vivo (F, G) studies (sheep).
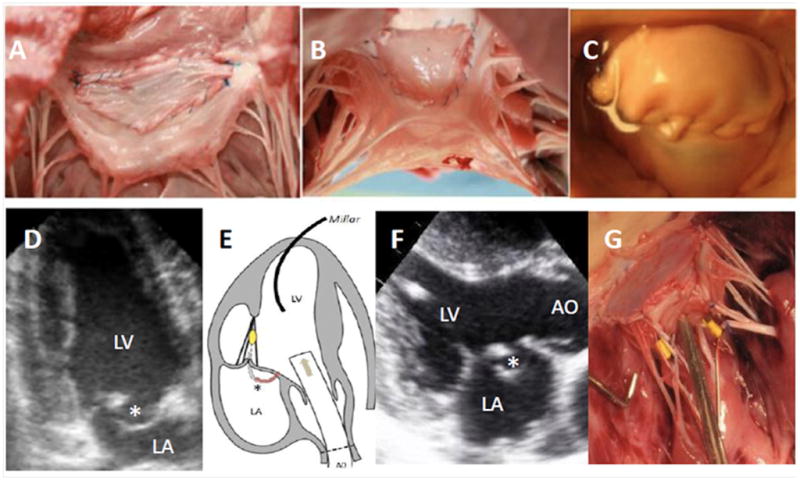
After patch insertion, surgical views from the left atrium (A) or ventricle (B) and from the atrium after injection of pressurized water with prolapse (C). Echographic apical two-chamber views of the closed mitral valve before and after insertion (D) with AML billowing (*). Measurements of LV pressures and chordal tensions (sensor in yellow), while changes in pressure originate from aortic (AO) injections (arrow) (E). Echographic parasternal long-axis view in systole (F) showing AML billowing (*) after insertion. The sensors (in yellow) are sutured onto the strut chordae of the AML (G).
Echocardiographic data
In vivo, 6 adult sheep (45 kg) underwent epicardial echocardiography (Sequoia 516®, Acuson®) before and after patch insertion to generate the following measurements: LV ejection fraction (EF, biplane Simpson’s rule), maximal length of the AML (apical 3-chamber view), maximal systolic annulus diameter, maximal prolapse extent into the left atrium beyond a line connecting the annular hinge points. Color Doppler MR >1+ was considered significant.
CTF measurements
CTFs were measured before and after patch insertion ex vivo in 11 excised pig hearts at 3 specified levels of LV pressure (50, 75 and 100 mmHg) and in vivo in 4 Danish landrace/Yorkshire pigs (80 kg). Dedicated miniature c-shaped strain gauge force transducers (1) were sutured onto the middle part of each of the two main strut (secondary) chordae of the AML. LV pressures were recorded using Millar® catheters. Transducers were connected to a module rack (NI cDAQ 9172, National Instruments) with two input modules (NI 9215, NI 9237). Recordings were performed with virtual instrumentation utilizing an in-house build data-acquisition program (LabVIEW 8.6; National Instruments).
Statistics
Results are expressed as mean±SD. We compared (SAS 9.2) tension measurements using a non-parametric Friedman’s test, variations of mean tensions using a Wilcoxon’s test, changes in CT forces and LV pressures by a non parametric Wilcoxon’s test and ultrasonic measurements by a paired Wilcoxon’s test.
RESULTS
MVP pattern
In vivo, patch insertion resulted in a marked bulge of the AML reaching 4.9±2.4 mm with a parallel increase of AML length (16.6±1.5 to 27.3±4.6 mm, p=.03) without significant MR (fig. 1); LV volumes, EFs and annulus diameters remained unchanged.
Ex vivo CTFs
Among the 48 measurements performed, 2 failed due to sensor attachment problems. CTFs increased linearly with increasing LV pressure before and after insertion (p<.0001) (Table I) with excellent mean correlation coefficients (.98±.02 and .97±.03 for the posteromedial and anterolateral chordae, respectively). After MVP, CTFs decreased significantly at all pressure levels to a similar extent for both chordae (Table I); total CTFs (sum of anterior and posterior CTFs) decreased to similar extents (fig. 4).
Table I. Ex vivo study, chordal tension measurements.
The decrease in tensions (in Newton, N) before and after MVP creation in 11 pig hearts for 3 pre-specified LV pressure levels (50, 75, 100 mmHg) is demonstrated for the anterior (A) and posterior (P) AML secondary chordae as well as for the total chordal tension (A+P).
| Chordae | Before (N) | After (N) | Decrease (%) | P |
|---|---|---|---|---|
| 50 mmHg | ||||
| Anterior | .35±.25 | .21±.13 | 34.7±27.9 | .005 |
| Posterior | .31±.21 | .20±.16 | 31.5±26.8 | .014 |
| Total | .63±.36 | .41±.27 | 39.0±15.1 | <.001 |
| 75 mmHg | ||||
| Anterior | .61±.36 | .36±.22 | 36.4±20.5 | <.001 |
| Posterior | .50±.33 | .34±.26 | 31.2±28.4 | .01 |
| Total | 1.1±.58 | .69±.44 | 36.5±16.0 | <.001 |
| 100 mmHg | ||||
| Anterior | .83±.41 | .54±.31 | 36.1±15.4 | <.001 |
| Posterior | .69±.46 | .49±.37 | 29.3±23.9 | .005 |
| Total | 1.52±.74 | 1.02±.62 | 34±13.0 | <.001 |
In vivo CTFs
Among the 8 implanted transducers, 5 transmitted data both before and after MVP, yielding a total of 24 measurements for the different LV pressures. Individual CTFs constantly decreased after MVP [.66±.24 (.28–.93) N to .41±.16 (.15–.60) N, mean 37.9% (p<.0001)]. When indexed to concomitant LV pressures, a dramatic 41% decrease was still noted (.68 to .40 N, p<.0001).
DISCUSSION
A large animal model of MVP without MR was created by implanting a large oval-shaped autologous pericardial patch within the AML. Absence of MR is mandatory to preclude the influence of any regurgitation per se on chordal tension (2). The procedure dramatically decreased the tensions exerted on both strut chordae; it does not however necessarily reflect decreased leaflet wall stress, but might actually reduce stresses on the belly of the patch and conversely increase stresses at the hinge points (the chordae/suture line), as previously described in ischemic MR experimentally or after mitral ring annuloplasty (3).
This preliminary study represents a first step towards the creation of a large animal model of MVP and also be viewed as a description of force redistribution following surgical techniques that utilize AML patch repair for MR (4). The surgeons should be aware of redistribution of leaflet stresses with increased load on the patch area that has been shown to promote fibrosis and calcification.
Finally, as a redistribution of chordal tension may lead to spatially-limited biological changes in the mitral valve apparatus (5), the long-term influence of such a redistribution of CTF on valve biology necessitates further studies.
Acknowledgments
This research project was funded by grant 07CVD04 (M Granier, Morten O. Jensen, Jesper L. Honge, Sten L. Nielsen, Alain Carpentier, RA Levine, AA Hagège) from the Leducq Foundation, Paris, France, for the Leducq MITRAL Transatlantic Network. M Granier was also funded by a grant from the French Society of Cardiology and the French Federation of Cardiology. JL Honge and MØ Jensen were funded by the Danish Heart Foundation Grant #07-4-B248-A1380-22362, the Central Denmark Region Health Science Research Fund, the A.P. Møller Foundation for the Advancement of Medical Science, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsens Fond, Hørslev Fonden. Dr. Robert A. Levine’s participation was supported by NHLBI grant # K24 HL67434. We also acknowledge the expert technical contribution of Marie-Cécile Perier, statistician, INSERM U 970, and Julie Piquet, laboratory technician, Paris Cardiovascular Research Center, Paris, France.
Footnotes
Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nielsen SL, Soerensen DD, Libergren P, Yoganathan AP, Nygaard H. Miniature c-shaped transducers for chordae tendineae force measurements. Ann Biomed Eng. 2004;32:1050–1057. doi: 10.1114/b:abme.0000036641.69903.62. [DOI] [PubMed] [Google Scholar]
- 2.Stephens EH, Nguyen TC, Itoh A, Ingels NB, Jr, Miller DC, Grande-Allen KJ. The effects of mitral regurgitation alone are sufficient for leaflet remodeling. Circulation. 2008;118(Suppl 14):S243–9. doi: 10.1161/CIRCULATIONAHA.107.757526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen SL, Lomholt M, Johansen P, Hansen SB, Andersen NT, Hasenkam JM. Mitral ring annuloplasty relieves tension of the secondary but not primary chordae tendineae in the anterior mitral leaflet. J Thorac Cardiovasc Surg. 2011;141:732–7. doi: 10.1016/j.jtcvs.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Romano MA, Patel HJ, Pagani FD, Prager RL, Deeb GM, Bolling SF. Anterior leaflet repair with patch augmentation for mitral regurgitation. Ann Thorac Surg. 2005;79:1500–4. doi: 10.1016/j.athoracsur.2004.08.086. [DOI] [PubMed] [Google Scholar]
- 5.Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM, Vesely I. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. J Am Coll Cardiol. 2003;42:271–7. doi: 10.1016/s0735-1097(03)00626-0. [DOI] [PubMed] [Google Scholar]


