Abstract
Although methamphetamine and alcohol are commonly used together in a binge-like pattern, there is a dearth of empirical data investigating the repeated effects of this drug combination. The current study examined acute and residual mood, performance, and physiological effects of methamphetamine alone, alcohol alone, and the combination. Nine adult male volunteers completed this 20-day within-participant, residential laboratory study. During four 5-day blocks of sessions, participants were administered oral methamphetamine (0, 10 mg) combined with alcohol (0, 0.375, 0.75 g/kg) three times (day 2: AM, day 2: PM, and day 3: PM). Breath alcohol concentrations, cardiovascular, subjective, and cognitive/psychomotor performance effects were assessed before drug administration and repeatedly thereafter. Subjective and objective sleep measures were also assessed; residual effects were assessed on days 3–5 of each block. Following the first drug administration, the methamphetamine–alcohol combination produced greater elevations of heart rate and ratings of “good drug effect” compared to either drug alone. Methamphetamine attenuated alcohol-related performance decrements and feelings of intoxication, whereas alcohol attenuated methamphetamine-related sleep disruptions. By the third administration, many of these effects were significantly diminished, suggesting that participants developed tolerance. Few residual effects were observed. These data show that methamphetamine combined with alcohol produced a profile of effects that was different from the effects of either drug alone. The largely positive effects of the drug combination (i.e., greater euphoria, and fewer performance and sleep disruptions) might explain why these drugs are often used in combination.
Keywords: Methamphetamine, Alcohol, Psychomotor performance, Subjective effects, Sleep, Tolerance, Humans
Introduction
Data from epidemiological studies indicate that club drugs, such as MDMA (3-methylenedioxymethamphetamine, ‘ecstasy’) and methamphetamine, are often used in combination with other drugs of abuse. For example, several researchers have reported that novice and experienced illicit methamphetamine users frequently use the stimulant in combination with alcohol (Semple et al. 2003; Parsons et al. 2007). For instance, Halkitis et al. (2005) observed that more than 60% of methamphetamine users in New York City reported regular use of the methamphetamine – alcohol combination. Additionally, Furr et al. (2000) observed that, relative to non- and moderate alcohol drinkers, individuals with daily episodes of alcohol intoxication were five times more likely to have used methamphetamine. Despite its relatively common use among methamphetamine users, this drug combination may have dangerous consequences. Data from hospital emergency departments indicate that alcohol is frequently mentioned in combination with methamphetamine (SAMHSA 2010); in 2008, this drug combination accounted for 24% of all methamphetamine-related emergency department episodes. Nevertheless, although these drugs are frequently used together, there is little empirical evidence investigating the interactive effects of methamphetamine and alcohol.
By comparison, the interactive effects of alcohol and other stimulants have received considerable experimental attention. For instance, researchers have observed that single-dose combinations of cocaine and alcohol produced relatively greater increases in heart rate and measures of euphoria (Foltin and Fischman 1988; Foltin et al. 1993; Perez-Reyes and Jeffcoat 1992; Farre et al. 1993, 1997; Higgins et al. 1993; McCance-Katz et al. 1993, 1998, 2005). In contrast, on several other measures cocaine attenuated the effects of alcohol. For example, cocaine reduced alcohol-related psychomotor performance decrements and subjective feelings of inebriation (Foltin et al. 1993; Farre et al. 1993; Higgins et al. 1993). Similar effects have been observed in investigations of the effects of alcohol combined with caffeine (e.g., Kerr et al. 1991; Drake et al. 2003) and D-amphetamine (e.g., Perez-Reyes et al. 1992). Given these findings, it is possible that individuals who use alcohol and stimulants do so in an effort to enhance the total level of intoxication and/or reduce the undesirable depressant effects of alcohol.
To our knowledge, there is only one published investigation of the effects of the methamphetamine–alcohol combination in humans. Mendelson and colleagues (1995) assessed the acute effects of a single dose of intravenous methamphetamine (30 mg) combined with oral alcohol (1 gm/kg) on physiological measures, mood, and psychomotor performance. Consistent with the data obtained from investigations of other stimulants, methamphetamine combined with alcohol increased heart rate and euphoria more than either drug alone and attenuated alcohol-related subjective feelings of intoxication; there were no drug-related effects on psychomotor performance. It is important to note, however, that drug effects were only assessed following a single drug administration of the drug combination. Anecdotally, methamphetamine users often take the drugs in a binge-like pattern (i.e., multiple doses over the course of an evening and/or several days: Cho et al. 2001; Semple et al. 2003). Given this situation, it is surprising that there are no published data assessing the consequences of repeated administration of the methamphetamine – alcohol combination on a wider range of measures, such as sleep and next-day mood and physiological effects.
Therefore, this study characterized methamphetamine and alcohol interactive effects following repeated administrations on physiological and subjective measures, cognitive/psychomotor performance, and sleep. During this 20-day residential study, participants received oral methamphetamine (0, 10 mg) and/or alcohol (0, 0.375, 0.75 g/kg) on three consecutive occasions (i.e., the morning of day 2, the evening of day 2, and the evening of day 3). We hypothesized that the drug combination would (1) produce greater “positive” subjective effects compared to either drug alone; (2) decrease alcohol-related performance impairments and feelings of intoxication; and (3) increase sleep disruptions and residual effects compared to either drug alone.
Methods and materials
Participants
Adult stimulant and alcohol users were recruited via word-of-mouth referral and newspaper and online advertisement in New York City. All potential participants completed an initial telephone and an in-person medical and psychiatric evaluation. Eligibility criteria included a minimum of 21 years of age, reports of past year amphetamine use (i.e., D-amphetamine, methamphetamine, or MDMA) and current (i.e., past 30 days) alcohol use. Exclusion criteria included significant medical history (e.g., cardiovascular, neurological, or major psychiatric illnesses, excluding stimulant dependence) or any other condition that would increase risk for study participation
Nine male research volunteers (six Black, one Hispanic, two White) completed this 20-day inpatient study; Table 1 provides individual participant demographic information. As a group, they were 41.1 ± 3.9 (mean ± SD) years old and had completed 13.6 ± 1.6 (mean ± SD) years of formal education. All passed comprehensive medical examinations and psychiatric interviews (i.e., Structured Clinical Interviews for DSM-IV) and were within normal weight ranges according to the 1983 Metropolitan Life Insurance Company height/weight table (body mass index: 26.4 ± 3.5 [mean ± SD]). All participants reported current alcohol use (1–5 days/week; 3–7 drinks/day), and current cocaine use (1–6 days/week). Two participants reported current marijuana use (1–7 days/week), one reported current methamphetamine use (3 days/week), six smoked 5–10 tobacco cigarettes/day, and eight reported current caffeine use (1–9 cups/day). Three participants met criteria for cocaine dependence but no one was seeking treatment for his drug use and no one met criteria for any other Axis I disorder.
Table 1.
Participant demographics and current drug use (past 30 days)
| Age (years) |
Race/ ethnicity |
Education (years) |
Tobacco use (cig/day) |
Alcohol use (days/week) |
Alcohol use (drinks/day) |
Marijuana use (days/week) |
Cocaine use (days/week) |
MA use (days/week) |
|---|---|---|---|---|---|---|---|---|
| 40 | Black | 12 | 7 | 2 | 3 | – | 3 | – |
| 45 | Black | 12 | 5 | 3 | 4 | – | 4a | – |
| 34 | Hispanic | 14 | 10 | 1 | 6 | – | 3 | – |
| 39 | Black | 15 | – | 3 | 4 | – | 2a | 3 |
| 39 | Black | 15 | 10 | 3 | 5 | 1 | 3a | – |
| 42 | White | 16 | – | 3 | 5 | – | 4 | – |
| 40 | Black | 14 | – | 5 | 3 | – | 1 | – |
| 44 | Black | 12 | 10 | 5 | 4 | 7 | 2 | – |
| 47 | White | 12 | 5 | 3 | 6 | – | 5 | – |
Note that an important inclusion criteria was that all participants had to report at least past year amphetamine use
Endorsed criteria for current cocaine dependence (DSM-IV-TR)
Participants were told that the purpose of the study was to evaluate the effects alcohol and other medications (i.e., an FDA-approved stimulant and/or sedative) on performance and subjective effects of volunteers living in a residential laboratory. Before study enrollment, each signed a consent form that was approved by the Institutional Review Board of The New York State Psychiatric Institute (NYSPI). Upon discharge, each participant was informed about experimental and drug conditions and was paid for participation at a rate of $70 per day.
Pre-study training
Prior to study enrollment, participants completed two training sessions (3–4 h each) on the computerized cognitive/psychomotor tasks that would be used during the study. On a separate day, they received oral methamphetamine (10 mg) in combination with the largest alcohol dose (0.75 g/kg) to be tested in order to monitor any adverse reactions and provide them with experience with the study drug combination. Because this was a double-blind study, participants were not informed of the actual medication or dose until study completion.
Design
During this 20-day study participants were housed in a residential laboratory at the New York State Psychiatric Institute in three groups of 3–4 individuals. The study consisted of four 5-day blocks of sessions, during which participants completed visual analog mood scales and cognitive/psychomotor task batteries and had physiological measures assessed. Table 2 provides the study design. Briefly, a capsule (either placebo or active methamphetamine) and a liquid (either placebo or active alcohol) were administered daily, once in the morning and once in the evening. The first day of each block served as a baseline period; only placebo was administered. On the second day of each block, active drug was administered both in the morning and evening. In the morning on the third day of each block, placebo was administered to provide the opportunity to investigate residual drug effects. In the evening on the third day, active drug was administered in order to investigate potential evidence of tolerance. On the fourth and fifth days of each block, participants were administered placebo. These days served as washout a period and also provided the opportunity to investigate residual drug effects. The dosing order was counterbalanced so that no two participants received the same drug combination during the same block.
Table 2.
Study design
| Time | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0900/1000 | P/P | MA/A2 | P/P | P/P | P/P | P/P | MA/P | P/P | P/P | P/P |
| 2100/2200 | P/P | MA/A2 | MA/A2 | P/P | P/P | P/P | MA/P | MA/P | P/P | P/P |
| Time | Day 11 | Day 12 | Day 13 | Day14 | Day15 | Day 16 | Day 17 | Day 18 | Day19 | Day 20 |
| 0900/1000 | P/P | P/A2 | P/P | P/P | P/P | P/P | MA/A1 | P/P | P/P | P/P |
| 2100/2200 | P/P | P/A2 | P/A2 | P/P | P/P | P/P | MA/A1 | MA/A1 | P/P | P/P |
Capsule dosing occurred at 0900 and 2100 h; liquid dosing at 1000 and 2200 h
All participants completed four 5-day blocks of sessions, one for each dosing condition (i.e., MA/P, MA/A1, MA/A2, and P/A2). Dosing order was varied across participants
MA 10 mg methamphetamine, A1 0.375 g/kg alcohol, A2 0.75 g/kg alcohol, P placebo
Procedure
Participants moved into the laboratory on the day before the study began so that they could receive further training on tasks and experimental procedures. The first experimental day began the following morning at 0800 h. Fifteen minutes after awakening at 0815 h, participants completed a visual analog sleep questionnaire and the baseline task battery, composed of the cognitive/psychomotor tasks and the subjective-effects questionnaire (described below). Then, they were weighed (but were not informed of their weight) and given time to eat breakfast. At 0900 h, participants were administered an active methamphetamine or placebo capsule in a private vestibule. At 1000 h, they completed the subjective-effects questionnaire and then were administered an active alcohol or placebo liquid. Then, participants completed three task batteries from 1045 to 1315 h, took a 1.5-h lunch break period, and completed three additional task batteries from 1515 to 1715 h. Participants were given a 15-min break between each task battery. Cardiovascular measures and breath alcohol levels were obtained at baseline and 0.75, 1.5, 2.25, 3, 4, 5, and 6 h post-capsule administration.
Beginning at 1715 h, participants had access to the social area, where they could interact with other study participants and engage in recreational activities. During this period, social behavior was recorded as previously described (e.g., Haney et al. 2007). Briefly, a computerized observation program prompted recording of each participant’s behavior every 2.5 min. Behaviors were classified into private (time spent in bathroom/bedroom) and social (time spent in the recreational area). Time spent in the social area was further divided into time spent talking and time spent in silence. Outcomes were total minutes spent engaging in each behavior per day.
Two films were shown daily, beginning at 1715 and 2000 h. At 2045, participants completed the subjective-effects questionnaire and cardiovascular measures and breath alcohol levels were obtained. At 2100 h, participants were administered an active methamphetamine or placebo capsule; at 2200 h, participants completed the subjective-effects questionnaire and then were administered an active alcohol or placebo liquid. Participants had access to the social area except for brief moments during which they completed the subjective-effects questionnaire and cardiovascular effects and breath alcohol levels were obtained (i.e., 0.75, 1.5, 2, and 3 h post-capsule administration). Lights were turned out at 2400 h for an 8-h sleep period. Participants could smoke tobacco cigarettes ad libitum from 0800 to 2330 h.
Subjective-effects and psychomotor battery
The computerized visual analog questionnaire consisted of a series of 100-mm lines labeled “not at all” at one end and “extremely” at the other end (Hart et al. 2003). The lines were labeled with adjectives describing a mood (e.g., “I feel…” “irritable,” “talkative,” “unmotivated”), a drug effect (e.g., “I feel…,” “stimulated,” “a good drug effect,” “a bad drug effect”), or a physical symptom (“I feel nauseous,” “I have a headache,” “My heart is beating faster than usual”). Additionally, at various time points participants completed a drug-effect questionnaire (DEQ), during which they were required to rate “good effects” and “bad effects” on a 5-point scale: 0 = “not at all” and 4 = “very much.” They were also asked to rate the drug strength as well as their desire “to take the drug again.” Lastly, participants were asked to rate how much they liked the drug effect on a 9-point scale: −4 = “disliked very much,” 0 = “feel neutral, or feel no drug effect,” and 4 = “liked very much.”
The computerized psychomotor task battery consisted of five tasks: (1) the Digit Recall Task; (2) the digit-symbol substitution task (DSST); (3) the divided attention task (DAT); (4) the rapid information task (RIT); and (5) the Repeated Acquisition Task (RA task).
During the Digit Recall task, an eight-digit number was displayed for 3 s on the computer screen. Participants were instructed to enter the number correctly while it was on the screen and again after it had disappeared from the screen. They were also told that they would be asked to reproduce and recognize the number near the end of the battery. This task was designed to assess changes in immediate and delayed recall (see Hart et al. 2001).
The DSST is 3-min task (McLeod et al. 1982) that consisted of nine random three-row, three-column squares (one square blackened/row) displayed across the top of the computer screen. Each array was associated with a number (1–9). A randomly generated number appeared at the bottom of the screen, indicating which of the arrays should be reproduced on the nine-key keypad attached to the computer. Participants were instructed to reproduce as many patterns as possible by entering the patterns associated with the randomly generated numbers. This task was designed to assess changes in visuospatial processing.
The DAT is 5-min task that combines concurrent pursuit-tracking and vigilance tasks (Miller et al. 1988). Participants tracked a moving circle on the video screen using the mouse, and also had to signal when a small black square appeared at any of the four corners of the screen. Accurate tracking of the moving stimulus increased its speed proportionately. This task was designed to assess changes in vigilance and inhibitory control.
During the RIT, a series of digits was presented at the rate of 100 digits per min, and subjects were instructed to press a response button as quickly as possible whenever they detected sequences of three consecutive odd or three consecutive even digits (Wesnes and Warburton 1983). A point was earned for each correct “hit” and a point was deducted for each “miss” or “false alarm.” Participants were instructed to earn as many points as possible during the task. This task was designed to assess changes in sustained concentration and inhibitory control.
At the start of the 3-min RA task, participants were instructed to learn a ten-response sequence of button presses. A position counter incremented by one each time a correct button was pressed, and remained unchanged after an incorrect response. The points counter increased by one each time the ten-response sequence was correctly completed. The sequence remained the same throughout the task, but a new random sequence was generated for each subsequent task battery. Participants were instructed to earn as many points during the task as possible by pressing the buttons in the correct sequence. This task was designed to assess changes in learning and memory (see Kelly et al. 1993).
Sleep monitoring
Subjective sleep experience from the immediately preceding sleep period was measured by a visual analog sleep questionnaire completed shortly after waking. The questionnaire consisted of a series of 100-mm lines labeled “not at all” at one end and “extremely” at the other end. The lines were labeled: “I slept well last night,” “I woke up early this morning,” “I fell asleep easily last night,” “I feel clear-headed this morning,” “I woke up often last night,” “I am satisfied with my sleep last night,” and a fill-in question in which participants were asked to estimate the number of hours they slept the previous night (Haney et al. 2001). Objective sleep was measured by tracking gross motor activity using the Actiwatch® Activity Monitoring System (Actiwatch®; Respironics Company, Bend, OR). This system allowed for calculation of total sleep time, sleep onset latency, sleep efficiency (total sleep time as a percentage of time in bed), and number of wake bouts (Kushida et al. 2001).
Drug
Tablets of methamphetamine hydrochloride (Desoxyn, Abbot Laboratories, North Chicago, IL) were repackaged by the Pharmacy Department of the New York State Psychiatric Institute by placing tablets into a white no. 00 opaque capsule and adding lactose filler. Placebo consisted of white no. 00 capsules containing only lactose. Alcohol dose was calculated based on the estimated total body water (TBW) of each participant (Watson et al. 1980). The volume of all beverages was held constant at 500 ml to control for any alcohol expectancy effects. The placebo beverage consisted of Canada Dry Tonic® water and Ocean Spray Cranberry Juice CocktailR in a 3:1 ratio. The alcohol beverage consisted of the same mixture, with 100 proof Absolut® vodka added, as needed. The placebo and active beverage for a given individual were isocaloric (no more than a 10-calorie difference between beverages), using regular or low calorie tonic and juice and dextrose or Equal® sweetener. Each beverage was topped with 1 ml of vodka and one drop of peppermint oil. Participants were given 5 min to consume the beverage (Evans and Levin 2003).
The four drug conditions were: 10 mg methamphetamine combined with a placebo liquid (MA/P), 10 mg methamphetamine combined with 0.375 g/kg alcohol (MA/A1), 10 mg methamphetamine combined with 0.75 g/kg alcohol (MA/A2), and a placebo capsule combined with 0.75 g/kg alcohol (P/A2). These alcohol doses were selected because they reliably increase breath alcohol levels as well as subjective and objective behavioral measures of intoxication in experienced alcohol users (e.g., Evans and Levin 2003). Additionally, in order to reduce the time burden of participation and because the aim of this study was to compare the effects of the methamphetamine – alcohol combination to the effects of each drug alone, a placebo/placebo condition was not included.
Data analysis
Acute effects (day 2), sleep (days 2–5), and residual effects (days 3–5)
Cardiovascular effects, breath alcohol levels, subjective ratings, and cognitive/psychomotor performance data were analyzed using two-factor repeated-measures analyses of variances (ANOVAs): the first factor was drug condition (MA/P, MA/A1, MA/A2, and P/A2) and the second factor was time (time and number of assessments varied depending on the measure, e.g., subjective ratings were assessed at time points baseline, 1.0, 1.75, 2.5, 3.5, 6.25, 7, and 7.75 h after capsule administration). Peak cardiovascular and subjective-effects data were also analyzed, for the sake of brevity, these analyses are included in Table 3 only. Cigarette consumption, social interaction measures, and sleep data were analyzed using a single-factor ANOVA; the factor was drug condition.
Table 3.
Acute (day 2) drug-related effects on subjective-effect ratings
| Drug conditions | |||||||
|---|---|---|---|---|---|---|---|
| MA/P | MA/A1 | MA/A2 | P/A2 | ||||
| Mean(SEM) | Mean(SEM) | F, d | Mean(SEM) | F, d | Mean(SEM) | F, d | |
| Peak subjective-effect ratings (VAS max = 100) | |||||||
| Content | 12.2 (5.6) | 35.4 (9.5) | 14.1, 1.0* | 40.6(13.9) | 21.1,1.0* | 30.9 (11.0) | 9.1, 0.8* |
| Drunk | 1.1 (0.8) | 5.1 (3.5) | 0.5 | 13.9 (8.7) | 5.5 | 44.2(13.5) | 62.2, 2.0**** |
| Energetic | 15.9 (9.5) | 38.3(12.5) | 6.9, 0.7* | 44.8(11.9) | 11.5, 0.9* | 44.2(11.9) | 11.1, 0.9* |
| Good drug effect | 6.0 (5.8) | 45.2(14.2) | 24.7, 1.3* | 62.9(13.5) | 52.0, 2.0*** | 33.9(12.8) | 12.5, 1.0**** |
| High | 12.2 (7.4) | 29.6(12.8) | 7.3, 0.6* | 49.7(11.1) | 34.1, 1.4*** | 26.9 (9.0) | 5.2, 0.6**** |
| Mellow | 20.0 (8.7) | 37.0(12.8) | 4.8, 0.5* | 59.3(12.1) | 25.5, 1.3*** | 39.8(14.2) | 6.5, 0.6**** |
| Sedated | 3.3 (3.2) | 6.2 (4.4) | 0.2 | 10.6 (7.0) | 1.3 | 34.3(11.1) | 23.9,1.4**** |
| Social | 22.1 (6.9) | 52.3 (9.2) | 17.3, 1.3* | 48.2(13.1) | 12.9, 0.9* | 48.6(11.6) | 13.3, 1.0* |
| Stimulated | 12.8 (8.2) | 40.7(14.0) | 17.4, 0.8* | 42.4(12.6) | 19.7, 0.9* | 26.2 (9.5) | 5.9, 0.5*** |
| Talkative | 10.7 (5.3) | 44.4 (9.5) | 22.6, 1.5* | 35.2(12.6) | 11.9, 0.9* | 29.8 (11.1) | 7.2, 0.8* |
| Want alcohol | 1.1 (0.8) | 13.0(10.6) | 2.7 | 37.9(15.7) | 25.5, 1.5* | 31.8(13.9) | 17.7, 1.4* |
| Peak subjective-effect ratings (DEQ) | |||||||
| Good drug effect | 1.0 (0.4) | 2.2 (0.4) | 22.1, 1.0* | 2.7 (0.4) | 41.1, 1.4* | 1.9 (0.4) | 11.7, 0.8**** |
| Drug liking | −0.4 (0.7) | 1.7 (0.8) | 21.4, 0.9* | 1.6 (0.9) | 19.2, 0.8* | 1.7 (0.7) | 21.4, 1.0* |
| Drug strength | 1.0 (0.3) | 2.4 (0.3) | 15.2, 1.6* | 2.9 (0.2) | 29.1, 2.5* | 2.2 (0.4) | 10.9, 1.1**** |
| Desire to Take Again | 0.7 (0.3) | 2.3 (0.4) | 12.5,1.5* | 2.1 (0.5) | 37.3, 1.2* | 2.1 (0.5) | 37.7,1.2* |
p < 0.05, significantly different from MA/P
p < 0.05, significantly different from MA/A1
p < 0.05, significantly different from MA/A2
Tolerance
Cardiovascular effects, breath alcohol levels, and subjective ratings following the evening dosing were analyzed using a two-factor ANOVA: the first factor was drug condition and the second factor was drug administration number (Admin 1 and Admin 3). The area under the curve (AUC) for the subjective-effects and cardiovascular data was determined using the trapezoidal method (Tallarida and Murray 1981).
For all analyses, ANOVAs provided the error terms needed to calculate between-drug planned comparisons (MA/P vs. all other doses, MA/A1 vs. MA/A2, and MA/A2 vs. P/A2) and within-drug planned comparisons (acute effects: peak vs. baseline time points; tolerance: Admin 1 vs. Admin 3). p Values were considered statistically significant at less than 0.05, using Huynh – Feldt corrections when appropriate.
Results
Acute effects (day 2)
Breath alcohol levels
Figure 1 (top left panel) shows that alcohol systematically increased breath alcohol levels (BAL). Peak BAL were observed 2.25 h after capsule administration (i.e., 1.25 h after alcohol administration) and declined over the next several hours. The effect of alcohol was dose-related in that all alcohol doses caused a significantly greater BAL increase compared to methamphetamine alone and the P/A2 and MA/A2 doses produced larger increases than the MA/A1 dose (F 21,168 = 14.5 – 366.8, p < 0.0001 for all comparisons, d = 6.7–33.3). There was no significant difference between the P/A2 and MA/A2 doses.
Fig. 1.
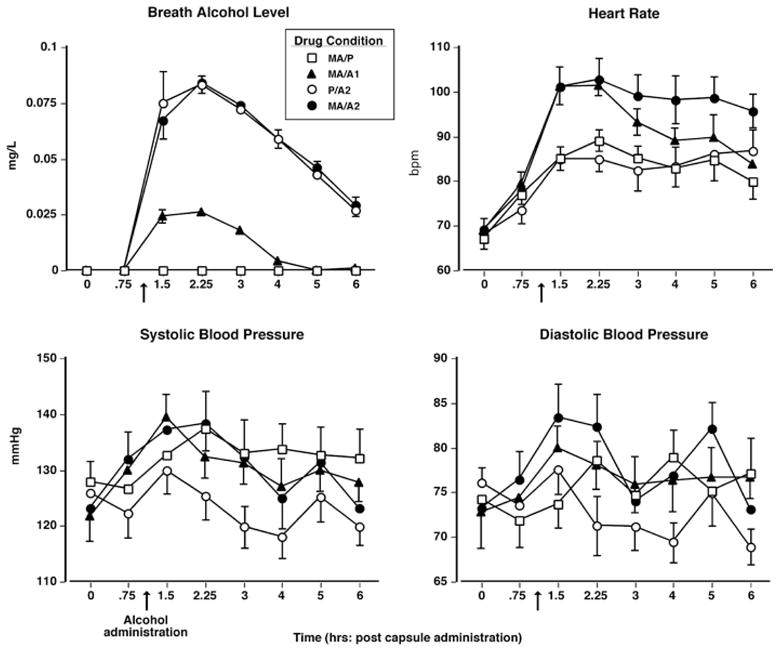
Upper panel(left): breath alcohol level as a function of drug condition and time. Upper panel (right): heart rate as a function of drug condition and time. Lower panels: systolic and diastolic pressure as a function of drug condition and time. Error bars represent 1 SEM. Overlapping error bars were omitted for clarity. The arrow denotes time of alcohol administration
Cardiovascular effects
Figure 1 (top right and bottom panels) displays cardiovascular measures as a function of drug condition and time. All active drug conditions increased heart rate (HR) above baseline measurements (F 21,168 = 35.1 – 127.1, p < 0.0001 for all comparisons, d = 2.1 – 3.3). Furthermore, both MA/A1 and MA/A2 produced significantly greater increases in HR compared to either methamphetamine or alcohol alone and MA/A2 produced larger increases than MA/A1 (F 21,168 = 10.9 – 30.2, p < 0.005 for all comparisons, d = 1.5 – 2.3). Compared to baseline, all active methamphetamine conditions produced increases on systolic pressure (SP) and the methamphetamine – alcohol combination (both MA/A1 and MA/A2) increased diastolic pressure (DP: F 21,168 = 6.6 – 23.2, p < 0.05 for all comparisons, d = 0.7 – 3.3). Alcohol alone did not significantly elevate blood pressure.
Subjective effects
Figure 2 illustrates the effects of methamphetamine and alcohol on selected subjective-effect ratings over time. The methamphetamine – alcohol combination (MA/A1 and MA/A2) produced larger increases on ratings of “good drug effect” compared to either drug alone, and P/A2 produced a greater effect than MA/P (F 21,168 = 12.5 – 52.0, p < 0.05 for all comparisons, d = 1.0 – 2.0). Conversely, compared to both MA/A2 and MA/P, alcohol alone produced greater increases on ratings of “drunk” (F 21,168 = 9.1 – 17.9, p < 0.05 for all comparisons, d = 1.5 – 2.6). Table 3 summarizes other significant acute drug effects observed on the visual analog questionnaire and the DEQ.
Fig. 2.
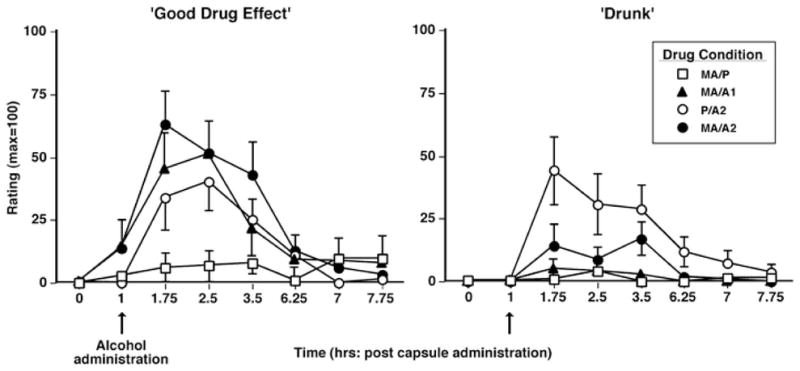
Selected subjective-effect ratings as a function of drug condition and time. Error bars represent 1 SEM. Overlapping error bars were omitted for clarity. The arrow denotes time of alcohol administration
Cognitive/psychomotor performance effects
Figure 3 shows how the first administration of methamphetamine and alcohol altered performance over time on selected measures. On the DAT (left panel), P/A2 decreased the maximum tracking speed compared to all other drug conditions (F 18,144 = 6.3 – 12.9, p < 0.05 for all comparisons, d = 2.4 – 2.7). Similarly, on the RIT (middle panel), P/A2 decreased the number of hits compared to MA/P (F(18,144) = 6.7, p < 0.05, d = 1.3). Conversely, the MA/A2 produced increases in the percentage of errors on the RA task compared to MA/P and MA/A1 (F 18,144 = 14.4 – 18.2, p < 0.001 for all comparisons, d = 1.5 – 1.7; right panel). No other significant acute drug effects on performance were noted.
Fig. 3.
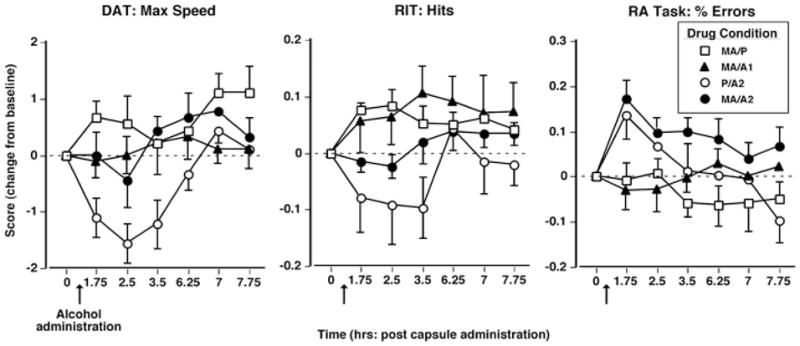
Selected cognitive/psychomotor performance effects as a function of drug condition and time. Error bars represent 1 SEM. Overlapping error bars were omitted for clarity. The arrow denotes time of alcohol administration
Effects of cigarette smoking
For the six participants who smoked during the study, MA/A2 increased the daily number of cigarettes smoked (F 12,60 = 24.1, p < 0.05, d = 0.9); the average increase was approximately 4–5 cigarettes (day 0: 9.5 ± 1.4 versus day 1: 14.2 ± 1.9). Furthermore, participants smoked more cigarettes under the MA/A2 condition compared to all other drug conditions (F 12,60 = 10–19.2, p < 0.05 for all comparisons, d = 0.4–0.8; data not shown). No other significant drug effects on cigarette smoking were observed.
Effects on social interactions
There were no significant differences between drug conditions on the total amount of time spent in the social area. However, participants spent a smaller proportion of time talking to other participants following the MA/P dose compared to all active alcohol conditions (MA/P: 10.9% versus MA/A1: 24.8%, P/A2: 28.5%, and MA/A2: 25.2%; F 12,96 = 5.8–9.3, p < 0.05 for all comparisons, d = 1.3–1.7). No other significant drug effects on social interaction measures were observed on days 1–5.
Tolerance
Cardiovascular and BAL effects
Figure 4 (left panel) shows the effects of dosing condition on HR AUC following the first drug administration (Admin 1) and the third drug administration (Admin 3). After Admin 1, both methamphetamine–alcohol combinations (MA/A1 and MA/A2) produced greater increases in HR AUC compared to either drug alone (F 3,24 = 26.3–47.0, p < 0.0001 for all comparisons, d = 1.3–1.9). By Admin 3, however, only MA/A2 produced greater increases in HR AUC compared to MA/P and P/A2 (F 3,24 = 11.3–14.5, p < 0.005 for all comparisons, d = 0.7–0.9). Furthermore, both MA/A1 and MA/A2 produced significantly lesser effects in HR AUC when Admin 3 was compared to Admin 1 (F 3,24 = 15.4–26.5, p < 0.0001 for all comparisons, d = 0.8–1.6). Drug-related effects on blood pressure were unaltered over time.
Fig. 4.
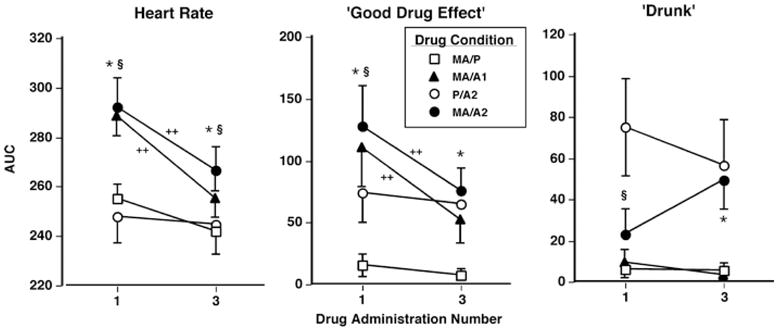
Selected tolerance-related cardiovascular effects and subjective-effects ratings (AUC) as a function of drug condition and day. Error bars represent 1 SEM. Overlapping error bars were omitted for clarity. *MA/A2 significantly different from MA/P (p < 0.05). §MA/A2 significantly different from P/A2 (p < 0.05). ++Admin 1 is significantly different from Admin 3 (p < 0.05)
Subjective effects
Figure 4 (middle and right panels) illustrates the effects of dosing condition on selected subjective-effect ratings across drug administrations. After Admin 1, the MA/A2 dose produced greater ratings of “good drug effect” AUC and smaller ratings of “drunk” AUC compared to P/A2 (F 3,24 = 10.7–12.7, p < 0.005 for all comparisons, d = 0.6–0.8). By Admin 3, however, there were no significant differences between P/A2 and MA/A2. Additionally, regarding “good drug effect,” both MA/A1 and MA/A2 produced significantly smaller ratings when Admin 3 was compared to Admin 1 (F 3,24 = 12.1–14.9, p < 0.005 for all comparisons, d = 0.7–0.8). Table 4 illustrates other tolerance-related effects on the visual analog scales and the DEQ.
Table 4.
Tolerance-related effects on subjective ratings of mood (AUC)
| Drug conditions by day | |||||||
|---|---|---|---|---|---|---|---|
| P/A2(Admin 1) | P/A2 (Admin 3) | MA/A2 (Admin1) | MA/A2 (Admin 3) | ||||
| Mean(SEM) | Mean(SEM) | F value | Mean(SEM) | F value | Mean(SEM) | F value | |
| Visual Analog Scales (VAS max= 100) | |||||||
| Content | 107.6 (36.2) | 100.3(29.9) | 0.3 | 122.6(40.3) | 1.2 | 72.6(26.1) | 13.4***** |
| Energetic | 112.6 (27.6) | 40.0(15.3) | 10.4* | 118.2(31.5) | 0.1 | 70.1(23.0) | 4.6*** |
| High | 57.2 (19.2) | 31.0(13.9) | 2.9 | 96.6(27.0) | 6.5* | 56.0(18.3) | 6.9*** |
| Drug effects questionnaire | |||||||
| Good Drug Effect | 3.8 (0.6) | 3.6 (0.5) | 0.2 | 4.8 (0.9) | 3.4 | 3.2 (0.6) | 8.2*** |
| Drug Liking | 3.6 (1.1) | 1.3 (1.3) | 7.3* | 3.1 (1.7) | 0.3 | 1.1 (1.5) | 5.9*** |
p < 0.05, significantly different from P/A2 (Admin 1)
p < 0.05, significantly different from P/A2 (Admin 3)
p < 0.05, significantly different from MA/A2 (Admin 1)
Sleep effects
Figure 5 displays the effects of methamphetamine and alcohol administration on objective and subjective sleep measures across days of each study block. Regarding objective sleep measures (Fig. 5, left panels), on day 2, participants’ actual hours slept and sleep efficiency were significantly decreased by all active methamphetamine doses compared with P/A2 (F 12,96 = 11.6–30.7, p < 0.005 for all comparisons, d = 1.2–2.5). Methamphetamine alone reduced both of these measures to a greater extent compared to MA/A2 (F 12,96 = 4.6, p < 0.05 for all comparisons, d = 0.7–0.8). On day 3, MA/P significantly decreased these measures compared to P/A2 (F(12,96) = 9.9, p < 0.005 for all comparisons, d = 1.2–1.3). Similarly, participants’ estimated number of hours slept and ratings of “slept well last night” on day 2 were decreased by all active methamphetamine doses compared with P/A2 (F 12,96 = 4.5–45.2, p < 0.05 for all comparisons, d = 0.8–3.5; Fig. 5, right panels). Methamphetamine alone reduced both of these subjective measures to a greater extent compared to MA/A2 (F 12,96 = 5.0–8.8, p < 0.05 for all comparisons, d = 0.9–1.3). Table 5 shows additional significant effects produced by methamphetamine and alcohol on objective and subjective sleep measures.
Fig. 5.
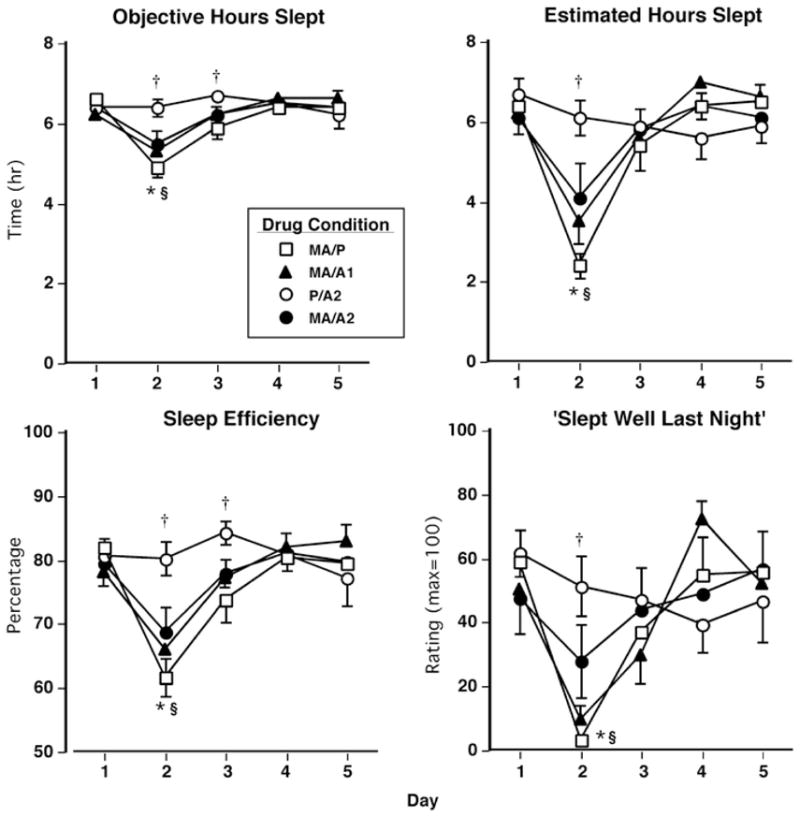
Left panels selected objective measures sleep as a function of drug condition and day. Right panels: selected subjective measures sleep as a function of drug condition and day. Error bars represent 1 SEM. Overlapping error bars were omitted for clarity. *MA/A2 significantly different from MA/P (p < 0.05). §MA/A2 significantly different from P/A2 (p < 0.05). †MA/P significantly different from P/A2 (p < 0.05)
Table 5.
Drug-related effects on sleep measures (day 2) and residual drug effects on subjective ratings of mood (days 3–4)
| Drug conditions | |||||||
|---|---|---|---|---|---|---|---|
| MA/P | MA/A1 | MA/A2 | P/A2 | ||||
| Mean(SEM) | Mean(SEM) | F value | Mean(SEM) | F value | Mean(SEM) | F value | |
| Sleep questionnaire (max = 100) | |||||||
| Fell asleep easily | 9.8 (8.4) | 26.1 (11.2) | 1.5 | 22.1 (11.9) | 0.8 | 56.6 (11.4) | 12.0**** |
| Satisfied with Sleep | 3.3 (2.0) | 4.8 (3.4) | 0.0 | 22.1(10.6) | 2.6 | 57.7(10.8) | 21.8**** |
| Woke often | 85.6 (10.6) | 77.0 (12.0) | 0.4 | 66.2 (13.1) | 2.0 | 40.9 (12.7) | 10.5* |
| Actiwatch measures | |||||||
| Sleep latency(min) | 53.4 (13.1) | 38.7 (11.1) | 1.5 | 37.0 (9.0) | 1.9 | 20.6 (3.9) | 7.5* |
| # of awakenings per hour | 6.9 (0.9) | 5.2 (0.9) | 10.1* | 4.8 (0.5) | 16.0* | 3.5 (0.6) | 42.8* |
| Subjective-effect ratings (AUC: day 3) | |||||||
| Friendly | 196.3 (48.1) | 324.2 (49.1) | 10.6* | 217.8 (70.6) | 7.4** | 284.2 (73.3) | 5.1* |
| Headache | 121.9 (64.0) | 3.3 (2.1) | 9.1* | 157.6 (83.5) | 15.4** | 30.6 (17.0) | 5.4**** |
| Jittery | 41.7 (25.6) | 22.4 (12.0) | 0.6 | 88.4 (62.6) | 6.8** | 3.2 (2.9) | 11.3*** |
| Nauseous | 11.9 (7.3) | 2.0 (1.4) | 0.3 | 68.4(31.5) | 9.2*** | 38.6(18.1) | 2.0 |
| Sleepy | 410.9(66.7) | 360.9(70.6) | 0.8 | 299.1(48.7) | 3.9 | 279.4(76.2) | 5.5* |
| Social | 178.6 (50.3) | 189.0 (36.1) | 0.1 | 89.8 (33.9) | 6.0*** | 178.1 (40.6) | 5.9*** |
| Subjective-effect ratings (AUC: day 4) | |||||||
| Social | 212.1 (43.3) | 152.7 (44.3) | 2.7 | 128.4 (42.0) | 5.3* | 209.9 (37.9) | 5.1 |
| Talkative | 141.6 (40.1) | 122.0 (40.4) | 0.4 | 58.0 (25.0) | 8.1*** | 122.3 (45.9) | 4.8*** |
p < 0.05, significantly different from MA/P
p < 0.05, significantly different from MA/A1
p < 0.05, significantly different from MA/A2
Residual effects
Cardiovascular effects
Figure 6 (top panels) displays mean cardiovascular measures across the work period on the days following active drug administration. On the day following two active drug administrations (day 3 of each block), heart rate remained significantly elevated by MA/P and MA/A2 compared to P/A2 (F 12,96 = 7.3–19.4, p < 0.01 for all comparisons, d = 0.5–0.8). Conversely, on day 4, SP and DP were significantly elevated by P/A2 compared with both MA/P and MA/A2 (F 12,96 = 4.1–8.3, p < 0.05 for all comparisons, d = 0.3–0.5). No other significant drug effects on cardiovascular measures were observed on days 2–4.
Fig. 6.
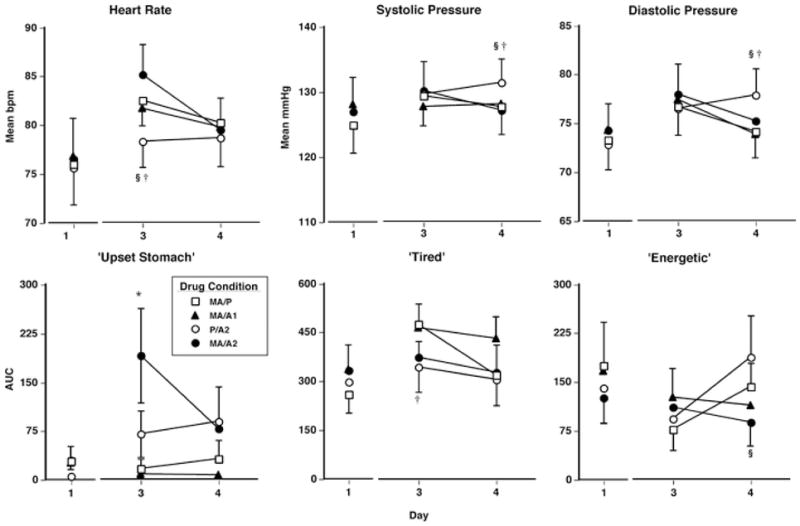
Upper panels residual effects on cardiovascular measures (mean values over time) as a function of drug condition and day. Lower panels: Residual effects on subjective-effects ratings (AUC) as a function of drug condition and day. Error bars represent 1 SEM. Overlapping error bars were omitted for clarity. *MA/A2 significantly different from MA/P (p < 0.05). §MA/A2 significantly different from P/A2 (p < 0.05). †MA/P significantly different from P/A2 (p < 0.05)
Subjective effects
Figure 6 (bottom panels) displays residual effects on selected subjective-effects ratings over the course of the work period. On day 3, ratings of “upset stomach” were significantly increased by MA/A2 compared with MA/P and ratings of “tired” were significantly elevated by MA/P compared with P/A2 (F 12,96 = 4.4–10.5, p < 0.05 for all comparisons, d = 0.6–1.3). On day 4, ratings of “energetic” were significantly decreased under the MA/A2 condition compared with the P/A2 condition (F 12,96 = 5.1, p < 0.01, d = 0.7). Table 5 shows additional significant residual effects produced by methamphetamine and alcohol on subjective-effect ratings.
Cognitive/psychomotor performance effects
There were no significant residual cognitive/psychomotor effects.
Discussion
The present results show that single-dose, co-administration of methamphetamine and alcohol produced greater increases on cardiovascular measures and feelings of euphoria (i.e., ratings of “good drug effect” and “high”) than single doses of either drug alone. Furthermore, the drug combination decreased alcohol-specific feelings of intoxication (i.e., ratings of “drunk” and “sedated”) and performance decrements observed with alcohol alone, while attenuating the magnitude of methamphetamine-related sleep disruptions. Overall, the above drug effects were diminished following repeated administrations, indicating that tolerance may have developed to some effects produced by methamphetamine–alcohol combinations. While, for the most part, these results are consistent with data from previous investigations of alcohol combined with various stimulants, including caffeine (e.g., Kerr et al. 1991), cocaine (e.g., Foltin and Fischman 1988; Foltin et al. 1993), D-amphetamine (e.g., Perez-Reyes et al. 1992), and intravenous methamphetamine (Mendelson et al. 1995), this is the first study of the consequences of repeated administrations of the methamphetamine–alcohol combination on a wider range of behaviors.
As hypothesized, methamphetamine attenuated many alcohol-related effects. For example, participants performed worse on measures of divided attention and vigilance following administration of alcohol alone. Conversely, on the same tasks, the methamphetamine–alcohol combination caused no such performance decrements. Furthermore, following administration of the drug combination, participants reported feeling less “drunk” and “sedated.” These results suggest a potential concern because some methamphetamine-using individuals might increase their alcohol consumption in an attempt to achieve the accustomed level of alcohol intoxication, thus leading to a potential increase in alcohol toxicity. Another concern is that some individuals might underestimate their level of alcohol-related impairment on more complicated tasks after ingesting the methamphetamine–alcohol combination. Of course, this is speculative; therefore, future studies should investigate choice to self-administer alcohol following methamphetamine administration as well as the effects of the drug combination on more complicated tasks.
The above concern should not be overstated, however, because of the observation that many of the acute effects produced by the methamphetamine–alcohol combination were dampened following the third drug administration. For example, following the first drug administration, the methamphetamine–alcohol combination produced significantly greater increases on feelings of euphoria (i.e., ratings of “good drug effect”) and methamphetamine attenuated alcohol-related increases in ratings of “drunk.” Conversely, following the third administration, ratings of “good drug effect” and “drunk” produced by alcohol alone and the methamphetamine alcohol combination were similar, suggesting that methamphetamine’s effects were diminished. This is consistent with an earlier investigation showing that tolerance occurred to many methamphetamine-related mood and sleep effects following repeated administrations of low oral doses (Comer et al. 2001). Of course, several alternative possibilities could explain these tolerance-like effects. For example, it is possible that differences between drug administrations could be due to differences in the time of day (i.e., the first drug administration occurred in the morning on day 2; the third administration occurred in the evening on day 3). It is also possible that a dampened response to the drug combination’s mood effects was influenced by increased fatigue due to the sleep disruptions that occurred during the previous night. Future studies investigating possible tolerance to the methamphetamine–alcohol combination might address the limitations of the current study by administering the drugs at the same time of day over the course of several days.
Based on data from previous studies indicating that methamphetamine reliably disturbed the sleep experience (Comer et al. 2001; McGregor et al. 2005; Perez et al. 2008), we predicted that methamphetamine administered both alone and in combination with alcohol would disrupt sleep. Although we received support for this hypothesis (i.e., objective and subjective sleeps measures were disrupted under all active methamphetamine conditions), an interesting observation was that the large alcohol dose attenuated methamphetamine-related sleep decrements. For example, compared to the methamphetamine alone, the methamphetamine–alcohol combination produced significantly smaller decreases in the number of hours participants slept. Because this is the first published investigation of the sleep-related effects of oral methamphetamine combined with alcohol, it is difficult to relate the present findings with previous research. Nevertheless, it is important to note that there is a large body of evidence indicating that a range of alcohol doses decreases sleep latency and increases total sleep time (for discussion, see Roehrs and Roth 2001); thus, it is likely that alcohol counteracted the sleep-reducing effects of methamphetamine. This effect, in addition to the increased euphoria produced by methamphetamine and alcohol, may partially explain why some individuals ingest the drugs simultaneously. That is, methamphetamine users may use alcohol not only to augment the acute subjective drug experience but also to relieve stimulant-associated sleep disturbances. This finding, however, should be interpreted within the context of an important limitation. Most of the current participants did not report regular use of methamphetamine. Therefore, future studies examining the repeated administration of the methamphetamine–alcohol combination should include regular methamphetamine users.
We predicted that the methamphetamine–alcohol combination would produce greater residual cardiovascular effects and mood and performance disturbances than either drug alone; we received partial support for this hypothesis. Overall, we observed few residual effects. Although, methamphetamine combined with the large alcohol dose produced sustained increases in heart rate and increased ratings of “upset stomach” on day 3 (i.e., the day following the first and second drug administrations), on other measures, the drug combination seemed to prevent deleterious next-day effects. For example, ratings of “tired” were greater following methamphetamine alone compared to the methamphetamine–alcohol combination. Furthermore, next-day cognitive/psychomotor performance was unaffected by all drug conditions. It is important to note, however, that the dosing procedure in the current study may not mimic the way the drugs are used in the natural ecology. For instance, illicit methamphetamine is commonly used in substantially larger doses via routes of administration other than oral (e.g., Cho et al. 2001). It is possible that we would have seen a different pattern of residual effects had we administered larger doses of methamphetamine via a route of administration commonly associated with abuse.
Another potential limitation of the current study is that participants were permitted to smoke tobacco cigarettes ad libitum. Because nicotine can acutely affect mood and cognitive/psychomotor performance (Myers et al. 2008; Heishman et al. 2010), it is possible that the psychoactive effects of nicotine influenced the present results. Our analysis of the number of cigarettes smoked indicated that this variable did not impact response to any subjective, cardiovascular, or performance measures. A related interesting finding was that the methamphetamine–alcohol combination significantly increased the number of cigarettes smoked. This observation is consistent with previous data showing that amphetamine and alcohol alone increases cigarette consumption (e.g., Tidey et al. 2000; Mitchell et al. 1995). One potential implication of these findings is that individuals who simultaneously ingest methamphetamine and alcohol may be at risk of becoming heavy smokers. Future investigations should directly examine the effects of this drug combination on choice to self-administer tobacco cigarettes.
In conclusion, this study provides evidence that oral methamphetamine combined with alcohol produced a profile of effects that was different from either drug alone. Methamphetamine attenuated many alcohol-related effects on performance and mood, whereas alcohol attenuated some methamphetamine-related effects on sleep. Furthermore, the drug combination produced greater increases on heart rate and subjective ratings of euphoria compared to either drug alone. This constellation of effects might explain why these drugs are often used in combination. One potential concern is that there may be an increased risk for alcohol toxicity if individuals attempt to drink more alcohol in order to achieve the accustomed effects. Another potential concern is that individuals might underestimate their level of alcohol-related impairment and thus might be more likely to engage in risky behaviors such as driving while intoxicated. Therefore, future studies should investigate the effects of the methamphetamine–alcohol combination on (1) performance on more complex cognitive tasks and (2) subsequent choice to consume alcohol.
Acknowledgments
The medical assistance of Drs. Elias Dakwar and David Mysels and the technical assistance of Audrey Perez, Michaela Bamdad, Marc Scullin, Mabel Torres, Laura Rolfe, and Christina Hadzitheodorou are gratefully acknowledged. This research was supported by grant number DA-03746 from the National Institute on Drug Abuse.
References
- Cho AK, Melega WP, Kuczenski R, Segal DS. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse. 2001;39:161–166. doi: 10.1002/1098-2396(200102)39:2<161::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Effects of repeated oral methamphetamine administration in humans. Psychopharmacology. 2001;155:397–404. doi: 10.1007/s002130100727. [DOI] [PubMed] [Google Scholar]
- Drake CL, Roehrs T, Turner L, Scofield HM, Roth T. Caffeine reversal of ethanol effects on the multiple sleep latency test, memory, and psychomotor performance. Neuropsychopharmacology. 2003;28:371–378. doi: 10.1038/sj.npp.1300026. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR. Response to alcohol in females with a paternal history of alcoholism. Psychopharmacology. 2003;169:10–20. doi: 10.1007/s00213-003-1474-2. [DOI] [PubMed] [Google Scholar]
- Farre M, de la Torre R, Llorente M, Lamas X, Ugena B, Segura J, Cami J. Alcohol and cocaine interactions in humans. J Pharmacol Exp Ther. 1993;266:1364–1373. [PubMed] [Google Scholar]
- Farre M, de la Torre R, Gonzalez ML, Teran MT, Roset PN, Menoyo E, Cami J. Cocaine and alcohol interactions in humans: neuroendocrine effects and cocaethylene metabolism. J Pharmacol Exp Ther. 1997;283:164–176. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Ethanol and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1988;31:877–883. doi: 10.1016/0091-3057(88)90399-1. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pippen PA, Kelly TH. Behavioral effects of cocaine alone and in combination with ethanol or marijuana in humans. Drug Alcohol Depend. 1993;32:93–106. doi: 10.1016/0376-8716(93)80001-u. [DOI] [PubMed] [Google Scholar]
- Furr CD, Delva J, Anthony JC. The suspected association between methamphetamine (‘ice’) smoking and frequent episodes of alcohol intoxication: data from the 1993 National Household Survey on Drug Abuse. Drug Alcohol Depend. 2000;59:89–93. doi: 10.1016/s0376-8716(99)00078-2. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Green KA, Mourgues P. Longitudinal investigation of methamphetamine use among gay and bisexual men in New York City: findings from Project BUMPS. J Urban Health. 2005;82:i18–i25. doi: 10.1093/jurban/jti020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology. 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood and sleep. JAIDS. 2007;45:545–554. doi: 10.1097/QAI.0b013e31811ed205. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Methamphetamine self-administration by humans. Psychopharmacology. 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Nasser J, Foltin RW. Methamphetamine attenuates disruptions in performance and mood during simulated night-shift work. Psychopharmacology. 2003;169:42–51. doi: 10.1007/s00213-003-1464-4. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Rush CR, Bickel WK, Hughes JR, Lynn M, Capeless MA. Acute behavioral and cardiac effects of cocaine and alcohol combinations in humans. Psychopharmacology. 1993;111:285–294. doi: 10.1007/BF02244943. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: dose and time profiles of marijuana, amphetamine, alcohol, and diazepam. J Anal Toxicol. 1993;17:264–272. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Sherwood N, Hindmarch I. Separate and combined effects of the social drugs on psychomotor performance. Psychopharmacology. 1991;104:113–119. doi: 10.1007/BF02244564. [DOI] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Price LH, McDougle CJ, Kosten TR, Black JE, Jatlow PI. Concurrent cocaine-ethanol ingestion in humans: pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology. 1993;111:39–46. doi: 10.1007/BF02257405. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone—a multiple-dose study. Biol Psychiatry. 1998;44:250–259. doi: 10.1016/s0006-3223(97)00426-5. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst Use Misuse. 2005;40:511–528. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrum Comput. 1982;14:463–466. [Google Scholar]
- Mendelson J, Jones RT, Upton R, Jacob P., 3rd Methamphetamine and ethanol interactions in humans. Clin Pharmacol Ther. 1995;57:559–568. doi: 10.1016/0009-9236(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Miller TP, Taylor JL, Tinklenberg JR. A comparison of assessment techniques measuring the effects of methylphenidate, secobarbital, diazepam and diphenhydramine in abstinent alcoholics. Neuropsychobiology. 1988;19:90–96. doi: 10.1159/000118441. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, de Wit H, Zacny JP. Effects of varying ethanol dose on cigarette consumption in healthy normal volunteers. Behav Pharmacol. 1995:359–365. [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Kelly BC, Weiser JD. Initiation into methamphetamine use for young gay and bisexual men. Drug Alcohol Depend. 2007;90:135–144. doi: 10.1016/j.drugalcdep.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez AY, Kirkpatrick MG, Gunderson EW, Marrone G, Silver R, Foltin RW, Hart CL. Residual effects of intranasal methamphetamine on sleep, mood, and performance. Drug Alcohol Depend. 2008;94:258–262. doi: 10.1016/j.drugalcdep.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes M, Jeffcoat AR. Ethanol/cocaine interaction: cocaine and cocaethylene plasma concentrations and their relationship to subjective and cardiovascular effects. Life Sci. 1992;51:553–563. doi: 10.1016/0024-3205(92)90224-d. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, White WR, McDonald SA, Hicks RE. Interaction between ethanol and dextroamphetamine: effects on psychomotor performance. Alcohol Clin Exp Res. 1992;16:75–81. doi: 10.1111/j.1530-0277.1992.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Res Health. 2001;25:101–109. [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. Binge use of methamphetamine among HIV-positive men who have sex with men: pilot data and HIV prevention implications. AIDS Educ Prev. 2003;15:133–147. doi: 10.1521/aeap.15.3.133.23835. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of pharmacologic calculations. Springer; Heidelberg, Germany: 1981. [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST. d-amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology. 2000;153:85–92. doi: 10.1007/s002130000600. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]


