Abstract
Human mesenchymal stem cells (hMSC) have proven beneficial in the repair and preservation of infarcted myocardium. Unfortunately, MSCs represent a small portion of the bone marrow and require ex vivo expansion. To further advance the clinical usefulness of cellular cardiomyoplasty, derivation of “MSC-like” cells that can be made available “off-the-shelf” are desirable. Recently, human embryonic stem cell-derived mesenchymal cells (hESC-MC) were described. We investigated the efficacy of hESC-MC for cardiac repair after myocardial infarction (MI) compared to hMSC. Because of increased efficacy of cell delivery, cells were embedded into collagen patches and delivered to infarcted myocardium. Culture of hMSC and hESC-MCs in collagen patches did not induce differentiation or significant loss in viability. Transplantation of hMSC and hES-MC patches onto infarcted myocardium of athymic nude rats prevented adverse changes in infarct wall thickness and fractional area change compared to a non-viable patch control. Hemodynamic assessment showed that hMSCs and hES-MC patch application improved end diastolic pressure equivalently. There were no changes in systolic function. hES-MC and hMSC construct application enhanced neovessel formation compared to a non-viable control and each cell type had similar efficacy in stimulating endothelial cell growth in vitro. In summary, the use of hES-MC provides similar efficacy for cellular cardiomyoplasty as compared to hMSC and may be considered a suitable alternative for cell therapy.
Keywords: Tissue Engineering [E05.200.249.750], Mesenchymal Stem Cells [A11.872.580], Myocardial Infarction [C14.280.647.500], Embryonic Stem Cells [A11.872.190], Cell Transplantation [E04.936.225]
INTRODUCTION
Heart failure represents a leading cause of death in the United States. Recently, cellular cardiomyoplasty has been explored as a potential therapeutic option for patients at risk for heart failure. One issue limiting advancements in clinical cellular cardiomyoplasty is determining an optimal cell source. Traditionally, cells intended for human use have been limited to those that can be isolated in large quantities. As a result, bone marrow mononuclear cells have become popular in clinical studies (Assmus et al. 2006; Diederichsen et al. 2008; Hamano et al. 2001; Lunde et al. 2006; Patel et al. 2005; Perin et al. 2003; Schachinger et al. 2004; Tse et al. 2003; Wollert et al. 2004). The use of this heterogeneous population of cells imparts difficulties in trying to assess which cell types within the mix contribute to improved function. One population within unfractionated bone marrow that may be useful is mesenchymal stem cells (MSC). This cell population has proven successful in both preclinical and clinical studies (Dai et al. 2005; Jiang et al. 2006; Simpson et al. 2007; Tang et al. 2005; Tang et al. 2004). Unfortunately, MSCs represent a small portion of the bone marrow and require invasive procedures for removal and subsequent ex vivo expansion to maximize their efficacy. An alternate solution would be to pool several allogeneic cell sources before cellular cardiomyoplasty. Unfortunately, the need to perform invasive procedures still exists. Thus, the derivation of MSC-like cells that can be made available “off-the-shelf” without the need for intrusive surgery might help address this problem.
Boyd et al. recently described the derivation of human embryonic stem cell-derived mesenchymal cells (hESC-MC) (Boyd et al. 2009). H9 hESC and BG01 hESC were cultured in EGM2-MV for 20–30 days until epithelial outgrowths formed a confluent sheet within the culture dish. After several passages, MSC markers began to appear including CD73, CD90, CD105 and CD166. Pluripotent markers such as Oct4 and TRA-1-60 demonstrated significant downregulation with continued passage and culture. hESC-MC exhibited osteogenic and chondrogenic differentiation and the ability to contract a collagen lattice, properties similar to those of MSCs. Moreover, hES-MCs had ex vivo expansion characteristics analogous to MSCs, possibly making them suitable as an alternative to MSC-based therapies. Therefore, we tested whether hES-MCs would have similar utility in cardiac cell replacement therapy as do MSCs.
MATERIALS AND METHODS
Animal Handling
Male athymic RNU nude rats (250–350 g) obtained from Charles River (Wilmington, MA) were allowed to acclimate to housing conditions for at least one week before use. All animals received care in compliance with federal and institutional guidelines with approval from the Institutional Animal Care and Use Committee.
Cell Culture
CD34 negative female hMSCs (three different donors) obtained from Lonza were cultured in complete media consisting of Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% MSC qualified serum, L-glutamine and penicillin/streptomycin at 37°C in 5% CO2. hES-MC and human microvascular endothelial cells-cardiac (hMVEC-C) were cultured in complete EGM2-MV medium containing 5% FBS at 37°C in 5% CO2. Cells were used for in vitro or in vivo experiments once they attained 80–90% confluency.
Formation of Cell Seeded Collagen Patches
Because of our previous experience showing increased cell delivery efficacy, hMSC (female) expanded to passage P3 – P6 or hES-MC (female) expanded to P4 – P12 were embedded into a rat tail type I collagen matrix to form cardiac patches (Simpson et al. 2007). To produce cardiac patches for progenitor cell delivery, 0.2 million hMSC or 0.2 million hES-MC were mixed in a solution of rat tail type I collagen, 5x DMEM, sodium hydroxide and 10% fetal bovine serum such that the final collagen concentration was 2 mg/mL. The solution was placed in individual wells of a non-tissue culture-treated 48-well plate in order to create a patch that was between 0.3 – 0.7 cm in diameter after compaction. Patches were cultured at 37°C in 5% CO2 for 1 d before in vitro or in vivo usage. For the control experiments, non-viable (NV) cardiac patches were prepared by freezing 1 d old patches overnight in phosphate buffered saline at −80°C. The patches were thawed at room temperature and used for subsequent experiments. Non-viable patches were only exposed to a single freeze-thaw cycle. We cannot rule out that the freeze-thaw process altered the patches in some unknown way, but construction of patches with nonviable cells did not allow for sufficient mechanical integrity of the patches.
Viability Assays
To assess cell viability within the construct, patches containing hMSC or hES-MC were digested 3 d after production in type I collagenase (500 U/mL) diluted in DMEM for 30 min at 37°C with intermittent mixing to remove cells. Viability was measured using a 1:10 dilution of the cell suspension by trypan blue exclusion. Counts were made using a hemocytometer. Viability was recorded as the number of live cells divided by the number of total cells.
Proliferation
Proliferation was determined by measuring the incorporation of 5-ethynyl-2′-deoxyuridine (EdU). Cellularized constructs were pulsed with 10 mM EdU (Invitrogen; Carlsbad, CA) for 72 h after their initial formation. Afterwards, constructs were digested using collagenase to isolate cells as described above. Next, cells were fixed, permeabilized and stained with anti-EdU using the Click-iT proliferation kit (Invitrogen; Carlsbad, CA). Afterwards, cells were washed with 1% BSA/PBS and used for flow cytometry. Samples were run and analyzed for positive fluorescein isothiocyanate (FITC) signal using FCS Express 3.0 software and the histogram subtraction function (De Novo Software; Los Angeles, CA).
Assessment of Cell Differentiation
Differentiation of hMSCs and hES-MCs within the patch was measured by monitoring the expression of markers for stem cell potency over several days. hMSCs and hES-MCs isolated from the patch were stained and analyzed for the expression of CD105 and CD73 by flow cytometry. After cells were isolated from the patch, they were fixed using a 4% paraformaldehyde (PFA) for 15 min on ice. Next, cells were stained with the appropriate primary antibodies for 30 min on ice. If necessary, fluorescent conjugated secondary antibodies were added afterwards for 25 min at 4°C (Santa Cruz Biotechnology; Santa Cruz, CA). Cells were washed in a 0.3% BSA/PBS solution and analyzed via flow cytometry.
Real Time RT-PCR
RNA was isolated from cellularized constructs 48 h after initial seeding using a commercial RNeasy kit (Qiagen; Valencia, CA). RNA concentration and purity were measured using a spectrophotometer. Afterwards, mRNA was converted into cDNA using an Applied Biosystems cDNA synthesis kit (Applied Biosystems, Foster City, CA). The reaction mixture was run for 5 min at 25°C, 30 min at 42°C and lastly, 5 minutes at 85°C. Real Time RT-PCR was run using a total of 5 ng template cDNA for each sample. Each sample was run in duplicate using ABI FAST SYBR green supermix (Applied Biosystems) for multiple genes including: angiogenin (ANG), platelet derived growth factor-B (PDGF-B), vascular endothelial growth factor (VEGF), C-X-C motif ligand 1 (CXCL1), ribosomal protein L13A (RPL13A), β-actin and ribosomal protein 18s (R18s). Primer assays for each primer set were obtained from Qiagen. The fast PCR protocol consisted of an initial denaturing step at 95°C for 20 min. Next, samples were run at 95°C for 3 s and 60°C for 30 s for 40 cycles. Relative RNA abundance was calculated using the following equation: 2ΔΔcT. cDNA from hMSC monolayers was used as the internal control.
Endothelial Cell Growth Assay
To assess the mechanism whereby the progenitor cells increased vascular formation in the injured area, hMSCs and hES-MCs were cultured under hypoxic conditions (1% O2) for 1 d as a patch in DMEM supplemented with L-glutamine and penicillin/streptomycin (Maintenance Media; MM). All data were taken from cells/patches exposed to hypoxia (1% O2) to mimic likely conditions in the infarct region. Afterwards the conditioned medium was removed from the cells or patches, spun at 5000 rpm, filtered to remove debris, and placed on hMVEC-C for 3 d. After 3 d, conditioned medium was removed from hMVEC-C, and cell counts were performed. Trypan exclusion was used to determine the total number of cells and viability. Viability was recorded as the number of live cells divided by the number of total cells.
Infarct Model and Patch Application
Myocardial infarction (MI) was induced by permanent ligation of the left anterior descending (LAD) coronary artery in athymic nude male rats. Rats were anesthetized with 5% isoflurane in pure oxygen. After endotracheal intubation and initiation of ventilation the heart was exposed via a left thoracotomy, and the proximal LAD coronary artery was ligated using 6-0 silk suture. Ten minutes after ligation, either viable or non-viable cardiac patches were applied onto the anterior wall of the infarct site and secured with fibrin glue (Baxter; Deerfield, IL; Figure 3.3). Cardiac patches were secured to the heart directly beneath the ligation site and covered approximately 30–40% of the LV wall. Rats with induced infarction with or without a non-viable construct served as controls. Buprenorphine (0.1 mg/kg) was injected subcutaneously after surgery (and as necessary), and rats were allowed to recover under close supervision.
Figure 3.
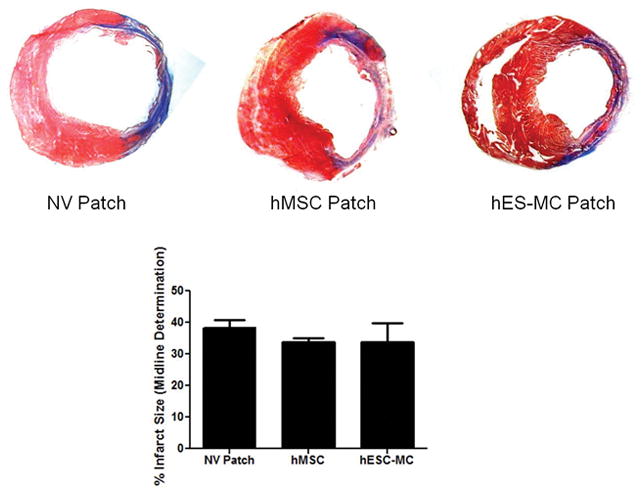
No change in infarct size with cardiac patch transplantation. Infarct size was determined by measuring the infarct midline circumference of Masson’s Trichrome stained tissue sections. Analysis revealed no change in infarct size four weeks after initial LAD ligation when comparing MI controls, hearts treated with MSC patches, or hearts treated hES-MC patches.
Echocardiography
Transthoracic echocardiograms were performed on rats using a VisualSonics Vevo 770 ultrasound unit (VisualSonics, Toronto, Canada). The VisualSonics RMV 716 Scanhead with center frequency 17.5 MHz, frequency band 11.5–23.5 MHz, and focal length 17.5 mm was used for echo acquisition in rats. The animals were maintained lightly anesthetized during the procedure with 1.5% isoflurane delivered through a face mask at a rate of 0.3–0.5 L/min. The animals were kept warm on a heating pad, and the body temperature was continuously monitored using a rectal thermometer probe and maintained between 35 and 37°C. Two-dimensional and M-mode echocardiography were used to assess LV wall thickness, end diastolic diameter (EDD) and fractional area change (FAC). Images were obtained from the parasternal long axis, and parasternal short axis at the mid-papillary level. Baseline echocardiograms were acquired at 3 d after infarction with additional echocardiograms acquired at 28 d after infarction. Rats with a baseline ejection fraction of 50% or more were excluded from the study.
Cardiac Hemodynamics
Cardiac hemodynamics were measured after the final echocardiographic examination. Rats were anesthetized with 1% isoflurane, and a 2F Millar Mikro-Tip catheter (SPR-869, Millar Instruments, Houston, TX) was inserted into the right carotid artery and advanced into left ventricle. Left ventricle (LV) pressures were recorded on a PowerLab system and analyzed using Chart v6.1 software (ADinstruments, Colorado Springs, CO). After the procedure, rats were intravenously injected with 20% potassium chloride to paralyze/relax the heart before excision for histology.
Myocardial Histology
After the hemodynamic studies, hearts were excised, perfused with 4% paraformaldehyde and then cryo-protected by immersion in 30% sucrose for 24–96 h. Isopentane cooled in liquid nitrogen was used to freeze hearts immersed in optimal cutting temperature (OCT) medium. Sections were cut to 7 μm using a commercial cyrostat and used for either isolectin B4, α-smooth muscle actin, or Masson’s Trichrome staining. Isolectin B4 diluted 1:250 in Tris buffered saline with 0.1% Tween-20 was added to sections for 1 h at 37°C. Afterwards, sections were washed in PBS, counterstained with DAPI and mounted with an anti-fade aqueous mounting media (Vector Labs, Burlingame, CA). Immunohistochemical staining for α-smooth muscle actin (α-SMA, Sigma, St. Louis, MO; 1:200) was performed to detect myofibroblast presence within and around the infarct zone. Non-specific binding was blocked by incubation with 5% goat serum (Sigma) for 1 h. The primary antibody was then added for 2 h at room temperature or overnight at 4°C. Slides were then counterstained with DAPI and mounted with an anti-fade aqueous mounting media (Vector Labs, Burlingame, CA). α-SMA positive pixels were counted using Image J software. α-SMA positive muscle and pericytes in mature arterioles were discarded before calculation. To calculate infarct size, at least four Masson’s Trichrome stained sections at various levels along the long axis were analyzed for collagen deposition. The midline technique for infarct size determination was used as described previously (Takagawa et al. 2007). Briefly, the LV midline was drawn at the center of the anterior (lateral) wall along the length of the infarct. This circumference was divided by the total midline circumference of the heart to determine infarct size.
Statistical Analysis and Interpretation
A one-way ANOVA with appropriate post-hoc testing was used for the interpretation of in vivo and histological data sets. A p value less than 0.05 indicated statistical significance. A Student’s t-test was used to determine changes in pro-angiogenic growth factor mRNA abundance between hMSCs and hES-MCs as well as changes in viability and proliferation in vitro.
RESULTS
hMSCs and hES-MCs perform similarly in collagen patches
Because of previous experience with increased efficacy of progenitor cell delivery, we embedded hMSCs and hES-MCs in collagen patches before application to the infarcted heart. This gave us the opportunity to compare the behavior of each cell type in a three dimensional culture system. Stem cell potency for cells cultured within collagen patches was determined by monitoring the expression levels of two antigens within the progenitor/stem cell populations. For both hMSCs and hES-MCs, CD105 and CD73 were used to determine the extent of differentiation at 3 d. hMSCs and hES-MCs embedded within collagen patches displayed similar expression levels for CD105 (hMSCs: 76% vs. hES-MCs: 81% expression; p > 0.05). CD73 expression was higher in hES-MCs, however (hMSCs: 75% vs. hES-MCs: 95% expression; p = 0.0002). This suggests similar differentiation existed between the two cell types in 3D culture (Figure 1A) although hES-MCs maintain higher basal expression of CD73 over long periods in culture.
Figure 1.
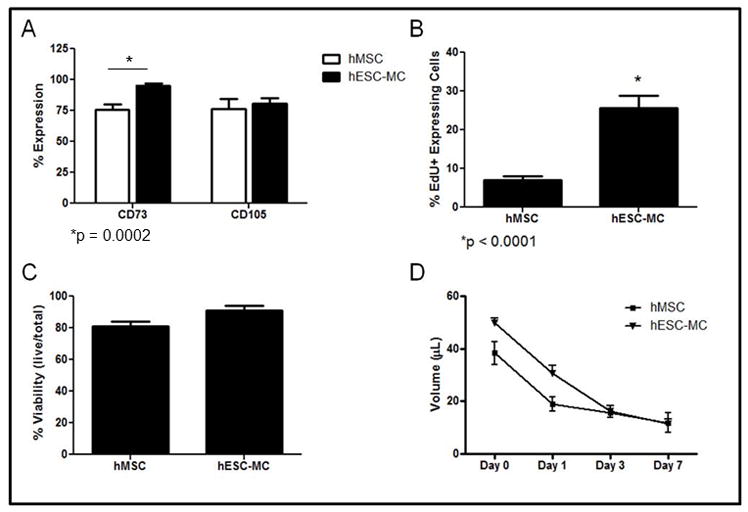
hMSCs and hES-MCs respond similarly in 3D culture. A) hMSCs and hES-MCs displayed similar potency over 3 d. B) hES-MCs maintained a higher proliferative capacity than hMSCs over 3 d. C) hES-MCs and hMSCs displayed no difference in viability over 3 d. D) Patch compaction was similar between hMSCs and hES-MCs.
To determine the proliferative capacity of hMSCs and hES-MCs in collagen patches, the extent of EdU incorporation was measured using a standard pulse-chase protocol. For both cell types, there was a noticeable reduction in EdU incorporation after cardiac patch formation. hMSCs showed an 86% reduction in EdU incorporation while hES-MCs showed a 57% reduction in EdU incorporation (p < 0.05 for both). hES-MCs maintained a higher proliferative capacity when compared to hMSCs after 3 d (25.5 ± 3.2%, vs. 7.0 ± 0.9% p < 0.01); Figure 1B).
Cell viability was comparable between cell types. To determine cell viability within collagen patches, hMSCs and hES-MCs were isolated using a collagenase solution after 3 d in culture. Both hMSCs and hESC-MCs maintained viability above 80% after culture in collagen patches (Figure 1C). There was no significant difference in the viability of hMSCs compared to hESC-MCs. (hMSC Patch: 81 ± 3% vs. hESC-MC Patch: 91 ± 3% viability).
The ability of stem cells to compact a collagen lattice was also used to measure cell function in 3D culture. Patch volume was used as a measure of compaction. Both hMSCs and hES-MCs displayed similar levels of compaction over 7 d (Day 7: hMSC: 12 ± 4; vs. hES-MC: 12 ± 2 μL; Figure 1D).
Taken together this data shows that hMSCs and hES-MCs respond similarly when cultured in collagen patches and supports previous reports concerning the similarity of these cell types. Given both cell types remain viable and maintain potency in 3D culture, in vivo investigations were performed to determine their efficacy in improving cardiac function after MI.
hMSCs and hES-MCs Improve Cardiac Remodeling and Function after Myocardial Infarction
To determine the effect of cardiac patch transplantation on cardiac remodeling and function after MI, echocardiography and invasive hemodynamics were performed. Both hMSC and hES-MC cardiac patches had a beneficial role in preventing adverse remodeling. In particular, hMSC patches helped to maintain myocardial wall thickness (MI: 0.6 ± 0.1 and NV: 0.7 ± 0.01 vs. hMSCs: 1 ± 0.1 and hES-MCs: 1 ± 0.1mm; *p < 0.05 vs. MI and NV Patch) LV EDD (MI: 9.1 ± 0.1 and NV: 9.2 ± 0.2 vs. hMSCs: 8.2 ± 0.1 and hES-MCs: 8 ± 0.2mm *p < 0.05 vs. MI and NV Patch) and the LV FAC (MI: 31 ± 4 and NV: 33 ± 3 vs. hMSCs: 51 ± 3 and hES-MCs: 47 ± 2%; *p < 0.05 vs. MI and NV Patch) over 4 wks after infarction compared to MI and NV patch controls (Figure 2A–C). Representative M-mode images show improved changes in myocardial remodeling with hMSC or hES-MC patch application (Figure 2D–G). There was no difference between hMSC or hES-MC patch treatments.
Figure 2.
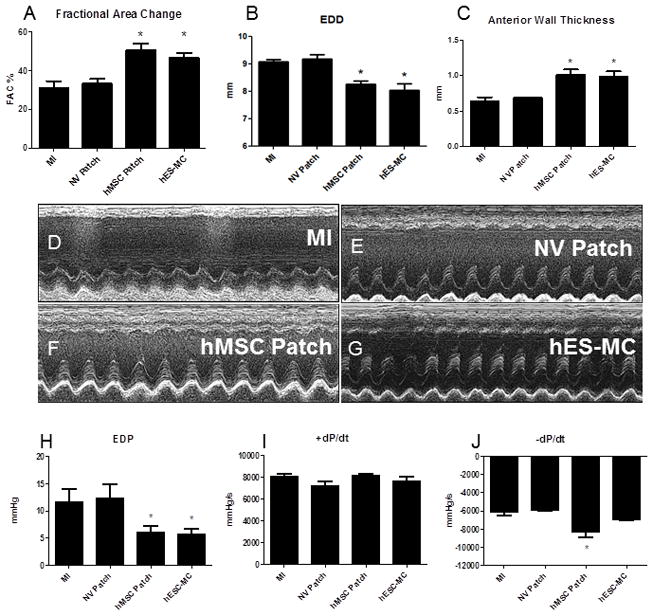
hMSCs and hES-MCs improve cardiac remodeling and function after myocardial infarction. A) Left ventricle blood pool fractional area change (FAC), B) left ventricular end diastolic diameter and C) infarct wall thickness were significantly improved after hMSC and hES-MC treatments as compared to MI and non-viable (NV) patch controls (*p < 0.05). Representative echocardiographic M-mode images showing LV function and properties for MI control (D), NV patch control (E), hMSC patch (F) and hES-MC (G). Invasive hemodynamic analysis for H) Left ventricle end diastolic pressure (EDP), I) +dP/dt and J) −dP/dt show improvement in diastolic parameters with no change in systolic parameters after hMSC and hES-MC patch application compared to MI and NV patch controls (*p < 0.05).
Invasive hemodynamics showed that hMSC and hES-MC applications after MI improved LV end diastolic pressure (EDP) compared to MI and NV patch controls at 4 weeks (MI: 12 ± 2 and NV: 12 ± 3 vs. hMSCs: 6 ± 1 and hES-MC: 6 ± 1 mmHg; *p < 0.05 vs. MI and NV Patch controls; Figure 2J). There was no significant change in systolic function as assessed by +dP/dt (MI: 8030 ± 318 and NV: 7226 ± 415 vs. hMSCs: 8145 ± 158 and hES-MC: 7664 ± 355 mmHg/s; Figure 2I). Additionally, although −dP/dt was significantly increased with hMSC patch application, hES-MC patch application did not induce a similar effect (MI: −6186 ± 284 and NV: −5882 ± 63 vs. hMSCs: −8252 ± 640 and hES-MC: −7009 ± 59 mmHg *p < 0.05 vs MI and NV Patch controls; Figure 2H). For all parameters there were no significant differences between hMSC and hES-MC patches. Infarct size was also unchanged between groups (NV Patch: 38 ± 3%, hMSC Patch: 34 ± 1%, and hES-MC Patch: 34 ± 6%; p = 0.71; Figure 3).
hMSCs and hES-MCs Alter Neo-vessel Formation after Myocardial Infarction
One possible explanation as to why hMSCs and hES-MCs improve cardiac remodeling and function is through the release of beneficial paracrine factors. Such factors can act by decreasing cardiomyocyte apoptosis, preventing adverse fibrosis, or increasing angiogenesis (Hung et al. 2007; Tang et al. 2005; Tang et al. 2004). To determine whether cardiac patch application had an effect on neovessel formation after myocardial infarction, histological evaluation for isolectin B4 was performed. The data indicated that hMSC and hES-MC patches augmented neo-vessel formation compared to controls (non-viable patch: 3 ± 0.3 vs. hMSC: 11 ± 2 vs. hES-MC: 11 ± 2; p < 0.05; Figure 4A–D). Neovessels were generally found on the epicardial side of the anterior infarct and peri-infarct wall, though fewer neovessels were also found spanning the infarct within the lateral and posterior walls. Additionally, histological evaluation was completed to determine the extent of myofibroblast presence. There were no significant differences between the non-viable control, hMSC, or hES-MC groups (Figure 4E–H).
Figure 4.
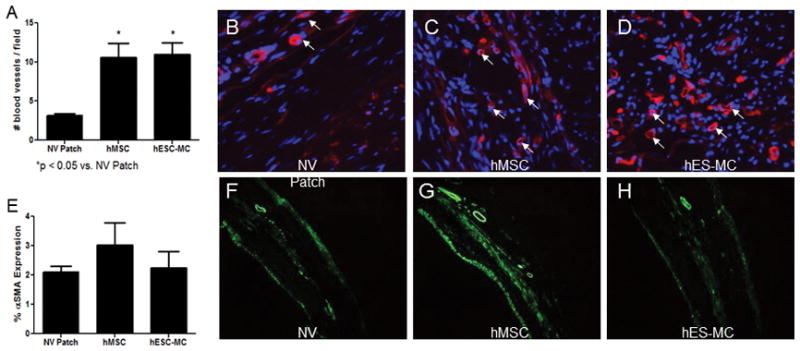
Application of hES-MC patches improves neo-vessel formation. A) hMSC and hES-MC applications increase neo-vessel formation compared to controls (*p < 0.05). B–D). Neo-vessel presence was detected using isolectin B4 and appears red (white arrows). DAPI (blue) was used as a nuclear counter stain (Magnification 40X). E) Myofibroblast abundances were unchanged between groups (F–H) Anti-αSMA (green) was used to detect myofibroblasts (10X).
hMSCs and hES-MCs show different cytokine patterns
Despite similarities in neovessel induction, the two cell types showed differences in the relative amount of pro-angiogenic factor mRNA abundances. hMSCs displayed increased mRNA abundance of VEGF (Figure 5A; hMSCs: 16 ± 4 vs. hES-MCs: 1.0 ± 0.2; *p < 0.05) and PDGF-B (Figure 5D; hMSCs: 332 ± 87 vs. hES-MCs: 4.0 ± 0.5; *p < 0.05) when compared to hES-MCs. On the other hand, hES-MCs displayed increased abundance of CXCL1 (Figure 5B; hMSCs: 3.0 ± 0.3 vs. hES-MCs: 41 ± 4; *p < 0.05) when compared to hMSCs. There was no difference in the relative mRNA abundance of ANG between hMSCs and hES-MCs (Figure 5C; hMSCs: 0.5 ± 0.2 vs. hES-MCs: 0.8 ± 0.1; p = 0.2). Despite these differences, hMSCs and hES-MCs conditioned media showed equivalent effects on endothelial cell growth and proliferation in an in vitro trypan blue exclusion assay (Figure 6).
Figure 5.
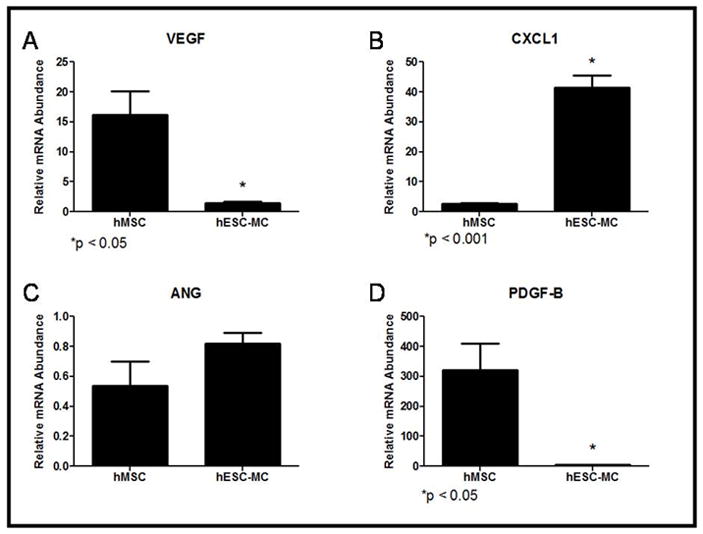
Angiogenic growth factor mRNA abundances varied in hMSC and hES-MC progenitor cells: A) Vascular Endothelial Growth Factor (VEGF; *p < 0.05) B) CXCL1 (*p < 0.05) C) Angiogenin (ANG) and D) Platelet Derived Growth Factor-B (PDGF-B; *p < 0.05).
Figure 6.
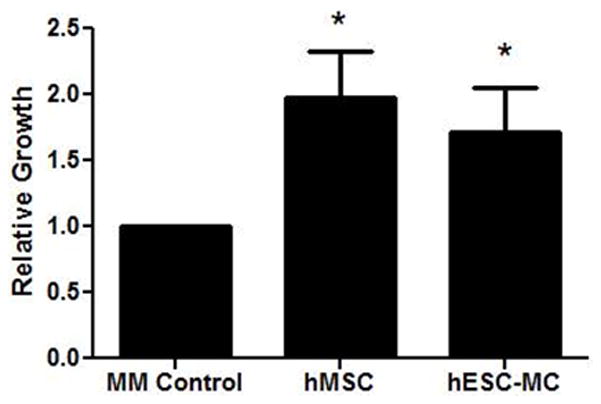
hMSC and hES-MC conditioned media support endothelial cell proliferation. Exposure of hMVEC-C to hMSC or hES-MC conditioned media results in increased relative cell counts compared to a maintenance media control (*p < 0.01).
DISCUSSION
The choice of cell source used in human clinical trials is limited by the need to expand specific cell populations after isolation. This is the case with MSCs, which have to be purified and expanded before transplantation into patients. This delay represents a potential problem when treating patients with cellular cardiomyoplasty. Given the difficulties of harvesting mesenchymal stem cells for preventive therapy, it is desirable to develop novel techniques for deriving “off-the-shelf” hMSC substitutes. hES-MCs represent one possible solution. It is reported that these cells display a similar phenotype to hMSCs, but the reparative potential of hES-MCs in cellular cardiomyoplasty had not been evaluated. In this study, we compared hMSCs to hES-MCs delivered to infarcted myocardium.
A tissue engineered approach to cell delivery was chosen given evidence that cardiac patches provide increased cell engraftment efficiency (Simpson et al. 2007). Constructing patches provided an opportunity to study the two cell types in 3D culture conditions. In general, the behavior of hMSCs and hES-MCs was similar when embedded in collagen patches. Both cell types demonstrated a relatively high level of stem cell potency, sustained viability, and reduced cell proliferation. A similar response was seen by Liu et al., in which mouse MSCs displayed reduced proliferation and differentiation potential when cultured in the presence of carbon nanotubes (Liu et al.). The parallels in behavior between the two cells types in collagen patches adds to previous evidence that hES-MCs are similar in phenotype to hMSCs (Boyd et al. 2009) and further supports their role as a suitable alternative to hMSC therapy.
In order to determine the effectiveness of these cell types in mediating cardiac repair, we observed cardiac function after MI with and without the application of hMSC or hES-MC patches. Overall, hES-MCs provided a similar level of structural and functional preservation compared to hMSCs. Several reports using hMSCs as a cell source for cellular cardiomyoplasty have also observed improvement in cardiac function after cell delivery (Dai et al. 2005; Simpson et al. 2007; Tang 2005). In our study, hMSC and hES-MC cardiac patch transplantations prevented adverse wall thinning, adverse LV remodeling, and adverse changes in diastolic function. It is likely that cardiac patch application modulated the fibrovascular repair response by promoting angiogenesis and possibly an extracellular matrix (ECM) composition that favors maintenance of diastolic function and LV structure. The data support a comparable angiogenic response from both cells types. Given the effect of hES-MCs is similar to that of hMSCs for MI repair, it is likely that neovessel formation is playing a role in MI repair and preservation. The similar effect on LV diastolic pressure may represent a similar role in altering ECM content after MI (Berry et al. 2006). Although several parameters are similar between the two cell groups, hES-MC patch application does not favorably affect −dP/dt comparable to hMSC. We speculate that changes in ECM turnover and/or composition may account for such differences. Future experiments that investigate the ECM composition during infarct repair would aid in uncovering beneficial mechanisms to preserving myocardial mechanics and function.
Patch application appears to have little effect on endogenous cardiomyocyte survival or the prevention of infarct expansion. Indeed, treatment with cardiac patches showed no difference in infarct size compared to controls. Previous investigations using hMSCs have shown improvements in these parameters (Amado et al. 2005; Simpson et al. 2007). Such differences are possibly because of the delivery strategy or number of cells delivered. The current study used an initial seeding density of 2 × 105 cells. Other reports typically use cell numbers in excess of 1 × 106 cells for studies involving a rat model (Dai et al. 2005; Grinnemo et al. 2006; Hou et al. 2007; Nagaya et al. 2005; Tang et al. 2005).
The effects of hMSCs and hES-MCs on cardiac remodeling, function, and neovessel formation after MI may be the result of paracrine actions on endogenous cells such as myofibroblasts and endothelial cells (Dai et al. 2005; Li et al. 2008; Tang et al. 2005). The angiogenic effect is thought to be the result of secreted paracrine factors, such as VEGF (Potapova et al. 2007; Tang et al. 2004), IGF-1 (Chen et al. 2008), SDF-1 (Zhang et al. 2007) and MCP-1 (Hung et al. 2007). Despite an expectation that paracrine factor profiles would be similar, given the analogous response to each cell type, there were differential secretory profiles that were cell specific. hMSCs displayed heightened mRNA abundance of VEGF and PDGF-B while hES-MCs displayed heightened mRNA abundance of CXCL1. Despite these differences, the local angiogenic effects of hES-MCs were similar to hMSCs in vitro and in vivo. This implies that there may be multiple ways to achieve the same angiogenic effect. Consistent with this implication, the increased abundance of CXCL1 in hES-MC can lead to VEGF secretion (Scapini et al. 2004), ECM degradation (Breland et al. 2008), and subsequent changes in infarct morphology and mechanical properties. Another possible explanation is that the relevant growth factors involved in myocardial repair and/or preservation were not measured in this study. Several other proangiogenic factors such as interleukins, fibroblast growth factor or matrix metalloproteinases may also explain the mechanisms of repair involved in this study. Future studies that involve in depth investigation of an array of paracrine factors and subsequent trophic responses are essential to the progression of the field.
Although the hESC-MCs respond similarly to hMSCs in vitro and in vivo, the clinical usefulness of this approach may be diminished given the possibility of rejection. The data presented, however, represents a proof of concept that can be applied to a more suitable cell type such as induced pluirpotent stem cells, which may offer a scalable autologous source for MSC-like cells with cardiomyoplastic potential. Future studies will address the derivation, differentiation and bioprocessing of induced pluripotent stem cells for cell therapy. Additionally, the use of a homogenous population of cultured cells offers the possibility of efficient genetic tracking once delivered to the recipient. This may allow for the altering of major histocompatibility complexes, using genetic engineering approaches that can eliminate issues of rejection.
CONCLUSIONS
In conclusion, hES-MC displayed similar responses to hMSCs in 3D culture, retaining pluripotency and longevity. Additionally, hES-MCs displayed similar efficacy at attenuating adverse LV remodeling and maintaining diastolic function as hMSCs. Given similar angiogenic effects, it is likely that neovessel formation plays a vital role in cardiac repair. Therefore, cellular cardiomyoplasty with the use of hES-MC represents a potential alternative to hMSC therapy.
Acknowledgments
We would like to thank Baxter, Inc for their generous contribution of fibrin glue. Supported by NIH R01 HL085558, R01 HL073753, and P01 HL058000 (SCD)
Footnotes
Disclaimers: None
Author Contributions:
David L. Simpson: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing
Nolan L. Boyd: Provision of study materials
Sunjay Kaushal: Provision of study materials
Steve L. Stice: Provision of study materials
Samuel C. Dudley: Conception and design, financial support, manuscript writing final approval of manuscript
References
- Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102(32):11474–9. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355(12):1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290(6):H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- Boyd NL, Robbins KR, Dhara SK, West FD, Stice SL. Human Embryonic Stem Cell-Derived Mesoderm-like Epithelium Transitions to Mesenchymal Progenitor Cells. Tissue Eng Part A. 2009;15:1897–1907. doi: 10.1089/ten.tea.2008.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland UM, Halvorsen B, Hol J, Oie E, Paulsson-Berne G, Yndestad A, Smith C, Otterdal K, Hedin U, Waehre T, et al. A potential role of the CXC chemokine GROalpha in atherosclerosis and plaque destabilization: downregulatory effects of statins. Arterioscler Thromb Vasc Biol. 2008;28(5):1005–11. doi: 10.1161/ATVBAHA.108.162305. [DOI] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112(2):214–23. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- Diederichsen AC, Moller JE, Thayssen P, Junker AB, Videbaek L, Saekmose SG, Barington T, Kristiansen M, Kassem M. Effect of repeated intracoronary injection of bone marrow cells in patients with ischaemic heart failure the Danish stem cell study--congestive heart failure trial (DanCell-CHF) Eur J Heart Fail. 2008;10(7):661–7. doi: 10.1016/j.ejheart.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Grinnemo KH, Mansson-Broberg A, Leblanc K, Corbascio M, Wardell E, Siddiqui AJ, Hao X, Sylven C, Dellgren G. Human mesenchymal stem cells do not differentiate into cardiomyocytes in a cardiac ischemic xenomodel. Ann Med. 2006;38(2):144–53. doi: 10.1080/07853890500422982. [DOI] [PubMed] [Google Scholar]
- Hamano K, Nishida M, Hirata K, Mikamo A, Li TS, Harada M, Miura T, Matsuzaki M, Esato K. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: clinical trial and preliminary results. Jpn Circ J. 2001;65(9):845–7. doi: 10.1253/jcj.65.845. [DOI] [PubMed] [Google Scholar]
- Hou M, Yang KM, Zhang H, Zhu WQ, Duan FJ, Wang H, Song YH, Wei YJ, Hu SS. Transplantation of mesenchymal stem cells from human bone marrow improves damaged heart function in rats. Int J Cardiol. 2007;115(2):220–8. doi: 10.1016/j.ijcard.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25(9):2363–70. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang Y, Dong A, Du Y, Huang X, Wang J, et al. Homing and differentiation of mesenchymal stem cells delivered intravenously to ischemic myocardium in vivo: a time-series study. Pflugers Arch. 2006;453(1):43–52. doi: 10.1007/s00424-006-0117-y. [DOI] [PubMed] [Google Scholar]
- Li JH, Zhang N, Wang JA. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J Endocrinol Invest. 2008;31(2):103–10. doi: 10.1007/BF03345575. [DOI] [PubMed] [Google Scholar]
- Liu D, Yi C, Zhang D, Zhang J, Yang M. Inhibition of proliferation and differentiation of mesenchymal stem cells by carboxylated carbon nanotubes. ACS Nano. 4(4):2185–95. doi: 10.1021/nn901479w. [DOI] [PubMed] [Google Scholar]
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112(8):1128–35. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- Patel AN, Geffner L, Vina RF, Saslavsky J, Urschel HC, Jr, Kormos R, Benetti F. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J Thorac Cardiovasc Surg. 2005;130(6):1631–8. doi: 10.1016/j.jtcvs.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107(18):2294–302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, Doronin SV. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25(7):1761–8. doi: 10.1634/stemcells.2007-0022. [DOI] [PubMed] [Google Scholar]
- Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, Albini A, Lowell C, Berton G, Noonan DM, et al. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol. 2004;172(8):5034–40. doi: 10.4049/jimmunol.172.8.5034. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44(8):1690–9. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Simpson D, Liu H, Fan TH, Nerem R, Dudley SC., Jr A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25(9):2350–7. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol. 2007;102(6):2104–11. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL. Autologous mesenchymal stem cells for post-ischemic myocardial repair. Methods Mol Med. 2005;112:183–92. doi: 10.1385/1-59259-879-x:183. [DOI] [PubMed] [Google Scholar]
- Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips MI. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80(1):229–36. doi: 10.1016/j.athoracsur.2005.02.072. discussion 236–7. [DOI] [PubMed] [Google Scholar]
- Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117(1):3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361(9351):47–9. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21(12):3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]


