Abstract
Aims
Point-of-care HbA1c is routine in clinical practice. Comparison of point-of-care HbA1c against laboratory measurements across sites and over time is warranted.
Methods
One hundred and twenty-one young persons with Type 1 diabetes from four centres provided 450 paired samples collected over 10 months for point-of-care HbA1c and central laboratory-based high-performance liquid chromatography (HPLC) HbA1c determinations. Change in HbA1c over time was assessed by difference from initial to final HbA1c and by growth modelling with annualized slope calculation. Change in HbA1c was categorized as improved (decrease of ≥ 0.5% or negative slope), no change (± 0.4% of initial HbA1c or slope = 0) or worsened (increase of ≥ 0.5% or positive slope).
Results
The 450 paired samples (median of four pairs/patient) were highly correlated (r = 0.97, P < 0.0001), as were time-specific and site-specific pairs (r = 0.94 to 0.98, P < 0.0001). Initial-to-final point-of-care HbA1c and HPLC HbA1c changes were 0.3 ± 1.1% (range −2.7 to 4.1) and 0.4 ± 1.2% (–3.9 to 4.5), respectively, with 21% of patients (n = 26) discordant for change categories. ΔHbA1c by point-of-care HbA1c vs. HPLC HbA1c differed across the HbA1c range and by ≥ 0.5% absolute difference in ΔHbA1c in 14 (54%) of the 26 patients discordant for HbA1c change categories. Mean annual HbA1c slope was 0.4 ± 1.5% (−5.4 to 4.8) for point-of-care HbA1c and 0.4 ± 1.6% (−6.9 to 5.2) for HPLC HbA1c, with 18% (n = 22 pairs) discordant for change categories.
Conclusions
Assessment of absolute HbA1c change may not be different for point-of-care HbA1c compared with HPLC HbA1c; however, misclassification of patients by discrete cut-off values may occur with point-of-care HbA1c compared with HPLC HbA1c determinations.
Keywords: children, HbA1c, point-of-care, Type 1 diabetes
Introduction
HbA1c is the standard measure of glycaemic control. Provider and patient knowledge of HbA1c is associated with improved outcomes [1–4]. High-performance liquid chromatography (HPLC), the assay used in the Diabetes Control and Complications Trial (DCCT) [5,6] has been the standard for measuring HbA1c. In clinical settings, point-of-care instruments have become routine because of their ease of use, finger-stick sampling and rapid turnaround. Immediate HbA1c results help guide diabetes management, especially when the patient provides no blood glucose data [2,4]. Cross-sectional validation studies indicate that point-of-care HbA1c values are equivalent to laboratory measurements [7–9]. Point-of-care devices are waived under Clinical Laboratory Improvement Amendments (CLIA) and certified by the National Glycohemoglobin Standardization Program (NGSP).
Clinical research, particularly longitudinal multi-centre trials, generally employs central laboratory HPLC HbA1c determinations. In clinical trials, HbA1c outcomes often include mean change in HbA1c from baseline, as well as the proportion of subjects achieving either target HbA1c levels, an absolute HbA1c decrease of ≥ 0.5% or a relative decrement in HbA1c of ≥ 10% [10–13]. As point-of-care HbA1c measurements are correlated with laboratory assays, some research studies have utilized point-of-care HbA1c assays as the primary HbA1c outcome [14,15]. Thus, there is a need to determine the utility of point-of-care HbA1c c assays for longitudinal assessments and multi-centre clinical investigations. Our aim was to compare changes in point-of-care HbA1c with HPLC HbA1c over time and across sites in a longitudinal study.
Patients and methods
Young people at four geographically distinct paediatric diabetes centres participated in a family-based pilot study [16]. Eligibility criteria included age 9.0–14.5 years, Type 1 diabetes duration ≥ 1 year, insulin dose > 0.5 units kg−1 day−1 and HbA1c ≤ 119 mmol/mol (13.0%). Blood samples for point-of-care HbA1c and HPLC HbA1c measurements were simultaneously drawn by finger stick every 3 months for four sequential visits over 9.7 ± 2.2 (mean ± SD) months. Only visits with both point-of-care HbA1c and HPLC HbA1c for a subject were included. Written informed consent/assent was obtained from parents/young person. The study was approved by the Institutional Review Boards at participating institutions.
HPLC HbA1c samples, obtained by research assistants who received standardized training in sample processing, were shipped to a central laboratory (Joslin Diabetes Center) for HbA1c assay (Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer™; Tosoh Medics, South San Francisco, CA, USA). Quality control procedures were performed daily and met the requirements of the College of American Pathologists. The National Glycohemoglobin Standardization Program reference range for HbA1c is 20–42 mmol/mol (4.0–6.0%) [assay range 22–204 mmol/mol (4.2–20.8%)]. The interassay coefficient of variation was < 5% for both low and high controls. The coefficient of variation was 4.8% at an HbA1c value of 5.55% per Diabetes Control and Complications Trial [low control limit 36–39 mmol/mol (5.4–5.7%)] and 2.6% at an HbA1c value of 10.9% per Diabetes Control and Complications Trial [high control limit 85–107 mmol/mol (9.9–11.9%)].
Point-of-care HbA1c samples were analysed by immunoassay using the DCA 2000®+ Analyzer (previously Bayer Healthcare, Elkhart, IN, USA, now Siemens Healthcare Diagnostics, Deerfield, IL, USA). All sites used identical protocols for calibration, control and testing procedures. In addition, all sites obtained almost exclusively the same lots for low and high control solutions and assay cartridges. Reference range for point-of-care HbA1c was 23–39 mmol/mol (4.3–5.7%) [assay range 4–130 mmol/mol (2.5–14.0%)]. The coefficient of variation was 5.6% for the low control [control limit 25–49 mmol/mol (4.4–6.6%)] and 6.0% for the high control [control limit 70–116 mmol/mol (8.6–12.8%)].
For both HPLC and point-of-care determinations, change in HbA1c over time was calculated by two methods: (1) change from initial to final HbA1c (ΔHbA1c) and (2) growth modelling with annualized slope calculation (ΔHbA1c /year). Change in HbA1c was categorized as improved (decrease of ≥ 0.5% or negative slope), no change (± 0.4% of initial HbA1c or slope = 0) or worsened (increase of ≥ 0.5% or positive slope). We also determined the proportion of participants achieving age-specific HbA1c targets recommended by the American Diabetes Association (for ≤12 years old, HbA1c < 8%; for ≥ 13 years old, HbA1c < 7.5%) by each assay.
Analyses included Pearson correlations, linear mixed models, t-tests and χ2-tests using SAS 9.2 software (SAS Institute, Cary, NC, USA). Growth modelling, assuming linearity with a short follow-up period, provided annualized slope calculations. Data are presented as means ± SD (range) or percentages. P-values < 0.05 were considered statistically significant.
Results
A total of 121 young persons (29–31 young persons/site) with Type 1 diabetes comprised the sample. Patients had a mean age of 12.2 ± 1.6 years and a mean duration of diabetes of 5.5 ± 3.2 years. Fifty per cent of the young persons were male and 27% represented ethnic/racial minorities.
There were 450 paired point-of-care HbA1c and HPLC HbA1c samples with a median of four pairs/patient (mean 3.7 ± 0.6 pairs). Initial mean point-of-care HbA1c and HPLC HbA1c values were 65 mmol/mol (8.1 ± 1.2%) and 68 mmol/mol (8.4 ± 1.4%) (P < 0.0001), respectively, and final mean values were 68 mmol/mol (8.4 ± 1.4%) and 73 mmol/mol (8.8 ± 1.6%) (P < 0.0001), respectively (see Table 1). Point-of-care HbA1c values ranged from 33 to 130 mmol/mol (5.2–14.0%) and HPLC HbA1c values ranged from 33 to 147 mmol/mol (5.2–15.6%). Only one point-of-care HbA1c and six HPLC HbA1c results were ≥ 14%. In cross-sectional analyses, the 450 paired samples were highly correlated (r = 0.97, P < 0.0001). Correlation by visit across sites (n = 109–118) and within site across visits (n = 102–120) were equally high (r = 0.94–0.98, P < 0.0001).
Table 1.
Comparison of HbA1c values obtained by HPLC and point of care*
| Point-of-care HbA1c (%) |
HPLC HbA1c (%) |
Correlation | P-value | |
|---|---|---|---|---|
| Initial HbA1c (n = 121) |
8.1 ± 1.2 65 (40–104) |
8.4 ± 1.4 68 (38–113) |
0.97 | < 0.0001 |
| Final HbA1c (n = 121) |
8.4 ± 1.4 68 (38–130) |
8.8 ± 1.6 73 (38–147) |
0.97 | < 0.0001 |
| Absolute ΔHbA1c (n = 121) |
0.3 ± 1.1 | 0.4 ± 1.2 | 0.93 | < 0.0001 |
| Slope (ΔHbA1c/year) (n = 121) |
0.4 ± 1.5 | 0.4 ± 1.6 | 0.91 | < 0.0001 |
HbA1c data presented as % with standard deviation and in mmol/mol with range.
In longitudinal analyses, mean ΔHbA1c from initial to final visit was 0.3 ± 1.1% (range −2.7 to 4.1%) for point-of-care HbA1c and 0.4 ± 1.2% (−3.9 to 4.5%) for HPLC HbA1c. Point-of-care HbA1c and HPLC HbA1c change categories were discordant for ΔHbA1c in 21% of patients (n = 26) (P < 0.0001) (Fig. 1). In seven patients, point-of-care HbA1c improved while HPLC HbA1c showed no change. In four patients, point-of-care HbA1c worsened while HPLC HbA1c showed no change. In 10 patients, point-of-care HbA1c showed no change while HPLC HbA1c worsened. In five patients, point-of-care HbA1c showed no change while HPLC HbA1c improved. Discordant ΔHbA1c classification occurred across sites and the entire HbA1c range.
Figure 1.
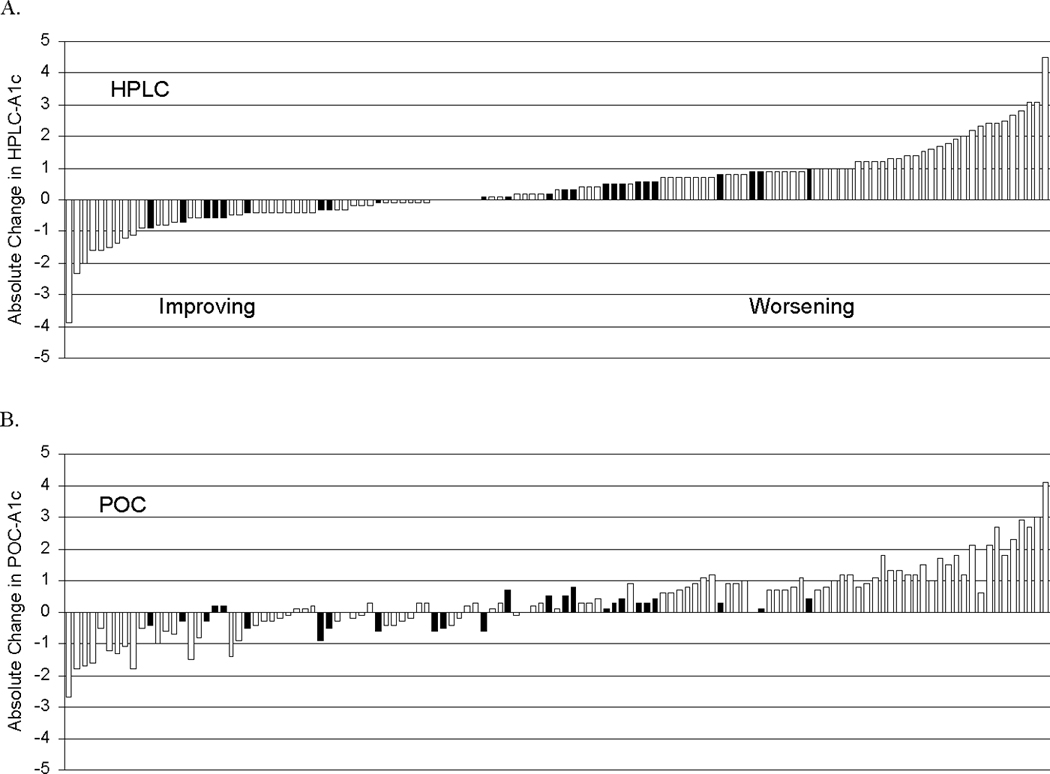
Change from initial to final HbA1c for HPLC HbA1c (a) and point-of-care HbA1c (b). In both figure parts, patients are rank ordered according to change in HPLC HbA1c. Discordant patients are noted with darker bars.
Mean ΔHbA1c/year was 0.4 ± 1.5% (−5.4 to 4.8) for point-of-care HbA1c and 0.4 ± 1.6% (−6.9 to 5.2) for HPLC HbA1c. Point-of-care HbA1c and HPLC HbA1c were discordant for ΔHbA1c/year in 18% of patients (n = 22) (P < 0.0001). In 11 patients, point-of-care HbA1c improved while HPLC HbA1c worsened. In 10 patients, point-of-care HbA1c worsened while HPLC HbA1c improved. In one patient, point-of-care HbA1c worsened while HPLC HbA1c showed no change.
Because of the negative bias of the point-of-care HbA1c assay compared with the HPLC HbA1c assay, at initial visit, 41% (n = 40) and 31% (n = 38) of patients achieved age-specific HbA1c targets by point-of-care HbA1c and HPLC HbA1c, respectively. At final visit, 37% (n = 43) and 28% (n = 34) achieved targets by point-of-care HbA1c and HPLC HbA1c, respectively. At initial visit, one patient attained the goal by HPLC HbA1c but not by point-of-care HbA1c and, at final visit, three patients attained the goal by HPLC HbA1c but not by point-of-care HbA1c. There was similar misclassification in 20% (n = 24) of patients, demonstrating a 10% change from initial to final HbA1c by the two assay methods.
Discussion
Consistent with previous studies, point-of-care HbA1c and HPLC HbA1c values were highly correlated in cross-sectional analyses. The strong cross-sectional correlations between point-of-care HbA1c and HPLC HbA1c assays have been demonstrated by others and support the utility of point-of-care HbA1c in clinical care [4,7–9]. DirecNet investigators showed high cross-sectional correlations (r = 0.94, P < 0.001) between point-of-care HbA1c values (using DCA 2000®+) and HPLC HbA1c values (performed by a central laboratory), although their point-of-care HbA1c values were biased significantly higher than the HPLC HbA1c values [7]. Interestingly, our HPLC HbA1c values were biased consistently higher than point-of-care HbA1c results. However, the bias does not alter our assessment of discordance in change in HbA1c over time between assays, although the bias does account for much of the difference in proportions of patients achieving HbA1c target values.
In longitudinal analyses, change in HbA1c from initial to final visit showed similar means and standard deviations by point-of-care HbA1c or HPLC HbA1c. When classifying by change ≥0.5%, 21% of patients were discordant between point-of-care HbA1c and HPLC HbA1c. We selected to define change in HbA1c from initial to final by a difference of ≥ 0.5%, as any result within 0.4% of the previous value might be considered within the assay’s error range [17]. Using slope calculations, we found discordance between point-of-care HbA1c and HPLC HbA1c in 18% of patients.
Our population was diverse (27% minority) and offered a wide range of HbA1c values, supporting the potential for generalizability. In addition, our study aimed to reduce variability in point-of-care HbA1c measurements by standardizing the lots used across sites. If such care had not been taken, it is possible we might have encountered greater discordance in point-of-care HbA1c results compared with the HPLC HbA1c results [18].
Clinical trials often declare a priori outcomes that include absolute or relative change in HbA1c from baseline to endpoint [10]. For example, a multi-centre trial might compare the proportion of patients who demonstrate a change in HbA1c of ≥ 0.5% or relative change in HbA1c of ≥ 10% in order to assess alteration in risk for microvascular complications as reported by the Diabetes Control and Complications Trial [5].
Our findings suggest that assessment of absolute HbA1c change may not be different for point-of-care HbA1c compared with HPLC HbA1c. However, classification of patients by discrete cut-off values differs between point-of-care HbA1c and HPLC HbA1c determinations in one out of five patients followed longitudinally, resulting in potential misclassification of individual patient outcomes. These data support a need for a central reference laboratory for longitudinal observations in multi-site clinical research studies that include categories of HbA1c change in addition to mean HbA1c outcomes.
Acknowledgements
This work was supported by the intramural research programme of the National Institute of Child Health and Human Development (NICHD), NIH grants P30DK036836 and T32DK007260, the Maria Griffin Drury Pediatric Fund, the Katherine Adler Astrove Youth Education Fund and the Charles H. Hood Foundation. The following investigators and institutions made up the steering committee of the Family Management of Childhood Diabetes multi-site trial: Jill Weissberg-Benchell PhD, Grayson Holmbeck PhD (Children’s Memorial Hospital, Chicago, Contract N01-HD-4-3363); Barbara Anderson PhD (Texas Children’s Hospital, Houston, Contract N01-HD-4-3362); Tim Wysocki PhD, Amanda Lochrie PhD (Nemours Children’s Clinic, Jacksonville, FL, Contract N01-HD-4-3361); Lori Laffel MD MPH, Deborah Butler MSW, Lisa Volkening MA (Joslin Diabetes Center, Boston, MA, Contract N01-HD-4-3364); Tonja Nansel PhD, Ronald Iannotti PhD (NICHD, Bethesda, MD); Cheryl McDonnell PhD, MaryAnn D’Elio (James Bell Associates, Arlington, VA, Contract N01-HD-3-3360).
Footnotes
Competing interests
Nothing to declare.
Portions of this manuscript were presented at the 70th Scientific Sessions of the American Diabetes Association (2010).
References
- 1.Larsen ML, Horder M, Mogensen EF. Effect of long-term monitoring of glycosylated hemoglobin levels in insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:1021–1025. doi: 10.1056/NEJM199010113231503. [DOI] [PubMed] [Google Scholar]
- 2.Cagliero E, Levina EV, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin-treated type 2 diabetic patients. Diabetes Care. 1999;22:1785–1789. doi: 10.2337/diacare.22.11.1785. [DOI] [PubMed] [Google Scholar]
- 3.Ginde AA, Cagliero E, Nathan DM, Camargo CA., Jr Point-of-care glucose and hemoglobin A1c in emergency department patients without known diabetes: implications for opportunistic screening. Acad Emerg Med. 2008;15:1241–1247. doi: 10.1111/j.1553-2712.2008.00240.x. [DOI] [PubMed] [Google Scholar]
- 4.Agus MS, Alexander JL, Wolfsdorf JI. Utility of immediate hemoglobin A1c in children with type I diabetes mellitus. Pediatr Diabetes. 2010;11:450–454. doi: 10.1111/j.1399-5448.2009.00635.x. [DOI] [PubMed] [Google Scholar]
- 5.The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Tran DV, Lyon AW, Higgins TN, et al. Use of serial patient hemoglobin A1c differences to determine long-term imprecision of immunoassay and high-performance liquid chromatography analyzers. J Diabetes Sci Technol. 2009;3:424–428. doi: 10.1177/193229680900300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Research in Children Network (DirecNet) Study Group. Performance of the DCA2000 for measurement of HbA1c levels in children with T1DM in a DirecNet outpatient clinical trial. Pediatr Diabetes. 2005;6:13–16. doi: 10.1111/j.1399-543X.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy L, Herman WH. Glycated hemoglobin assessment in clinical practice: comparison of the A1cNow point-of-care device with central laboratory testing (GOAL A1C Study) Diabetes Technol Ther. 2005;7:907–912. doi: 10.1089/dia.2005.7.907. [DOI] [PubMed] [Google Scholar]
- 9.Fonfrede M, Grimaldi A. Evaluation of the DCA 2000 system for glycated haemoglobin measurement. Diabetes Metab. 1998;24:66–67. [PubMed] [Google Scholar]
- 10.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 11.The DCCT Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- 12.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial—revisited. Diabetes. 2008;57:995–1001. doi: 10.2337/db07-1618. [DOI] [PubMed] [Google Scholar]
- 13.Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosino JM, Fennie K, Whittemore R, et al. Short-term effects of coping skills training in school-age children with type 1 diabetes. Pediatr Diabetes. 2008;9:74–82. doi: 10.1111/j.1399-5448.2007.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wysocki T, Harris MA, Buckloh LM, et al. Randomized trial of behavioral family systems therapy for diabetes: maintenance of effects on diabetes outcomes in adolescents. Diabetes Care. 2007;30:555–560. doi: 10.2337/dc06-1613. [DOI] [PubMed] [Google Scholar]
- 16.Nansel TR, Anderson BJ, Laffel LM, et al. A multisite trial of a clinic-integrated intervention for promoting family management of pediatric type 1 diabetes: feasibility and design. Pediatr Diabetes. 2009;10:105–115. doi: 10.1111/j.1399-5448.2008.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodall I, Colman PG, Schneider HG, McLean M, Barker G. Desirable performance standards for HbA1c analysis - precision, accuracy and standardisation: consensus statement of the Australasian Association of Clinical Biochemists (AACB), the Australian Diabetes Society (ADS), the Royal College of Pathologists of Australasia (RCPA), Endocrine Society of Australia (ESA), and the Australian Diabetes Educators Association (ADEA) Clin Chem Lab Med. 2007;45:1083–1097. doi: 10.1515/CCLM.2007.158. [DOI] [PubMed] [Google Scholar]
- 18.Lenters-Westra E, Slingerland RJ. Hemoglobin A1c point-of-care assays; a new world with a lot of consequences! J Diabetes Sci Technol. 2009;3:418–423. doi: 10.1177/193229680900300303. [DOI] [PMC free article] [PubMed] [Google Scholar]


