Highlights
► Outlined the need of novel strategies for cancer therapies that can counteract problems arising particularly in chemotherapy due to resistance to current drugs and their low specificity. ► Elaborated the differences in membrane composition and properties between cancer and non-cancer cells, the basis for the use of anticancer peptides derived from host defense peptides as new weapons against cancer. ► Described the current knowledge on the mode of action of these peptides and the status of in vivo studies. ► Summarized the challenges and perspectives for the development of host defense peptides as novel anticancer agents.
Abbreviations: PS, phosphatidylserine; bLFcin, bovine lactoferricin; pHLIP, pH (low) insertion peptide
Keywords: Anticancer peptides, Cancer cell membrane, Cancer selective toxicity, Targeted cancer therapy, Membrane permeabilization, Phosphatidylserine exposure
Abstract
Although much progress has been achieved in the development of cancer therapies in recent decades, problems continue to arise particularly with respect to chemotherapy due to resistance to and low specificity of currently available drugs. Host defense peptides as effector molecules of innate immunity represent a novel strategy for the development of alternative anticancer drug molecules. These cationic amphipathic peptides are able to discriminate between neoplastic and non-neoplastic cells interacting specifically with negatively charged membrane components such as phosphatidylserine (PS), sialic acid or heparan sulfate, which differ between cancer and non-cancer cells. Furthermore, an increased number of microvilli has been found on cancer cells leading to an increase in cell surface area, which may in turn enhance their susceptibility to anticancer peptides. Thus, part of this review will be devoted to the differences in membrane composition of non-cancer and cancer cells with a focus on the exposure of PS on the outer membrane. Normally, surface exposed PS triggers apoptosis, which can however be circumvented by cancer cells by various means.
Host defense peptides, which selectively target differences between cancer and non-cancer cell membranes, have excellent tumor tissue penetration and can thus reach the site of both primary tumor and distant metastasis. Since these molecules kill their target cells rapidly and mainly by perturbing the integrity of the plasma membrane, resistance is less likely to occur. Hence, a chapter will also describe studies related to the molecular mechanisms of membrane damage as well as alternative non-membrane related mechanisms. In vivo studies have demonstrated that host defense peptides display anticancer activity against a number of cancers such as e.g. leukemia, prostate, ascite and ovarian tumors, yet so far none of these peptides has made it on the market. Nevertheless, optimization of host defense peptides using various strategies to enhance further selectivity and serum stability is expected to yield novel anticancer drugs with improved properties in respect of cancer cell toxicity as well as reduced development of drug resistance.
1. Introduction
Cancer is a leading cause of death worldwide representing about one-eighth of all deaths (http://www.who.int/mediacentre/factsheets/fs297/en/) and being strongly affected by demographic changes especially ageing of the population. In 2008, more than 12.7 million people were newly diagnosed with cancer accounting for 7.6 million deaths, with over one quarter of the global cancer incidences occurring in Europe. Furthermore, cancer has also emerged as a major public health problem in developing countries. According to the World Health Organization new cases of cancer impairment will strongly increase with estimated death rates of up to 11 million in the year 2030. Although in recent decades much progress has been achieved in respect of therapies, like surgery, chemotherapy, radiation or hormone ablation therapy, they are not successful in more than 50% of cases (http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=900). Furthermore, for those who survive the risk of reoccurrence of the disease is a major problem. If the tumor progresses or reoccurs, chemotherapy is the standard treatment. However, resistance as well as potential toxicity and the many side effects of chemotherapeutics, which are mainly due to inadequate specificity for tumor cells, represent a major limitation in this type of therapy. Hence, increasingly the identification of more specific targets generally expressed within a certain tumor type is a major issue in anticancer research. While considerable attention is paid to receptors of growth factors and proteins involved in cell–cell signaling relatively little effort has been devoted to targeting cancer cell membranes per se. Thus, this review will focus on a promising new approach in cancer therapy which is based on host defense peptides – effector molecules of innate immunity that specifically target cancer cells and destroy them within minutes, mostly by damaging cell membrane without binding to specific receptors.
2. Deficits and drawbacks of conventional cancer therapies – need for novel treatment strategies
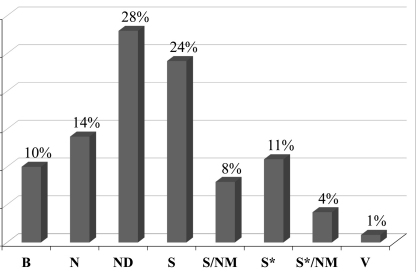
Before development of chemotherapeutics in the 1940s (Shewach and Kuchta, 2009), surgical removal of tumor tissue was the only available treatment and can still often be successfully applied for localized cancers for which radiation therapy is also widely used to target cells by directly damaging DNA (Tofilon and Camphausen, 2009). Nevertheless, many cancer types advance rapidly, reoccur or tend to metastasize making chemotherapy the treatment of choice (Espinosa et al., 2003). Currently used anticancer drugs are mostly based on alkylating agents, antimetabolites as well as natural products and derivatives thereof. Between 1940 and 2006, 175 new anticancer drugs were approved (Newman and Cragg, 2007). More than 50% thereof are biological (10% are usually large peptides and proteins), natural (14%) or naturally derived products (28% are semisynthetic modifications) (Fig. 1). Early compounds were based on nitrogen mustards, a powerful class of agents that alkylate bases of DNA (Shore, 1947), leading to cell death. A second class of chemotherapeutics, the antimetabolites, such as aminopterin and amethopterin, do not directly interact with DNA bases but interfere with synthesis of precursors as folate and cause mistakes during DNA replication of cancer cells (Shewach and Kuchta, 2009). In the 1960s, natural-product anticancer drugs started to be developed such as e.g. Vinca alkaloids that inhibit microtubule polymerization and thereby cell division or antracyclines that promote cell death upon intercalation in DNA and inhibition of replication (Chaires, 1985; Capranico et al., 1990). In the decades that followed a large number of further chemotherapeutics such as taxol or halychondrines and E7389 were developed that interfere with a variety of biological targets (Jackson et al., 2009).
Fig. 1.
New anticancer drugs (1940s-06/2006) subdivided by source adapted from (Newman and Cragg, 2007): (B) biological; (N) natural product; (ND) derived from natural product – semisynthetic modifications; (S) synthetic drug; (S/NM) synthetic/natural drug mimic; (S*) made by total synthesis; (S*/NM) made by total synthesis/natural drug mimic; (V) vaccine.
2.1. Side effects owing to insufficient selectivity
Most conventional chemotherapeutics, however, exhibit insufficient selectivity against healthy mammalian cells causing deleterious side effects (Smith et al., 2000; Cassidy and Misset, 2002; Kalyanaraman et al., 2002). Commonly, these agents act on cells that divide rapidly, one of the main properties of cancer cells. Therefore, damage can be expected in particular in rapidly dividing normal cells, like bone marrow, gastrointestinal mucosa and hair follicles, resulting in the most common side effects of chemotherapy like myelosuppression (decreased production of blood cells), mucositis (inflammation of the lining of the digestive tract) and alopecia (hair loss). Newer chemotherapies, such as the use of monoclonal antibodies (Nabholtz and Slamon, 2001), immunotoxins (Pastan and Kreitman, 1998) or angiogenesis inhibitors (Miller et al., 2009), target specific differences between tumors and healthy cells and tissues, thereby exhibiting lower toxicity. However, side effects have also been observed.
2.2. Formation of resistance
Besides toxicity, resistance to treatment with anticancer drugs is a huge problem. Resistance can be intrinsic and can result from individual variations in patients and somatic cell genetic differences in tumors (Gottesman, 2002). Furthermore, acquired resistance has become common, by e.g. expression of one or more energy-dependent transporters that detect and eject anticancer drugs from cells before interaction with intracellular targets can occur, or acquired insensitivity to drug induced apoptosis and induction of drug-detoxifying mechanism (Gottesman, 2002; Gillet and Gottesman, 2010; Perez-Tomas, 2006). Hence, many cancer types e.g. malignant melanoma (Schadendorf et al., 1994; Helmbach et al., 2001) show only slight sensitivity to anticancer drugs. Treatment with methylating antitumor agent dacarbazine and its oral equivalent temozolomide exhibits a response of only 15% (Chapman et al., 1999; Middleton et al., 2000). In metastatic melanoma, resistance is reported to be linked to defective apoptosis due to inhibition of expression of a gene encoding Apaf-1, the apoptotic protease activating factor-1 (Soengas et al., 2001). A number of multidrug-resistant cancers have been detected and thus various approaches including the development of drugs that engage, evade or exploit efflux by ABC transporters have been described (Szakacs et al., 2006).
Despite all the progress in the design of cancer therapy there is still no cancer treatment that is 100% effective against disseminated cancer (Gottesman, 2002). By contrast, the overall contribution of curative and adjuvant cytotoxic chemotherapy to 5-year survival in adults was estimated to be only 2.3% in Australia and 2.1% in the USA (Morgan et al., 2004). Therefore, the development of new anticancer drugs based on novel mode of actions with improved cancer cell selectivity is urgently needed. Furthermore, patients suffering from cancer with poor treatability like glioblastoma, a cancer of the brain, do not survive longer than 1–2 years even if the cancer is detected early and treated with surgery, chemotherapy, and radiotherapy, (CBTRUS, 2008). Stomach, ovarian and other cancers also demand new effective treatment.
3. Host defense peptides selectively targeting differences between cancer and non-cancer cell membranes
In the search for novel anticancer agents, host defense peptides have emerged as potential alternative anticancer therapeutics offering many advantages over other therapies. Because of their mode of action and specificity – the cell membrane being the major target – resistance and cytotoxicity are less likely to occur (Hoskin and Ramamoorthy, 2008; Papo and Shai, 2005; Mader and Hoskin, 2006; Schweizer, 2009) and thus, they are also expected to cause fewer side effects. Furthermore, these peptides mostly damage cell membranes within minutes (Cruciani et al., 1991), which would hinder formation of resistance (Papo and Shai, 2005). Host defense peptides being part of the innate immune system of many diverse species (e.g. mammals, insects, amphibians) were initially discovered because of their antimicrobial activity (Zasloff, 2002). Currently, the antimicrobial peptide database lists more than 100 natural host defense peptides with antitumor activity (Wang and Wang, 2004; Wang et al., 2009b). Selected host defense peptides for which in vitro studies were reported are listed in Table 1. The most intensively studied antimicrobial peptides that also exhibit cytotoxic activity against cancer cells were described in detail in terms of structure, mode of action and anticancer activity in a recent review by Hoskin and Ramamoorthy (2008). These host defense peptides are characterized by low molecular weight (in the majority of cases less than 30 amino acids) as well as low antigenicity (Iwasaki et al., 2009) and exhibit a predominantly cationic amphipathic structure making them prone to interact with anionic cell membrane surfaces (Lohner and Blondelle, 2005; Lohner, 2009). Indeed, various anionic molecules, discussed in the following, are more abundant on the surface of cancer cells as compared to non-cancer cells. This increased level of negative charge seemingly accounts for the selectivity of these membrane-active peptides towards cancer cells. In addition, one can envisage that anticancer peptides, which translocate to the cytosol, interact preferentially with the mitochondrial membrane owing to its high content of negatively charged lipids such as cardiolipin. As a consequence, the peptides may trigger apoptosis. Of note, mitochondrial membranes of different melanoma variants were shown to also contain significant amounts of phosphatidylserine (Schroeder and Gardiner, 1984). Furthermore, the susceptibility of cancer and non-cancer cells towards anticancer peptides is governed by differences in their membrane fluidity and/or by morphological changes such as the increased number of microvilli found on cancer cells. Fig. 2 outlines these and additional parameters such as acidification of cancer cell environment that may be of benefit in the anticancer activity of histidin-rich peptides as well as some important processes that suppress apoptosis in cancer cells.
Table 1.
Selected host defense peptides tested in in vitro studies for anticancer activity.a
| Peptide/Source | Sequence | In vitro study |
|---|---|---|
| α-Helical | ||
| Aureins Southern bell frog |
GLFDIIKKIAESF (aurein 1.2) |
60 cancer cell lines tested (human tumor line testing program of the US National Cancer Institute) (∼50 active) (Rozek et al., 2000) |
| BMAP-27/BMAP-28 Bovine |
GRFKRFRKKFKKLFKKLSPVIPLLHLG/ GGLRSLGRKILRAWKKYGPIIVPIIRIG |
Human tumor cells, leukemic cells (Risso et al., 1998) |
| Cecropin A Hyalophora cecropia Cecropin B Chinese oak silk moth |
KWKLFKKIEKVGQNIRDGIIKAGPAVAVVGQATQIAK KWKIFKKIEKVGRNIRNGIIKAGPAVAVLGEAKAL |
Bladder cancer (Suttmann et al., 2008) Leukemia (Chen et al., 1997), bladder cancer (Suttmann et al., 2008) |
| Citropins Australian blue mountains tree frog |
GLFDVIKKVASVIGGL (Citropin 1.1) |
60 human cancer cell lines (human tumor line testing program of the US National Cancer Institute) (Doyle et al., 2003); human histiocytic lymphoma cell line (Koszalka et al., 2011) |
| Epinicidin-1 Fish |
GFIFHIIKGLFHAGKMIHGLV | Human lung carcinoma, cervix adenocarcinoma, hepatocellular carcinoma, fibrosarcoma, histiocytic lymphoma cells (Lin et al., 2009) |
| Gaegurin-6/-5 Korean wrinkled frog |
FLPLLAGLAANFLPTIICKISYKC | Human lung, prostate, liver, kidney and breast cancer cell lines (Kim et al., 2003) |
| LL-37 Homo sapiens |
LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | Jurkat human T leukemia cells, HeLa cells (Mader et al., 2009) |
| Magainins African clawed frog Pexiganan (MSI-78) |
GIGKFLHSAKKFGKAFVGEIMNS (Magainin 2) GIGKFLKKAKKFGKAFVKILKK |
Hematopoietic cell lines (Cruciani et al., 1991), Ehrlich ascites tumor cells, human adenocarcinoma (Baker et al., 1993), bladder cancer line (Lehmann et al., 2006), histiocytic lymphoma cell line (Koszalka et al., 2011) |
| Melittin Honey bee venom |
GIGAVLKVLTTGLPALISWIKRKRQQ | Human hepatocellular carcinoma (Wang et al., 2009a); osteosarcoma (Chu et al., 2007); leukemic cells (Moon et al., 2008); prostate and ovarian (Holle et al., 2003), neuroblastoma cancer cells (Drechsler and Andrä, 2011) |
| NK-2 Porcine leukocytes NKCS and derivatives |
KILRGVCKKIMRTFLRRISKDILTGKK KILRGVSKKIMRTFLRRISKDILTGKK |
Human lymphocytes and seven human cancer cell lines (Schröder-Borm et al., 2005); human neuroblastoma cancer cells (Drechsler and Andrä, 2011) Human prostate carcinoma cells (Manavbasi et al., 2010) |
| Pep27 Strep. pneumonia |
MRKEFHNVLSSGQLLADKRPARDYNRK | Leukemia cell lines (Lee et al., 2005) |
| Polybia-MPI P. paulista |
IDWKKLLDAAKQIL | Human bladder cancer and prostate cancer cell line (Wang et al., 2008) |
| Temporin A Frog Rana temporaria |
FLPLIGRVLSGIL | Human histiocytic lymphoma cell line (Koszalka et al., 2011) |
| β-sheet | ||
| Buforin II Bufo bufo gargarizans |
TRSSRAGLQFPVGRVHRLLRK | 62 cell lines (Lee et al., 2008); human histiocytic lymphoma cell line (Koszalka et al., 2011) |
| Human α-defensins Homo sapiens Defensins |
ACYCRIPACIAGERRYGTCIYQGRLWAFCC (HNP1) Diverse sequences |
Human lung adenocarcinoma (Xu et al., 2008) HeLa, glioma, kidney cancer, lung cancer, myeloma (Iwasaki et al., 2009) |
| Gomesin A. gomesiana |
ECRRLCYKQRCVTYCRGR | Breast and colon adenocarcinoma, HeLa (Rodrigues et al., 2002) |
| Lactoferricin B Bovine |
FKCRRWQWRMKKLGAPSITCVRRAF | Neuroblastoma (Eliassen et al., 2006), melanoma, mammary carcinoma, colorectal adenocarcinoma (Eliassen et al., 2003) |
| Protegrin-1 porcine |
RGGRLCYCRRRFCVCVGR | Human histiocytic lymphoma cell line (Koszalka et al., 2011) |
| Tachyplesin I Southeast Asian horseshoe crab |
KWCFRVCYRGICYRRCR | Human gastric adenocarcinoma (Shi et al., 2006); human hepatocarcinoma cells (Li et al., 2003); |
| Others | ||
| PR-39 Porcine small intestine |
RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPPRFPPRFP | Human hepatocellular carcinoma cells (Ohtake et al., 1999) |
| Alloferon-1/-2 Insects |
HGVSGHGOHGVHG/GVSGHGVHG | Mice (IFN-production) (Chernysh et al., 2002) |
| Dolastatin 10 D. auricularia |
Dov-Val-Dil-Dap-Doeb | Murine PS leukemia cells (Pettit et al., 1987) |
For a complete list of anticancer peptides check http://aps.unmc.edu/AP/database/antiC.php.
Dolavaline (Dov), dolaisoleuine (Dil), dola-proine (Dap), dolaphenine (Doe).
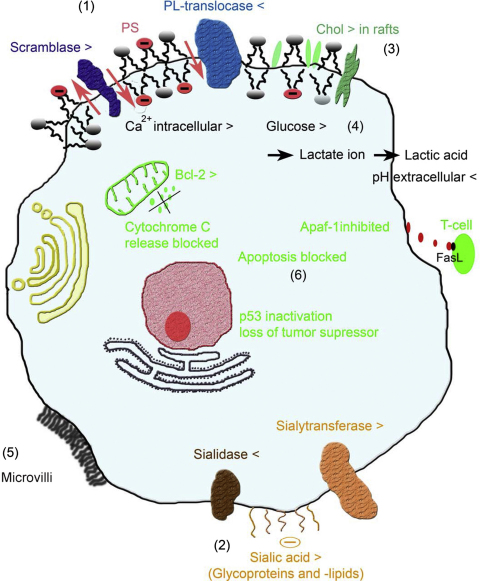
Fig. 2.
Sketch of specific characteristics of cancer cells concerning membrane and genetic changes. Selectivity of cationic anticancer peptides can be driven by direct interaction with anionic target molecules exposed on the cell surface such as PS. Exposure of PS (1) is related to several cell processes, which involves activation of a putative scramblase and inactivation of a putative ATP-dependent phospholipid (PL) – translocase. Increased levels of sialic acid of glycolipids and proteins on cancer surfaces (2), normally providing a protective shield, can also be a target for the positively charged peptides. Furthermore, changes in membrane fluidity (3) and pH (4) influence susceptibility and activity of peptides, which may also be affected by the increased surface area of cancer cells owing to the higher number of microvilli (5) present. Finally, peptides, which translocate into the cytosolic compartment, may interact with anionic lipids of mitochondria, triggering apoptosis, a process normally blocked by several changes in tumor cells such as e.g. inactivation of the tumor suppressor p53 (6). For details see main text.
3.1. Anionic phosphatidylserine exposed on the outer membrane leaflet of cancer cells
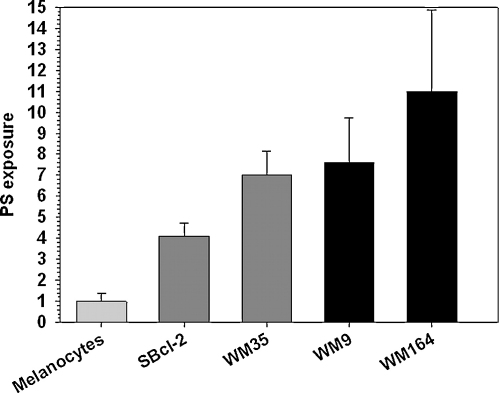
Indeed, one of the major differences between cancer and non-cancer cell-surfaces is the exposure of the negatively charged lipid phosphatidylserine (PS) on the outer leaflet of the cancer cell membrane, which in non-cancer cells exhibits an overall neutral charge due to the zwitterionic phosphatidylcholine and sphingomyelin (Bevers et al., 1996). Phosphatidylserine together with the major part of phosphatidylethanolamine is normally located in the inner leaflet of eukaryotic plasma membranes (Bevers et al., 1996). This asymmetric distribution of the major phospholipids between the two membrane leaflets is well documented (Zwaal and Schroit, 1997; Bevers et al., 1998) and assumed to be maintained by ATP-dependent aminophospholipid translocase activity (Seigneuret and Devaux, 1984) and/or abolished by a Ca2+-dependent scramblase activity, which triggers non-specific movement of phospholipids to both leaflets (Zwaal and Schroit, 1997). The loss of membrane asymmetry was demonstrated for several cancer types, revealing a promising uniform marker for cancer. Within some studies, PS exposed on the surface could even be visualized in vitro and in vivo by specific interaction with Annexin A5 and linked to a fluorophore (Dobrzynska et al., 2007; Szachowicz-Petelska et al., 2002; Riedl et al., 2011b). Since the pioneering work of Utsugi et al. (1991) showing that tumourigenic cells expose 3–7-fold more PS than normal keratinocytes, PS exposure has been described for human ovarian carcinoma (Rao et al., 1992), human gastric carcinoma (Woehlecke et al., 2003), different melanomas (Kirszberg et al., 2009; Cichorek et al., 2000) and leukemias (Connor et al., 1989; Schröder-Borm et al., 2005). In an extension of these studies, our laboratory focused on cancer with poor outcome and treatment efficacy including metastases as well as primary cell cultures and demonstrated that differently tumorigenic melanoma cell lines expose 4–11 times more PS than melanocytes (Fig. 3), whereby the amount of exposed PS correlated with tumor progression (Zweytick et al., 2011a; Riedl et al., 2011a). Furthermore, we proved that not only do cell lines derived from primary lesions and metastasis show PS on the outer leaflet of the cell membrane but also cells from primary cancer cell cultures. The number of different cancer types used in our study including (malignant) melanoma, a rhabdomyosarcoma, a malignant soft tissue tumor of mesenchymal origin, prostate cancer, renal cell cancer and a glioblastoma extended and strongly supported earlier findings, suggesting that PS exposure is a general phenomenon of cancer cells. In general, cells expressing PS in the outer leaflet of the plasma membrane are specifically recognized by the presence of macrophages (Fadok et al., 1992; Martin et al., 1995) and dendritic cells (Albert et al., 1998) triggering apoptosis. However tumor cells, although exposing PS, circumvent apoptosis and prevent recognition by macrophages (Fig. 2). For example, lung and colon cancer cells secrete elevated levels of a molecule that binds to FasL, a death activator on T-cells, and prevents them being killed. While melanoma inhibit expression of a gene encoding Apaf-1, the apoptotic protease activating factor-1, certain B-cell leukemias and lymphomas express high levels of Bcl-2, a protein that blocks apoptotic signals (Soengas et al., 2001; Miyashita and Reed, 1993). Therefore, cationic host defense peptides, which show an excellent tumor tissue penetration reaching the site of both primary tumor and distant metastasis (Shadidi and Sioud, 2003) and specifically interact with anionic membrane lipids (Zweytick et al., 2006, 2011b; Lohner et al., 1997), represent a promising approach in the development of novel anticancer drugs (Hoskin and Ramamoorthy, 2008; Papo and Shai, 2005; Mader and Hoskin, 2006; Schweizer, 2009). It is also of interest to note that anionic phospholipids are also present on the surface of tumor blood vessels connected to tumor tissue (Ran et al., 2002). The observation of negatively charged tumor vasculature provides an additional potential target for anticancer peptides, which may be also used for imaging. For example, selective staining of vascular endothelium of different tumors and also tumor cells in and around regions of necrosis was shown using 9D2, a monoclonal antibody for anionic phospholipids or Annexin A5 (Ran et al., 2002). Furthermore, it is reported that radio-labeled Annexin A5 may allow for repeated and selective in vivo identification of apoptotic cell death without the need for invasive biopsy (Van de et al., 2003), which should also be applicable in the imaging of tumor tissue.
Fig. 3.
PS exposure of melanoma cell lines: correlation of PS exposure with tumor progression of melanoma cell lines expressed as multiples of PS exposure of normal melanocytes as determined by Annexin A5 binding (SBcl-2, WM35 from primary and WM9, WM164 from metastatic lesions; error bars resulting from four independent experiments).
Data adopted from Riedl et al. (2011a) and Zweytick et al. (2011a).
3.2. Enhancement of sialic acid residues on cancer cell surface
Another source of negative charges on the surface of human cells is represented by sialic acid residues, which are linked to glycoproteins (e.g. mucins) and glycolipids. An overexpression of transmembrane mucins was shown e.g. for carcinoma cells from epithelia (Kufe, 2009; Carraway et al., 1999) and transmembrane mucin 1 (MUC1) was overexpressed in more than 90% of breast carcinomas and frequently in others such as ovarian, lung, colon and pancreatic carcinoma (Bafna et al., 2010). The extent of surface sialylation seems to be directly correlated with the metastatic potential of different cancer cells exhibiting a protective function against the host immune system and facilitating migration and invasion via modulation of adhesiveness (Wang et al., 2009a; Litynska et al., 2001; Cazet et al., 2010). The effect of different degrees of sialylation in terms of peptide interaction has been studied less intensively. For example, BMAP-27 and BMAP-28, host defense peptides from the cathelicidin family, exhibited lower activity on cancer cells when sialic acid had been cleaved off (Risso et al., 1998) suggesting that the outside charge seems to act as an initial interaction site for the peptides. By contrast, in a preliminary study performed in our laboratory the activity of peptides derived from a fragment of human lactoferricin, was unaffected by cleavage of sialic acid moieties of a rhabdomyosarcoma (unpublished data), suggesting something other than sialic acid as a preferred target for this tumor type. Summarizing, it is obvious that glycosylation plays an important role in the interaction with peptides. For more details about changes in the glycosylation pattern in cancer cells and impact on cell processes like cancer progression and impairment of signaling pathways see Hoskin and Ramamoorthy (2008).
3.3. Heparan sulfate
In addition to the above mentioned sources of negative charges on the surface of cancer cells, the role played by proteoglycans should also be noted (Kjellen and Lindahl, 1991). These proteins contain highly negatively charged glycosaminoglycan side chains mainly in the form of heparan sulfate and chondroitin sulfate composed of linear repeats of sulfated disaccharides (Sugahara et al., 2003; Tkachenko et al., 2005). These disaccharides contain a high number of sulfate groups yielding highly negatively charged molecules (Rabenstein, 2002). It has been reported for several cancer cells that proteoglycan expression and sulfation is altered (Jayson et al., 1998; Kleeff et al., 1998; Safaiyan et al., 1998; Matsuda et al., 2001; Sanderson, 2001; Aviel-Ronen et al., 2008; Nakatsura et al., 2004). Fadnes et al. (2009) reported that chondoitrin sulfate had no effect on the activity of bovine lactoferricin (bLFcin), which however was improved after depletion of heparan sulfate on cancer cells and lowered upon addition of exogenous heparan sulfate. Although this observation seemingly implies that heparan sulfate may inhibit the activity of anticancer peptides, the same group showed recently that small lytic peptides derived from bLFcin are not affected by heparan sulfate or even possess increased activity in their presence (Fadnes et al., 2011).
3.4. Altered membrane fluidity in cancer cell membranes
Apart from changes in the pattern of the surface charge, membrane fluidity also appears to be altered in tumorigenic cells, though it is not clear if this alteration is uniform throughout all carcinomas. The majority of studies indicate increased fluidity of the cancer cell membrane as described for lymphomas, lung carcinomas and neural tumors (Sherbet, 1989; Sok et al., 2002; Campanella, 1992; Rodrigues et al., 2002). By contrast, it has been reported that cells from solid tumor tissues (e.g. hepatoma) exhibit a lower membrane fluidity than their healthy counterparts (van Blitterswijk, 1984). In this context it is important to note that Sherbet et al. (Sherbet and Jackson, 1986) already suggested that membrane fluidity is not uniform all over the cell itself. This is in accordance with more recent observations showing that some breast and prostate cancer cell lines possess elevated levels of cholesterol-rich lipid rafts, raising the question if in fact only some parts of the cancer membrane comprise increased fluidity by different lateral lipid arrangement (Li et al., 2006). In the case of unaltered total cholesterol content, this would imply a cholesterol-depleted bulk membrane harboring PS that may exhibit higher susceptibility to peptide attack because of its increased fluidity and hence less tightly packed lipids. Interestingly, the metastatic cascade also seems to be influenced by alterations in membrane fluidity. Thus, increased fluidity was reported for metastatic cells (Nakazawa and Iwaizumi, 1989) due to differences in the cholesterol to phospholipid ratio (Schroeder and Gardiner, 1984). According to some reports, resistance to drugs also seems to be related to cell membrane fluidity. May et al. (1988) for example reported that vinblastine resistant leukemic cells possess 50% more cholesterol, corresponding to decreased fluidity, than vinblastine sensitive cells. This finding is supported by higher cholesterol levels in multidrug-resistant ovarian cancer cells (Mazzoni and Trave, 1993). It is evident that knowledge of membrane fluidity (with cholesterol content only being one parameter alongside degree of hydrocarbon chain saturation etc.) should be regarded as an important physical membrane parameter that interferes with insertion of amphipathic peptides being facilitated in more fluid membranes.
3.5. Microvilli increasing the cell surface area of cancer cells
Another important difference between cancer and non-cancer cells is the increase in surface area of tumorigenic cells, triggered by the increase of microvilli on these cells (Zwaal and Schroit, 1997; Papo and Shai, 2005; Domagala and Koss, 1980; Chaudhary and Munshi, 1995). This should be beneficial in the application of membrane-active peptides since efficiency can be increased by enabling the binding of a larger amount of peptides per cell (Zwaal and Schroit, 1997; Papo and Shai, 2005). Support for this notion comes from a comparative study on the cytolytic effect of cecropin B on tumor cells such as KG-1 leukemia and Ags stomach carcinoma and non-tumor cells like fibroblasts and red blood cells, which demonstrated that the peptide was more effective on the cancer than normal cells (Chan et al., 1998). Based on scanning electron microscopy data, it was suggested that this may mainly be due to the high population of irregular microvilli on the cell surface of the cancer cells and that the attraction of peptides by microvilli may be one of the main driving forces before membranolysis can be efficiently initiated.
In summary, accumulation of cationic amphipathic peptides will be triggered by the presence of anionic components on the cell surface as well as by the increase in cell surface owing to increased number of microvilli, while insertion of peptides will be mainly governed by membrane fluidity, which in eukaryotic membranes is largely regulated by its cholesterol content. Therefore, the differences in lipid composition and morphology of cell membranes govern the interaction with membrane-active peptide and furthermore, may also be related to the varying susceptibility of different types of cancer cells against a certain antitumor peptide (Leuschner and Hansel, 2004; Mader et al., 2005).
4. Mode of action of anticancer peptides
Two general mechanisms, triggering necrosis or apoptosis by anticancer peptides as a consequence of their membrane related mode of killing of cancer cells, have been discussed (Papo and Shai, 2005; Bhutia and Maiti, 2008; Schweizer, 2009). Both killing based on necrosis via cell membrane lysis and apoptosis via the mitochondrial lytic effect seems to be dependent on the presence of anionic lipids. As described in Section 3.1, negatively charged PS located in the outer leaflet of cancer cells can represent such a specific target, e.g. cardiolipin, a major anionic phospholipid of the mitochondrial membrane. Other sources of negatively charged molecules which are also exposed and often overexpressed on non-cancer plasma membranes such as sialylated glycoproteins or heparan sulfate proteoglycans should also be considered when considering the activity and efficacy of cationic anticancer peptides. However, their influence has been studied less systematically and seemingly contradictory effects have been reported in terms of anticancer activity of the peptides (see Sections 3.2 and 3.3). A major role of PS may be deduced from the selective binding of the synthetic host defense-like membranolytic peptide, [d]-K6L9, to PS, which was demonstrated by co-localization of PS and peptides on the outside of cancer cells (Papo et al., 2006). The authors thus suggested that the selectivity of the peptide towards cancer cells stems largely from its binding to surface exposed PS and that killing of the cancer cells occurs via cytoplasmic membrane depolarization. Consequently, it appears that for effective disruption of the cell membrane, peptide–lipid interaction is the crucial step which the peptides need in order to meet the proper structural requirements. Thus, in order to engineer more potent antitumor peptides in respect of efficacy as well as selectivity and thus reduced toxicity, it is necessary to further unravel their molecular mode of action.
Furthermore, non-membrane related mechanisms have been considered. For example, stimulation of NK cells as well as interferon synthesis by the insect antimicrobial peptide alloferon (Chernysh et al., 2002) or the transcriptional inhibitory effect of melittin and cecropin (Winder et al., 1998) were regarded as being probably due to an indirect mechanism, which is mediated by the ability of the cytolytic peptide to interfere with signal transduction pathways. Tachyplesin conjugated to an integrin homing domain (Park et al., 1992; Chen et al., 2001) was further shown to interact with the mitochondrial membranes of cancer cells and to up-regulate members of the death receptor pathway, which suggests that this peptide-conjugate also has more than one effect on cancer cells. Therefore, Papo and Shai (2005) proposed that these observations may imply that in addition to their being active on their own cytolytic peptides may activate or act synergistically with other host defense components in order to clear tumors. Nevertheless, since killing takes place very rapidly it seems quite reasonable to assume that a non-receptor mediated membranolytic killing mechanism is responsible for the majority of anticancer peptides derived from host defense peptides.
4.1. Membrane related molecular mechanisms of cell killing
The discovery that naturally occurring antimicrobial peptides mostly kill bacteria by damaging their cell membrane has initiated much effort in the detailed study of peptide–membrane interaction. The concept of a characteristic lipid composition for a given cell membrane is well accepted and hence the different physicochemical properties of the lipids found in biological membranes allow host defense peptides to discriminate between e.g. bacterial, cancer and non-cancer cell membranes (Lohner et al., 1997; Riedl et al., 2011b; Lohner, 2001). In a simplified view, these cationic amphipathic peptides will exhibit a higher affinity to cell membranes that expose negatively charged lipids on their outer membrane leaflet. The molecular mechanism(s) of membrane damage mutually depends on the nature of both peptides and membrane lipids (Sevcsik et al., 2008).
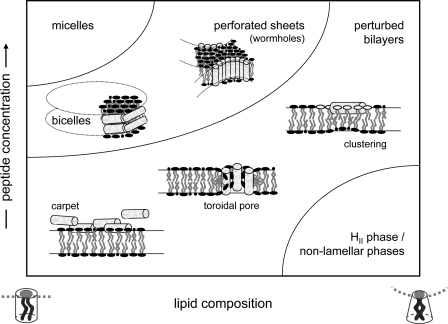
Several models (Fig. 4) have been suggested to explain their biological activity (for reviews see e.g. Lohner and Blondelle, 2005; Lohner, 2009; Bechinger, 2009; Wimley and Hristova, 2011). The most frequently discussed mode of action includes the formation of toroidal pores (Matsuzaki et al., 1996; Ludtke et al., 1996) and the carpet model (Shai, 2002). In the toroidal pore model peptides, together with the lipid, assemble into a supra-molecular arrangement of high curvature forming a water-filled pore. Although a number of molecules including α-helical and β-sheet type peptides have been shown to adopt such a pore under the given experimental conditions (Huang, 2006), there is little evidence that the majority of these peptides act in vivo via transmembrane pores (Wimley, 2010). The carpet model is most commonly cited when explaining membrane disintegration by a non-pore model. In this model, the amphipathic peptides accumulate at the membrane being aligned parallel to the bilayer surface. Once this “peptide carpet” becomes too dense, membrane permeabilization occurs via destabilization of the membrane bilayer (Shai, 2002). Thereby, at the molecular level different scenarios may apply, which were recently summarized in an excellent review by Wimley and Hristova (2011). Briefly explained, membrane permeabilization has been proposed as being due to (a) peptide induced clustering or phase separation of lipids creating defects at the peptide-rich and peptide-poor domains (Lohner, 2009; Epand et al., 2010; Epand and Epand, 2009, 2011; Lohner and Prenner, 1999; Zweytick et al., 2011b), (b) the sinking raft model, which allows a formal thermodynamic analysis to predict membrane perturbation (Pokorny and Almeida, 2004; Pokorny et al., 2008; Gregory et al., 2008; Almeida and Pokorny, 2009), (c) disruption of the normally strict segregation of polar and non-polar groups of the lipids driven by the partitioning of an imperfectly amphipathic peptide into the bilayer interface (Rathinakumar and Wimley, 2008, 2010) or (d) a detergent-like action in particular at high peptide concentration (Hristova et al., 1997; Bechinger and Lohner, 2006; Ostolaza et al., 1993). Furthermore, a complex generic phase diagram has recently been developed rationalizing the membrane interactions of cationic amphipathic peptides by their molecular shape to explain in a more general sense the mode of action of these cationic amphipathic peptides (Bechinger and Lohner, 2006; Bechinger, 2009). Besides electrostatic interactions, molecular properties of lipids such as their molecular shape and being related to the spontaneous curvature are known to play a significant role for the aggregation state of lipids (Israelachvili et al., 1980; Marsh, 1996; Bechinger, 2009) and, thus, also to affect interactions of these assemblies with membrane-active peptides. In fact in terms of structural membrane changes, insertion of antimicrobial peptide molecules, some of them also exhibiting antitumor activity such as NK-2 (Schröder-Borm et al., 2005) or lactoferricin derivatives (Riedl et al., 2011b), promoted the formation of inverted hexagonal or bi-continuous cubic phases in membrane mimetic systems (Staudegger et al., 2000; Willumeit et al., 2005; Hickel et al., 2008; Zweytick et al., 2008, 2011b).
Fig. 4.
Mode of action of membrane-active host defense peptides: boundaries of the schematic phase diagram of amphipathic peptide/phospholipid aggregates are given as a function of the concentration of membrane-associated peptide and the composition of the membrane from mixtures of cylindrical (phosphatidylserine, phosphatidylcholine) and truncated inverted cone lipids (cardiolipin, phosphatidylethanolamine); modified from Bechinger (2009). Membrane association of the cationic peptides is strongly increased in the presence of anionic phospholipids such as PS or cardiolipin. In the presence of cholesterol, an abundant membrane component in mammalian cells, the bilayer will be stabilized, i.e. the phase region of stable bilayers will be enhanced. Some molecular mechanisms of bilayer perturbation are schematically shown (for details see Section 4.1).
Furthermore, solid tumors are discussed as exhibiting an acidic extracellular environment (for a review see Griffiths, 1991). This acidification seems to be a consequence of lactic acid accumulation, which is produced during aerobic and anaerobic glycolysis and, due to inadequate vasculature in growing tumors, its insufficient removal. However, lactic acid accumulation does not seem to be the only reason for the acidification of the extracellular environment of tumors. Newell et al. (1993) reported that glycolysis-deficient cells are also more acidic than cells from normal tissues without accumulating lactic acid above serum levels. In any case, an acidic tumor environment might be a useful “activation” switch for anticancer peptides comprised of amino acids like histidine, which are only positively charged and thus active at low pH (Makovitzki et al., 2009). Such an approach was reported for the synthetic host defense-like lytic peptide [d]-K6L9, where the lysine residues were exchanged by histidine, resulting in reduced systemic toxicity compared to the parent peptide. It was suggested that the pH-dependent activity and lytic mode of action of the His-rich peptide, in hindering development of resistance, will make them potential candidates for treatment of solid tumors. In fact, intra-tumor and systemic inoculation of ([d]-H6L9) significantly reduced the 22RV1 prostate cancer tumor size in a xenograft mice model (Makovitzki et al., 2009).
In the context of taking advantage of the acidic environment of solid tumors it is worthwhile reporting on pHLIP (pH (Low) Insertion Peptide), a peptide derived from the C-helix of bacteriorhodopsin, although this peptide neither displays a membranolytic mechanism nor is it derived from a host defense peptide. pHLIP shows interesting pH-sensitive properties in that it only forms a transmembrane helix in the acidic environment of solid tumors, whereas at neutral or high pH it is in equilibrium between an unstructured form and bound to the surface of a lipid bilayer (Hunt et al., 1997; Reshetnyak et al., 2007). pHLIP itself does not exhibit direct toxicity to cells (Reshetnyak et al., 2006) or mice (Andreev et al., 2007) at concentrations below 50 μM, however, the peptide in its helical form possesses useful drug delivery efficiency facilitating translocation of cell-impermeable polar molecules into the cytoplasm (Reshetnyak et al., 2006, 2007; Andreev et al., 2007; Zoonens et al., 2008; Thévenin et al., 2009). One example of efficient drug delivery into cancer cells through translocation with pHLIP is phalloidin toxin, which inhibits cancer cell proliferation after cleavage from pHLIP inside the cells (An et al., 2010).
4.2. Non-membrane related mechanisms
Here, we briefly summarize some mechanisms, which do not involve permeabilization/disruption of plasma or mitochondrial membranes in cancer cells. There is growing evidence that the antimicrobial activity of host defense peptides is not solely related to cell membrane damage but some act caused by immunomodulatory effects (Hancock and Sahl, 2006; Easton et al., 2009; Wieczorek et al., 2010). Little knowledge is so far available as to whether this may also be the case for the antitumor activity of these cationic amphipathic peptides. In particular in the case of cancers where the immune response is often diminished or strongly suppressed, it was suggested that cancer therapies might be supported by the simultaneous administration of immunoadjuvants, which activate both an innate and acquired immune system response (Dredge et al., 2002). Although many small peptides of microbial origin possess a non-specific immunostimulatory response (for a review see Schweizer, 2009), reports on an immunomodulatory effect of host defense peptides in cancer cells are rare. One example are alloferons, histidine-rich peptides from insects shown to stimulate natural killer cells of human peripheral blood lymphocytes and to induce synthesis of interferon in mice models (Chernysh et al., 2002). This cytokine-like modulating property resulted in antitumor resistance.
Specific targets have so far only been reported for a few anticancer peptides. PR-39, a peptide of the cathelicidin family which is rich in proline and arginine residues, can translocate into the cytosolic compartment of cells without permeabilizing the plasma membrane where it interacts with proteins containing SH3-binding motifs (Chan and Gallo, 1998; Tanaka et al., 2001). Experiments using derivatives of PR-39 indicated that the N-terminal arginine is crucial for binding to the SH3-domain and subsequent induction of syndecan-1 production (Chan et al., 2001). Indeed, elevated levels of syndecan-1 were observed in treatment of human hepatocellular carcinoma cells with either PR-39 or upon transfection of this cell line with the PR-39 gene (Ohtake et al., 1999). Expression of syndecan-1 has been related to inhibition of cell invasion (Liebersbach and Sanderson, 1994) and thus it was suggested that this linear cationic peptide may suppress the invasive activity of these cancer cells therefore preventing metastasis (Ohtake et al., 1999). Another target of many anticancer drugs is tubulin, which was also shown to be the site of action for linear peptides derived from the host defense-like peptide dolastatin 10 (US Pat. No. 4,816,444) or dolastatin 15 (EP-A-398558) (Simmons et al., 2005). Thereby, the mechanism of action, as for all antitumor tubulin ligands, involves the perturbation of microtubule dynamics during the G2/M phase of cell division and subsequent entry into apoptosis (Davis et al., 1999).
Finally, a specific case was reported for bee venom melittin, which preferentially counter-selects mammalian cells with the overexpressed ras oncogene, where it acts through hyperactivation of phospholipase A2 (Sharma, 1992). It should be noted, however, that melittin is also known for its toxicity to mammalian cells (e.g. (Habermann and Jentsch, 1967)). In fact, for in vivo studies a melittin/avidin conjugate was used, which did not exert its anticancer activity by membranolysis but by targeting cancer cells with high matrix metalloproteinase 2 (Holle et al., 2003) as described in Section 5.2.
5. In vivo studies of anticancer peptides
Based on successful in vitro experiments, a number of peptides have also been tested in vivo to explore their therapeutic potential (Table 2). In addition to the administration of anticancer peptides per se, strategies have been developed that may improve pharmacokinetics and/or selectivity and hence reduce toxicity. This includes the application of vector-mediated delivery of genes encoding membranolytic peptides and the use of homing domains transporting the anticancer peptides into the cytosolic compartment of cancer cells. Some of the respective in vivo studies are briefly described below.
Table 2.
In vivo studies of anticancer peptides and proposed mode of action.
| Peptidea | Mode of action | Type of tumor |
|---|---|---|
| α-Helical | ||
| Magainin and analogues | Lytic | Murine ascites tumors (Baker et al., 1993); melanoma xenograft in nude mice (Soballe et al., 1995) |
| Melittin/avidin-conjugate | Lytic | Melanoma in mice (Holle et al., 2003) |
| Polybia-MPI/MPI-1 | Necrosis | Sarcoma xenograft tumors in mice (Zhang et al., 2010) |
| β-sheet | ||
| Human α-defensins | Apoptosis | Human lung adenocarcinoma xenograft in nude mice (Xu et al., 2008) |
| Gomesin | Necrosis | Murine melanoma (Rodrigues et al., 2008) |
| Lactoferricin B | Necrosis/Apoptosis | Fibrosarcoma, melanoma, colon carcinoma (Eliassen et al., 2002); Xenografting of SH-SY-5Y cells in nude rats (Eliassen et al., 2006); inhibition of metastasis of melanoma and lymphoma in mice (Yoo et al., 1997) |
| RGD-tachyplesin | Apoptosis | Melanoma mice (Chen et al., 2001) |
| Others | ||
| Dolastatin 10 | Anti-proliferative | Indolent lymphoma, Waldenstrom's macroglobulinemia and chronic lymphocytic leukemia (Simmons et al., 2005) |
| Synthetic | ||
| [d]-K6L9 | Necrosis | Breast cancer in SCID/NCr mice (Papo et al., 2006) |
| [d]-H6L9 | Lytic | 22RV1 prostate cancer in mice (Makovitzki et al., 2009) |
Source and amino acid composition of peptides are listed in Table 1.
5.1. Anticancer activity of peptides related to membrane damage
Magainins, originally isolated from the skin of the African clawed frog, Xenopus laevis (Zasloff, 1987), were among the first peptides to be tested for their in vivo anticancer activity. Magainin 2 was shown to exhibit an amphiphilic α-helical structure that facilitates the formation of ion-permeable pores in membranes, consequently inducing depolarization and cytolysis (Cruciani et al., 1991). Baker et al. (1993) demonstrated that magainin analogues were able to elevate the survival level of animals with ascites-producing tumors. Furthermore, magainin 2 has been tested for local treatment in a subcutaneous xenograft model of melanoma in nude mice, where it completely eliminated the tumor with only minimal damage to surrounding tissue (Soballe et al., 1995).
Another prominent member of anticancer peptides is bovine lactoferricin (Gifford et al., 2005), which is generated from lactoferrin through pepsin cleavage. Unlike magainins, bLFcin possesses an acyclic twisted antiparallel β-sheet structure due to a disulfide bridge between two cysteine residues (Vogel et al., 2002). Systematic studies on bLFcin derivatives revealed some parameters of importance for antitumor activity such as the need for high net positive charge (+7 as compared to +4 for antimicrobial activity) and clear cationic and hydrophobic sectors (amphipathicity) (Yang et al., 2004, 2003). Furthermore, the requirement of a stabilized secondary structure may be deduced from the higher activity of the cyclic peptide (Eliassen et al., 2002), which showed a clearer partitioning of the cationic and hydrophobic faces (Nguyen et al., 2005). As this property is less important for antimicrobial activity, it was suggested that it indicates a constraint for the peptide to traverse mammalian membranes (Gifford et al., 2005). Yoo et al. (1997) demonstrated that the peptide inhibits liver and lung metastasis in mice. Additionally, bLFcin showed antiangiogenic activity leading to suppression of tumor growth. In vivo studies with bLFcin on fibrosarcoma, melanoma and colon carcinoma tumors revealed massive necrosis of the tumor tissue after exposure to the peptide (Eliassen et al., 2002). Eliassen et al. (2006) also showed that bLFcin significantly inhibits tumor growth of neuroblastoma xenografts in nude rats. Clarification of the mechanism revealed that bLFcin induces apoptosis in human tumor cells through a pathway mediated by production of the intracellular ROS and activation of Ca2+/Mg2+-dependent endonucleases (Yoo et al., 1997), which was partially confirmed by studies carried out by Mader et al. (2005), who also documented the mitochondrial pathway of apoptosis for bLFcin. Other studies reported that induction of apoptotic or necrotic cell death was dependent on the concentration of the peptide (Onishi et al., 2008; Eliassen et al., 2006). Recently, it was shown that in vitro treatment with bLFcin killed Raji and Ramos human B-lymphoma cells via a caspase-independent apoptotic pathway being more efficient at low serum concentration of the peptide (Furlong et al., 2010). Notably, in the presence of exogenous bovine serum albumin the activity was inhibited, which suggests partial neutralization of the cationic peptide by anionic serum components as described earlier for the inhibition of the tumoricidal activity of amphiphilic alpha helical peptides (Peck-Miller et al., 1993). Nevertheless, immune-deficient SCID/beige mice exhibited extended survival using systemic administration of bLFcin underlining the potential of this peptide for further development as novel anticancer agent.
Gomesin (Gm), an antimicrobial peptide isolated from the hemocytes of the unchallenged Brazilian spider Acanthoscurria gomesiana, also exhibits anticancer activity (Silva et al., 2000). This cationic peptide adopts a hairpin-like two-stranded antiparallel β-sheet structure, which is maintained by two internal disulfide bridges (Mandard et al., 2002). Topical treatment of subcutaneous murine melanoma with gomesin significantly retarded tumor growth and increased the number of animals with tumors below a critical size (Rodrigues et al., 2008). Importantly, Gm did not exhibit any toxic effect on the peripheral healthy skin of mice after repeated applications of the peptide. Gm does not kill tumor cells through apoptosis but rather through induction of morphologic cell alterations with increased granularity, loss of cytoplasmic content by membrane permeabilization, and partial collapse of the proton gradient (Rodrigues et al., 2008).
Polybia-MPI (MPI) is an anticancer peptide derived from the venom of social wasp Polybia paulista. In order to improve the activity and stability of MPI, the C-terminal amide of the peptide was replaced by a thioamide group (MPI-1) (Zhang et al., 2010). Indeed, MPI-1 is more efficient in suppresssing tumor growth of sarcoma xenograft in mice than MPI, which was attributed to its higher serum stability. However, the modification did not affect the cytotoxic behavior of the peptide. In vitro studies provide evidence of killing caused by necrosis as a result of acute cell injury, swelling and bursting via disruption of the membrane or formation of transmembrane pores (Wang et al., 2009c).
An alternative approach for in vivo use of membranolytic peptides to maintain therapeutic levels and reduce cytotoxicity was suggested by Winder et al. (1998) and involving the introduction of vector-mediated delivery of genes encoding cecropin or melittin into a human bladder carcinoma derived cell line (Winder et al., 1998). The resultant cell clones were analyzed for tumorigenicity in nude mice showing that expression of cecropin resulted in either a complete loss of tumorigenicity in some clones or reduced tumorigenicity as measured by latency of tumor formation. Similarly, a plasmid that expressed secretable human α-defensin-1, also known as the human neutrophil peptide-1 (HNP1) was used in gene therapy of a human lung adenocarcinoma xenograft in nude mice (Xu et al., 2008). In this study, Xu et al. (2008) showed that HNP1 can be intracellularily expressed in tumor cells resulting in significant inhibition of tumor growth by induction of apoptosis.
5.2. Anticancer agents based on anticancer peptide-conjugates
Melittin, extracted from the European honey bee Apis mellifera, is a mostly α-helical peptide assumed to induce cell lysis by pore formation (Tosteson and Tosteson, 1981; Matsuzaki et al., 1997). Since melittin is highly toxic to red blood cells and untransformed cells, a melittin/avidin conjugate was designed to gain an inactive peptide towards healthy cells as it specifically targets cancer cells with high matrix metalloproteinase 2 (MMP2) activity (Holle et al., 2003). MMP2 is known to be overexpressed in several cancers and tumor microvascular endothelial cells and has the ability to reactivate peptide activity by cleavage of the conjugate at the tumor site. In vivo studies showed that the size of B16 tumors in mice decreased significantly after treatment with the melittin/avidin conjugate. Activity of the conjugate most probably arises from its ability to target tumor vasculature since the level of cell lysis in cultured cells was relatively low (Holle et al., 2003).
Tachyplesin, a peptide isolated from hemocytes of the horseshoe crab (Tachypleus tridentatus), exhibits a structure with exposed basic amino acids on the peptide surface (Nakamura et al., 1988), which enables binding to anionic phospholipids (Park et al., 1992; Katsu et al., 1993). The peptide was shown to have anticancer activity in vivo in a B16 mouse model (Chen et al., 2001). In this study, a synthetic tachyplesin was conjugated to the integrin homing domain RGB, which enables internalization of the peptide.
5.3. Anti-proliferative peptides
Dolastatin 10 (US Pat. No. 4,816,444) derived from the family of dolastatins, a peptide isolated from the marine shell-less mollusk Dolabela auricularia, completed Phase II trial against indolent lymphoma, Waldenstrom's macroglobulinemia and chronic lymphocytic leukemia in 2000 (Simmons et al., 2005). At the time of discovery it was shown to be the most potent anti-proliferative agent against murine PS leukemia cells (Pettit et al., 1987). Eventually, the peptide had to be dropped from clinical trials because of moderate peripheral neuropathy in 40% of patients. Therefore, derivatives of dolastatin 10, like TZT-1027 (Soblidotin) (Miyazaki et al., 1995) or of dolastatin 15 (antimitotic), like ILX-651 (Synthadotin) (Ebbinghaus et al., 2004), which show reduced toxicity but yet potent antitumor activity have been further explored in clinical studies.
6. Challenges for the development of host defense peptides as novel anticancer agents
Interestingly, despite numerous successful in vivo studies none of the membrane-active host defense peptides has so far made it on to the market. There may be several reasons for this and their development as novel anticancer agent may predominantly be hampered by the poor bioavailability and potential toxicity of such peptides (Hancock and Sahl, 2006). Peptide degradation in serum owing to the presence of proteases is a major obstacle, which besides unspecific binding to serum components also limits the half time of these molecules in serum. In order to improve the pharmacodynamic properties, different strategies may be applied to achieve enhanced cell specificity and serum stability.
6.1. Enhancing selectivity to further reduce cytotoxicity
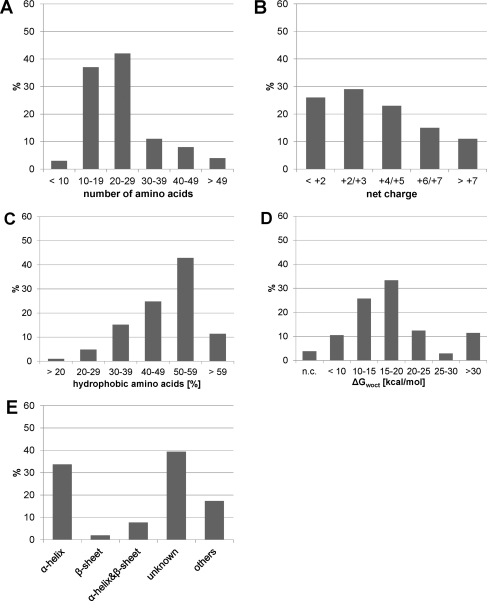
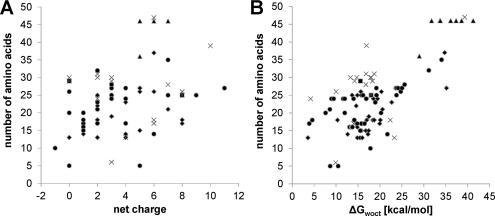
Not all host defense peptides showing antitumor activity are sufficiently selective against cancer cells. Accordingly, a more detailed knowledge of their structure–activity relationship is required in order to understand the molecular peptide parameters for the design of promising novel antitumor agents. As outlined above, host defense peptides exhibit great structural diversity yet share some common features. They are typically 10–40 amino acids long and positively charged (net charge of +2 to +9). While differing in the percentage of hydrophobic residues, these peptides share an amphiphilic character, i.e. they have the ability to adopt a structure in which hydrophobic and polar/charged residues are spatially organized in discrete sectors of the molecule. Although host defense peptides belong to different structural classes, they often exhibit an intrinsic flexibility and structural adaptability, which are thought to be responsible for different modes of interaction with membrane bilayers (Joanne et al., 2009). Thus net charge, amphipathicity, hydrophobicity, and structural propensity are among the most important physicochemical and structural parameters that govern the ability of these peptides to interact with cell membranes. A statistical analysis of the antitumor peptides listed in the Antimicrobial Peptide Data Base (Wang and Wang, 2004; Wang et al., 2009b) in terms of secondary structure, length of the peptide, distribution of charged and hydrophobic amino acid residues and determination of hydrophobicity expressed as transfer free energy of peptides from water to n-octanol (ΔGwoct) (Wimley et al., 1996) show that these parameters can vary within a wide range (Fig. 5). It is interesting to note that there is still a large fraction of peptides with unknown structure (∼39%) being comparable to the fraction of peptides with α-helical structure (∼34%), which seems to be the predominant one. However, as can be deduced from Tables 1 and 2 there have been not less peptides tested successfully in in vitro and in vivo studies, which exhibit a β-sheet structure, although these peptides account only for ∼2% of the total antitumor peptides listed. Pair correlations between peptide length and charge as well as peptide length and hydrophobicity indicate again that even for a given peptide length these parameters can vary widely being more apparent for the distribution of the net charge (Fig. 6). For a meaningful interpretation of these data one would also need to consider the antitumor activity and cell specificity of the peptides. However, this cannot be performed on this set of data, as the peptides listed in the Antimicrobial Peptide Data Base (Wang and Wang, 2004; Wang et al., 2009b) were tested against different cancer cells and healthy mammalian cells using also different protocols. But a cluster analysis of a database of 158 amphipathic α-helical peptides, from which the activity spectrum was determined, revealed structural characteristics of anticancer peptides (Dennison et al., 2010). This database was constructed using data presented by Owen (US Patent 6,875,744), and contained 14 peptides, which showed selectivity for cancer cell lines only, 123 peptides showing cytotoxicity also to non-cancer cells and 21 inactive peptides. Three-dimensional clustering techniques were used to group these peptides with respect to similarity in net positive charge, hydrophobicity and hydrophobic moment producing 21 clusters. The observation that the inactive peptides were found across seven clusters highlights the fact that these three parameters alone cannot be used as predictors of activity. However, as hydrophobicity of peptides is important for membrane penetration, an extended hydrophobic moment plot analysis was also performed. Although peptides with arc sizes >270° appeared to be less toxic, statistical analysis was unable to establish a direct correlation between arc size and toxicity. Furthermore, using this approach it was predicted that over 50% of the active peptides would be surface active. This suggests that amphiphilicity is a key driver of the membrane interactions for these peptides. Thereby, peptides showing cancer cell specificity fell within a narrow range of amphiphilicity of 0.53–0.78 and had no tilted helical peptide structure, which within the analysis was more commonly found for peptides being toxic to both cancer and non-cancer cells. Such a tilted structure has already been previously associated with relatively non-specific means of cell membrane lysis (Hoskin and Ramamoorthy, 2008). This cluster analysis in conjunction with an earlier study carried out by the same group (Dennison et al., 2006) suggests that the interplay of a range of physicochemical characteristics rather than any single overriding factor determines the biological activity of these peptides. Furthermore, to add to the complexity of the system one must bear in mind that the lipid composition of the target membrane should not be neglected as it in turn strongly affects the penetration behavior of peptides. Therefore, application of newly developed technologies for high-throughput screening as recently described in regard to antimicrobial peptides (Blondelle and Lohner, 2010) may be beneficial in unraveling the molecular parameters making anticancer peptides more suitable for clinical use, i.e. exhibiting high specificity and activity, low susceptibility to proteolysis and reduced toxicity.
Fig. 5.
Characteristics of anticancer peptides: (A) peptide length; (B) net charge; (C) hydrophobic content (% hydrophobic amino acids; http://aps.unmc.edu/AP/design/design_improve.php); (D) hydrophobicity of peptides expressed as transfer free energy of peptides from water to n-octanol (ΔGwoct) taking into account the contribution of both endgroups (COO− and NH3+), in case of C-amidation or N-acetylation ΔGwoct decreases by 3.6 kcal/mol and 2.3 kcal/mol, respectively (http://blanco.biomol.uci.edu/mpex/) (Wimley et al., 1996), n.c., not calculated for peptides >70 amino acids; (E) structural characteristics.
Fig. 6.
Pair correlations between peptide length and net charge (A) and hydrophobicity (B), respectively, for antitumor peptides listed in the Antimicrobial Peptide Database, APD2 (Wang et al., 2009b) excluding the three peptides >70 amino acids for which no ΔGwoct can be calculated. The secondary structure of the individual peptides is indicated in the panels by the following symbols: ♦, α-helix; ■, β-sheet; ▴, α-helix and β-sheet; ●, unknown; ×, others.
6.2. Improving serum stability
The in vivo potency of antitumor peptides is often hindered by proteolysis of the peptides, which reduces their half time in serum and hence bioavailability. One strategy used to overcome this problem is the application of vector-mediated delivery of genes that encode the active anticancer peptide (Winder et al., 1998) as outlined in Section 5.1. Another simple but efficient modification is the incorporation of d-amino acids or the replacement of all naturally occurring l-amino acids by its diastereomer (Papo and Shai, 2005). Early studies on magainin 2 and its all d-amino acid analogue MSI-238 demonstrated the feasibility of this approach (Baker et al., 1993). Both peptides were found to be active against P388 leukemia, S180 ascites and spontaneous ovarian tumor of mice upon intra-peritoneal injection of peptides, but with the all-D peptide showing significantly higher in vitro activity than the all-L peptide (Baker et al., 1993). A series of studies were initiated using systematically de novo-designed antimicrobial peptides composed of both d- and l-amino acids mimicking the lytic activity of host defense peptides (Papo and Shai, 2003). The resulting diastereomeric peptides lost their cytotoxic effect against normal cells but preserved anticancer activity with reduced serum inactivation and enzymatic degradation in vivo. For instance, one specific diastereomer composed of 15 amino acids ([d]-K6L9) showed selectivity to primary and metastatic tumors in human prostate and breast xenografts when administered systemically (Papo et al., 2004, 2006). A histopathological evaluation revealed that the diastereomer did not cause any damage to organs of mice emphasizing the potential for such peptides to be developed for therapeutic application.
7. Perspectives
Cancer treatment with chemotherapeutics still has many severe side effects and can induce drug resistance thus adversely affecting their successful use. Therefore, novel therapeutic approaches with improved cancer cell selectivity and thus reduced toxicity as well as hindered resistance development are urgently needed as has been observed for membrane-active host defense peptides in a number of in vivo studies (see Section 5). It is also advantageous that they have excellent tumor tissue penetration, which enables them not only to reach primary but also metastatic tumor sites. However, despite the wealth of information the molecular basis for selective targeting of cancer cells and the mechanism of cell killing by host defense peptides remain questions to be solved. Since there is a wide variety of host defense peptides yet many of them do not possess sufficient antitumor activity, optimization of peptides including innovative types of peptide modification to fine-tune their selective interaction with their target cell membrane together with a profound knowledge of their characteristic lipid composition are needed in order to attain a highly active and specific peptide. Biophysical studies considering the peptides as well as membrane characteristics will provide new insights for the rationale design of anticancer peptides. The progress may be facilitated by recent advances in high-throughput screening techniques that can also be implemented in academic laboratories. Based on such knowledge, the field of peptidomimetics may offer alternative means for the development of anticancer drugs with optimized pharmacodynamic profiles. In conclusion, these strategies are expected to yield novel anticancer drugs with improved properties with respect to selectivity and thus reduced toxicity, as well as to hindered development of drug resistance. These peptides may also be used in combination with chemotherapeutics as some peptides have shown synergy with classical chemotherapy (Papo and Shai, 2005). Achieving this goal would represent a major advancement in cancer treatment.
Acknowledgments
The authors gratefully acknowledge financial support from the Austrian Science Fund (FWF grant no. P20760-B11) and from the Sonnleitner as well as the Oelzelt Stiftung of the Austrian Academy of Sciences.
References
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Almeida P.F., Pokorny A. Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry. 2009;48:8083–8093. doi: 10.1021/bi900914g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An M., Wijesinghe D., Andreev O.A., Reshetnyak Y.K., Engelman D.M. pH-(low)-insertion-peptide (pHLIP) translocation of membrane impermeable phalloidin toxin inhibits cancer cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20246–20250. doi: 10.1073/pnas.1014403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev O.A., Dupuy A.D., Segala M., Sandugu S., Serra D.A., Chichester C.O., Engelman D.M., Reshetnyak Y.K. Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proc. Natl. Acad. Sci. U.S.A. 2007;104:7893–7898. doi: 10.1073/pnas.0702439104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviel-Ronen S., Lau S.K., Pintilie M., Lau D., Liu N., Tsao M.S., Jothy S. Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Mod. Pathol. 2008;21:817–825. doi: 10.1038/modpathol.2008.37. [DOI] [PubMed] [Google Scholar]
- Bafna S., Kaur S., Batra S.K. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M.A., Maloy W.L., Zasloff M., Jacob L.S. Anticancer efficacy of Magainin2 and analogue peptides. Cancer Res. 1993;53:3052–3057. [PubMed] [Google Scholar]
- Bechinger B., Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim. Biophys. Acta. 2006;1758:1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Bechinger B. Rationalizing the membrane interactions of cationic amphipathic antimicrobial peptides by their molecular shape. Curr. Opin. Colloid Interface Sci. 2009;14:349–355. [Google Scholar]
- Bevers E.M., Comfurius P., Dekkers D.W., Harmsma M., Zwaal R.F. Regulatory mechanisms of transmembrane phospholipid distributions and pathophysiological implications of transbilayer lipid scrambling. Lupus. 1998;7(suppl. 2):S126–S131. doi: 10.1177/096120339800700228. [DOI] [PubMed] [Google Scholar]
- Bevers E.M., Comfurius P., Zwaal R.F. Regulatory mechanisms in maintenance and modulation of transmembrane lipid asymmetry: pathophysiological implications. Lupus. 1996;5:480–487. doi: 10.1177/096120339600500531. [DOI] [PubMed] [Google Scholar]
- Bhutia S.K., Maiti T.K. Targeting tumors with peptides from natural sources. Trends Biotechnol. 2008;26:210–217. doi: 10.1016/j.tibtech.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Blondelle S.E., Lohner K. Optimization and high-throughput screening of antimicrobial peptides. Curr. Pharm. Des. 2010;16:3204–3211. doi: 10.2174/138161210793292438. [DOI] [PubMed] [Google Scholar]
- Campanella R. Membrane lipids modifications in human gliomas of different degree of malignancy. J. Neurosurg. Sci. 1992;36:11–25. [PubMed] [Google Scholar]
- Capranico G., Kohn K.W., Pommier Y. Local sequence requirements for DNA cleavage by mammalian topoisomerase II in the presence of doxorubicin. Nucleic Acids Res. 1990;18:6611–6619. doi: 10.1093/nar/18.22.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway K.L., Price-Schiavi S.A., Zhu X., Komatsu M. Membrane mucins and breast cancer. Cancer Control. 1999;6:613–614. doi: 10.1177/107327489900600616. [DOI] [PubMed] [Google Scholar]
- Cassidy J., Misset J.L. Oxaliplatin-related side effects: characteristics and management. Semin. Oncol. 2002;29:11–20. doi: 10.1053/sonc.2002.35524. [DOI] [PubMed] [Google Scholar]
- Cazet A., Julien S., Bobowski M., Krzewinski-Recchi M.A., Harduin-Lepers A., Groux-Degroote S., Delannoy P. Consequences of the expression of sialylated antigens in breast cancer. Carbohydr. Res. 2010;345:1377–1383. doi: 10.1016/j.carres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- CBTRUS, 2008. Statistical Report: Primary Brain Tumors in the United States, 2000-2004. Central Brain Tumor Registry of the United States, 2008.
- Chaires J.B. Thermodynamics of the daunomycin-DNA interaction: ionic strength dependence of the enthalpy and entropy. Biopolymers. 1985;24:403–419. doi: 10.1002/bip.360240208. [DOI] [PubMed] [Google Scholar]
- Chan S.C., Hui L., Chen H.M. Enhancement of the cytolytic effect of anti-bacterial cecropin by the microvilli of cancer cells. Anticancer Res. 1998;18:4467–4474. [PubMed] [Google Scholar]
- Chan Y.R., Gallo R.L. PR-39, a syndecan-inducing antimicrobial peptide, binds and affects p130Cas. J. Biol. Chem. 1998;273:28978–28985. doi: 10.1074/jbc.273.44.28978. [DOI] [PubMed] [Google Scholar]
- Chan Y.R., Zanetti M., Gennaro R., Gallo R.L. Anti-microbial activity and cell binding are controled by sequence determinants in the anti-microbial peptide PR-39. J. Invest. Dermatol. 2001;116:230–235. doi: 10.1046/j.1523-1747.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- Chapman P.B., Einhorn L.H., Meyers M.L., Saxman S., Destro A.N., Panageas K.S., Begg C.B., Agarwala S.S., Schuchter L.M., Ernstoff M.S., Houghton A.N., Kirkwood J.M. Phase III multicenter randomized trial of the dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J. Clin. Oncol. 1999;17:2745–2751. doi: 10.1200/JCO.1999.17.9.2745. [DOI] [PubMed] [Google Scholar]
- Chaudhary J., Munshi M. Scanning electron microscopic analysis of breast aspirates. Cytopathology. 1995;6:162–167. doi: 10.1111/j.1365-2303.1995.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Chen H.M., Wang W., Smith D., Chan S.C. Effects of the anti-bacterial peptide cecropin B and its analogs, cecropins B-1 and B-2, on liposomes, bacteria, and cancer cells. Biochim. Biophys. Acta. 1997;1336:171–179. doi: 10.1016/s0304-4165(97)00024-x. [DOI] [PubMed] [Google Scholar]
- Chen Y., Xu X., Hong S., Chen J., Liu N., Underhill C.B., Creswell K., Zhang L. RGD-tachyplesin inhibits tumor growth. Cancer Res. 2001;61:2434–2438. [PubMed] [Google Scholar]
- Chernysh S., Kim S.I., Bekker G., Pleskach V.A., Filatova N.A., Anikin V.B., Platonov V.G., Bulet P. Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12628–12632. doi: 10.1073/pnas.192301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S.T., Cheng H.H., Huang C.J., Chang H.C., Chi C.C., Su H.H., Hsu S.S., Wang J.L., Chen I.S., Liu S.I., Lu Y.C., Huang J.K., Ho C.M., Jan C.R. Phospholipase A2-independent Ca2+ entry and subsequent apoptosis induced by melittin in human MG63 osteosarcoma cells. Life Sci. 2007;80:364–369. doi: 10.1016/j.lfs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Cichorek M., Kozlowska K., Witkowski J.M., Zarzeczna M. Flow cytometric estimation of the plasma membrane diversity of transplantable melanomas, using annexin V. Folia Histochem. Cytobiol. 2000;38:41–43. [PubMed] [Google Scholar]
- Connor J., Bucana C., Fidler I.J., Schroit A.J. Differentiation-dependent expression of phosphatidylserine in mammalian plasma membranes: quantitative assessment of outer-leaflet lipid by prothrombinase complex formation. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3184–3188. doi: 10.1073/pnas.86.9.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani R.A., Barker J.L., Zasloff M., Chen H.C., Colamonici O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc. Natl. Acad. Sci. U.S.A. 1991;88:3792–3796. doi: 10.1073/pnas.88.9.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A., Jang J., Middleton K.M., Wang Y., Weisz I., Ling Y., Bekesi G. Novel suicide ligands of tubulin arrest cancer cells in S-phase. Neoplasia. 1999;1:498–507. doi: 10.1038/sj.neo.7900066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison S.R., Whittaker M., Harris F., Phoenix D.A. Anticancer alpha-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr. Protein Pept. Sci. 2006;7:487–499. doi: 10.2174/138920306779025611. [DOI] [PubMed] [Google Scholar]
- Dennison S., Harris F., Bhatt T., Singh J., Phoenix D. A theoretical analysis of secondary structural characteristics of anticancer peptides. Mol. Cell. Biochem. 2010;333:129–135. doi: 10.1007/s11010-009-0213-3. [DOI] [PubMed] [Google Scholar]
- Dobrzynska I., Kotynska J., Figaszewski Z. Changes in electrical charge of phosphatidylcholine and phosphatidylserine liposomal membranes caused by adsorption of monovalent ions. Chem Analityczna. 2007;52:931–944. [Google Scholar]
- Domagala W., Koss L.G. Surface configuration of human tumor cells obtained by fine needle aspiration biopsy. Scan. Electron. Micros. 1980;3:101–108. [PubMed] [Google Scholar]
- Doyle J., Brinkworth C.S., Wegener K.L., Carver J.A., Llewellyn L.E., Olver I.N., Bowie J.H., Wabnitz P.A., Tyler M.J. nNOS inhibition, antimicrobial and anticancer activity of the amphibian skin peptide, citropin 1.1 and synthetic modifications. The solution structure of a modified citropin 1.1. Eur. J. Biochem. 2003;270:1141–1153. doi: 10.1046/j.1432-1033.2003.03462.x. [DOI] [PubMed] [Google Scholar]
- Drechsler S., Andrä J. Online monitoring of metabolism and morphology of peptide-treated neuroblastoma cancer cells and keratinocytes. J. Bioenerg. Biomembr. 2011;43:275–285. doi: 10.1007/s10863-011-9350-y. [DOI] [PubMed] [Google Scholar]
- Dredge K., Marriott B., Todryk S., Dalgleish A. Adjuvants and the promotion of Th1-type cytokines in tumour immunotherapy. Cancer Immunol. Immunother. 2002;51:521–531. doi: 10.1007/s00262-002-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton D.M., Nijnik A., Mayer M.L., Hancock R.E.W. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009;27:582–590. doi: 10.1016/j.tibtech.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbinghaus S., Hersh E., Cunningham C.C., O’Day S., McDermott D., Stephenson J., Richards D.A., Eckardt J., Haider O.L., Hammon L.A. Phase II study of synthadotin (SYN-D; ILX651) administered daily for 5 consecutive days once every 3 weeks (qdx5q3w) in patients (Pts) with inoperable locally advanced or metastatic melanoma. J. Clin. Oncol. 2004;22(suppl.):7530. [Google Scholar]
- Eliassen L.T., Haug B.E., Berge G., Rekdal O. Enhanced antitumour activity of 15-residue bovine lactoferricin derivatives containing bulky aromatic amino acids and lipophilic N-terminal modifications. J Pept. Sci. 2003;9:510–517. doi: 10.1002/psc.472. [DOI] [PubMed] [Google Scholar]
- Eliassen L.T., Berge G., Leknessund A., Wikman M., Lindin I., Lokke C., Ponthan F., Johnsen J.I., Sveinbjornsson B., Kogner P., Flaegstad T., Rekdal O. The antimicrobial peptide, lactoferricin B, is cytotoxic to neuroblastoma cells in vitro and inhibits xenograft growth in vivo. Int. J. Cancer. 2006;119:493–500. doi: 10.1002/ijc.21886. [DOI] [PubMed] [Google Scholar]
- Eliassen L.T., Berge G., Sveinbjornsson B., Svendsen J.S., Vorland L.H., Rekdal O. Evidence for a direct antitumor mechanism of action of bovine lactoferricin. Anticancer Res. 2002;22:2703–2710. [PubMed] [Google Scholar]
- Epand R.M., Epand R.F., Arnusch C.J., Papahadjopoulos-Sternberg B., Wang G., Shai Y. Lipid clustering by three homologous arginine-rich antimicrobial peptides is insensitive to amino acid arrangement and induced secondary structure. Biochim. Biophys. Acta. 2010;1798:1272–1280. doi: 10.1016/j.bbamem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Epand R.M., Epand R.F. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim. Biophys. Acta. 2009;1788:289–294. doi: 10.1016/j.bbamem.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Epand R.M., Epand R.F. Bacterial membrane lipids in the action of antimicrobial agents. J. Pept. Sci. 2011;17:298–305. doi: 10.1002/psc.1319. [DOI] [PubMed] [Google Scholar]
- Espinosa E., Zamora P., Feliu J., González Barón M. Classification of anticancer drugs – a new system based on therapeutic targets. Cancer Treat. Rev. 2003;29:515–523. doi: 10.1016/s0305-7372(03)00116-6. [DOI] [PubMed] [Google Scholar]
- Fadnes B., Rekdal O., Uhlin-Hansen L. The anticancer activity of lytic peptides is inhibited by heparan sulfate on the surface of the tumor cells. BMC Cancer. 2009;9:183. doi: 10.1186/1471-2407-9-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadnes B., Uhlin-Hansen L., Lindin I., Rekdal O. Small lytic peptides escape the inhibitory effect of heparan sulfate on the surface of cancer cells. BMC Cancer. 2011;11:116. doi: 10.1186/1471-2407-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Voelker D.R., Campbell P.A., Cohen J.J., Bratton D.L., Henson P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Furlong S.J., Mader J.S., Hoskin D.W. Bovine lactoferricin induces caspase-independent apoptosis in human B-lymphoma cells and extends the survival of immune-deficient mice bearing B-lymphoma xenografts. Exp. Mol. Pathol. 2010;88:371–375. doi: 10.1016/j.yexmp.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Gifford J.L., Hunter H.N., Vogel H.J. Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005;62:2588–2598. doi: 10.1007/s00018-005-5373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet J.P., Gottesman M.M. Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- Gottesman M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- Gregory S.M., Cavenaugh A., Journigan V., Pokorny A., Almeida P.F.F. A quantitative model for the all-or-none permeabilization of phospholipid vesicles by the antimicrobial peptide Cecropin A. Biophys. J. 2008;94:1667–1680. doi: 10.1529/biophysj.107.118760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J.R. Are cancer cells acidic? Br. J. Cancer. 1991;64:425–427. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E., Jentsch T.J. Sequenzanalyse des Melttins aus den tryptischen und peptischen Spaltsstücken des Bienengiftes, Hoppe Seylers. Z. Physiol. Chem. 1967;348:37–50. [PubMed] [Google Scholar]
- Hancock R.E.W., Sahl H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Helmbach H., Rossmann E., Kern M.A., Schadendorf D. Drug-resistance in human melanoma. Int. J. Cancer. 2001;93:617–622. doi: 10.1002/ijc.1378. [DOI] [PubMed] [Google Scholar]
- Hickel A., Danner-Pongratz S., Amenitsch H., Degovics G., Rappolt M., Lohner K., Pabst G. Influence of antimicrobial peptides on the formation of nonlamellar lipid mesophases. Biochim. Biophys. Acta. 2008;1778:2325–2333. doi: 10.1016/j.bbamem.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Holle L., Song W., Holle E., Wei Y., Wagner T., Yu X. A matrix metalloproteinase 2 cleavable melittin/avidin conjugate specifically targets tumor cells in vitro and in vivo. Int. J. Oncol. 2003;22:93–98. [PubMed] [Google Scholar]
- Hoskin D.W., Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta. 2008;1778:357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristova K., Selsted M.E., White S.H. Critical role of lipid composition in membrane permeabilization by rabbit neutrophil defensins. J. Biol. Chem. 1997;272:24224–24233. doi: 10.1074/jbc.272.39.24224. [DOI] [PubMed] [Google Scholar]
- Huang H.W. Molecular mechanism of antimicrobial peptides: the origin of cooperativity. Biochim. Biophys. Acta. 2006;1758:1292–1302. doi: 10.1016/j.bbamem.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hunt J.F., Rath P., Rothschild K.J., Engelman D.M. Spontaneous, pH-dependent membrane insertion of a transbilayer alpha-helix. Biochemistry. 1997;36:15177–15192. doi: 10.1021/bi970147b. [DOI] [PubMed] [Google Scholar]
- Israelachvili J.N, Marcelja S., Horn R.G. Physical principles of membrane organization. Q. Rev. Biophys. 1980;13:121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Ishibashi J., Tanaka H., Sato M., Asaoka A., Taylor D., Yamakawa M. Selective cancer cell cytotoxicity of enantiomeric 9-mer peptides derived from beetle defensins depends on negatively charged phosphatidylserine on the cell surface. Peptides. 2009;30:660–668. doi: 10.1016/j.peptides.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Jackson K.L., Henderson J.A., Phillips A.J. The halichondrins and E7389. Chem. Rev. 2009;109:3044–3079. doi: 10.1021/cr900016w. [DOI] [PubMed] [Google Scholar]
- Jayson G.C., Lyon M., Paraskeva C., Turnbull J.E., Deakin J.A., Gallagher J.T. Heparan sulfate undergoes specific structural changes during the progression from human colon adenoma to carcinoma in vitro. J. Biol. Chem. 1998;273:51–57. doi: 10.1074/jbc.273.1.51. [DOI] [PubMed] [Google Scholar]
- Joanne P., Nicolas P., El Amri C. Antimicrobial peptides and viral fusion peptides: how different they are? Protein Pept. Lett. 2009;16:743–750. doi: 10.2174/092986609788681814. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B., Joseph J., Kalivendi S., Wang S., Konorev E., Kotamraju S. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol. Cell. Biochem. 2002;234–235:119–124. [PubMed] [Google Scholar]
- Katsu T., Nakao S., Iwanaga S. Mode of action of an antimicrobial peptide, tachyplesin I, on biomembranes. Biol. Pharm. Bull. 1993;16:178–181. doi: 10.1248/bpb.16.178. [DOI] [PubMed] [Google Scholar]
- Kim S., Kim S.S., Bang Y.J., Kim S.J., Lee B.J. In vitro activities of native and designed peptide antibiotics against drug sensitive and resistant tumor cell lines. Peptides. 2003;24:945–953. doi: 10.1016/s0196-9781(03)00194-3. [DOI] [PubMed] [Google Scholar]
- Kirszberg C., Lima L.G., Da Silva d.O., Pickering W., Gray E., Barrowcliffe T.W., Rumjanek V.M., Monteiro R.Q. Simultaneous tissue factor expression and phosphatidylserine exposure account for the highly procoagulant pattern of melanoma cell lines. Melanoma Res. 2009;19:301–308. doi: 10.1097/CMR.0b013e32832e40fe. [DOI] [PubMed] [Google Scholar]
- Kjellen L., Lindahl U. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Kleeff J., Ishiwata T., Kumbasar A., Friess H., Büchler M.W., Lander A.D., Korc M. The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J. Clin. Invest. 1998;102:1662–1673. doi: 10.1172/JCI4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszalka P., Kamysz E., Wejda M., Kamysz W., Bigda J. Antitumor activity of antimicrobial peptides against U937 histiocytic cell line. Acta Biochimica Polonica. 2011;58:111–117. [PubMed] [Google Scholar]
- Kufe D.W. Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Retz M., Sidhu S.S., Suttmann H., Sell M., Paulsen F., Harder J., Unteregger G., Stockle M. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur. Urol. 2006;50:141–147. doi: 10.1016/j.eururo.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Lee D., Hahm K., Park Y., Kim H., Lee W., Lim S., Seo Y., Choi C. Functional and structural characteristics of anticancer peptide Pep27 analogues. Cancer Cell Int. 2005;5:21–34. doi: 10.1186/1475-2867-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.S., Park C.B., Kim J.M., Jang S.A., Park I.Y., Kim M.S., Cho J.H., Kim S.C. Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett. 2008;271:47–55. doi: 10.1016/j.canlet.2008.05.041. [DOI] [PubMed] [Google Scholar]
- Leuschner C., Hansel W. Membrane disrupting lytic peptides for cancer treatments. Curr. Pharm. Des. 2004;10:2299–2310. doi: 10.2174/1381612043383971. [DOI] [PubMed] [Google Scholar]
- Li Q., Ouyang G., Peng X., Hong S. Effects of tachyplesin on the regulation of cell cycle in human hepatocarcinoma SMMC-7721 cells. World J. Gastroenterol. 2003;9:454–458. doi: 10.3748/wjg.v9.i3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Park M.J., Ye S.K., Kim C.W., Kim Y.N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am. J. Pathol. 2006;168:1107–1118. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebersbach B.F., Sanderson R.D. Expression of syndecan-1 inhibits cell invasion into type I collagen. J. Biol. Chem. 1994;269:20013–20019. [PubMed] [Google Scholar]
- Lin W.J., Chien Y.L., Pan C.Y., Lin T.L., Chen J.Y., Chiu S.J., Hui C.F. Epinecidin-1, an antimicrobial peptide from fish (Epinephelus coioides) which has an antitumor effect like lytic peptides in human fibrosarcoma cells. Peptides. 2009;30:283–290. doi: 10.1016/j.peptides.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Litynska A., Przybylo M., Pochec E., Hoja-Lukowicz D., Ciolczyk D., Laidler P., Gil D. Comparison of the lectin-binding pattern in different human melanoma cell lines. Melanoma Res. 2001;11:205–212. doi: 10.1097/00008390-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Lohner K. The role of membrane lipid composition in cell targeting of antimicrobial peptides. In: Lohner K., editor. Development of Novel Antimicrobial Agents: Emerging Strategies. Horizon Scientific Press; Wymondham, Norfolk, U.K.: 2001. pp. 149–165. [Google Scholar]
- Lohner K. New strategies for novel antibiotics: peptides targeting bacterial cell membranes. Gen. Physiol. Biophys. 2009;28:105–116. doi: 10.4149/gpb_2009_02_105. [DOI] [PubMed] [Google Scholar]
- Lohner K., Blondelle S.E. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb. Chem. High Throughput Screen. 2005;8:241–256. doi: 10.2174/1386207053764576. [DOI] [PubMed] [Google Scholar]
- Lohner K., Latal A., Lehrer R.I., Ganz T. Differential scanning microcalorimetry indicates that human defensin, HNP-2, interacts specifically with biomembrane mimetic systems. Biochemistry. 1997;36:1525–1531. doi: 10.1021/bi961300p. [DOI] [PubMed] [Google Scholar]
- Lohner K., Prenner E.J. Differential scanning calorimetry and X-ray diffraction studies of the specificity of the interaction of antimicrobial peptides with membrane-mimetic systems. Biochim. Biophys. Acta. 1999;1462:141–156. doi: 10.1016/s0005-2736(99)00204-7. [DOI] [PubMed] [Google Scholar]
- Ludtke S.J., He K., Heller W.T., Harroun T.A., Yang L., Huang H.W. Membrane pores induced by magainin. Biochemistry. 1996;35:13723–13728. doi: 10.1021/bi9620621. [DOI] [PubMed] [Google Scholar]
- Mader J.S., Hoskin D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs. 2006;15:933–946. doi: 10.1517/13543784.15.8.933. [DOI] [PubMed] [Google Scholar]
- Mader J.S., Salsman J., Conrad D.M., Hoskin D.W. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol. Cancer Ther. 2005;4:612–624. doi: 10.1158/1535-7163.MCT-04-0077. [DOI] [PubMed] [Google Scholar]
- Mader J.S, Mookherjee N., Hancock R.E.W., Bleackley R.C. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving bax activity. Mol. Cancer Res. 2009;7:689–702. doi: 10.1158/1541-7786.MCR-08-0274. [DOI] [PubMed] [Google Scholar]
- Makovitzki A., Fink A., Shai Y. Suppression of human solid tumor growth in mice by intratumor and systemic inoculation of histidine-rich and pH-dependent host defense-like lytic peptides. Cancer Res. 2009;69:3458–3463. doi: 10.1158/0008-5472.CAN-08-3021. [DOI] [PubMed] [Google Scholar]
- Manavbasi Y., Gofman Y., Ben Tal N., Willumeit R., Zweytick D., Lohner K. Structural aspects of the interaction of Nk-2 derived peptides with cancer cells. Biophys. J. 2010;98:277a. [Google Scholar]
- Mandard N., Bulet P., Caille A., Daffre S., Vovelle F. The solution structure of gomesin, an antimicrobial cysteine-rich peptide from the spider. Eur. J. Biochem. 2002;269:1190–1198. doi: 10.1046/j.0014-2956.2002.02760.x. [DOI] [PubMed] [Google Scholar]
- Marsh D. Lateral pressure in membranes. Biochim. Biophys. Acta. 1996;1286:183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
- Martin S.J., Reutelingsperger C.P., McGahon A.J., Rader J.A., van Schie R.C., LaFace D.M., Green D.R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Maruyama H., Guo F., Kleeff J., Itakura J., Matsumoto Y., Lander A.D., Korc M. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 2001;61:5562–5569. [PubMed] [Google Scholar]
- Matsuzaki K., Murase O., Fujii N., Miyajima K. An antimicrobal peptide, Magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 1996;35:11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K., Yoneyama S., Miyajima K. Pore formation and transition of melittin. Biophys. J. 1997;73:831–838. doi: 10.1016/S0006-3495(97)78115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May G.L., Wright L.C., Dyne M., Mackinnon W.B., Fox R.M., Mountford C.E. Plasma membrane lipid composition of vinblastine sensitive and resistant human leukaemic lymphoblasts. Int. J. Cancer. 1988;42:728–733. doi: 10.1002/ijc.2910420517. [DOI] [PubMed] [Google Scholar]
- Mazzoni A., Trave F. Cytoplasmic membrane cholesterol and doxorubicin cytotoxicity in drug-sensitive and multidrug-resistant human ovarian cancer cells. Oncol. Res. 1993;5:75–82. [PubMed] [Google Scholar]
- Middleton M.R., Lee S.M., Arance A., Wood M., Thatcher N., Margison G.P. O6-methylguanine formation, repair protein depletion and clinical outcome with a 4 h schedule of temozolomide in the treatment of advanced melanoma: results of a phase II study. Int. J. Cancer. 2000;88:469–473. [PubMed] [Google Scholar]
- Miller T.W., Isenberg J.S., Roberts D.D. Molecular regulation of tumor angiogenesis and perfusion via redox signaling. Chem. Rev. 2009;109:3099–3124. doi: 10.1021/cr8005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T., Reed J.C. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151–157. [PubMed] [Google Scholar]
- Miyazaki K., Kobayashi M., Natsume T., Gondo M., Mikami T., Sakakibara K., Tsukagoshi S. Synthesis and antitumor activity of novel dolastatin 10. Chem. Pharm. Bull. 1995;43:1706–1718. doi: 10.1248/cpb.43.1706. [DOI] [PubMed] [Google Scholar]
- Moon D.O., Park S.Y., Choi Y.H., Kim N.D., Lee C., Kim G.Y. Melittin induces Bcl-2 and caspase-3-dependent apoptosis through downregulation of Akt phosphorylation in human leukemic U937 cells. Toxicon. 2008;51:112–120. doi: 10.1016/j.toxicon.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Morgan G., Ward R., Barton M. The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clin. Oncol. 2004;16:549–560. doi: 10.1016/j.clon.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Nabholtz J.M., Slamon D. New adjuvant strategies for breast cancer: meeting the challenge of integrating chemotherapy and trastuzumab (Herceptin) Semin. Oncol. 2001;28:1–12. doi: 10.1016/s0093-7754(01)90187-3. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Furunaka H., Miyata T., Tokunaga F., Muta T., Iwanaga S., Niwa M., Takao T., Shimonishi Y. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J. Biol. Chem. 1988;263:16709–16713. [PubMed] [Google Scholar]
- Nakatsura T., Kageshita T., Ito S., Wakamatsu K., Monji M., Ikuta Y., Senju S., Ono T., Nishimura Y. Identification of glypican-3 as a novel tumor marker for melanoma. Clin. Cancer Res. 2004;10:6612–6621. doi: 10.1158/1078-0432.CCR-04-0348. [DOI] [PubMed] [Google Scholar]
- Nakazawa I., Iwaizumi M. A role of the cancer cell membrane fluidity in the cancer metastases: an ESR study, Tohoku. J. Exp. Med. 1989;157:193–198. doi: 10.1620/tjem.157.193. [DOI] [PubMed] [Google Scholar]
- Newell K., Franchi A., Pouysségur J., Tannock I. Studies with glycolysis-deficient cells suggest that production of lactic acid is not the only cause of tumor acidity. Proc. Natl. Acad. Sci. U.S.A. 1993;90:1127–1131. doi: 10.1073/pnas.90.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Nguyen L.T., Schibli D.J., Vogel H.J. Structural studies and model membrane interactions of two peptides derived from bovine lactoferricin. J. Pept. Sci. 2005;11:379–389. doi: 10.1002/psc.629. [DOI] [PubMed] [Google Scholar]
- Ohtake T., Fujimoto Y., Ikuta K., Saito H., Ohhira M., Ono M., Kohgo Y. Proline-rich antimicrobial peptide, PR-39 gene transduction altered invasive activity and actin structure in human hepatocellular carcinoma cells. Br. J. Cancer. 1999;81:393–403. doi: 10.1038/sj.bjc.6690707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi J., Roy M.K., Juneja L.R., Watanabe Y., Tamai Y. A lactoferrin-derived peptide with cationic residues concentrated in a region of its helical structure induces necrotic cell death in a leukemic cell line (HL-60) J. Pept. Sci. 2008;14:1032–1038. doi: 10.1002/psc.1039. [DOI] [PubMed] [Google Scholar]
- Ostolaza H., Bartolomé B., de Zárate I.O., de la Cruz F., Goni F.M. Release of lipid vesicle contents by the bacterial protein toxin [alpha]-haemolysin. Biochim. Biophys. Acta. 1993;1147:81–88. doi: 10.1016/0005-2736(93)90318-t. [DOI] [PubMed] [Google Scholar]
- Papo N., Braunstein A., Eshhar Z., Shai Y. Suppression of human prostate tumor growth in mice by a cytolytic d-, l-amino acid peptide: membrane lysis, increased necrosis, and inhibition of prostate-specific antigen secretion. Cancer Res. 2004;64:5779–5786. doi: 10.1158/0008-5472.CAN-04-1438. [DOI] [PubMed] [Google Scholar]
- Papo N., Shai Y. New lytic peptides based on the d,l-amphipathic helix motif preferentially kill tumor cells compared to normal cells. Biochemistry. 2003;42:9346–9354. doi: 10.1021/bi027212o. [DOI] [PubMed] [Google Scholar]
- Papo N., Shai Y. Host defense peptides as new weapons in cancer treatment. Cell. Mol. Life Sci. 2005;62:784–790. doi: 10.1007/s00018-005-4560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papo N., Seger D., Makovitzki A., Kalchenko V., Eshhar Z., Degani H., Shai Y. Inhibition of tumor growth and elimination of multiple metastases in human prostate and breast xenografts by systemic inoculation of a host defense-like lytic peptide. Cancer Res. 2006;66:5371–5378. doi: 10.1158/0008-5472.CAN-05-4569. [DOI] [PubMed] [Google Scholar]
- Park N.G., Lee S., Oishi O., Aoyagi H., Iwanaga S., Yamashita S., Ohno M. Conformation of tachyplesin I from Tachypleus tridentatus when interacting with lipid matrixes. Biochemistry. 1992;31:12241–12247. doi: 10.1021/bi00163a038. [DOI] [PubMed] [Google Scholar]
- Pastan I., Kreitman R.J. Immunotoxins for targeted cancer therapy. Adv. Drug Deliv. Rev. 1998;31:53–88. doi: 10.1016/s0169-409x(97)00094-x. [DOI] [PubMed] [Google Scholar]
- Peck-Miller K.A., Darveau R.P., Fell H.P. Identification of serum components that inhibit the tumoricidal activity of amphiphilic alpha helical peptides. Cancer Chemother. Pharmacol. 1993;32:109–115. doi: 10.1007/BF00685612. [DOI] [PubMed] [Google Scholar]
- Perez-Tomas R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr. Med. Chem. 2006;13:1859–1876. doi: 10.2174/092986706777585077. [DOI] [PubMed] [Google Scholar]
- Pettit G.R., Kamano Y., Herald C.L., Tuinman A.A., Boettner F.E., Kizu H., Schmidt J.M., Baczynskyj L., Tomer K.B., Bontems R.J. The isolation and structure of a remarkable marine animal antineoplastic constituent: dolastatin 10. J. Am. Chem. Soc. 1987;109:6883–6885. [Google Scholar]
- Pokorny A., Almeida P.F.F. Kinetics of dye efflux and lipid flip-flop induced by δ-lysin in phosphatidylcholine vesicles and the mechanism of graded release by amphipathic, α-helical peptides. Biochemistry. 2004;43:8846–8857. doi: 10.1021/bi0497087. [DOI] [PubMed] [Google Scholar]
- Pokorny A., Kilelee E.M., Wu D., Almeida P.F.F. The activity of the amphipathic peptide δ-lysin correlates with phospholipid acyl chain structure and bilayer elastic properties. Biophys. J. 2008;95:4748–4755. doi: 10.1529/biophysj.108.138701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein D.L. Heparin and heparan sulfate: structure and function. Nat. Prod. Rep. 2002;19:312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- Ran S., Downes A., Thorpe P.E. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132–6140. [PubMed] [Google Scholar]
- Rao L.V., Tait J.F., Hoang A.D. Binding of annexin V to a human ovarian carcinoma cell line (OC-2008). Contrasting effects on cell surface factor VIIa/tissue factor activity and prothrombinase activity. Thromb. Res. 1992;67:517–531. doi: 10.1016/0049-3848(92)90013-z. [DOI] [PubMed] [Google Scholar]
- Rathinakumar R., Wimley W.C. Biomolecular engineering by combinatorial design and high-throughput screening: small, soluble peptides that permeabilize membranes. J. Am. Chem. Soc. 2008;130:9849–9858. doi: 10.1021/ja8017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinakumar R., Wimley W.C. High-throughput discovery of broad-spectrum peptide antibiotics. FASEB J. 2010;24:3232–3238. doi: 10.1096/fj.10-157040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshetnyak Y.K., Andreev O.A., Lehnert U., Engelman D.M. Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6460–6465. doi: 10.1073/pnas.0601463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshetnyak Y.K., Segala M., Andreev O.A., Engelman D.M. A monomeric membrane peptide that lives in three worlds: in solution, attached to, and inserted across lipid bilayers. Biophys. J. 2007;93:2363–2372. doi: 10.1529/biophysj.107.109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl S., Rinner B., Asslaber M., Schaider H., Walzer S., Novak A., Lohner K., Zweytick D. In search of a novel target – phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochim. Biophys. Acta. 2011;1808:2638–2645. doi: 10.1016/j.bbamem.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl S., Rinner B., Tumer S., Schaider H., Lohner K., Zweytick D. Targeting the cancer cell membrane specifically with human lactoferricin derivatives. Ann. Oncol. 2011;22(suppl. 3):iii31–iii34. [Google Scholar]
- Risso A., Zanetti M., Gennaro R. Cytotoxicity and apoptosis mediated by two peptides of innate immunity. Cell. Immunol. 1998;189:107–115. doi: 10.1006/cimm.1998.1358. [DOI] [PubMed] [Google Scholar]
- Rodrigues C.M., Sola S., Castro R.E., Laires P.A., Brites D., Moura J.J. Perturbation of membrane dynamics in nerve cells as an early event during bilirubin-induced apoptosis. J. Lipid Res. 2002;43:885–894. [PubMed] [Google Scholar]
- Rodrigues E.G., Dobroff A.S., Cavarsan C.F., Paschoalin T., Nimrichter L., Mortara R.A., Santos E.L., Fazio M.A., Miranda A., Daffre S., Travassos L.R. Effective topical treatment of subcutaneous murine B16F10-Nex2 melanoma by the antimicrobial peptide gomesin. Neoplasia. 2008;10:61–68. doi: 10.1593/neo.07885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozek T., Wegener K.L., Bowie J.H., Olver I.N., Carver J.A., Wallace J.C., Tyler M.J. The antibiotic and anticancer active aurein peptides from the Australian Bell Frogs Litoria aurea and Litoria raniformis the solution structure of aurein 1.2. Eur. J. Biochem. 2000;267:5330–5341. doi: 10.1046/j.1432-1327.2000.01536.x. [DOI] [PubMed] [Google Scholar]
- Safaiyan F., Lindahl U., Salmivirta M. Selective reduction of 6-O-sulfation in heparan sulfate from transformed mammary epithelial cells. Eur. J. Biochem. 1998;252:576–582. doi: 10.1046/j.1432-1327.1998.2520576.x. [DOI] [PubMed] [Google Scholar]
- Sanderson R.D. Heparan sulfate proteoglycans in invasion and metastasis. Semin. Cell Dev. Biol. 2001;12:89–98. doi: 10.1006/scdb.2000.0241. [DOI] [PubMed] [Google Scholar]
- Schadendorf D., Worm M., Algermissen B., Kohlmus C.M., Czarnetzki B.M. Chemosensitivity testing of human malignant melanoma. A retrospective analysis of clinical response and in vitro drug sensitivity. Cancer. 1994;73:103–108. doi: 10.1002/1097-0142(19940101)73:1<103::aid-cncr2820730119>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Schröder-Borm H., Bakalova R., Andrä J. The NK-lysin derived peptide NK-2 preferentially kills cancer cells with increased surface levels of negatively charged phosphatidylserine. FEBS Lett. 2005;579:6128–6134. doi: 10.1016/j.febslet.2005.09.084. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Gardiner J.M. Membrane lipids and enzymes of cultured high- and low-metastatic B16 melanoma variants. Cancer Res. 1984;44:3262–3269. [PubMed] [Google Scholar]
- Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009;625:190–194. doi: 10.1016/j.ejphar.2009.08.043. [DOI] [PubMed] [Google Scholar]
- Seigneuret M., Devaux P.F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc. Natl. Acad. Sci. U.S.A. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevcsik E., Pabst G., Richter W., Danner S., Amenitsch H., Lohner K. Interaction of LL-37 with model membrane systems of different complexity: influence of the lipid matrix. Biophys. J. 2008;94:4688–4699. doi: 10.1529/biophysj.107.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadidi M., Sioud M. Selective targeting of cancer cells using synthetic peptides. Drug Resist. Updat. 2003;6:363–371. doi: 10.1016/j.drup.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- Sharma S.V. Melittin resistance: a counterselection for ras transformation. Oncogene. 1992;7:193–201. [PubMed] [Google Scholar]
- Sherbet G.V. Membrane fluidity and cancer metastasis. Exp. Cell Biol. 1989;57:198–205. doi: 10.1159/000163526. [DOI] [PubMed] [Google Scholar]
- Sherbet G.V., Jackson S. Temperature-dependent surface charge modulation, membrane fluidity, and metastatic ability of L51178Y lymphoma. Anticancer Res. 1986;6:129–134. [PubMed] [Google Scholar]
- Shewach D.S, Kuchta R.D. Introduction to cancer chemotherapeutics. Chem. Rev. 2009;109:2859–2861. doi: 10.1021/cr900208x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Wang Y., Liang Y., Li Q. Effects of tachyplesin and n-sodium butyrate on proliferation and gene expression of human gastric adenocarcinoma cell line BGC-823. World J. Gastroenterol. 2006;12:1694–1698. doi: 10.3748/wjg.v12.i11.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. Review of the nitrogen mustards. Hahnemann Mon. 1947;82:461–470. [PubMed] [Google Scholar]
- Silva P.I., Daffre S., Bulet P. Isolation and characterization of gomesin, an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the Tachyplesin family. J. Biol. Chem. 2000;275:33464–33470. doi: 10.1074/jbc.M001491200. [DOI] [PubMed] [Google Scholar]
- Simmons T.L., Andrianasolo E., McPhail K., Flatt P., Gerwick W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005;4:333–342. [PubMed] [Google Scholar]
- Smith L.L., Brown K., Carthew P., Lim C.K., Martin E.A., Styles J., White I.N. Chemoprevention of breast cancer by tamoxifen: risks and opportunities. Crit. Rev. Toxicol. 2000;30:571–594. doi: 10.1080/10408440008951120. [DOI] [PubMed] [Google Scholar]
- Soballe P.W., Maloy W.L., Myrga M.L., Jacob L.S., Herlyn M. Experimental local therapy of human melanoma with lytic magainin peptides. Int. J. Cancer. 1995;60:280–284. doi: 10.1002/ijc.2910600225. [DOI] [PubMed] [Google Scholar]
- Soengas M.S., Capodieci P., Polsky D., Mora J., Esteller M., Opitz-Araya X., McCombie R., Herman J.G., Gerald W.L., Lazebnik Y.A., Cordon-Cardo C., Lowe S.W. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- Sok M., Sentjurc M., Schara M., Stare J., Rott T. Cell membrane fluidity and prognosis of lung cancer. Ann. Thorac. Surg. 2002;73:1567–1571. doi: 10.1016/s0003-4975(02)03458-6. [DOI] [PubMed] [Google Scholar]
- Staudegger E., Prenner E.J., Kriechbaum M., Degovics G., Lewis R.N.A.H., McElhaney R.N., Lohner K. X-ray studies on the interaction of the antimicrobial peptide gramicidin S with microbial lipid extracts: evidence for cubic phase formation. Biochim. Biophys. Acta. 2000;1468:213–230. doi: 10.1016/s0005-2736(00)00260-1. [DOI] [PubMed] [Google Scholar]
- Sugahara K., Mikami T., Uyama T., Mizuguchi S., Nomura K., Kitagawa H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Suttmann H., Retz M., Paulsen F., Harder J., Zwergel U., Kamradt J., Wullich B., Unteregger G., Stockle M., Lehmann J. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 2008;8:5. doi: 10.1186/1471-2490-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szachowicz-Petelska B., Dobrzynska I., Figaszewski Z., Sulkowski S. Changes in physico-chemical properties of human large intestine tumour cells membrane. Mol. Cell. Biochem. 2002;238:41–47. doi: 10.1023/a:1019946718876. [DOI] [PubMed] [Google Scholar]
- Szakacs G., Paterson J.K., Ludwig J.A., Booth-Genthe C., Gottesman M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Fujimoto Y., Suzuki M., Suzuki Y., Ohtake T., Saito H., Kohgo Y. PI3-kinase p85α is a target molecule of proline-rich antimicrobial peptide to suppress proliferation of ras-transformed cells. Jpn. J. Cancer Res. 2001;92:959–967. doi: 10.1111/j.1349-7006.2001.tb01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenin D., An M., Engelman D.M. pHLIP-mediated translocation of membrane-impermeable molecules into cells. Chem. Biol. 2009;16:754–762. doi: 10.1016/j.chembiol.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachenko E., Rhodes J.M., Simons M. Syndecans: new kids on the signaling block. Circ. Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- Tofilon P.J., Camphausen K. Molecular targets for tumor radiosensitization. Chem. Rev. 2009;109:2974–2988. doi: 10.1021/cr800504x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson M.T., Tosteson D.C. The sting. Melittin forms channels in lipid bilayers. Biophys. J. 1981;36:109–116. doi: 10.1016/S0006-3495(81)84719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi T., Schroit A.J., Connor J., Bucana C.D., Fidler I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51:3062–3066. [PubMed] [Google Scholar]
- van Blitterswijk W.J. Alteration in lipid fluidity in the plasma membrane of tumor cells. In: Shinitzky M., editor. Physiology of Membrane Fluidity. CRC Press; Boca Raton, FL, USA: 1984. pp. 53–84. [Google Scholar]
- Van de W.C., Lahorte C., Vermeersch H., Loose D., Mervillie K., Steinmetz N.D., Vanderheyden J.L., Cuvelier C.A., Slegers G., Dierck R.A. Quantitative tumor apoptosis imaging using technetium-99m-HYNIC annexin V single photon emission computed tomography. J. Clin. Oncol. 2003;21:3483–3487. doi: 10.1200/JCO.2003.12.096. [DOI] [PubMed] [Google Scholar]
- Vogel H.J., Schibli D.J., Jing W., Lohmeier-Vogel E.M., Epand R.F., Epand R.M. Towards a structure-function analysis of bovine lactoferricin and related tryptophan- and arginine-containing peptides. Biochem. Cell. Biol. 2002;80:49–63. doi: 10.1139/o01-213. [DOI] [PubMed] [Google Scholar]
- Wang F.L., Cui S.X., Sun L.P., Qu X.J., Xie Y.Y., Zhou L., Mu Y.L., Tang W., Wang Y.S. High expression of alpha 2,3-linked sialic acid residues is associated with the metastatic potential of human gastric cancer. Cancer Detect. Prev. 2009;32:437–443. doi: 10.1016/j.cdp.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Wang G., Li X., Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37:D933–D937. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.R., Zhang B.Z., Zhang W., Yan J.X., Li J., Wang R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides. 2008;29:963–968. doi: 10.1016/j.peptides.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Wang K.R., Yan J.X., Zhang B.Z., Song J.J., Jia P.f., Wang R. Novel mode of action of polybia-MPI, a novel antimicrobial peptide, in multi-drug resistant leukemic cells. Cancer Lett. 2009;278:65–72. doi: 10.1016/j.canlet.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wang G. APD: the antimicrobial peptide database. Nucleic Acids Res. 2004;32:D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek M., Jenssen H., Kindrachuk J., Scott W.R.P., Elliott M., Hilpert K., Cheng J.T.J., Hancock R.E.W., Straus S.K. Structural studies of a peptide with immune modulating and direct antimicrobial activity. Chem. Biol. 2010;17:970–980. doi: 10.1016/j.chembiol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Willumeit R., Kumpugdee M., Funari S.S., Lohner K., Pozo-Navas B., Brandenburg K., Linser S., Andrä J. Structural rearrangement of model membranes by the peptide antibiotic NK-2. Biochim. Biophys. Acta. 2005;1669:125–134. doi: 10.1016/j.bbamem.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wimley W., Hristova K. Antimicrobial peptides: successes, challenges and unanswered questions. J. Membr. Biol. 2011;239:27–34. doi: 10.1007/s00232-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimley W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010;5:905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimley W.C., Creamer T.P., White S.H. Solvation energies of amino acid side chains and backbone in a family of host-guest pentapeptides. Biochemistry. 1996;35:5109–5124. doi: 10.1021/bi9600153. [DOI] [PubMed] [Google Scholar]
- Winder D., Günzburg W.H., Erfle V., Salmons B. Expression of antimicrobial peptides has an antitumour effect in human cells. Biochem. Biophys. Res. Commun. 1998;242:608–612. doi: 10.1006/bbrc.1997.8014. [DOI] [PubMed] [Google Scholar]
- Woehlecke H., Pohl A., Alder-Baerens N., Lage H., Herrmann A. Enhanced exposure of phosphatidylserine in human gastric carcinoma cells overexpressing the half-size ABC transporter BCRP (ABCG2) Biochem. J. 2003;376:489–495. doi: 10.1042/BJ20030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Wang Y.S., Pan W.B., Xiao B., Wen Y.J., Chen X.C., Chen L.J., Deng H.X., You J., Kan B., Fu A.F., Li D., Zhao X., Wei Y.Q. Human alpha-defensin-1 inhibits growth of human lung adenocarcinoma xenograft in nude mice. Mol. Cancer Ther. 2008;7:1588–1597. doi: 10.1158/1535-7163.MCT-08-0010. [DOI] [PubMed] [Google Scholar]
- Yang N., Lejon T., Rekdal O. Antitumour activity and specificity as a function of substitutions in the lipophilic sector of helical lactoferrin-derived peptide. J. Pept. Sci. 2003;9:300–311. doi: 10.1002/psc.457. [DOI] [PubMed] [Google Scholar]
- Yang N., Strom M.B., Mekonnen S.M., Svendsen J.S., Rekdal O. The effects of shortening lactoferrin derived peptides against tumour cells, bacteria and normal human cells. J. Pept. Sci. 2004;10:37–46. doi: 10.1002/psc.470. [DOI] [PubMed] [Google Scholar]
- Yoo Y.-C., Watanabe S., Watanabe R., Hata K., Shimazaki K., Azuma I. Bovine lactoferrin and lactoferricin, a peptide derived from bovine lactoferrin, inhibit tumor metastasis in mice. Jpn. J. Cancer Res. 1997;88:184–190. doi: 10.1111/j.1349-7006.1997.tb00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. U.S.A. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhang W., Li J., Liu L.W., Wang K.R., Song J.J., Yan J.X., Li Z.Y., Zhang B.Z., Wang R. A novel analog of antimicrobial peptide Polybia-MPI, with thioamide bond substitution, exhibits increased therapeutic efficacy against cancer and diminished toxicity in mice. Peptides. 2010;31:1832–1838. doi: 10.1016/j.peptides.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Zoonens M., Reshetnyak Y.K., Engelman D.M. Bilayer interactions of pHLIP, a peptide that can deliver drugs and target tumors. Biophys. J. 2008;95:225–235. doi: 10.1529/biophysj.107.124156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal R.F., Schroit A.J. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- Zweytick D., Pabst G., Abuja P.M., Jilek A., Blondelle S.E., Andrä J., Jerala R., Monreal D., Martinez de Tejada G., Lohner K. Influence of N-acylation of a peptide derived from human lactoferricin on membrane selectivity. Biochim. Biophys. Acta. 2006;1758:1426–1435. doi: 10.1016/j.bbamem.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Zweytick D., Riedl S., Rinner B., Asslaber M., Schaider H., Walzer S., Novak A., Lohner K. In search of new targets – the membrane lipid phosphatidylserine – the underestimated Achilles’ heel of cancer cells. Ann. Oncol. 2011;22(suppl. 3):iii42–iii43. [Google Scholar]
- Zweytick D., Tumer S., Blondelle S.E., Lohner K. Membrane curvature stress and antibacterial activity of lactoferricin derivatives. Biochem. Biophys. Res. Commun. 2008;369:395–400. doi: 10.1016/j.bbrc.2008.01.176. [DOI] [PubMed] [Google Scholar]
- Zweytick D., Deutsch G., Andrä J., Blondelle S.E., Vollmer E., Jerala R., Lohner K. Studies on lactoferricin derived E. coli membrane active peptides reveal differences in the mechanism of N-acylated versus non-acylated peptides. J. Biol. Chem. 2011;24:21266–21276. doi: 10.1074/jbc.M110.195412. [DOI] [PMC free article] [PubMed] [Google Scholar]