Abstract
High resolution manometry (HRM) has demonstrated two distinct smooth muscle contraction segments in the esophageal body; changes in these segments typify certain esophageal disorders.
We investigated segmental characteristics in subgroups of non-cardiac chest pain (NCCP).
METHODS
32 NCCP subjects were segregated into a GERD group (ambulatory pH testing off antisecretory therapy showing elevated total acid exposure time, AET ≥4.0% and positive symptom association probability, SAP) and an acid sensitive group (normal AET and positive SAP). HRM Clouse plots were analyzed; smooth muscle segment lengths, pressure amplitude peaks were measured for segment 2 and segment 3 (proximal and distal smooth muscle segments). Pressure volumes were determined in mmHg/cm/sec for each peristaltic segment, and ratios of segment 3: segment 2 calculated. Values were compared to a cohort of 14 normal controls.
RESULTS
A distinctive shift in peak contraction amplitude to segment 3 was evident in the acid sensitive group (segment 2, 100.03±11.06 mmHg, segment 3, 145.23±10.29 mmHg, p=0.006). Pressure volumes were similarly shifted to segment 3 (segment 2: 855.3±135.1 mmHg/cm/sec, segment 3: 2115.2±218.6 mmHg/cm/sec, p<0.005). In contrast, peak amplitude and pressure volume were near equal in the two segments in GERD and control groups. A threshold segment 3: segment 2 pressure volume ratio of 1.9 had the best performance characteristic for segregating acid sensitivity subjects from all GERD and control subjects.
CONCLUSIONS
Shift in contractile vigor to the third peristaltic segment may be seen in acid sensitive subjects. HRM characteristics of smooth muscle contraction segments are of value in making this determination.
INTRODUCTION
Noncardiac chest pain (NCCP) is a common clinical entity in gastroenterologic practice, and accounts for significant morbidity and healthcare costs.[1, 2] Patients with NCCP demonstrate a spectrum of abnormalities on pH monitoring [3, 4]. At one end of the spectrum, evidence of GERD is strong with elevated acid exposure times and frequently, positive symptom association testing (SAP). The other end of the spectrum consists of a hypersensitivity pattern, chest pain being triggered by demonstrable noxious chemical and/or distension stimuli such as reflux events, in the setting of normal or low acid exposure times.[3] Patients with such hypersensitivity have traditionally been further characterized by provocative testing with balloon distension and acid perfusion.[4, 5] More recently, emergent research techniques including impedance planimetry, high frequency ultrasonography and multimodality testing have been used, but high resolution manometry (HRM) has not been studied to make this distinction to date.[6–8]
HRM methods have demonstrated that peristalsis is comprised of a chain of coordinated contraction segments.[9–11] The first segment (S1) consists of skeletal muscle esophagus. Two dominant contraction segments of nearly equal size (segment 2 and segment 3) comprise the majority of the esophageal body (Figure 1), presumably representing the transition to cholinergic and noncholinergic control in the smooth muscle esophagus; this has been observed in humans as well as other mammals.[12–14] Changes in these contraction segments have now been identified in esophageal disease states. For instance, in distal esophageal obstruction, second segment dominance may suggest mechanical obstruction, while third segment dominance is seen in functional obstruction.[13] A relationship between unexplained chest pain and segmental changes in peristalsis was first suggested by Clouse et al, who observed increased contractile amplitude and double peak wave frequency in the third segment in patients with unexplained chest pain.[15] These observations point to the fact that third segment dominance may be a marker for functionality and esophageal hypervigilance.
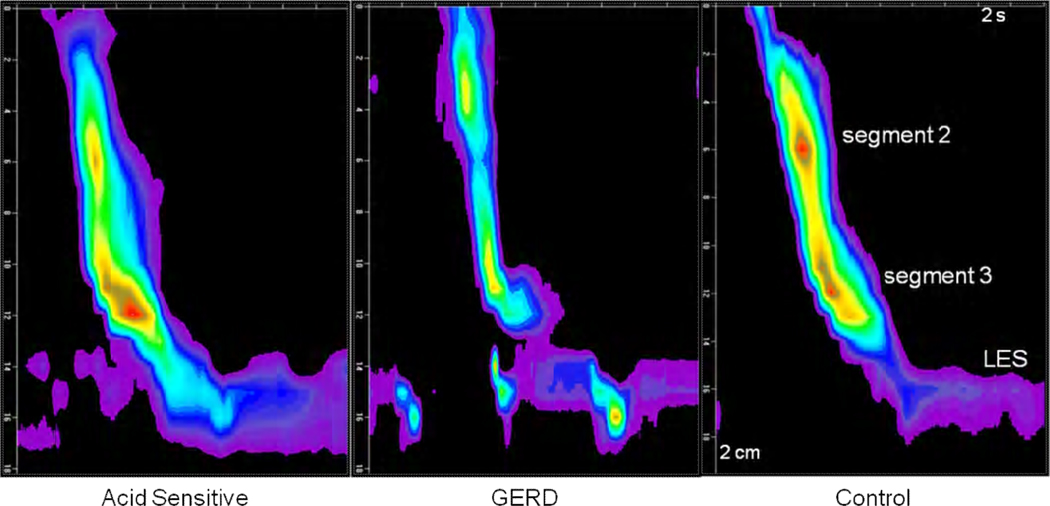
FIGURE 1.
Examples of Clouse plots showing the two smooth muscle contraction segments (segment 2 and segment 3) and the lower esophageal sphincter (LES) in the three subject groups. The two segments are symmetrical in length and amplitude in GERD and control subjects. In contrast, there is a visually conspicuous shift in peak contraction amplitude and segment length to segment 3 in the example from an acid sensitive subject.
Our objective was to characterize segmental alterations in the esophageal smooth muscle contraction segments in subjects with NCCP in this proof of concept study. We hypothesized that patients with acid hypersensitivity in the setting of NCCP would demonstrate increased contractile amplitude in the distal smooth muscle segment, but not patients with GERD triggered NCCP. Consequently, we wanted to determine if a GERD predominant NCCP pattern can be distinguished from an acid hypersensitive pattern by evaluation of segmental changes on HRM.
METHODS
Subjects
The subjects for this study were extracted from review of manometric and pH monitoring data obtained from adult outpatients (≥18 yr) referred for evaluation of unexplained chest pain at the gastrointestinal motility center at Washington University in St. Louis. Study subjects were identified by interrogating the computerized esophageal physiology database and extracting records of patients who underwent manometry and pH testing while off acid suppression for the evaluation of NCCP. Included were 16 consecutive subjects with NCCP and GERD, who had both elevated acid exposure times and statistically significant symptom reflux associations on ambulatory pH testing performed off antisecretory therapy, fulfilling criteria for ‘strong GERD evidence’.[16] The acid hypersensitivity group was composed of 16 consecutive NCCP subjects who had normal acid exposure times and a normal upper endoscopy, but statistically significant symptom reflux associations on pH testing, again off antisecretory therapy. All included patients had a negative cardiac evaluation prior to undergoing esophageal physiology testing. To qualify for inclusion, the manometric pattern had to be read as normal, without named motor disorders and without consistent separation between the LES and the diaphragmatic crural pinch. Subjects with only intermittent separation of the diaphragmatic hiatus from the LES were included. Exclusion criteria therefore included manometric diagnoses of achalasia, incomplete LES relaxation with preserved peristalsis, diffuse esophageal spasm, nutcracker esophagus, ineffective esophageal motility and aperistalsis as defined by standard criteria.[17–19]. The comparison group consisted of 14 asymptomatic institutional controls studied with the same HRM system.
Clinical information was extracted from data accompanying the referral, our institution’s electronic medical record and from symptom self-reporting sheets completed by the patients immediately prior to HRM. These self-report sheets recorded symptom frequency and severity on 5-point Likert scales, the product of which was calculated to determine the symptom severity index. Global symptomatic status was recorded on 10 cm visual analog scales. Final exclusion of alternate diagnosis for NCCP was established from interrogation of our institution’s electronic medical record. The review of clinical data was approved by the Human Research Protection Office of Washington University School of Medicine.
Manometric Methods
Esophageal HRM was performed on each patient using a prototype water perfused HRM system that predated the current solid-state HRM systems. The use of these methods for clinical esophageal manometry has been reported previously.[13, 20] In brief, a water perfused, silicone catheter with 21 recording sites spaced at 1 cm intervals was passed transnasally in the fasted subject. The catheter was advanced such that the recording ports had an intragastric location, and a pull-through maneuver was performed determine the resting lower esophageal sphincter (LES) pressure and LES location. The catheter was then positioned such that >2 recording sites remained in an intragastric position while the more proximal sites recorded from the LES and approximately 80% of the esophageal body. Ten swallows with 4 ml ambient temperature water were spaced at 30 second intervals. The catheter was repositioned such that the proximal recording site rested in the upper esophageal sphincter (UES), and an additional ten swallows were taken. Data were displayed and analyzed as HRM Clouse plots using a computerized data acquisition and display system capable of topographic analysis methods (Medical measurement Systems, Enchede, Holland).
Characteristics of peristalsis were determined for this study when the catheter was in the distal position. HRM Clouse plots from the 10 distal swallows were analyzed together to determine the best pressure trough locations separating the peristaltic sequence into individual segments. Locations and amplitudes of the intrasegment pressure troughs were collected, thereby also allowing extraction of the length of each pressure segment (Figure 2). Troughs were identified from HRM Clouse plots, using the pressure interrogation tool to complement visual identification of the nadir trough pressure between segments [11,13]. A pressure decline of at least 10 mmHg compared to adjacent contraction areas was required, but trough amplitudes were typically well separated from peak contraction amplitudes. Peak segmental amplitude and location were determined by locating the highest amplitude within each segment. Segmental pressure volume measurements in mmHg.cm.second were determined for each smooth muscle segment from an on-screen pressure volume measurement tool using methods described previously.[11, 13] Using this tool, a region of interest is selected using an onscreen tool; the lowest pressure of interest is designated, and the cumulative pressure above this plane is recorded in mmHg.cm.second. A similar measurement has been described encompassing both smooth muscle peristaltic segments, when it is termed the distal contraction integral (DCI).[21]
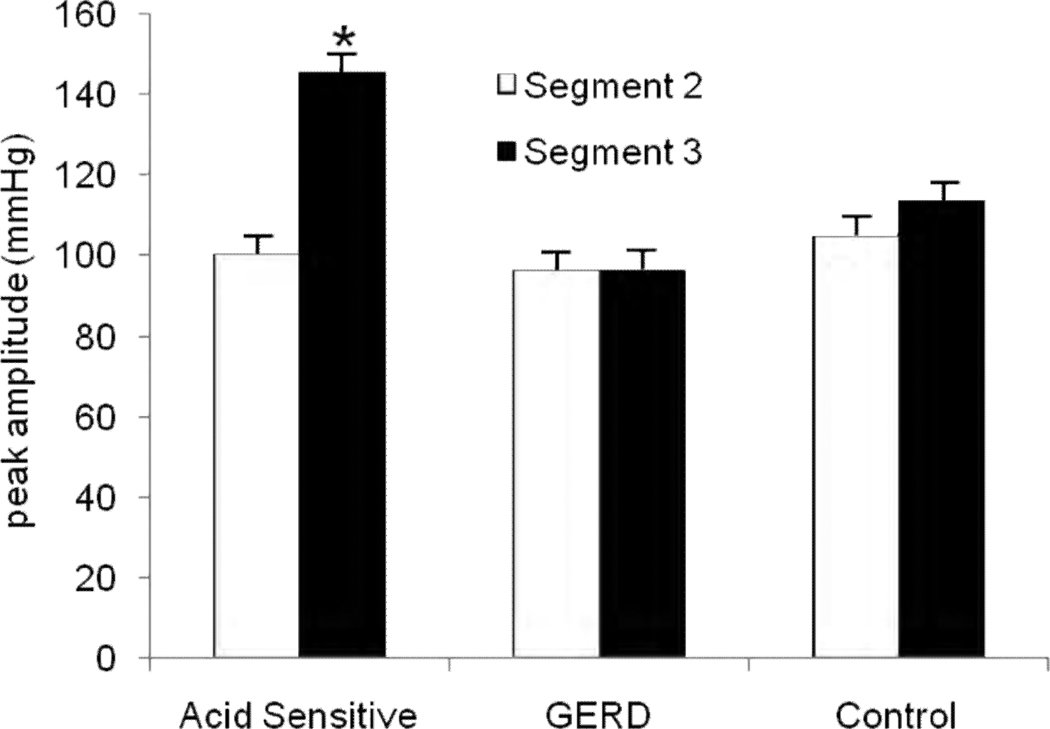
FIGURE 2.
Averaged peak contraction amplitudes in the three subject groups. Amplitudes overall were lowest in the GERD group and highest in the acid sensitive group. While amplitudes were similar between segment 2 and segment 3 in GERD subjects and controls, values were significantly higher in segment 3 in the acid sensitive group. (* p=0.006 compared to segment 2)
pH Monitoring
Placement of the pH probes was performed at the outpatient endoscopy and motility facility at our medical center following HRM. Subjects were evaluated off PPI and were asked to discontinue proton pump inhibitors at least 7 days prior and histamine-2 receptor antagonists (H2RAs), prokinetic agents, and antacids at least 3 days prior to the pH study. Following discharge from the endoscopy facility, all subjects were instructed to resume normal activity and diet, maintain a daily diary that included symptoms, activities, and meal periods, and to activate the symptom indicator button of the pH recorder every time they experienced chest pain.
Analyses of pH data included quantification of acid exposure time (AET) and determination of reflux–symptom association through probability testing. AET was defined as the percent time the esophageal pH remained below 4 in the distal esophagus. The SI was calculated as the proportion of reflux symptoms while pH<4 within the total number of symptom episodes recorded, expressed as a percentage; according to the method of Weiner et al. [22] A value of ≥50% was considered indicative of a high proportion of reflux-associated symptoms. [22] The SAP was calculated using the Ghillebert probability estimate (GPE), and a p value <0.05 was required for significance.[23–25] A detailed description of this method of symptom association has been reported elsewhere.[25] Like the SAP calculated by the Weusten method, the GPE determines the likelihood that a symptom and acid reflux events co-occur solely by chance.[26] The overall likelihood is calculated as a sum of partial probabilities for exact numbers of reflux-associated symptoms within the context of the total number of symptoms, the proportion of time “at risk” (2 minutes following a reflux event) for linking a symptom to a low pH value, and the total recording time.[23–25].
Statistical Methods
Grouped values are reported as mean ± standard error of mean, and 95% confidence intervals when appropriate. Comparisons between groups were performed using Student’s t-tests or χ2 analysis as appropriate. Receiver operating characteristic (ROC) analysis was performed to identify the ideal cutoff for discriminating acid sensitive from GERD subjects and controls. In each instance, p<0.05 was required for statistical significance.
RESULTS
The study groups consisted of 16 subjects with GERD (mean age 52 ±2.2 yr, 56.3% female), and 16 subjects with acid sensitivity (mean age 51 ±3.4 yr, 75.0% female). Manometric findings in the study subjects were compared to 14 asymptomatic controls (38.7±3.7 yr, 62.3% female). All study subjects presented with chest pain in accordance with study inclusion criteria; other symptoms included heartburn and regurgitation in 78.1% each of subjects. The symptom severity index for chest pain was 6.3±1.1, 5.0±1.4 in the GERD group, and 7.5±1.6 in the acid sensitivity group (p=0.04, Table 1). As would be expected, acid exposure times were significantly higher in the GERD group compared to the acid sensitive group (p <0.001). All study subjects had positive symptom association using the GPE, with a p value of <0.05 in all instances. The SI was positive in 20 subjects, 12 (75.0%) in the GERD group and 8 (50.0%) in the acid sensitivity group (p=0.27). The median SI values were higher in the GERD group (67%) compared to the acid sensitive group (45%) (p <0.001). Intermittent small (<2 cm) separation of the LES from the diaphragmatic hiatus was observed in 4 subjects in the GERD group and amounted to <1/3 of swallows in each instance. None of the subjects in the control or acid sensitive groups demonstrated such separation.
Table 1.
Demographic, Clinical and Manometric Characteristics
| GERD n=16 |
Acid Sensitive n=16 |
Normal Controls n=14 |
|
|---|---|---|---|
| Mean age | 51.7 | 51 | 38.7 ± 3.7 |
| Gender | 9 F/ 7 M | 12 F/ 4 M | 10 F/ 4 M |
| Chest Pain Severity Index* | 5.0 [2.3–8.0] | 7.5 [4.4–10.5] | n/a |
| Global Symptoms on VAS* | 7.3 [6.4–8.1] | 6.2 [4.6–7.8] | n/a |
| Acid exposure time* | 16.2 [12.4–19.9] | 2.1 [1.5–2.6] | n/a |
| Symptom association | |||
| GPE | 100% | 100% | n/a |
| SI* | 75.0% | 50.0% | n/a |
| Segment lengths | |||
| Segment 1 | 35.0% | 31.5% | 24.8% |
| Segment 2 | 39.5% | 26.5% | 44.4% |
| Segment 3† | 25.5% | 42.0% | 30.8% |
p<0.05 for comparisons between GERD and acid sensitive groups
p<0.05 across groups
Values in parenthesis indicate 95% confidence intervals
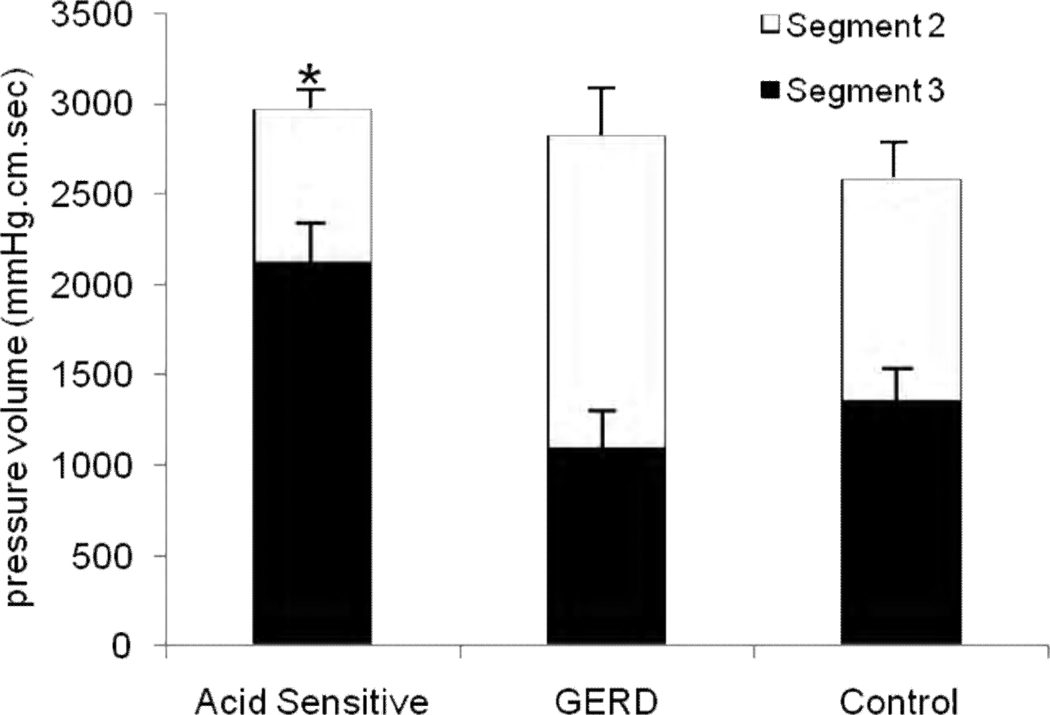
Esophageal contraction segments were identified and measured in all subjects and controls, and are reported as percent esophageal length. Proportionate lengths of the first and second segments were similar in all three groups, but segment 3 was shorter in the GERD group (Table 1, p<0.01 across groups). Next, esophageal contraction amplitudes and pressure volumes were examined. Averaged peak amplitudes were lowest in the GERD group and highest in the acid sensitive group (Figure 2). A visually conspicuous and distinctive shift in peak contraction amplitude to segment 3 was evident in the acid sensitivity group (segment 2, 100.03±11.06 mmHg, segment 3, 145.23±10.29 mmHg, p=0.006), a finding not seen in the GERD or control groups (Figure 2). Furthermore, pressure volumes were also significantly shifted to segment 3 in the acid sensitivity group (segment 2: 855.3±135.1 mmHg/cm/sec, segment 3: 2115.2±218.6 mmHg/cm/sec, p<0.005), but GERD subjects and controls maintained symmetric pressure volume relationship between the second and third segments (Figure 3).
FIGURE 3.
Comparison of pressure volume (mmHg.cm.sec) between segment 2 and segment 3 in the three groups. Segment 3 had a significantly higher pressure volume compared to segment 2 in the acid sensitive group (* p<0.005); values were proportionate in the other two groups.
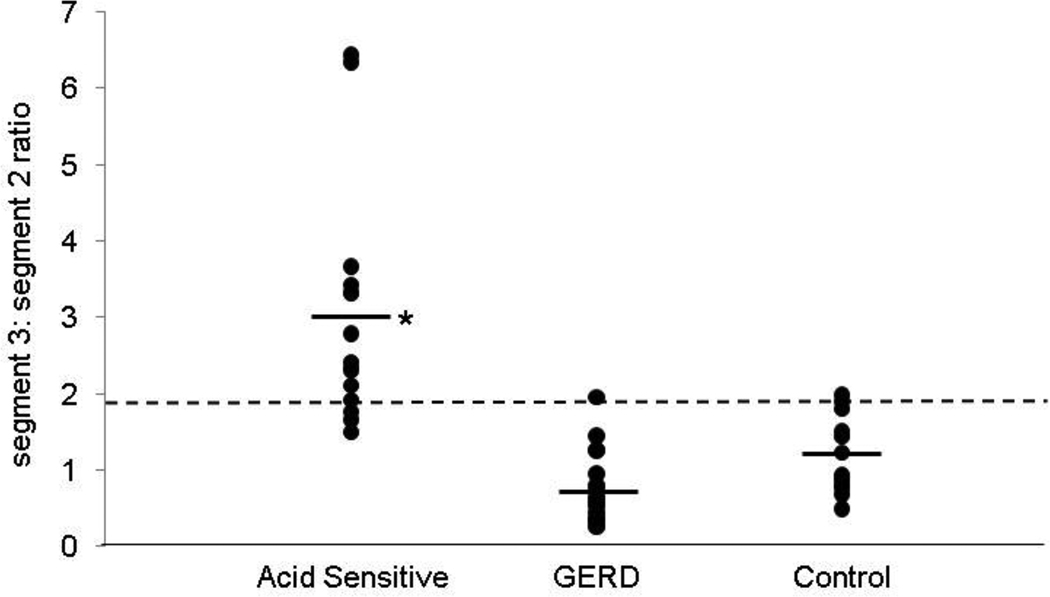
Segment length, peak amplitude and pressure volumes were further evaluated as segment 3 to segment 2 ratios (Table 2). Segment length ratio was significantly higher in the acid sensitive group, compared to both the GERD group and controls (p< 0.001 for each comparison). Peak amplitude ratios were also significantly higher in the acid sensitive group (p< 0.001 for each comparison with GERD group and controls). Pressure volume ratios were the most discriminant, and identified the acid sensitive group as distinct from the other two groups (Table 2). With each of these assessments, the findings supported a more prominent third segment in the acid sensitive group, in terms of segment length, peak amplitude and vigor of contraction as measured by pressure volume (Figure 4). These conclusions did not change when 4 subjects with intermittent hiatus hernia in the GERD group were separated from the analysis. Using ROC analysis, a pressure volume cut-off ratio of 1.9 was identified as the best threshold for discriminating acid sensitive subjects from control and GERD subjects, with a sensitivity of 87% and specificity of 90%, (Table 3). A higher sensitivity of 100% could be achieved with a threshold of 2, at the cost of a slightly lower specificity.
Table 2.
Comparison of Proximal and Distal Smooth Muscle Contraction Segments (Segment 2 and Segment 3)
| Segment 3:Segment 2 Ratio | Acid Sensitive* | GERD | Controls |
|---|---|---|---|
| Segment Length | 0.92 [0.75–1.08] | 0.47 [0.35–0.58] | 0.80 [0.66–0.93] |
| Peak Amplitude | 1.45 [1.28–1.62] | 0.99 [0.88–1,11] | 1.13 [0.97–1.29] |
| Pressure Volume | 3.05 [2.32–3.78] | 0.77 [0.53–1.01] | 1.20 [0.95–1.45] |
p<0.001 compared to GERD and controls for all categories
Values in parenthesis indicate 95% confidence intervals
FIGURE 4.
Comparison of pressure volume ratio (segment 3:segment 2) in the three groups. When averaged, this ratio was significantly higher in the acid sensitive group compared to the other two groups (*p<0.001). A threshold ratio of 2.0 had the highest sensitivity (100%) in segregating acid sensitive subjects from GERD and control groups, while a ratio of 1.9 provided better performance characteristics (sensitivity 87%, specificity 90%).
Table 3.
Receiver Operating Characteristic (ROC) Analysis Identifying Optimal Performance Characteristics Segregating Acid Sensitive from GERD and Control Groups
| Pressure Volume Ratio Segment 3:2 | Sensitivity | Specificity |
|---|---|---|
| 1.45 | 76.2 | 100 |
| 1.5 | 75 | 96.2 |
| 1.6 | 78.9 | 96.3 |
| 1.7 | 77.8 | 92.9 |
| 1.8 | 76.5 | 89.7 |
| 1.9 | 86.7 | 90.3 |
| 2.0 | 100 | 88.2 |
These observations translated into visually distinctive patterns on HRM Clouse plots upon comparison of acid sensitive and GERD subjects. Clouse plots of acid sensitive subjects demonstrated a conspicuous shift in strength of contraction to the third segment, in many instances associated with a diminutive second segment (Figure 1). A relatively symmetrical relationship between the two segments was evident in each of the other two groups.
DISCUSSION
In this study, we demonstrate that subjects with acid sensitivity have a distinctive pattern on HRM Clouse plots, with an identifiable shift in amplitudes and contractile vigor to the third peristaltic segment, the distal of the two smooth muscle contraction segments. In contrast, the two smooth muscle contraction segments are of similar relative proportions in normal subjects and those with GERD. These shifts in contractile amplitude and pressure volume can be distinguished easily by both visual and analytical inspection of HRM Clouse plots, especially by adjusting the sectors evaluated by the DCI algorithm currently available with HRM analysis software. These findings support previous reports of distal shift in contractile vigor in esophageal hypersensitivity and NCCP, and suggest that hypermotility and hypersensitivity could potentially be epiphenomena of the same neuromuscular pathophysiology.
The initial HRM analysis of smooth muscle peristalsis by Clouse et al demonstrated two approximately equal functional segments.[9] In normal subjects the two smooth muscle segments account for 41% and 31% of esophageal length respectively [13], with a demonstrable pressure trough between the segments [11,13]. The existence of the trough fits with our understanding of the gradient between excitatory and inhibitory influences in the smooth muscle esophagus [27, 28]. Cholinergic excitatory neurons appear to have a greater influence in the proximal smooth muscle and inhibitory NANC neurons predominate in the distal smooth muscle and in the LES.[14, 28] Inspection of HRM plots before and after administration of cisapride, a cholinomimetic agent, demonstrate augmentation of the second segment[14]. Neuro-physiologic studies have suggested that these motor phenomena may be the result of central separation of esophageal motor control into three distinct neuromuscular units: a) the UES and upper striated muscle esophagus, b) proximal smooth muscle of the esophageal body and c) distal smooth muscle of the esophageal body and LES.[29]
The potential relationship between unexplained chest pain and the segmental changes in peristalsis was first suggested by Clouse and Staiano in the 1990’s.[10] In a study of 10 patients with chest pain and nutcracker esophagus, they observed a marked increase in contractile volume in the distal smooth muscle segment (segment 3) in contrast to the proximal smooth muscle segment (segment 2). Further studies in patients with functional esophageal symptoms demonstrated that double peak waves were more frequently seen in the third contraction segment.[15, 17, 30], suggesting that these findings may be epiphenomena associated with esophageal hypersensitivity. We recently observed a similar shift of the peristaltic pressure-volume to the third segment in patients with dysphagia due to functional LES obstruction, but not with structural mechanical obstruction at the LES level.[13] Abnormal vagal function with a prominent vagal inhibitory response has been associated with esophageal hypersensitivity in acid sensitive patients, when esophageal acid infusion was utilized to induce noncardiac chest pain [34]. Taken together with these studies, our results lend support to the hypothesis that functional esophageal symptoms may be associated with exaggerated contraction patterns in the distal esophageal smooth muscle, potentially from abnormal function of NANC inhibitory neurons. Clouse et al have reported merging together of the two smooth muscle contraction segments to result in an exaggerated and hypercontractile pattern in nutcracker esophagus; our findings could represent a lesser variant in that only the distal smooth muscle contraction segment is exaggerated with acid sensitivity.[10] However, both these exaggerated contraction patterns can be seen in asymptomatic subjects, and unique clinical findings that separate these two patterns have not been systematically identified.
Early provocative studies by Rao, Richter and others suggested that in NCCP patients with normal esophagus on upper endoscopy and normal esophageal acid exposure, chest pain events were triggered by hypersensitivity of esophageal chemoreceptors to drops in pH and stretch receptors to luminal distention.[5, 7] Yang et al observed that individuals with nonerosive reflux disease and functional heartburn are significantly more sensitive to esophageal acid perfusion and balloon distention than patients with erosive esophagitis or healthy controls.[31] Additionally, our group has demonstrated that NCCP patients with strong GERD evidence (elevated AET and positive SAP) were more likely to achieve symptom relief with aggressive antireflux therapy compared to those with only positive SAP, limited GERD evidence, or no GERD evidence on pH monitoring.[3] When viewed in the light of the above literature, our findings in this report lend strength to the concept of a spectrum of ambulatory pH abnormalities in reflux triggered NCCP. The relevance of the spectrum is that patients with strong evidence of GERD (elevated acid exposure time, erosive esophagitis, Barrett’s esophagus) respond well to aggressive antireflux therapy [5]. This group may be distinguished by a hypomotile pattern in the distal smooth muscle esophagus, a pattern reported more frequently in the setting of GERD sequelae (peptic stricture and Barrett’s esophagus), and potentially part of the underlying pathophysiology of GERD in these patients.[32, 33] At the other end of the spectrum, hypermotility and hypersensitivity in the distal smooth muscle esophagus may be associated with suboptimal symptomatic outcomes when only acid reflux is treated without addressing the underlying esophageal hypersensitivity, but may respond to the use of antidepressants as neuromodulators.[34] We speculate that hypermotility patterns provide better acid clearance, hence lower acid exposure times and less likelihood of GERD complications.
Our study does have limitations. First, since we retrospectively identified our study subjects, our report lacks standardized pre-manometry evaluation of subjects beyond the described symptom assessment by questionnaire. Second, study numbers are small, mainly because we wanted to only include clean patients with clearly defined acid exposure and symptom association patterns, and similar motor patterns that qualified as ‘normal’ on standard analysis to maintain uniformity across groups. The generalized applicability of our conclusions to patients with incomplete or overlapping syndromes of reflux disease and acid sensitivity cannot be fully assessed by this report. However, it is our unpublished observation that shifts in contraction vigor to the third contraction segment are associated with heightened symptom reporting. Further, this project was not geared to determine outcome data; therefore the impact of management directed by HRM could not be accessed. Nevertheless, we believe these findings provide preliminary evidence that segmental contraction abnormalities in patients with NCCP can discriminate GERD from acid sensitivity. Future studies need to evaluate symptom burden in subjects with and without exaggerated distal smooth muscle contraction in blinded fashion to further address if our findings can predict symptomatic states and patient outcome. Development of software to ‘average’ all the wet swallows into one composite image may facilitate further analysis; such signal averaging has been shown in limited studies to provide a representative ‘fingerprint’ of each subject’s unique contraction pattern [11, 35]
In conclusion, we have demonstrated that HRM characteristics of smooth muscle peristaltic contraction segments can select out subjects with heightened esophageal sensory perception subjects presenting for evaluation of NCCP. These segmental contraction abnormalities lend further support to the notion that hypermotility and hypersensitivity could both be manifestations of a common neuromuscular pathophysiologic process. These findings underscore the value of evaluating and reporting changes in individual contraction segments while analyzing HRM Clouse plots. Further prospective and blinded studies are needed to replicate our results in larger patient cohorts, and to determine if patient outcome can be predicted by these segmental contraction abnormalities.
ACKNOWLEDGEMENTS
Roles: VMK: data collection, supervision of data analysis, subgroup analysis, manuscript preparation and revision. CPG: study concept and design, data collection, analysis and interpretation, manuscript preparation and revision;
The authors also acknowledge the Mentors in Medicine program, Department of Medicine, Washington University School of Medicine for providing time and support for VMK to participate in this project.
Partially funded by the Washington University Mentors in Medicine program, and by National Institutes of Health, National Research Service Award 5-T32-DK07301-35, from the National Institute of Digestive Diseases and Kidney (NIDDK).
Footnotes
Presented in preliminary form at the Annual Meeting of the American Gastroenterological Association Chicago, June 2009
No conflicts of interest exist. No writing assistance was obtained.
Competing interests: The authors have no competing interests.
REFERENCES
- 1.Oranu AC, Vaezi MF. Noncardiac chest pain: gastroesophageal reflux disease. Med Clin North Am. 2010;94(2):233–242. doi: 10.1016/j.mcna.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Eslick GD. Noncardiac chest pain: epidemiology, natural history, health care seeking, and quality of life. Gastroenterol Clin North Am. 2004;33(1):1–23. doi: 10.1016/S0889-8553(03)00125-0. [DOI] [PubMed] [Google Scholar]
- 3.Kushnir VM, Sayuk GS, Gyawali CP. Abnormal GERD parameters on ambulatory pH monitoring predict therapeutic success in noncardiac chest pain. Am J Gastroenterol. 2010;105(5):1032–1038. doi: 10.1038/ajg.2009.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barish CF, Castell DO, Richter JE. Graded esophageal balloon distention. A new provocative test for noncardiac chest pain. Dig Dis Sci. 1986;31(12):1292–1298. doi: 10.1007/BF01299805. [DOI] [PubMed] [Google Scholar]
- 5.Richter JE, Barish CF, Castell DO. Abnormal sensory perception in patients with esophageal chest pain. Gastroenterology. 1986;91(4):845–852. doi: 10.1016/0016-5085(86)90685-2. [DOI] [PubMed] [Google Scholar]
- 6.Mittal RK, et al. Sensory and motor function of the esophagus: lessons from ultrasound imaging. Gastroenterology. 2005;128(2):487–497. doi: 10.1053/j.gastro.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Rao SS, et al. Unexplained chest pain: the hypersensitive, hyperreactive, and poorly compliant esophagus. Ann Intern Med. 1996;124(11):950–958. doi: 10.7326/0003-4819-124-11-199606010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Sifrim D, Blondeau K. New techniques to evaluate esophageal function. Dig Dis. 2006;24(3–4):243–251. doi: 10.1159/000092877. [DOI] [PubMed] [Google Scholar]
- 9.Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261(4 Pt 1):G677–G684. doi: 10.1152/ajpgi.1991.261.4.G677. [DOI] [PubMed] [Google Scholar]
- 10.Clouse RE, Staiano A. Topography of normal and high-amplitude esophageal peristalsis. Am J Physiol. 1993;265(6 Pt 1):G1098–G1107. doi: 10.1152/ajpgi.1993.265.6.G1098. [DOI] [PubMed] [Google Scholar]
- 11.Clouse RE, Alrakawi A, Staiano A. Intersubject and interswallow variability in topography of esophageal motility. Dig Dis Sci. 1998;43(9):1978–1985. doi: 10.1023/a:1018838710214. [DOI] [PubMed] [Google Scholar]
- 12.Goyal RK, Rattan S. Nature of the vagal inhibitory innervation to the lower esophageal sphincter. J Clin Invest. 1975;55(5):1119–1126. doi: 10.1172/JCI108013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gyawali CP, Kushnir VM. High-resolution manometric characteristics help differentiate types of distal esophageal obstruction in patients with peristalsis. Neurogastroenterol Motil. 2011;23(6) doi: 10.1111/j.1365-2982.2011.01672.x. p 502-e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staiano A, Clouse RE. The effects of cisapride on the topography of oesophageal peristalsis. Aliment Pharmacol Ther. 1996;10(6):875–882. doi: 10.1046/j.1365-2036.1996.94266000.x. [DOI] [PubMed] [Google Scholar]
- 15.Clouse RE, Staiano A, Alrakawi A. Topographic analysis of esophageal double-peaked waves. Gastroenterology. 2000;118(3):469–476. doi: 10.1016/s0016-5085(00)70252-6. [DOI] [PubMed] [Google Scholar]
- 16.Kushnir VM, Sayuk GS, Gyawali CP. The Effect of Antisecretory Therapy and Study Duration on Ambulatory Esophageal pH Monitoring. Dig Dis Sci. 2011;56(5):1412–1419. doi: 10.1007/s10620-010-1443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter JE, et al. Esophageal manometry in 95 healthy adult volunteers. Variability of pressures with age and frequency of "abnormal" contractions. Dig Dis Sci. 1987;32(6):583–592. doi: 10.1007/BF01296157. [DOI] [PubMed] [Google Scholar]
- 18.Clouse RE, et al. Manometric findings during spontaneous chest pain in patients with presumed esophageal "spasms". Gastroenterology. 1983;85(2):395–402. [PubMed] [Google Scholar]
- 19.Aliperti G, Clouse RE. Incomplete lower esophageal sphincter relaxation in subjects with peristalsis: prevalence and clinical outcome. Am J Gastroenterol. 1991;86(5):609–614. [PubMed] [Google Scholar]
- 20.Alrakawi A, Clouse RE. The changing use of esophageal manometry in clinical practice. Am J Gastroenterol. 1998;93(12):2359–2362. doi: 10.1111/j.1572-0241.1998.00687.x. [DOI] [PubMed] [Google Scholar]
- 21.Pandolfino JE, et al. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103(1):27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 22.Wiener GJ, et al. The symptom index: a clinically important parameter of ambulatory 24-hour esophageal pH monitoring. Am J Gastroenterol. 1988;83(4):358–361. [PubMed] [Google Scholar]
- 23.Ghillebert G, et al. Ambulatory 24 hour intraoesophageal pH and pressure recordings v provocation tests in the diagnosis of chest pain of oesophageal origin. Gut. 1990;31(7):738–744. doi: 10.1136/gut.31.7.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hersh MJ, Sayuk GS, Gyawali CP. Long-term therapeutic outcome of patients undergoing ambulatory pH monitoring for chronic unexplained cough. J Clin Gastroenterol. 2010;44(4):254–260. doi: 10.1097/MCG.0b013e3181b8e97b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prakash C, Clouse RE. Wireless pH monitoring in patients with non-cardiac chest pain. Am J Gastroenterol. 2006;101(3):446–452. doi: 10.1111/j.1572-0241.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 26.Weusten BL, et al. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology. 1994;107(6):1741–1745. doi: 10.1016/0016-5085(94)90815-x. [DOI] [PubMed] [Google Scholar]
- 27.Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42(5):610–619. doi: 10.1097/MCG.0b013e31816b444d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crist J, Gidda JS, Goyal RK. Intramural mechanism of esophageal peristalsis: roles of cholinergic and noncholinergic nerves. Proc Natl Acad Sci U S A. 1984;81(11):3595–3599. doi: 10.1073/pnas.81.11.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol. 2003;114(12):2226–2244. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 30.Clouse RE, Staiano A. Contraction abnormalities of the esophageal body in patients referred to manometry. A new approach to manometric classification. Dig Dis Sci. 1983;28(9):784–791. doi: 10.1007/BF01296900. [DOI] [PubMed] [Google Scholar]
- 31.Yang M, et al. Quantitative assessment and characterization of visceral hyperalgesia evoked by esophageal balloon distention and acid perfusion in patients with functional heartburn, nonerosive reflux disease, and erosive esophagitis. Clin J Pain. 2010;26(4):326–331. doi: 10.1097/AJP.0b013e3181c8fc83. [DOI] [PubMed] [Google Scholar]
- 32.Allen ML, McIntosh DL, Robinson MG. Healing or amelioration of esophagitis does not result in increased lower esophageal sphincter or esophageal contractile pressure. Am J Gastroenterol. 1990;85(10):1331–1334. [PubMed] [Google Scholar]
- 33.Ang D, et al. The spectrum of motor function abnormalities in gastroesophageal reflux disease and Barrett's esophagus. Digestion. 2009;79(3):158–168. doi: 10.1159/000210265. [DOI] [PubMed] [Google Scholar]
- 34.Prakash C, Clouse RE. Long-term outcome from tricyclic antidepressant treatment of functional chest pain. Dig Dis Sci. 1999;44(12):2373–2379. doi: 10.1023/a:1026645914933. [DOI] [PubMed] [Google Scholar]
- 35.Roman S, et al. Weak peristalsis in esophageal pressure topography: classification and association with Dysphagia. Am J Gastroenterol. 106(2):349–356. doi: 10.1038/ajg.2010.384. [DOI] [PMC free article] [PubMed] [Google Scholar]