Abstract
Endogenous glucocorticoid negative feedback influence on the hypothalamic-pituitary-adrenal axis (HPAA) depends on glucocorticoid actions exerted on multiple glucocorticoid-sensitive tissues and differential glucocorticoid effects that are expressed within several distinct temporal domains. The relative contribution and underlying molecular mechanisms of action for the effects of location and timing of glucocorticoid exposure on HPAA activity remains to be determined. In this study, we examined the effects of acute exposure to corticosterone(CORT) at the level of the paraventricular nucleus (PVN)on the HPAA response to a subsequent stressor in a short-term (1hr) timeframe. Intra-PVN CORT microinjection one hour prior to restraint suppressed the adrenocorticotrophic hormone (ACTH) response and blunted restraint-induced corticotropin-releasing hormone (CRH) hnRNA expression in the PVN and pro-opiomelanocortin hnRNA expression in the anterior pituitary (AP), however it had no effect on restraint-induced plasma prolactin levels and c-fos mRNA expression (PVN and AP). This pattern of results suggests that CORT acts locally at the level of the PVN within a short-term timeframe to suppress stress-induced excitation-exocytosis coupling within CRH neurones and CRH gene induction without altering the stress-associated trans-synaptic input and intracellular signal transduction that converges on PVN c-fos gene induction. Our study is the first to demonstrate that an acute infusion of CORT into the PVN is sufficient to suppress the ACTH response to stress initiated 1 hr after CORT infusion.
Keywords: Glucocorticoids, Paraventricular Nucleus, c-fos, corticotropin releasing hormone (CRH), proopiomelanocortin (POMC)
Introduction
Proper neural and humoral control of adrenal glucocorticoid secretion is necessary for the optimal function of nearly every physiological system in the human body. The temporal pattern and amplitude of varying basal (circadian and ultradian) and stimulated glucocorticoid secretion influence many important systems-level states such as metabolism, immunity, cognition, and reproduction (1–3). In turn, the proper control of glucocorticoid secretion is substantially dependent upon the molecular and cellular effects of glucocorticoids on the neural and endocrine components of the hypothalamo-pituitary adrenal axis (HPAA) (4). Dysregulation of glucocorticoid negative feedback and the associated alteration of hypothalamic corticotropin-releasing hormone (CRH) and systemic glucocorticoid levels is observed in a variety of stress-related psychiatric disorders including major depressive disorder, anxiety, and post-traumatic stress disorder (5–8). The pioneering groundwork describing the phenomenon of glucocorticoid negative feedback was detailed some 40–60 years ago (9–11) and provides the basis for the current model of glucocorticoid negative feedback. This model suggests that acute endogenous or exogenous increases in glucocorticoids exert distinct effects upon the hormonal output of the HPAA that are discernable based upon discrete temporal domains. These time domains include 1) fast feedback (<10min), 2) intermediate feedback (30min–2hrs), and 3) slow feedback (>3hrs). However, it is unknown whether the inhibitory effect of glucocorticoids within each of these domains is a result of actions at anatomical sites intrinsic and/or extrinsic to the HPAA, and to what extent actions at each anatomical site comprise the overall effect within a given feedback time domain.
Studies have shown a clear inhibitory influence of systemically administered exogenous glucocorticoids on HPAA activity (12–14). Furthermore, data from our lab and others indicates that varying the interval between acute systemic CORT treatment and sampling of HPAA functional measures (both during basal and activated states) reveals unique profiles of glucocorticoid effects on hormonal and cellular responses of each intrinsic cellular component of the HPAA (13, 15–17). Interpretation of the underlying bases of these different response profiles is complicated by the likelihood that each measure reflects a combination of direct and indirect glucocorticoid effects exerted both intrinsic and extrinsic to the HPAA. For example, it is widely believed that glucocorticoids directly inhibit both the stress-induced transcriptional activation of the CRH gene and the secretion of CRH peptide into the hypopheseal portal system (4). This belief is supported by numerous studies that have manipulated acutely or chronically systemic glucocorticoid levels (18). However no study to date has determined whether inhibition of both stress-stimulated CRH peptide release and CRH gene induction is due to the effects of glucocorticoids acting directly within the PVN.
In order to determine the cellular and molecular mechanisms by which CORT produces negative feedback control of HPA axis activity it is necessary to identify: 1) the specific CORT target cells that participate in HPA axis negative feedback function, and 2) the specific CORT actions in those target cells that contributes to altered HPA axis hormonal secretion evident at a particular point in time. The focus of this study is to determine whether a component of the negative feedback that is evident 1 hr after a phasic increase in CORT (short-term delayed negative feedback) includes direct effects of CORT at the level of the PVN that influences stress-induced activity of CRH neurones, and the effect of that particular feedback element, if any, on downstream components of the HPAA (anterior pituitary). Although it is clear that a systemic increase in CORT can substantially suppress the ACTH response to an acute stress challenge initiated 1 hr later (13, 15, 16, 19), it remains undetermined whether that suppressive effect depends, at least in part, on direct CORT actions at the level of the PVN(20). Several studies examining basal and stress-induced CORT levels in mice lacking GR expression in the forebrain (GR expression in the PVN was spared) have concluded that brain sites with efferent connections to the PVN, such as the hippocampus and medial prefrontal cortex, are critical CORT feedback sites (21, 22). However, those studies have not directly tested phasic CORT feedback effects within a relatively short timeframe (less than 6 hr). Moreover, those forebrain GR knockout studies cannot rule out a role of tonic CORT effects on central brain sites that modulates the system level neural response to acute stress. Other studies support the possibility that the anterior pituitary is the primary functional target for short-term CORT feedback effects (15, 23).
The experimental strategy in this study was to mimic an acute stress-induced phasic rise in endogenous glucocorticoids solely at the level of the PVN followed by a subsequent stressor in a short-term (1hr) timeframe. Accordingly, CORT was infused acutely into the PVN one hour prior to a restraint stress challenge in adrenalectomised rats given low basal CORT replacement in the drinking water. One hour following PVN microinfusion, HPAA functional measures were analyzed from rats in their home cage (basal) or following a 15 minute restraint stress session (activated). Cellular (c-fos mRNA, CRH hnRNA, and POMC hnRNA expression) and hormonal (ACTH and prolactin) responses to the stressor were examined within intrinsic components of the HPAA (PVN and pituitary). This study demonstrates that glucocorticoids act in a short-term (1hr) fashion directly at the level of the PVN to 1) inhibit stress-induced CRH gene induction without altering the associated trans-synaptic input to the PVN that is responsible for c-fos gene induction, and to 2) inhibit CRH peptide release as suggested by the inhibition of stress-induced anterior pituitary POMC gene induction and plasma ACTH levels.
Materials and Methods
Animals
Young adult male Sprague-Dawley rats (250–300g) were obtained from Harlan Laboratories (Indianapolis, IN), housed individually in polycarbonate tubs in temperature and humidity controlled rooms, and maintained on a 12h:12h light:dark cycle (lights on at 0600h). All animals had ad libitum access to standard rodent chow (Harlan) and tap water, and were allowed to acclimate to the facility for at least one week prior to guide cannula implantation. All experimental procedures were completed within the first half of the light photoperiod. Animal protocols were pre-approved by the Institutional Animal Care and Use Committee at the University of Colorado.
Guide cannula implants
Under halothane anaesthesia, animals were fitted with a stainless steel indwelling bilateral guide cannula (26 gauge, 1.0 mm cannula-to-cannula spacing, cut to extend 7.0 mm from pedestal; Plastics One, Roanoke, VA) with the aid of a small animal stereotaxic instrument (David Kopf Instruments, Tujunga, CA). Coordinates to allow placement of the cannula tip to the region just dorsal to the paraventricular nucleus were: 1.8 mm posterior to bregma and 6.5 mm below the skull surface. The guide cannula was affixed to the skull via three small machine screws surrounded by dental acrylic. A bilateral dummy cannula extending 1.0 mm beyond the end of guide cannula was then inserted, and a dust cap was placed over the external end of the cannula. Animals were post-operatively administered buprenorphine (0.05 mg/kg, sc) for pain, and allowed to recover for one week, singly housed.
Adrenalectomy
One week after guide cannula implant, animals were adrenalectomised(ADX)under halothane anaesthesia to remove endogenous CORT and placed on CORT supplemented (25 μg/ml) drinking water to restore a circadian pattern of CORT exposure (24). On the experimental test day (three days following ADX) CORT supplemented water was replaced with saline water at 0600h. Animals had negligible plasma CORT at the time of tissue harvest as measured by CORT enzyme immunoassay (see below).
Experimental test procedure
On the first and second day following ADX, animals were handled in order to acclimate them to the microinjection procedure prior to test day. On the third day following ADX, between 0800h and 1200h, animals received an acute microinjection of CORT (Steraloids, Newport, RI; 10 ng in 0.5 μl at0.25 μl/min) or vehicle (aCSF + 0.2% EtOH) into the PVN 60 min prior to a 15 min restraint stress or home cage exposure(n=11–12 per group). The dose of CORT was selected based on other published reports of glucocorticoid microinfusion into the PVN(25, 26). This relatively high concentration of CORT (57.7 μM) is expected to fully occupy all glucocorticoid receptors within the most proximal field of diffusion. The CORT solution was made by dilution of a CORT ethanol solution (10 μg/μl) into a freshly made aCSF solution (140 mM NaCl, 3.35 mM KCl, 1.15 mM MgCl2, 1.26 mM CaCl2, 1.2 mM Na2HPO4, 0.3 mM NaH2PO4, pH 7.4) to a final concentration of 20 ng/μL, and subsequent filtration through a 0.22 um filter (Milipore, Bedford, MA). CORT or vehicle were infused through bilateral stainless steel injectors (33 gauge; Plastics One, Roanoke, VA), designed to project 1.0 mm past the end of the guide cannula to 7.5 mm below the skull surface. The infusion solution was delivered via PE20 tubing attached at one end to the injectors and at the other end to 10 μl Hamilton syringes. The infusion rate and duration (0.25 μL/min, 2 minutes) was controlled by an automated infusion pump (Stoelting, Model 101). Injectors were left in place for an additional minute to allow for diffusion of solution into parenchyma. One hour after microinfusion animals were restrained via placement in an adjustable-length Plexiglass tube containing air holes (23.5 cm length by 7.0 cm diameter) for 15 minutes. Rats were decapitated immediately following restraint or directly from home cage. Trunk blood was collected into chilled EDTA-coated tubes for subsequent ACTH and CORT analysis. Brainand pituitary were dissected and flash frozen in cold isopentane (−30C), and stored at −80C until sectioning. Tissue was sectioned on a Leica cryostat at 12μm thickness (brains: 3 series of 6 slides through the PVN; pituitary: 1 series of 6 slides), thaw-mounted on poly-L-lysine-coated slides, and stored at −80C until further processing. Cannula placement was verified via cresyl violet staining (Figure 1).
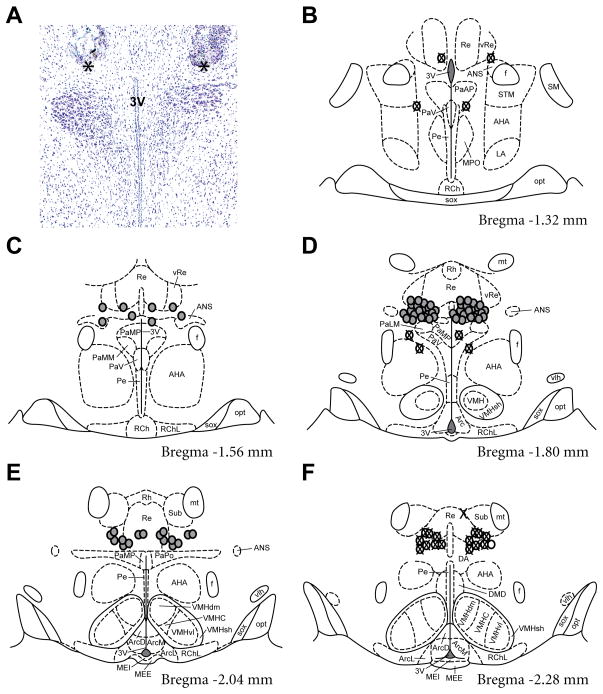
Figure 1.
Anatomical location of intracranial bilateral cannula. A) Representative photomicrograph of a cresyl violet stained coronal section through the PVN. Tissue damage from the cannula is present dorsal to the PVN proper. The ventral tip of the cannula at the rostral-caudal midpoint is denoted by an asterisk. B–F) Location of each cannula tip as determined from cresyl violet stained sections mapped on 5 serial coronal sections of the Paxinos and Watson atlas (Paxinos and Watson 2007). Cannula placements flanking the borders of the PVN were considered “hits” and are denoted by a grey circle. Cannula placements outside the rostral, caudal and dorsal borders of the PVN, or where placement resulted in damage within the PVN, were classified as “misses” and are indicated by a circle with an X. Abbreviations: 3V, third ventricle; AHA, anterior hypothalamic area; ANS, accessory neurosecretory nuclei; Arc, arcuate nucleus; ArcD, dorsal part of arcuate nucleus; ArcL, lateral part of arcuate nucleus; ArcM, medial part of arcuate nucleus; DA, dorsal hypothalamic area; DMD, dorsal part of dorsomedial hypothalamic nucleus; f, fornix; LA, lateroanterior hypothalamic nucleus; MEE, external layer of median eminence; MEI, internal layer of median eminence; MPO, medial preoptic nucleus; mt, mammillothalamic tract; opt, optic tract; PaAP, anterior parvocellular paraventricular nucleus; PaLM, lateral magnocellular paraventricular nucleus; PaMM, medial magnocellular paraventricular nucleus; PaMP, medial parvocellular paraventricular nucleus; PaPo, posterior paraventricular nucleus; PaV, ventral paraventricular nucleus; Pe, periventricular hypothalamic nucleus; RCh, retrochiasmatic area; RChL, lateral part of retrochiasmatic area; Re, reuniens thalamic nucleus; Rh, rhomboid thalamic nucleus; SM, nucleus of the stria medullaris; sox, supraoptic decussation; STM, medial division of the bed nucleus of the stria terminalis; Sub, submedius thalamic nucleus; vlh, ventrolateral hypothalamic tract; VMH, ventromedial hypothalamic nucleus; VMHC, central part of ventromedial hypothalamic nucleus; VMHdm, dorsomedial part of ventromedial hypothalamic nucleus; VMHsh, ventromedial hypothalamic nucleus shell; VMHvl, ventrolateral part ofventromedial hypothalamic nucleus; vRe, ventral reuniens thalamic nucleus.
ACTH radioimmunoassay
Plasma was assayed for ACTH concentration via a competitive radioimmunoassay as described elsewhere(27), using ACTH antiserum (rabbit antibody Rb7, courtesy of Dr. Bill Engeland, University of Minnesota, Minneapolis, MN). The samples were assayed in two assays with an intraassay coefficient of variance of 2.0% and 8.2%, and interassay coefficient of variance was 15.6%.
Corticosterone enzyme immunoassay
Plasma was assayed for CORT concentration via an enzyme immunoassay kit (Assay Design, Ann Arbor, MI) according to manufacturer’s protocol. The samples were assayed in one assay and the intraassay coefficient of variance was 7.2%. Animals with vehicle or CORT microinjection had (mean ± SEM) 0.63 +/−0.18 or 0.54 +/−0.13 μg/dl CORT, respectively.
Prolactin enzyme immunoassay
Plasma was assayed for rat prolactin via an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI, Number 589701) according to manufacturer’s protocol. Only plasma samples from rats with correct guide cannula placements were included in this assay. The sensitivity for this assay was ~2.0ng/ml, and the intra-assay coefficient of variance was 8.9%.
In situ hybridization
S-35 labelled antisense riboprobes were generated and in situ hybridization performed as previously described (13). Probes were generated using plasmids containing a portion of the cDNA sequences encoding for c-fos mRNA (courtesy of Dr. T. Curran, St. Jude Children’s Research Hospital, Memphis TN), POMC hnRNA(courtesy of Dr. Stanley Watson, University of Michigan, Ann Arbor MI), or CRH hnRNA (generated in-house). The Crh plasmid was prepared by subcloning a553 nucleotide sequence completely localised within the first intron into the pSC-A vector, which was subsequently linearised with the endonuclease EcoRV and transcribed using T7 RNA polymerase. Uncalibrated optical densities were obtained from digitised images of the x-ray film using Image J software (NIH). Three (PVN) or four (pituitary) bilateral measurements were made for each animal and subtracted from an adjacent background density measurement.
Statistics
All data was analyzed with Prism (v 5.0, GraphPad Software Inc., San Diego CA). One-way ANOVA followed by Newman-Keuls multiple comparison test was used for post hoc pairwise comparsons. Significance was determined as P ≤ 0.05. Data are expressed in graphs as group means +/− SEM.
Results
Microinjection was restricted to an area adjacent to the dorsal boundary of the PVN
Indwelling bilateral cannulae were directed to the dorsal border of the PVN in order to allow for microinfusion of vehicle or CORT into the region of the PVN without causing direct physical damage to the PVN itself. Tissue damage resulting from the bilateral cannula was visualised via microscopic analysis of cresyl violet-stained sections (Figure 1A), and was subsequently mapped onto the atlas of Paxinos and Watson (28)(Figure 1B–1F). Each dot represents the ventral border of an individual cannula tract at its rostrocaudal centre point (indicated by asterisk in Figure 1A). Rats with cannula placement adjacent to the borders of the PVN were labelled as “hits” and are represented by grey dots in Figure 1. Rats in which the cannula placement was 0.5 mm beyond the rostral, caudal, and/or dorsal borders of the PVN, or extended into the PVN resulting in deposition ventral to the PVN were labelled as “misses” for the purpose of statistical analysis (12/47 animals), and are represented by white dots with crosses in Figure 1(B–F). There were 6 misses in the home cage vehicle group, 2 misses in the home cage CORT group, 0 misses in the restraint vehicle group and 4 misses in the restraint CORT group. The data from restraint rats that had incorrect cannula placements and received a CORT infusion are included in the graphs and supporting statistical analyses to provide an indication of anatomical dependency of CORT infusion. There were no statistically significant differences between the homecage groups of rats identified with correct or incorrect cannula placements for any of the dependent measures (data not shown).
CORT microinfusion into the PVN differentially inhibits the subsequent hormonal response to restraint
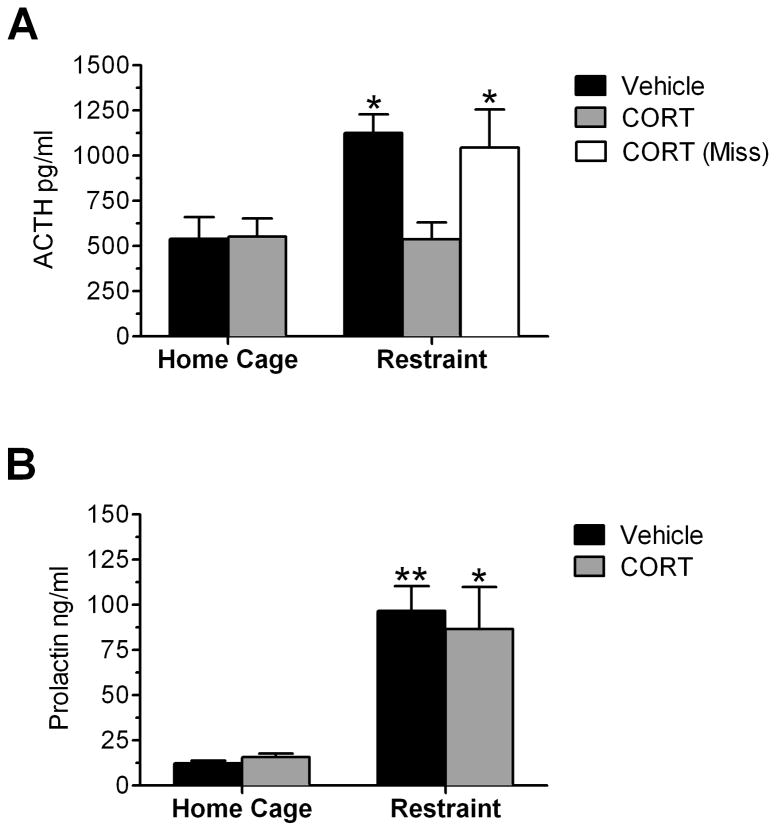
To examine the functional responsiveness of the HPA axis to an acute psychological stressor, plasma ACTH and prolactin concentrations were measured following a 15-minute restraint stress, or directly from home cage (Figure 2). Rats were given a microinfusion of CORT or vehicle into the PVN 60 minutes prior to stressor onset. Analysis by ANOVA indicated that there was a significant main effect of group (F(4,34) = 6.966; P = 0.0003) on plasma ACTH concentration. Post hoc pairwise analysis indicated that a 15 minute session of restraint significantly increased plasma ACTH in vehicle infused rats (P < 0.05), but had no effect in CORT infused rats (Figure 2A). Interestingly, in rats where cannula placement was not near the PVN (“miss”), a 15-minute restraint stress elicited an increase in plasma ACTH concentration similar to the vehicle infused rats (Figure 2). There was also a significant main effect of group on plasma prolactin concentration (F(4,27) = 8.763; P = 0.0003). In contrast to ACTH, Post hoc pairwise analysis indicated that a 15 minute session of restraint significantly increased plasma prolactin in both vehicle infused rats (P < 0.01) and CORT infused rats (P < 0.05) (Figure 2B). Therefore, a 60-minute intra-PVN pretreatment of CORT selectively blocked the restraint-induced increase in plasma ACTH concentration without altering restraint-induced prolactin secretion.
Figure 2.
Effect of 1hr CORT pretreatment within the PVN on restraint-induced plasma ACTH and prolactin concentration. A) CORT microinfusion into the PVN 1hr prior to restraint stress inhibits the restraint-induced rise in plasma ACTH levels measured 15 minutes after stress onset (grey bars). CORT infusion outside of the PVN (“Miss”) did not alter restraint-induced plasma ACTH concentration (white bar). B) CORT microinfusion into the PVN 1hr prior to restraint stress does not alter the restraint-induced increase in plasma prolactin levels. *, **: Significant difference compared to vehicle-infused home cage animal (*P<0.05, **P<0.01, n=7–12 for all groups except the vehicle home cage group (n=5) and restraint-CORT miss group (n=4)).
CORT microinfusion into the PVN inhibits restraint-induced CRH hnRNA expression, but has no effect on restraint-induced c-fos mRNA, within the PVN
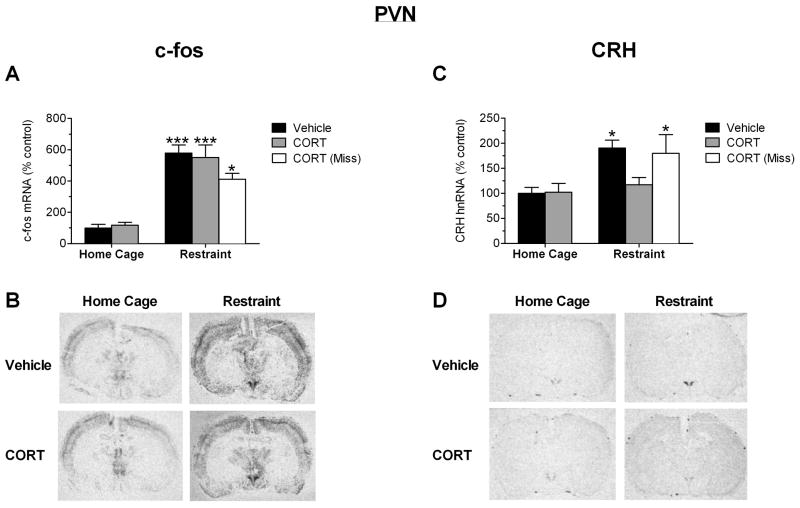
To examine the responsiveness of the PVN to an acute psychological stress at a gene expression level, c-fos and CRH transcript levels were quantified. Analysis by ANOVA showed that there was a significant main effect of group (F(4,34) = 19.93; P < 0.0001) on c-fos mRNA expression in the PVN (Figure 3A and 3B). Post hoc tests indicated that restraint led to an increase in c-fos mRNA (P < 0.001), but this increase was not affected by CORT treatment (P < 0.001). There was a significant main group effect (F(4,34) = 5.871; P = 0.0011) on CRH hnRNA expression in the PVN. Post hoc pairwise analysis indicated that a 15-minute session of restraint significantly increased CRH hnRNA in the PVN of vehicle infused rats (P < 0.05), but not in CORT infused rats (Figure 3C and D). In rats where cannula placement was not near the PVN (“miss”), a 15-minute restraint stress elicited an increase in CRH hnRNA expression within the PVN (P < 0.05) to a similar extent as vehicle infused rats. Therefore, a 60-minute pretreatment of CORT selectively targeting the PVN completely blocked the restraint-induced increase in CRH, but not c-fos, gene expression in the PVN.
Figure 3.
Effect of 1hr CORT pretreatment within the PVN on restraint-induced gene expression in the PVN as measured by in situ hybridization. A) CORT microinfusion into the PVN 1hr prior to restraint stress does not alter c-fos gene induction in the PVN in response to restraint. B) Autoradiograms of c-fos expression from representative sections through the PVN. C) CORT microinfusion within the PVN 1hr prior to restraint stress inhibits the restraint-induced increase in PVN CRH gene expression measured 15 minutes after stress onset (grey bars). CORT infusion outside of the PVN (“Miss”) did not alter the stress-induced increase in CRH gene expression (white bar). D) Autoradiograms of CRH expression from representative sections through the PVN. *, ***: Significant difference compared to vehicle-infused home cage animal (*P<0.05, ***P<0.001, n=8–12 for all groups except the vehicle home cage group (n=5) and restraint-CORT miss group (n=4)).
CORT microinfusion into the PVN inhibits restraint-induced POMC hnRNA expression, but has no effect on restraint-induced c-fos mRNA, within the anterior pituitary
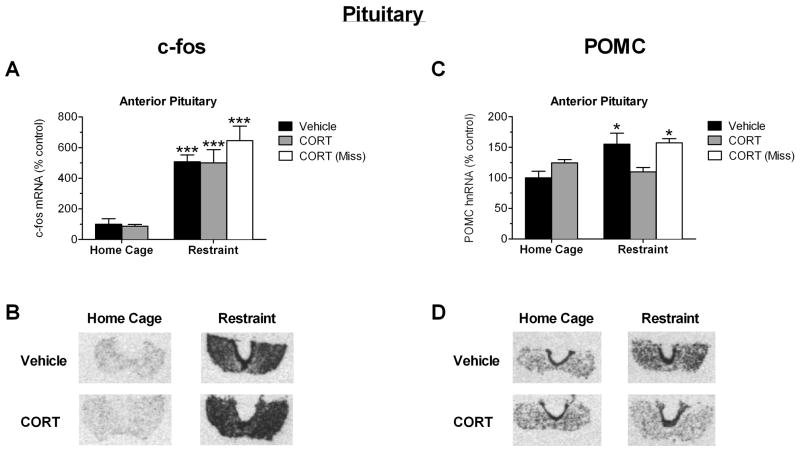
To examine the responsiveness of the anterior pituitary to an acute psychological stress at a gene expression level, c-fos and POMC transcript levels were measured. Analysis by ANOVA indicated that there was a significant main group effect (F(4,26) = 23.29; P < 0.0001). Post hoc pairwise analysis indicated that a 15-minute session of restraint significantly increased c-fos mRNA in the anterior pituitary of vehicle infused rats (P < 0.001), and in CORT infused rats (P < 0.001) (Figure 4A). There was also a main effect of group (F(4,26) = 3.579; P = 0.0302) on POMC hnRNA expression in the anterior pituitary. Post hoc pairwise analysis indicated that a 15-minute session of restraint significantly increased POMC hnRNA in the anterior pituitary of vehicle infused rats (P < 0.05), but not in CORT infused rats (Figure 4C). In rats where cannula placement was not near the PVN (“miss”), a 15-minute restraint stress elicited an increase in POMC hnRNA expression within the anterior pituitary (P < 0.05) to a similar extent as vehicle infused rats. Therefore, a 60-minute pretreatment of CORT selectively targeting the PVN completely blocked the restraint-induced increase in POMC, but not c-fos, gene expression in the anterior pituitary.
Figure 4.
Effect of 1hr CORT pretreatment within the PVN on restraint-induced gene expression in the anterior pituitary as measured by in situ hybridization. A) CORT microinfusion into the PVN 1hr prior to restraint stress does not alter c-fos gene induction in the anterior pituitary in response to restraint. B) Autoradiograms of c-fos expression from representative sections through the pituitary. C) CORT microinfusion within the PVN 1hr prior to restraint stress inhibits the restraint-induced increase in anterior pituitary POMC gene expression measured 15 minutes after stress onset (grey bars). CORT infusion outside of the PVN (“Miss”) did not alter the stress-induced increase in anterior pituitary POMC gene expression (white bar). D) Autoradiograms of POMC expression from representative sections through the pituitary. *, ***: Significant difference compared to vehicle-infused home cage animal (*P<0.05, ***P<0.001, n=8–12 for all groups except the vehicle home cage group (n=5) and restraint-CORT miss group (n=4)).
Discussion
The primary objective of this study was to identify a specific temporal(1 hr interval)and anatomically selective (PVN)component of phasic glucocorticoid negative feedback. This identification required careful consideration and control of location and timing of glucocorticoid exposure. In doing so, we have determined an impact of a phasic rise in CORT at the level of the PVN on the ability of the HPAA to respond at a cellular and hormonal level to a subsequent stressor within a short-term (1hr) timeframe. These data indicate that short-term glucocorticoid negative feedback at the level of the PVN includes two separate primary effects: 1) inhibition of stress-induced CRH gene expression, but not inhibition of some associated neural input to the PVN as shown by a lack of effect on c-fos gene induction, and 2) likely inhibition of CRH peptide release as revealed by suppression of downstream stress-induced ACTH plasma levels and suppression of stress-induced POMC gene expression in the anterior pituitary.
Studies have previously demonstrated that the tonic presence of the synthetic glucocorticoid dexamethasone in the vicinity of the PVN (steroid microimplants) suppresses basal and stress-induced HPA axis hormone secretion(26, 29). Our study is the first to demonstrate that an acute infusion of CORT into the PVN is sufficient to suppress the ACTH response to stress initiated 1 hr after CORT infusion. Detailed analysis of the guide cannula tip placements in our study indicate that the suppressive effect of CORT depended on a very restricted anatomical localization. Proximity to the third ventricle was not sufficient for this suppressive effect, rather CORT microinfusion had to be in close proximity to the PVN. Another study using a very similar microinfusion procedure determined that diffusion of dexamethasone conjugated to bovine serum albumin was restricted to the vicinity of the PVN and did not extend to the median eminence(25). In our study the off-target CORT microinfusions not only failed to inhibit stress-induced ACTH secretion, but also failed to inhibit stress-induced CRH hnRNA levels in the relatively nearby PVN. This result suggests that there was a very limited spatial spread of our CORT microinfusions. Thus, our results strongly point to an inhibitory effect of CORT on ACTH secretion as a result of the localized inhibition of stress-induced neurohormone secretion from PVN CRH neurones.
There are two other reports of PVN directed glucocorticoid microinfusion procedures similar to our own, but those studies examined different temporal domains. The most recent of those two studies demonstrated a very rapid suppressive effect of dexamethasone microinfusion into the PVN on the ACTH response to stress challenge that occurred immediately after microinfusion. In that study, the suppressive effect of dexamethasone was attenuated by concurrent PVN microinfusion of a CB1 receptor antagonist, suggesting that the glucocorticoid suppressive effect depended on a rapid increase of local endocannabinoid release (25). It is undetermined whether the glucocorticoid suppressive effect evident in our study depends on the same or similar mechanism. However, it should be noted that several studies provide support for the existence of separate negative feedback processes that underlie the fast feedback effect evident within 30sec–5 min and the delayed feedback evident 1 hr after a phasic increase in CORT. Fast feedback effects appear to require a very short interval between phasic glucocorticoid increase and stress onset (less than 15 min). One basis for this short interval requirement may be the apparent rate-sensitive dependence of fast feedback in which fast feedback is only expressed during the rising phase of altered glucocorticoid levels(17). There appears to be an interval of time (“silent period”) between glucocorticoid treatment and subsequent stress onset (approximately 15 min to 45 min) when glucocorticoid treatment fails to produce a negative feedback effect on stress-induced HPA axis hormone secretion (30). Further, the suppressive effects that emerge by 1 hr after phasic glucocorticoid elevation appear to require de novo protein synthesis(16). It should also be noted that a recent study has found that systemic CB1 receptor antagonist treatment of rats had a facilitatory rather than inhibitory effect on the suppressive effect of CORT when CB1 receptor antagonist was administered 1 hr before, rather than immediately before CORT and stress onset (31). Thus, there may be a complex temporal relationship between altered endocannabinoid signalling and CORT negative feedback.
The second reported study that is procedurally similar to ours found that PVN microinfusion of dexamethasone led to inhibition of stress-induced ACTH and CORT secretion initiated approximately 3 hr after dexamethasone infusion (32). The suppressive effect that we see 1 hr after CORT microinfusion in the PVN, advances the temporal window for this delayed glucocorticoid negative feedback effect, and extends the effect to the endogenous form of glucocorticoids. Demonstrating CORT dependency is important because another study found that the tonic implant of dexamethasone, but not CORT, in the PVN had an inhibitory effect on HPA axis hormone secretion (29).
Several other studies have attempted to assess the relatively short-term effect of systemic glucocorticoid treatment on basal or stress-induced CRH and arginine vasopressin (AVP) secretion by measuring neurohormone levels present in pituitary portal blood samples taken from anaesthetised rats. Fink et al. (33)found that 3 h after dexamethasone treatment there was a reduction of the high portal AVP, but not CRH, neurohormone levels present in ADX rats. Plotsky et al. (34)found that 2 h systemic CORT pretreatment inhibited hypotension induced increases in portal CRH. Several older studies used a different strategy to indirectly assess glucocorticoid inhibition of the secretion of ACTH secretagogues. Those studies performed ex-vivo bioassay on extracts of median eminence CRH and concluded that a systemic infusion of CORT or dexamethasone within a 1–2hr time-frame inhibited stimulus-induced CRH release(35, 36). Because those portal blood sampling and median eminence neuropeptide content studies used systemic glucocorticoid treatment, they were not ableto determine whether the suppressive effect of glucocorticoid on neurohormone secretion was due to an action at the level of the PVN, or at more central brain sites.
Although the focus of our study has been upon the effect of short-term glucocorticoid negative feedback on stress-induced HPA axis activity, it is noteworthy that intra-PVN CORT treatment did not affect the high basal ACTH present in ADX rats. We believe that these relatively high basal ACTH levels were due to insufficient normalization of basal CORT by the CORT drinking water treatment. Although the high basal ACTH levels could also reflect some residual stress from the microinfusion procedure, we note that CORT microinfusion did not affect these apparent basal levels while completely blocking the subsequent increase in ACTH produced by restraint challenge. In a separate study we found that systemic CORT treatment within this 1 hr timeframe did suppress the high basal ACTH levels present in ADX rats (37). Taken together, these results suggest that the inhibitory effect of systemic CORT treatment on elevated basal ACTH secretion in ADX rats is exerted directly at the level of the anterior pituitary.
Our study also shows that CORT at the level of the PVN was sufficient to suppress subsequent stress-induced CRH gene induction. Several studies have shown that systemic glucocorticoid treatment also suppresses stress-induced CRH gene induction within this time-frame (13, 15, 16, 38), although glucocorticoid elevation may be less effective if it occurs concurrently with stress onset (39). Our study examined the effect of short-term (1hr) intra-PVN CORT exposure on restraint-induced CRH primary transcript levels (hnRNA) and lends strong support to the prospect that the inhibitory effect of systemic glucocorticoids on CRH gene induction seen in previous studies is due to a direct action within the PVN. In vitro studies indicate that in certain cell lines glucocorticoids can inhibit the induction of endogenous CRH gene expression or transfected CRH gene promoter activity (40, 41). There is also evidence for a functional negative glucocorticoid response element in the human CRH gene(42), as well as evidence for glucocorticoid regulation of CRH mRNA stability(43). It is possible, however, that the inhibitory effect of CORT microinfusion on CRH gene expression observed in this study was due to an indirect effect of CORT acting on other cells present in close proximity to the PVN. It is likely that a significant component of stress-induced activation of CRH neurones depends on altered activity of glutamatergic and GABAergic neurones that surround the PVN and densely innervate CRH neurones(44, 45). However, it should be noted that there is considerable enrichment of GR protein expression within CRH neurones that is not evident in the surrounding region(46). Also, we did not see a suppression of stress-induced c-fos gene induction within the PVN, as would be expected if there was substantial inhibition of the transynaptic excitatory input to CRH neurones. There is, however, some support for CORT within this timeframe to inhibit the local GABAergic tone which could then contribute to increased c-fos gene expression(45). Bali and colleagues (47)have demonstrated in PVN containing hypothalamic organotypic cultures that CORT can suppress stimulus induced CRH gene induction even in the presence of tetrodotoxin. Tetrodotoxin blocks generation of action potentials and is believed to therefore prevent transynaptic activity in that culture system.
Although our CORT pretreatment blocked stress-induced CRH gene expression, we do not believe that this particular effect of CORT is responsible for the presumed inhibition in CRH neurohormone secretion. We observed complete inhibition of ACTH secretion within 15 min after stress onset. The CORT suppression of CRH gene induction that is triggered by stress onset cannot contribute to concurrent reductions in neurohormone secretion. Alterations in CRH gene transcription are estimated to require 1.5–2 hrs before affecting mature CRH peptide stores present in axon terminals (18). Moreover, in our study CORT pretreatment did not affect basal CRH hnRNA levels. If CORT suppression of CRH gene induction is due to a direct inhibitory action at the CRH gene promoter, then the parallel suppression of neurohormone secretion is due to a separate process. Alternatively, both suppression of CRH gene induction and neurohormone secretion could reflect a common process by which CORT inhibits an intracellular signalling factor that is upstream of both stimulus-induced exocytosis and gene induction.
It should also be noted that we observed a suppression of stress-induced POMC gene expression in the anterior pituitary. This gene expression has been shown to be rapidly induced by CRH stimulation of corticotrophs (48). Interestingly, AVP treatment, either alone or in combination with CRH, does not have an effect on rat anterior pituitary POMC hnRNA (49). Therefore, the inhibitory effect of PVN CORT microinfusion on stress-induced anterior pituitary POMC hnRNA in parallel with inhibition of ACTH secretion is consistent with the notion that CORT in the PVN suppressed stress-induced CRH secretion, thereby preventing stress-induced activation of corticotrophs. Paradoxically, there was not an inhibition of stress-induced c-fos mRNA within the anterior pituitary. We believe that this may represent stress-induced activation of other anterior pituitary cell types in addition to corticotrophs (e.g. lactotrophs; (50)). Corticotrophs comprise a relatively small proportion of anterior pituitary endocrine cells. Visual examination of the anterior pituitary autoradiograms from our study shows a much more wide spread expression of c-fos mRNA than POMC hnRNA throughout the anterior pituitary (Compare Fig. 4B and 4D). If the CORT inhibitory effect was selective to corticotroph secretagogues, then perhaps decreased c-fos gene expression in corticotrophs was masked by the absence of an inhibitory effect on the stress-induced activation of other anterior pituitary cell types. Accordingly, we observed that stress-induced plasma prolactin levels were not altered by the intra-PVN corticosterone microinfusion. These data lend support to the speculation that the stress-induced c-fos expression in the anterior pituitary is likely attributable to, but not necessarily restricted to, lactotropes. This also suggests that intra-PVN CORT, within the short-term feedback domain, does not inhibit the stress-induced alteration of prolactin secretagogue(s), but does inhibit the secretagogue for ACTH. Future anterior pituitary double-labeling studies will be necessary to test this speculation.
In summary, this study demonstrates that a phasic increase in CORT restricted to the PVN region of the hypothalamus was sufficient to suppress subsequent (1 hr) stress-induced ACTH hormone secretion and stress-induced POMC gene expression in the anterior pituitary. These results support the model that CORT can act directly on CRH neurones to inhibit stress-induced CRH secretion within a short-term delayed negative feedback timeframe (1 hr). Moreover, this CORT treatment selectively inhibited stress-induced CRH, but not c-fos, gene expression within the PVN. This latter result further suggests that CORT within this time-frame produces a direct inhibitory effect on CRH neurone function, without alteration of stress-induced neural afferent activity.
Acknowledgments
We would like to thank Mike VanElzakker for help with tissue collection and blood processing, and Julie Highland for help with image capture and quantification.
Support:
NIH
MH75968 and MH06597
References Cited
- 1.Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. Eur J Pharmacol. 2008;583(2–3):255–62. doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 4.Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987:43113–73. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- 5.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 6.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29(3):185–93. [PMC free article] [PubMed] [Google Scholar]
- 7.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 8.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4(2):141–94. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Hodges JR, Jones MT. Corticotrophin release in the cortisol-treated rat. J Physiol. 1962:163391–8. doi: 10.1113/jphysiol.1962.sp006984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayers G, Sayers MA. Regulation of pituitary adrenocorticotrophic activity during the response of the rat t oacute stress. Endocrinology. 1947;40(4):265–73. doi: 10.1210/endo-40-4-265. [DOI] [PubMed] [Google Scholar]
- 11.Yates FE, Leeman SE, Glenister DW, Dallman MF. Interaction between plasma corticosterone concentration and adrenocorticotropin-releasing stimuli in the rat: evidence for the reset of an endocrine feedback control. Endocrinology. 1961:6967–80. doi: 10.1210/endo-69-1-67. [DOI] [PubMed] [Google Scholar]
- 12.Akana SF, Scribner KA, Bradbury MJ, Strack AM, Walker CD, Dallman MF. Feedback sensitivity of the rat hypothalamo-pituitary-adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology. 1992;131(2):585–94. doi: 10.1210/endo.131.2.1322275. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg AB, Campeau S, Day HE, Spencer RL. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2003;15(11):1075–83. doi: 10.1046/j.1365-2826.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 14.Pace TW, Gaylord RI, Jarvis E, Girotti M, Spencer RL. Differential glucocorticoid effects on stress-induced gene expression in the paraventricular nucleus of the hypothalamus and ACTH secretion in the rat. Stress. 2009;12(5):400–11. doi: 10.1080/10253890802530730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsberg AB, Frank MG, Francis AB, Rubin BA, O’Connor KA, Spencer RL. Specific and time-dependent effects of glucocorticoid receptor agonist RU28362 on stress-induced pro-opiomelanocortin hnRNA, c-fos mRNA and zif268 mRNA in the pituitary. J Neuroendocrinol. 2006;18(2):129–38. doi: 10.1111/j.1365-2826.2005.01396.x. [DOI] [PubMed] [Google Scholar]
- 16.Osterlund C, Spencer RL. Corticosterone pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis activity via multiple actions that vary with time, site of action, and de novo protein synthesis. J Endocrinol. 2011;208(3):311–22. doi: 10.1530/JOE-10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5(1):1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 18.Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol. 2005;26(3–4):109–30. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Buckingham JC. The influence of corticosteroids on the secretion of corticotrophin and its hypothalamic releasing hormone. J Physiol. 1979:286331–42. doi: 10.1113/jphysiol.1979.sp012622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallman MF. Adrenocortical function, feedback, and alphabet soup. Am J Physiol Endocrinol Metab. 2005;289(3):E361–2. doi: 10.1152/classicessays.00033.2005. [DOI] [PubMed] [Google Scholar]
- 21.Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26(7):1971–8. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149(11):5482–90. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt MV, Sterlemann V, Wagner K, Niederleitner B, Ganea K, Liebl C, Deussing JM, Berger S, Schutz G, Holsboer F, Muller MB. Postnatal glucocorticoid excess due to pituitary glucocorticoid receptor deficiency: differential short-and long-term consequences. Endocrinology. 2009;150(6):2709–16. doi: 10.1210/en.2008-1211. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson L, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122(4):1343–8. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- 25.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151(10):4811–9. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman S, Weidenfeld J. The dorsal hippocampus modifies the negative feedback effect of glucocorticoids on the adrenocortical and median eminence CRF-41 responses to photic stimulation. Brain Res. 1993;614(1–2):227–32. doi: 10.1016/0006-8993(93)91039-u. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson WE, Davis DR, Sherrell BJ, Orth DN. Rapid radioimmunoassay for corticotropin in unextracted human plasma. Clin Chem. 1984;30(2):259–65. [PubMed] [Google Scholar]
- 28.Paxinos G, Watson CS. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- 29.Kovacs KJ, Makara GB. Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain Res. 1988;474(2):205–10. doi: 10.1016/0006-8993(88)90435-0. [DOI] [PubMed] [Google Scholar]
- 30.Jones MT, Hillhouse EW, Burden JL. Dynamics and mechanics of corticosteroid feedback at the hypothalamus and anterior pituitary gland. J Endocrinol. 1977;73(3):405–17. doi: 10.1677/joe.0.0730405. [DOI] [PubMed] [Google Scholar]
- 31.Ginsberg AB, Pecoraro NC, Warne JP, Horneman HF, Dallman MF. Rapid alteration of stress-induced hypothalamic-pituitary-adrenal hormone secretion in the rat: a comparison of glucocorticoids and cannabinoids. Stress. 2010;13(3):248–57. doi: 10.3109/10253890903336839. [DOI] [PubMed] [Google Scholar]
- 32.Feldman S, Weidenfeld J. Further evidence for the central effect of dexamethasone at the hypothalamic level in the negative feedback mechanism. Brain Res. 2002;958(2):291–6. doi: 10.1016/s0006-8993(02)03581-3. [DOI] [PubMed] [Google Scholar]
- 33.Fink G, Robinson IC, Tannahill LA. Effects of adrenalectomy and glucocorticoids on the peptides CRF-41, AVP and oxytocin in rat hypophysial portal blood. J Physiol. 1988:401329–45. doi: 10.1113/jphysiol.1988.sp017165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plotsky PM, Otto S, Sapolsky RM. Inhibition of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation by delayed glucocorticoid feedback. Endocrinology. 1986;119(3):1126–30. doi: 10.1210/endo-119-3-1126. [DOI] [PubMed] [Google Scholar]
- 35.Buckingham JC, Hodges JR. Production of corticotrophin releasing hormone by the isolated hypothalamus of the rat. J Physiol. 1977;272(2):469–79. doi: 10.1113/jphysiol.1977.sp012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takebe K, Kunita H, Sakakura M, Horiuchi Y, Mashimo K. Suppressive effect of dexamethasone on the rise of CRF activity in the median eminence induced by stress. Endocrinology. 1971;89(4):1014–9. doi: 10.1210/endo-89-4-1014. [DOI] [PubMed] [Google Scholar]
- 37.Spencer RL, Jarvis E, Leid L, Osterlund CD, Unnithan R. Regulation of stress-induced mitogen-activated protein kinase pathway activity in the hypothalamic paraventricular nucleus by tonic, but not phasic changes in corticosterone levels. Society for Neuroscience Annual Meeting Abstract #6782. 2008 [Google Scholar]
- 38.Jiang YQ, Kawashima H, Iwasaki Y, Uchida K, Sugimoto K, Itoi K. Differential effects of forced swim-stress on the corticotropin-releasing hormone and vasopressin gene transcription in the parvocellular division of the paraventricular nucleus of rat hypothalamus. Neurosci Lett. 2004;358(3):201–4. doi: 10.1016/j.neulet.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 39.Ma XM, Aguilera G. Differential regulation of corticotropin-releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology. 1999;140(12):5642–50. doi: 10.1210/endo.140.12.7214. [DOI] [PubMed] [Google Scholar]
- 40.Guardiola-Diaz HM, Kolinske JS, Gates LH, Seasholtz AF. Negative glucorticoid regulation of cyclic adenosine 3′, 5′-monophosphate-stimulated corticotropin-releasing hormone-reporter expression in AtT-20 cells. Mol Endocrinol. 1996;10(3):317–29. doi: 10.1210/mend.10.3.8833660. [DOI] [PubMed] [Google Scholar]
- 41.Yamamori E, Iwasaki Y, Taguchi T, Nishiyama M, Yoshida M, Asai M, Oiso Y, Itoi K, Kambayashi M, Hashimoto K. Molecular mechanisms for corticotropin-releasing hormone gene repression by glucocorticoid in BE(2)C neuronal cell line. Mol Cell Endocrinol. 2007;264(1–2):142–8. doi: 10.1016/j.mce.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Dostert A, Heinzel T. Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr Pharm Des. 2004;10(23):2807–16. doi: 10.2174/1381612043383601. [DOI] [PubMed] [Google Scholar]
- 43.Ma XM, Camacho C, Aguilera G. Regulation of corticotropin-releasing hormone (CRH) transcription and CRH mRNA stability by glucocorticoids. Cell Mol Neurobiol. 2001;21(5):465–75. doi: 10.1023/A:1013863205647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16(3):381–5. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- 45.Verkuyl JM, Karst H, Joels M. GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur J Neurosci. 2005;21(1):113–21. doi: 10.1111/j.1460-9568.2004.03846.x. [DOI] [PubMed] [Google Scholar]
- 46.Uht RM, McKelvy JF, Harrison RW, Bohn MC. Demonstration of glucocorticoid receptor-like immunoreactivity in glucocorticoid-sensitive vasopressin and corticotropin-releasing factor neurons in the hypothalamic paraventricular nucleus. J Neurosci Res. 1988;19(4):405–11. 68–9. doi: 10.1002/jnr.490190404. [DOI] [PubMed] [Google Scholar]
- 47.Bali B, Ferenczi S, Kovacs KJ. Direct inhibitory effect of glucocorticoids on corticotrophin-releasing hormone gene expression in neurones of the paraventricular nucleus in rat hypothalamic organotypic cultures. J Neuroendocrinol. 2008;20(9):1045–51. doi: 10.1111/j.1365-2826.2008.01759.x. [DOI] [PubMed] [Google Scholar]
- 48.Autelitano DJ, Lundblad JR, Blum M, Roberts JL. Hormonal regulation of POMC gene expression. Annu Rev Physiol. 1989:51715–26. doi: 10.1146/annurev.ph.51.030189.003435. [DOI] [PubMed] [Google Scholar]
- 49.Levin N, Blum M, Roberts JL. Modulation of basal and corticotropin-releasing factor-stimulated proopiomelanocortin gene expression by vasopressin in rat anterior pituitary. Endocrinology. 1989;125(6):2957–66. doi: 10.1210/endo-125-6-2957. [DOI] [PubMed] [Google Scholar]
- 50.Takigami S, Fujiwara K, Kikuchi M, Yashiro T. In vivo correlation between c-Fos expression and corticotroph stimulation by adrenocorticotrophic hormone secretagogues in rat anterior pituitary gland. Cell Tissue Res. 2008;331(3):589–94. doi: 10.1007/s00441-007-0547-7. [DOI] [PubMed] [Google Scholar]