Abstract
Severed tendons can undergo regenerative healing, intrinsic tendon repair. Fibrillogenesis of chick tendon involves “collagen fibril segments” (CFS), which are the building blocks of collagen fibers that make up tendon fascicles. The CFS are 10.5 micron in length, composed of tropocollagen monomers arranged in parallel arrays. Rather than incorporating single tropocollagen molecules into growing collagen fibers, incorporating large CFS units is the mechanism for generating collagen fibers. Is intrinsic tendon repair through the reestablishment of tendon embryogenesis? Gentamicin treated 10-day-old chick embryo tendons released CFS were fluorescently tagged with Rhodamine (Rh). Organ cultured severed 14-day-old embryo tendon explants received Rh tagged CFS. At day 4 auto fluorescent tagged CFS were identified at the severed tendon ends by fluorescent microscopy. Accumulation of fluorescent tagged CFS was exclusively localized to the severed ends of tendon explants. Parallels between collagen fiber growth during embryonic fibrillogenesis and tendon repair reveal CFS incorporation is responsible for collagen fibers growth. CFS incorporation into frayed collagen fibers from severed tendons is the proposed mechanism for intrinsic tendon repair, which is an example of regenerative repair.
Keywords: Collagen fibril segments, intrinsic tendon repair, organ culture, tendon healing
Introduction
Tendons are rigid cables that connect bone to muscle. The parallel arrangement of collagen fibers within fascicles is accountable for the rigidity of these cables. The major collagen type within tendon fibers is type I collagen. (Birk et al.,1989a). Robert Trelstad and coworkers studies of chick embryo tendon development showed banded collagen structures within the chick embryonic tendon fibroblast cytoplasmic-like vacuoles (Trelstad & Hayashi, 1979). These banded collagen fibril structures are not ingested fibrils destined for breakdown, rather they are newly synthesized collagen fibrils that will be utilized for generating new collagen fibers. The banded collagen fibrils are not in typical cytoplasmic vacuole, such as Golgi vesicles or lysosomes, but are specialized cell plasma membrane clefts that are specialized extracellular compartments. Newly synthesized procollagen released from the rough endoplasmic reticulum (RER) is cleaved into tropocollagen within these clefts (Birk & Trelstad; 1986). Tropocollagen is not directly incorporated into collagen fibers, but is initially incorporated into Collagen Fibril Segments (CFS) that are incorporated into expanding collagen fibers (Birk et al., 1989a; Franchi et al., 2007).
Damaged flexor tendon repair can terminate in scar, like other soft tissues, referred to as extrinsic tendon repair. Another option for tendon repair is intrinsic tendon repair, where regenerative healing occurs by a process that simulates embryonic fibrillogenesis. The termination of tendon repair by intrinsic tendon repair is a regenerated tendon. Extrinsic tendon repair is typical wound healing, characterized by the initial intrusion of inflammatory cells followed by fibroblasts derived from neighboring tissues, terminating in the deposition of a new disorganized connective tissue matrix, a scar. Such a repaired tendon may have deficiencies in strength as well as the ability to glide within its sheath. A major difference separating extrinsic and intrinsic tendon repair is the organization of the newly deposited connective tissue within tendon fascicles. In intrinsic tendon repair the fascicle collagen fibers run in parallel arrays, indistinguishable from the orientation of collagen fibers in uninjured tendon. The outcome of extrinsic tendon repair is disrupted fascicles and newly deposited connective tissue organized in random arrays showing variations in the collagen fiber diameters (Ehrlich et al., 2005).
Success in studying repair in isolated organs such as tendon is advanced by utilizing embryonic derived tissues. Because these tissues flourish in vivo in an environment deficient in oxygen, they can retain their viability in stationary organ culture. Embryonic chick explants remain viable in stationary organ culture for long periods of time in ambient air and 5% CO2 (Fell, 1956). Unlike metabolically active fibroblasts typical of the proliferative phase of wound repair, intrinsic tendon repair is completed in the absence of angiogenesis and developing a new blood supply. The restoration of normal function to a severed tendon by intrinsic tendon repair requires endogenous tendon fibroblasts bridging the gap between the severed tendon ends with the deposition of new connective tissue matrix. It is proposed that released CFS from severed collagen fibers generate a pool of CFS that supply a portion of the building blocks utilized at the healing site (Ingraham et al., 2003). Here the study of intrinsic tendon repair employs the introduction of fluorescent tagged CFS to 14 day old severed chick tendon explants maintained in organ culture. The pattern of fluorescence that appears within severed tendon explants is evaluated 4 days later by fluorescent microscopy.
Method
Isolated tendons, harvested from fertilized chicken eggs, were obtained from the GemWillow Farm (Grantville, PA). Eggs were incubated in a poultry incubator. Tendons from 10 day old chick embryos were isolated by pulling on the chick’s toes. The freed tendons were suspended in 5 ml of phosphate buffered saline (PBS) with gentamicin at 1 mg/mL (Sigma Chemical Co., St. Louis, MO) within a sealed 12 mL centrifuge tube for 90 days at 4° C with agitation. The tendon-antibiotic suspension was vortexed, then centrifuged at 10,000 × g for 2 min and the supernatant saved. The supernatant fraction contained the CCFS and the pellet fraction contained insoluble tissues that were discarded.
Transmission electron microscopy (TEM) confirmed the identity of CFS. Briefly a drop of supernatant was placed on a formvar coated copper grid for 10 min, followed by staining with 2% phosphotungstic acid and then viewed with a Philips 400 Electron Microscope. The CFS from the supernatant were fluorescently tagged with Lissamine rhodamine (Rh) B-200 on celite (USB Corp., Cleveland, OH). To obtain a minimal number of Rh covalently bound to the CFS so that steric hindrance was minimized, the following procedure was followed. The CFS suspension was placed on ice and the pH adjusted to 7.0. About 10 mg of Lissamine Rh on celite was added, the tube briefly vortexed then immediately centrifuged at 5000 × g for 1 min, the supernatant saved and the celite rich pellet discarded. The minimally tagged CFS are referred to as Rh-FS. A high density of Rh bound onto CFS would be expected to interfere with the association of Rh-FS with cells as well as with growing collagen fibers at tendon wound edges.
Chick embryos at 14 days were removed from their shells and their leg tendons harvested by pulling on their toes with a forceps. Isolated tendons were placed in Dulbecco’s modification of Eagles medium (DMEM) with 10% new born bovine serum and 5 µg gentamicin/mL, referred to as complete DMEM. Tendons were cut into 5 mm explants. Four tendon explants were placed on a Gelman Sciences filter membrane (Pall Corp. Port Washington, NY), which was centered on a stainless steel mesh triangle suspended over the central well of a 60×15mm Falcon #3037 Organ Culture Dish (BD Biosciences, Lincoln Park, NJ). The central well was filled with 1.0 ml complete DMEM, and the surrounding outer well received 2.5 ml of sterile water. Surface tension kept the tendon explants covered with thin film of culture medium, allowing a gas exchange between the ambient air and tendon explants. Each tendon explant was severed completely with a scalpel and the cut ends re-approximated by carefully bringing them together. The adherence of the tendon explant to the underlying membrane surface was sufficient for maintaining the 2 severed explants lengths in a fixed position. The fixation of the tendon lengths, which reestablished the continuity of the severed tendon, mimicked the stabilization of a suture repaired severed tendon. Rh-FS were introduced to wounded tendons by directly pipetting a 1µL suspension of Rh-FS onto tendon explants. Tendons were maintained in an incubator set at 37°C with 5% CO2 in a water saturated atmosphere.
Wounded 14 day chick embryo tendon explants maintained in organ culture with Rh-FS for 4 days were prepared for fluorescence microscopy. At 4 days tendon explants were carefully harvested by leaving the explant attached to the underlying membrane so as not to disrupt the repair region between explants. The healing tendon explants with associated membrane were fixed with 4% paraformaldehyde for 5 min and then rinsed with PBS. A pair of tendon explants was incubated with DAPI (Invitrogen, Carlsbad, CA) for 10 min to fluorescently stain nuclei blue. Unstained and DAPI blue stained tendon explants attached to membrane were transferred to glass slides, a drop of GelMount (Biomedia, Foster City, CA) places on top, followed with a coverslip. Slides were viewed with a Zeiss Inverted Fluorescent microscope equipped with epifluorescence and images recorded with a digital camera.
Results
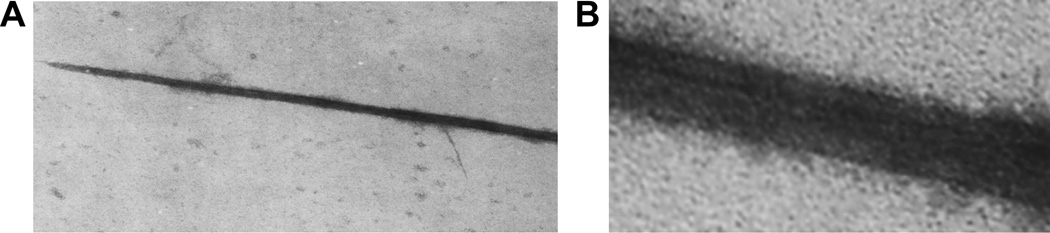
CFS were released by incubating isolated 10-day chick embryo tendons with a high concentration of gentamicin in the cold for 3 months. A centrifugal force of 10,000×g for 2 min was not sufficient to pellet the cfs, but sufficient to remove tendons and other debris. As shown in Fig. 1, a CFS identified by TEM imaging, showed a long CFS had a tapered end, which is the expected morphology of a CFS (Kadler et al., 1996). The insert panel in Fig. 2 taken at higher magnification showed the typical collagen like banding pattern along the length of the isolated CFS.
Figure 1.
A TEM of a fibril segment isolated from the supernatant of a long term incubation of a 10 day chick embryo tendon with 1 mg/mL gentamicin antibiotic. Released fibril segments were isolated by centrifugation and an aliquot subjected to TEM. The insert shows a higher power image with a banding pattern associated with a fibril segment.
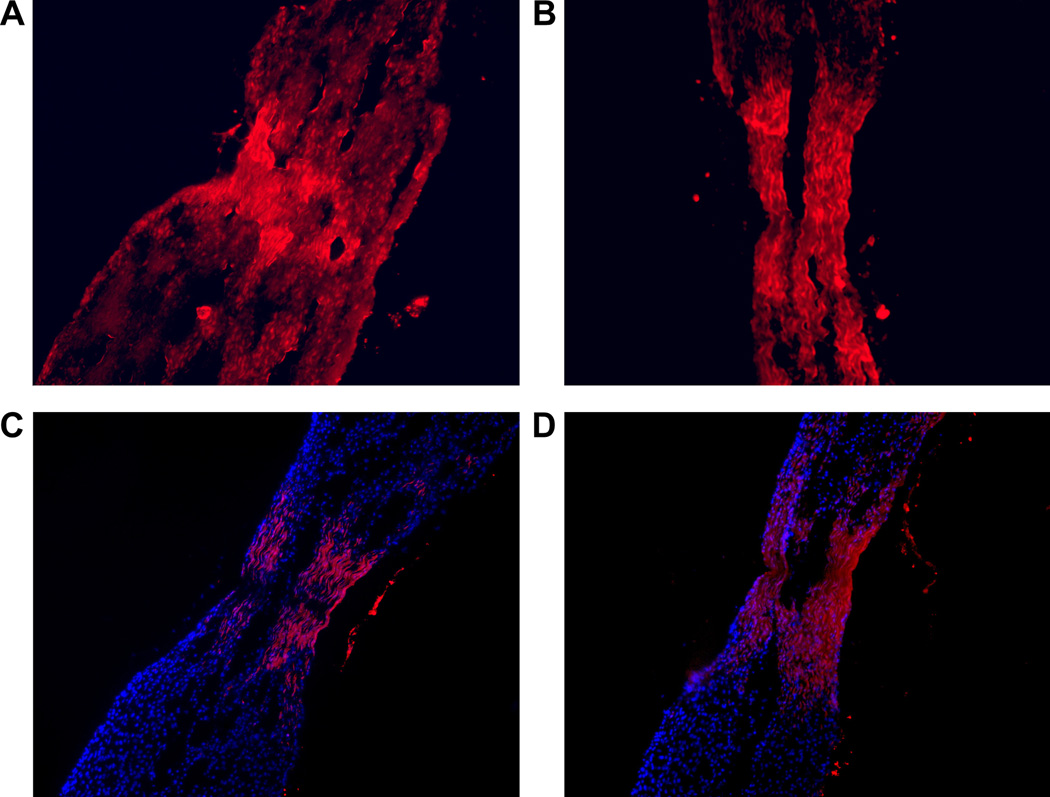
Figure 2.
Auto fluorescence micrographs from a 14 day severed tendon explant that was incubated with red fluorescent fibril segments for 4 days. Panels A and B show the accumulation of auto-fluorescence within the wound gap between organ cultured severed tendon explants. Panel C and D show DAPI blue stained fluorescence tendon fibroblast nuclei along with red auto-fluorescent fibril segments, which have amassed at the severed tendon wound gap. Note in panel D the absence of stained nuclei at the center of the wound gap, suggesting the accumulation of fibril segments on growing collagen fibers can be independent of direct association with tendon fibroblasts.
A 1µL Rh-FS suspension was added to organ cultured severed tendon explants and viewed at 4 days with a fluorescent microscope. The accumulation of Rh-FS with the tendon explants occurred in a specific manner. Minimal red fluorescence was localized along the uninjured intact length of tendon explants. Red fluorescence appeared at the distal cut ends of the explants (not shown) as well as within the gap between wound edges (Fig. 2). The red auto-fluorescence within the gap between the approximated wound edges of tendon explants presented in figure 2 demonstrated the specificity of that fluorescence accumulation. In Fig. 2, panels A and B, the typical pattern of red auto-fluorescence that filled the gap between the severed edges, showed a collagen fiber pattern that had organization. The accumulation of Rh-FS was limited to the gap and cut ends of the tendon explants. In addition to red auto-fluorescent fibers, blue fluorescent stained nuclei from tendon fibroblasts are presented in Fig. 2, panels C and D. DAPI stained tendon fibroblast nuclei identified the localization of tendon fibroblasts in the repair region of severed tendon explants. The blue fluorescent tendon fibroblasts were associated with red fluorescent Rh-FS fibers located at the wound edges. At the center of the gap between the severed tendon lengths, where there was the absence of red auto-fluorescent Rh-FS collagen fibers that was the deficiency of tendon fibroblasts. The absence of blue fluorescent nuclei in the central gap region confirmed the absence of tendon fibroblasts.
Discussion
When viewed at 4 days, introducing fluorescent tagged isolated collagen CFS to wounded tendon explants in organ culture produces a localized intense red fluorescence in the wound gap. The concept is the accumulation of fluorescent CFS at the wound gap results from their translocation by tendon fibroblasts to their eventual entrapment within the gap between a severed tendon explant. CFS, the building unit of tendon collagen fibers, is initially released from the edges at severed tendon site (Ehrlich et al., 2005), eventually are reincorporated into the healing tendon (Ingraham et al., 2003) The tendon explant scheme utilized in this study facilitates the investigation of the reutilization of preformed CFS in severed tendon repair. The regeneration of a severed tendon by intrinsic tendon repair requires a pool of free CFS from the release of damaged tendons as well as from the synthesis of new CFS by tendon fibroblasts near wound edges. It is proposed in vivo, initially exogenous CFS are released from the frayed ends of damaged tendons. These released CFS reduce the diameter of collagen fibers at the wound site (Ehrlich et al., 2005). The reduction in the collagen fiber diameters at the injury site is assumed responsible for the translucent appearance at the site of a damaged tendon undergoing wound healing.
The mechanism, by which the fibroblast transports CFS to the injury site and their trapping at the injury site, is unclear. It is established that collagen CFS are synthesized within specialized extracellular pockets, referred to as crypts, located on the surface of embryonic chick tendon fibroblast (Birk et al., 1989b). The arranging and packing of collagen monomers into very large highly organized CFS occurs within these crypts. These CFS are released and incorporated into growing tendon collagen fibers. In our study localization of exogenous added Rh-FS was monitored at day 4, at which time added fluorescent CFS were confined to the gap between severed tendon explants, as well as at the distal cut ends of the explants. The accumulation of fluorescent CFS at the gap involves the translocation of Rh-FS and their entrapment at the edges within the wound gap. The definition of translocation is the actual movement of fluorescent tagged CFS along the surface of tendon explants. An intact CFS, 10.5 µm in length (Kadler et al., 1996), would suggest that its translocation is along the surface of the tendon explant that engages the participation of the tendon fibroblast’s plasma membrane surface. By fluorescence microscopy no intracellular accumulation of fluorescence by introducing Rh-FS to wounded tendon explants is seen. It appears no intracellular uptake of CFS occurs during the translocation of CFS. The translocation of CFS is assumed to be an extracellular process.
It is hard to comprehend how the process of CFS translocation is unidirectional, where Rh-FS only move towards wound edges. The expectation is movement of CFS on the tendon surface is through random motion. The capture of CFS at wound edges reduces their concentration on the explant surface adjacent to wound edges. The removal of CFS at wound edges causes a local deficit of CFS, which will be repopulated with new CFS by their random movement on the tendon surface. The continued capture of CFS at wound edges that reduces their density adjacent to wound edges, followed by a reintroduction of new CFS to that area eventually will purge CFS from the surface of a wounded tendon. The mechanism for the fixation of Rh-FS at the wound edges is not obvious. The low density and absence of cells in the center of the wound gap supports the notion that the accumulation of auto-fluorescent fibers is associated with growing collagen fibers independent of their direct association with cells. By self-assembly CFS become incorporated on the surface of a growing collagen fiber, where the trapping phenomenon of Rh-FS is through their integration with an established growing collagen fiber.
In contrast to the typical wound healing process, where the restoration and enhancement of the vascular blood supply by angiogenesis is critical; with intrinsic tendon repair there is no introduction of a new vascular supply. Neovascularization, a component of extrinsic tendon repair, is not a component of intrinsic tendon repair. The absence of neovascularization in our organ culture tendon explant model is consistent with healing process that transpires with in vivo intrinsic tendon repair. A better understanding of the mechanisms for CFS translocation and accumulation at tendon wound sites is the focus of future studies.
Highlights.
The study investigates intrinsic tendon repair in organ culture.
Isolated fibril segments (FS), tendon collagen fiber building blocks were fluorescently tagged.
FS introduced to severed tendons exclusively localized to the frayed ends of the severed tendons.
Large FS units instead of incorporating tropocollagen units is the basis of intrinsic tendon repair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J. Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Southern JF, Zycband EI, Fallon JT, Trelstad RL. Collagen fibril bundles: a branching assembly unit in tendon morphogenesis. Development. 1989a;107:437–443. doi: 10.1242/dev.107.3.437. [DOI] [PubMed] [Google Scholar]
- Birk DE, Zycband EI, Winkelmann DA, Trelstad RL. Collagen fibrillogenesis in situ: fibril segments are intermediates in matrix assembly. Proc. Natl. Acad. Sci. U SA. 1989b;86:4549–4553. doi: 10.1073/pnas.86.12.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich HP, Lambert PA, Saggers GC, Myers RL, Hauck RM. Dynamic changes appearing in collagen fibers during intrinsic tendon repair. Ann. Plast. Surg. 2005;54:201–206. doi: 10.1097/01.sap.0000141380.52782.db. [DOI] [PubMed] [Google Scholar]
- Fell HB. The physiology of skeletal tissue in culture. Lect. Sci. Basis Med. 1956;6:28–45. [PubMed] [Google Scholar]
- Franchi M, Trirè A, Quaranta M, Orsini E, Ottani V. Collagen structure of tendon relates to function. Scientif. World J. 2007;7:404–420. doi: 10.1100/tsw.2007.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham JM, Hauck RM, Ehrlich HP. Is the tendon embryogenesis process resurrected during tendon healing? Plast. Reconstr. Surg. 2003;112:844–854. doi: 10.1097/01.PRS.0000070180.62037.FC. [DOI] [PubMed] [Google Scholar]
- Jaibaji M. Advances in the biology of zone II flexor tendon healing and adhesion formation. Ann. Plast. Surg. 2000;45:83–92. doi: 10.1097/00000637-200045010-00017. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P, Balint JB, Reffy A. Three-dimensional ultrastructure of human tendons. Acta Anat. 1991;142:306–312. doi: 10.1159/000147207. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem. J. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manske PR, Gelberman RH, Lesker PA. Flexor tendon healing. Hand Clin. 1985;1:25–34. [PubMed] [Google Scholar]
- Trelstad RL, Hayashi K. Tendon collagen fibrillogenesis: intracellular subassemblies and cell surface changes associated with fibril growth. Dev. Biol. 1979;71:228–242. doi: 10.1016/0012-1606(79)90166-0. [DOI] [PubMed] [Google Scholar]
- Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: regulation of collagen fibrillogenesis. J. Musculoskelet. Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]