Sir: The fragile X premutation, an expansion of a CGG triplet repeat in the 5′-untranslated region of the FMR1 gene (55–200 repeats), is one of the most common aetiologies of primary ovarian insufficiency: fragile X-associated primary ovarian insufficiency (FXPOI).1–3 Men who carry the premutation are at increased risk for a late-onset neurodegenerative disorder termed fragile X tremor/ataxia syndrome (FXTAS).4 In premutation carriers, FMR1 is transcribed at increased levels, and the large rCGG tracks may cause disease by acting in a toxic gain of function manner.5–8
The pathological correlate of FXTAS comprises ubiquitin staining intranuclear inclusions in neurons and astrocytes throughout the cerebrum and brainstem.9 The ubiquitin staining suggests a link to proteasome protein degradation. In addition, the inclusions contain FMR1 mRNA, which is postulated to bind and sequester RNA-binding proteins.8,9 We hypothesized that intranuclear inclusions would also be demonstrated in the ovaries of women with FXPOI.
We obtained ovaries from FMR1 premutation carriers (n = 5) and control subjects (n = 4) matched by age and menstrual cycle status (Table 1). Protocols were approved by the Partners Human Research Committee or the Institutional Review Board at Emory University.
Table 1.
Descriptive Summary of Clinical and Pathologic Findings.
| Subject | Age | Repeat Number |
Menstrual History at Time of Surgery |
Reason for Surgery |
Ovarian Pathology (H&E) | Ubiquitin Immunohistochemistry |
|---|---|---|---|---|---|---|
| Established Fragile X Premutation Carriers | ||||||
| Case 1 | 31 | 75 | Postmenopausal since age 20 yrs |
Ovarian cysts |
Follicles and Stroma: Atretic follicles; ovarian stroma present and evaluable. Epithelium: Cortical inclusion cysts. Mucinous cyst. |
Follicles and Stroma: Intranuclear inclusions in stromal cells: up to 3 per 10 high power fields. Non-specific nuclear staining seen in perivascular stromal cells. Epithelium: Non-specific nuclear staining. |
| Case 2 | 42 | 100 | 28 day cycle | Adenomyosis |
Follicles and Stroma: Atretic follicles; ovarian stroma present and evaluable. Epithelium: Cortical inclusion cysts. |
Follicles and Stroma: Intranuclear inclusions in stromal cells: up to 20 per 10 high power fields. Epithelium: Minimal to no non-specific staining. |
| Case 3 | 39 | 100 | Irregular, infertility, menometrorrhagia worsening with oral contraceptives. |
Menometror- rhagia, pelvic pain (Ovarian biopsy only) |
Follicles and Stroma: No follicles identified; ovarian stroma present and evaluable. Epithelium: Endometriosis. |
Follicles and Stroma: Intranuclear inclusions in stromal cells: more than 50 per 10 high power fields. Non-specific nuclear and cytoplasmic staining seen in ovarian stromal cells. Epithelium: Non-specific nuclear and cytoplasmic staining seen in endometriotic stromal and epithelial cells. |
| Case 4 | 72 | 79 | Postmenopausal | Uterine bleeding, and biopsy proven endometrial adenocarcinoma |
Follicles and Stroma: Atretic follicles; fibroma; ovarian stroma present and evaluable. Epithelium: Cortical inclusion cysts. |
Follicles and Stroma: Intranuclear inclusions in stromal cells: more than 50 per 10 high power fields. Non-specific nuclear and cytoplasmic staining seen in ovarian and perivascular stromal cells. Epithelium: Non-specific nuclear staining. |
| Case 5 | 44 | 62 | Irregular menses every 3 to 4 months, with 6 months of amenorrhea, infertility and FSH 34 IU/L |
Complex right ovarian cyst and BRCA1 mutation |
Follicles and Stroma: Cystic follicle (functional); ovarian stroma present and evaluable. Epithelium: Cortical inclusion cysts. |
Follicles and Stroma: Intranuclear inclusions in stromal cells: up to 7 per 10 high power fields. Non-specific cytoplasmic staining; granulosa > theca cells. Non-specific nuclear and cytoplasmic staining seen in ovarian and perivascular stromal cells. Epithelium: Non-specific nuclear staining. |
| Control Ovarian Tissue | ||||||
| Control 1 | 27 | Normal (31, 36) |
Regular 28 days | Ovarian cyst |
Follicles and Stroma: No follicles identified. Ovarian stroma present and evaluable. Epithelium: Mature cystic teratoma (squamous, sebaceous, thyroid, and glial-type tissue present). |
Follicles and Stroma: No intranuclear inclusions in stromal cells. Non-specific nuclear staining seen in ovarian and perivascular stromal cells. Epithelium: Non-specific nuclear and cytoplasmic staining seen in epithelial and ectodermal elements of the mature cystic teratoma. |
| Control 2 | 47 | Normal (31, 31) |
Unknown |
Follicles and Stroma: Cystic follicle (functional); ovarian stroma present and evaluable. Epithelium: Cortical inclusion cysts |
Follicles and Stroma: No intranuclear inclucions in stromal cells. Non-specific cytoplasmic staining; granulosa > theca cells. Non-specific nuclear and cytoplasmic staining seen in ovarian and perivascular stromal cells. Epithelium: Minimal non-specific nuclear staining. |
|
| Control 3 | 70 | Unknown | Postmenopausal |
Follicles and Stroma: Atretic follicles; ovarian stroma present and evaluable. Epithelium: Cortical inclusion cysts. |
Follicles and Stroma: No intranuclear inclusions in stromal cells. Non-specific nuclear staining seen in ovarian and perivascular stromal cells. Epithelium: Minimal non-specific nuclear staining. |
|
| Control 4 | 61 | Unknown | Postmenopausal | Prophylactic oophorectomy for strong family history of breast cancer |
Follicles and Stroma: Atretic follicles; ovarian stroma present and evaluable Epithelium: Ovarian surface and fallopian tube only. |
Follicles and Stroma: Intranuclear inclusions in stromal cells: up to 2 per 10 high power fields. Minimal non-specific staining seen in ovarian stromal cells. Epithelium: Minimal non-specific nuclear and cytoplasmic staining. |
Notes: Field diameter of high power field: 0.63 mm.
The ubiquitin-positive intranuclear inclusions observed measured 3 to 5 micrometers, and could not be seen on H&E sections.
Ovarian tissues were prepared by standard formalin fixation and paraffin embedding. Five-micrometre sections were cut and stained with haematoxylin and eosin (H&E), or deparaffinized and pretreated with aqueous H2O2 at 37°C for immunohistochemistry (no antigen retrieval). Slides were incubated with primary antibody (polyclonal anti-rabbit ubiquitin, 1:3000 dilution; DakoCytomation, Carpinteria, CA, USA) for 1 h, and then washed and incubated with peroxidase-labelled polymer-conjugated goat anti-rabbit immunoglobulin (Envision Plus; Dako, Carpinteria, CA, USA) for 30 min. Antibody was visualized by peroxidase reaction with 3,3′-diaminobenzidine tetrahydrochloride (DAB+; Dako), and slides were counterstained with haematoxylin.
A ‘ubiquitin-positive inclusion’ was defined as a discrete intranuclear body measuring 3–5 μm in diameter, and demonstrating strong immunoreactivity on ubiquitin staining. The density of the inclusions within the section was recorded by manual count under a high-power objective (0.63-μm field diameter).
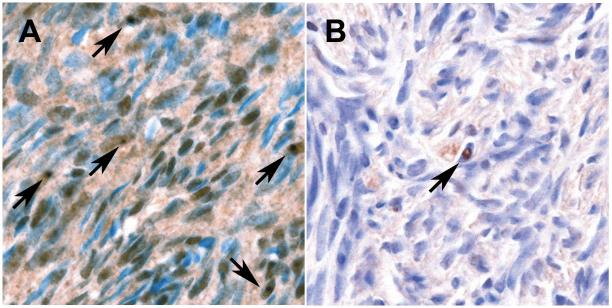
The general histopathological characteristics of ovaries from subjects with FXPOI wereindistinguishable from those of ovaries from the general population (Table 1; Figures 1 and 2). Ovaries from premutation carriers demonstrated ubiquitin-positive intranuclear inclusions in the stromal cells. Prominent staining and stromal ubiquitin-positive inclusions were numerous in the ovaries of both cases 3 (Figure 1A) and 4. The ovarian ubiquitin-positive inclusions were not appreciable on an H&E section.
Figure 1.
A, Ovary of a fragile X premutation carrier with 100 repeats demonstrating frequent ubiquitin-positive inclusions (arrows). Non-specific staining of stromal nuclei and cytoplasm is also seen. B, Ovary of a control case showing a rare ubiquitin-positive inclusion (arrow). (Ubiquitin stains, haematoxylin counterstain; photographed under ×40 objective and ×0.70 C-Mount adapter.)
Figure 2.
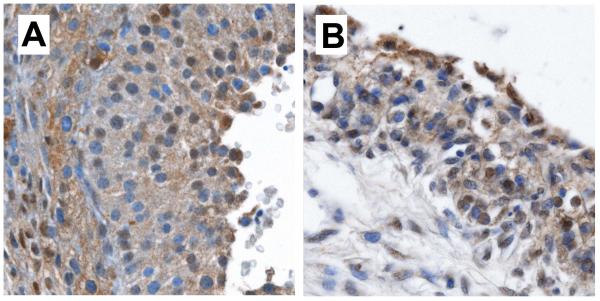
Ovarian follicles including granulosa and theca cells found in (A) a fragile X premutation carrier with 62 repeats and (B) a control patient, showing similar morphology and ubiquitin immunostaining patterns. (Ubiquitin stains, haematoxylin counterstain; photographed under ×40 objective and ×0.70 C-Mount adapter).
Rare ubiquitin-positive intranuclear inclusions were seen in one control ovary (Figure 1B). However, numerous inclusions were not seen in any of the control cases. In both FXPOI and control ovaries, non-specific nuclear and cytoplasmic ubiquitin immunoreactivity was seen in stromal and epithelial cells, including epithelial elements of a mature cystic teratoma from a control. The intensity of non-specific ubiquitin staining varied significantly, both within and between FXPOI and control groups. All of these non-specific findings probably reflect physiological protein degradation processes within the ovary.
FXTAS brain tissue was evaluated as a control, and, by contrast, demonstrated larger eosinophilic inclusions within neurons and astrocytes that were strongly immunoreactive for ubiquitin.9 This may represent different sizes and charges of protein degradation products in neurons than in the ovary (for example, myelin basic protein would be strongly eosinophilic). Non-specific (cytoplasmic) staining was also described in the neurons of FXTAS individuals and was postulated to be related to inability to degrade normal proteins.9
There were no ubiquitin-positive inclusions in granulosa or theca cells (Figure 2), and there was no difference in follicle number between FXPOI and control cases. The absence is surprising, because follicle cells, along with the oocyte (none observed), are the primary cells affected in FXPOI.10,11 The ubiquitin-positive stromal inclusions therefore do not apparently disrupt the normal structural development of the ovary. Rather, we hypothesize that inclusions seen in the stromal cells of otherwise normal-appearing ovaries reflect an underlying toxic gain-of-function relating to protein degradation. Within the follicles themselves, selective failure and loss of affected follicles may occur too quickly for the pathological inclusions to be visualized (analogous to the phenomenon seen in Purkinje cells of FXTAS patients).9 At present, the mechanisms of the observed changes remain unclear.
In summary, we demonstrated ubiquitin-positive inclusions within ovarian stromal cell nuclei with no correlate on H&E sections, occurring with a high frequency and density in women who carry the fragile X premutation as compared with ovaries from a control population. These inclusions appear to represent the ovarian analogues to similar structures seen in the neurons of FXTAS patients. Further studies are warranted to determine the components of these inclusions and their cellular effects.
Acknowledgements
We thank Dr Joseph Woodward Carlson and Dr Christopher P. Crum for their help in annotating and obtaining samples. We also thank the women who participated in our studies for their generous contributions and ongoing support. This work was supported, in part, by National Institutes of Health grant NIH RO1 HD29909.
References
- 1.Nolin SL, Brown WT, Glicksman A, et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am. J. Hum. Genet. 2003;72:454–464. doi: 10.1086/367713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am. J. Med. Genet. 1999;83:322–325. [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan AK, Marcus M, Epstein MP, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum. Reprod. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 4.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 5.Allen EG, He W, Yadav-Shah M, Sherman SL. A study of the distributional characteristics of FMR1 transcript levels in 238 individuals. Hum. Genet. 2004;114:439–447. doi: 10.1007/s00439-004-1086-x. [DOI] [PubMed] [Google Scholar]
- 6.Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum. Mol. Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 7.Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todd PK, Paulson HL. RNA-mediated neurodegeneration in repeat expansion disorders. Ann. Neurol. 2010;67:291–300. doi: 10.1002/ana.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greco CM, Hagerman RJ, Tassone F, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 10.Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J. Clin. Endocrinol. Metab. 2004;89:4569–4574. doi: 10.1210/jc.2004-0347. [DOI] [PubMed] [Google Scholar]
- 11.Rohr J, Allen EG, Charen K, et al. Anti-Mullerian hormone (AMH) indicates early ovarian decline in FMR1 premutation carriers: a preliminary study. Hum. Reprod. 2008;23:1220–1225. doi: 10.1093/humrep/den050. [DOI] [PubMed] [Google Scholar]