Abstract
Background/Aims
The mitochondrial permeability transition (MPT) and inflammation play important roles in liver injury caused by ischemia-reperfusion (IR). This study investigated the roles of sphingosine kinase-2 (SK2) in mitochondrial dysfunction and inflammation after hepatic IR.
Methods
Mice were gavaged with vehicle or ABC294640 (50 mg/kg), a selective inhibitor of SK2, 1 h before surgery and subjected to 1 h-warm ischemia to ~70% of the liver followed by reperfusion.
Results
Following IR, hepatic SK2 mRNA and sphingosine-1-phosphate (S1P) levels increased ~25-fold and 3-fold, respectively. SK2 inhibition blunted S1P production and liver injury by 54% to 91%, and increased mouse survival from 28% to 100%. At 2 h after reperfusion, mitochondrial depolarization was observed in 74% of viable hepatocytes, and mitochondrial voids excluding calcein disappeared, indicating MPT onset in vivo. SK2 inhibition decreased mitochondrial depolarization and prevented MPT onset. Inducible nitric oxide synthase, phosphorylated NFκB-p65, TNFα mRNA, and neutrophil infiltration all increased markedly after hepatic IR, and these increases were blunted by SK2 inhibition. In cultured hepatocytes, anoxia/reoxygenation resulted in increases of SK2 mRNA, S1P levels and cell death. SK2 siRNA and ABC294640 each substantially decreased S1P production and cell death in cultured hepatocytes.
Conclusions
SK2 plays an important role in mitochondrial dysfunction, inflammation responses, hepatocyte death and survival after hepatic IR and represents a new target for the treatment of IR injury.
Keywords: inflammation, ischemia/reperfusion, liver, mitochondrial permeability transition, sphingosine kinase, sphingosine-1-phosphate
INTRODUCTION
A variety of proliferative factors and cytokines rapidly increase the activity of sphingosine kinases (SK1 and SK2) that phosphorylate sphingosine (1–4). Spingosine-1-phosphate (S1P), the product of this reaction, regulates several cell processes including cell proliferation and death (1–4). Additionally, activation of SK results in proinflammatory processes, including activation of inflammatory cells and increases of nuclear factor-kappa B (NF-κB), toxic cytokines, cyclooxygenase-2 (COX-2), nitric oxide synthase (NOS), adhesion molecules and reactive oxygen species (3, 5–8).
Altered sphingolipid metabolism occurs in hypoxic and ischemic injury. For example, plasma S1P levels increase during myocardial infarction (9). Renal and pulmonary injuries are lower in S1P3−/− mice compared to the wild-types following ischemia-reperfusion (IR), suggesting that S1P increases IR injury by binding to S1P3 receptors (10, 11). Conversely, adenoviral gene transfer of SK1 and treatment with S1P have been reported to protect the heart against IR injury (12, 13). Therefore, the roles of SKs in IR injury may be organ specific, perhaps relating to the subtypes of S1P receptors. The role of SK2 activation in hepatic IR injury has not been previously studied.
A variety of pathophysiological processes, such as free radical and toxic cytokine formation, onset of the mitochondrial permeability transition (MPT), and inflammation contribute to IR injury to the liver (14–17). Because S1P formation plays an important role in toxic cytokine production and inflammation, we tested the affects of a specific SK2 inhibitor, ABC294640, on mitochondrial function and inflammatory processes after liver IR in vivo.
MATERIALS AND METHODS
Animals
Male C57BL/6 (8–9 weeks) mice were gavaged with 50 mg/kg of 3-(4-chlorophenyl)adamantine-1-carboxylic acid (pyridine-4-ylmethyl)amide (ABC294640, Apogee Biotechnology Corporation, Hummelstown, PA; see structure in Supplements), a specific SK2 inhibitor (18), or an equivalent volume of vehicle (0.375% Tween 80 in phosphate buffered saline, pH 7.1) 1 h before surgery. Under ether anesthesia, ischemia to 70% of the total liver was induced for 1 h as previously described (19). After opening the vascular clamp, the non-ischemic liver lobes were removed, and mice were observed 7 days for survival. Sham operation included equivalent anesthesia and laparotomy without ischemia. All animals were given humane care using protocols approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina.
Serum Transaminase, Bilirubin and Histology
Liver and blood samples were collected at 2 and 6 h after surgery. Serum alanine aminotransferase (ALT) and bilirubin were determined using analytical kits from Pointe Scientific (Lincoln Park, MI). Necrotic areas were quantified by image analysis of 10 randomly selected fields per liver in slides stained with hematoxylin-eosin in a blinded manner using a IPlab 3.7v software (BD Biosciences, Rockville, MD) (19). Apoptosis was assessed by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) (20). TUNEL-positive and negative cells were counted in a blinded manner in 10 randomly selected fields using a 40x objective.
Detection of MPT Onset in Isolated Mitochondria
Mitochondria were isolated from the liver of male Sprague Dawley rats (225–250 g) in 250 mM sucrose, 2 mM K+-HEPES buffer, pH 7.4, as previously described (21). Mitochondrial swelling was assessed from the decrease of absorbance at 540 nm using a NovoStar plate reader (BMG Labtech GmbH, Offenburg, Germany) (22). Mitochondrial suspensions were treated with 0 to 500 μM sphingosine-1-phosphate, 0 to 100 μM sphingosine in the presence or absence of 2 μM CsA. In some experiments, after mitochondrial suspensions were treated with various compounds aliquots of 50 μM CaCl2 were added every 5 minutes to induce the MPT.
Hepatocyte Culture, Anoxia/Reoxygenation, and siRNA Treatment
Hepatocytes were isolated from male C57BL/6 mice by collagenase digestion and purified as described (23). Viability was >90% as indicated by trypan blue exclusion. Hepatocytes were cultured in Waymouth’s MB-752/1 medium containing 27 mM NaHCO3, 2 mM L-glutathione, 10% fetal calf serum, 100 nM insulin, and 100 nM dexamethasone for 4 h at 37°C under normoxic conditions (5% CO2). To knockdown SK2, hepatocytes were transfected with specific SK2 siRNA (10 nM) or control, nonsilencing RNA (Applied Biosystems Inc., Foster City, CA) in the presence of Lipofectamine 2000 transfection reagent (6 μl/ml; Invitrogen Life Technologies, San Diego, CA). SK2 siRNA duplex targeted nucleotides 208–227 of the SK2 mRNA sequence (NM 203280) composed of sense, 5′-AAGACUGGGUGACAAUAGATT-3′ and antisense, 5′-UCU AUUGUCACCCAGUCUUGG-3′. The cells were incubated with oligonucleotide duplexes in serum free Waymouth’s MB-752/1 medium for 18 h at 37°C. Some cells without siRNA transfection were treated with ABC294640 (30 μM) just before incubation in KRH buffer at pH 6.2 in an anaerobic chamber (Coy Laboratory Products, Ann Arbor, MI) for 4 h followed by reoxygenation in normoxic KRH buffer at pH 7.4 for 2 h to simulate IR (23). Cell viability was assessed by propidium iodide (PI) fluorescence (23). Some hepatocytes were harvested after simulated IR to quantify S1P and SK2 mRNA levels.
Intravital Multiphoton Microscopy
At 2 h after IR or sham operation, hepatic mitochondrial polarization and cell death were monitored by intravital multiphoton microscopy using Rh123 (Sigma, St. Louis, MO) and PI, respectively (19). MPT onset was assessed by visualization of calcein entry into mitochondria by intravital multiphoton microscopy (19).
Assay of Sphingosine-1-Phosphate
Liver tissue was homogenized or hepatocytes were sonicated in ice-cold 50 mM Tris buffer (pH 7.4) containing 0.25 M sucrose, 25 mM KCl, 0.5 mM EDTA, and 1% phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Homogenates/lysates were centrifuged at 2,500×g for 10 minutes, and S1P in supernatants was determined using an enzyme-linked immunosorbent assay kit (Echelon Inc., Salt Lake City, UT). Protein was quantified using a Protein Assay Kit from Bio-Rad Laboratory (Hercules, CA).
Western Blotting
Livers were harvested at 2 or 6 h after IR or sham operation, and Western blotting was performed (19) using specific antibodies against cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, 1:1000), NF-κB p65, phosphorylated NF-κB p65 (Ser 536) (Santa Cruz Biotechnology, Santa Cruz, CA;1:500) or actin (Cell Signaling Technology; 1:3000), as described (19).
Assay of TNFα and SK2 mRNA by Quantitative Real-Time PCR
Livers were harvested at 2 h and 6 h after IR. TNFα mRNA was detected by quantitative real-time PCR, as described (20). SK2 mRNA in liver and hepatocytes exposed to IR was determined using a forward primer of 5′-TTCCACCCGACATCCCTTTCAGTT-3′ and a reverse primer of 5′-ACAAAGCAGCTACTGGCTCTGACT-3′. The abundance of mRNAs was normalized against hypoxanthine phospho-ribosyl-transferase (HPRT) using the ΔΔCt method.
Statistical Analysis
Groups were compared using ANOVA plus Student-Newman-Keul’s post-hoc test or Kaplan-Meier test using p<0.05 as the criterion of significance. Values are mean ± SEM. Group numbers are given in the figure legends.
RESULTS
ABC294640 and SK2 Knockdown Prevent Anoxia/Reoxygenation-Induced Death of Cultured Hepatocytes
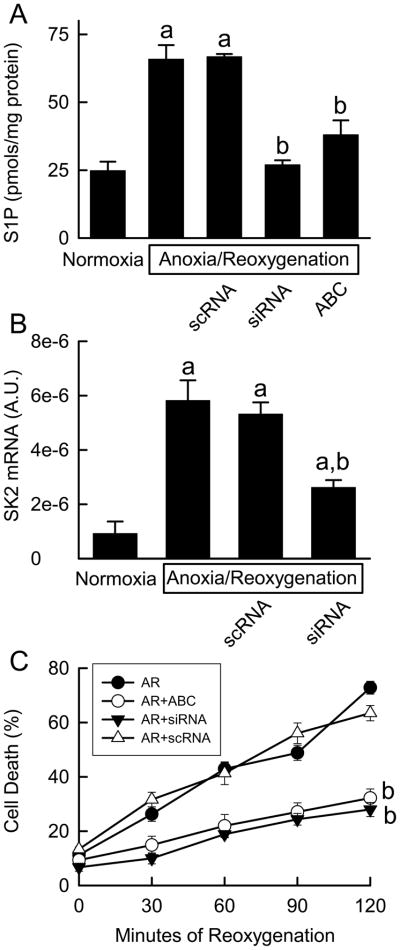
To investigate if SK2 is involved in IR injury, we assessed the effects of ABC294640, a specific SK2 inhibitor that does not affect SK1 and other kinases (18), on S1P production and cell death in cultured hepatocytes. After anoxia/reoxygenation, S1P levels increased substantially and cell death increased from the basal level of ~10% to 73% at 120 min (Fig. 1A and C). ABC294640 markedly decreased S1P formation and cell death (Fig. 1A and C). Heat treatment of cultured hepatocytes at 100°C for 10 min did not increase S1P, indicating that this increase of S1P was not merely a result of cell death.
Fig. 1. ABC294640 and SK2 siRNA prevent anoxia/reoxygenation-induced cell death of cultured hepatocytes.
Cultured hepatocytes were treated with ABC294640 and SK2 siRNA as indicated in “METHODS.” At 120 min after reoxygenation, S1P in hepatocytes was quantified by ELISA (A). SK2 mRNA was measured by real-time PCR (B). Cell death was detected by propidium iodide fluorescence at the indicated times (C). A.U., arbitrary unit; scRNA, scrambled RNA; siRNA, small interfering RNA. a, p<0.05 vs normoxia; b, p<0.05 vs anoxia/reoxygenation (n = 4 per group).
At 120 min after anoxia/reoxygenation, levels of SK2 mRNA were increased approximately 5-fold in hepatocytes (Fig. 1B). The increases in SK2 mRNA and S1P formation were not affected by treatment of the hepatocytes with scrambled RNA, but both responses were suppressed by SK2 siRNA (Fig. 1B). SK2 siRNA decreased cell death to 30% (Fig. 1C). Together, the pharmacologic and genetic data support the hypothesis that excessive SK2 and the S1P it produces play an important role in promoting hepatocyte death following anoxia/reoxygenation.
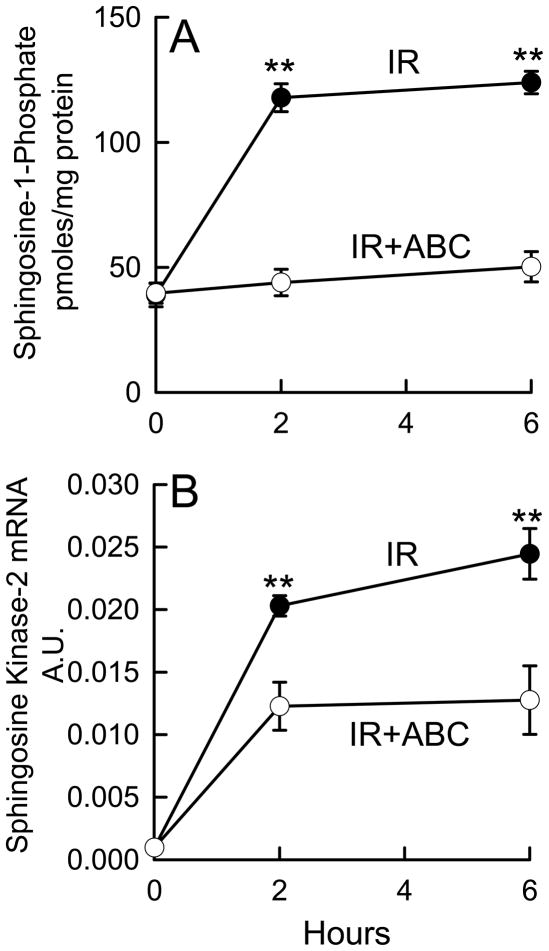
Warm IR Increases S1P Production in the Liver
Because IR increased SK2 expression in cultured cells, we investigated the affects of ABC 294640 on the production of S1P after hepatic IR in mice. As indicated in Figure 2A, S1P increased from ~40 pmol/mg protein before IR to 118 and 124 pmol/mg protein at 2 h and 6 h after reperfusion, respectively. ABC294640-treatment largely prevented the increase of S1P after IR in vivo. We also investigated if IR affects hepatic SK2 mRNA expression in vivo. At 2 and 6 h after hepatic IR, SK2 mRNA levels were increased 20~25-fold (Fig. 2B), suggesting increased SK2 expression, consistent with the above results with isolated hepatocytes and reports by others that SK expression is induced in hypoxic cells in culture (24). Although an enzyme inhibitor does not necessarily inhibit the expression of its target, ABC294640 partially blunted the upregulation of SK2 mRNA after hepatic warm IR (Fig. 2B). Thus, IR also activates SK2 in vivo leading to increased S1P production that can be blocked by ABC294640.
Fig. 2. ABC294640 prevents upregulation of SK2 and S1P production after hepatic IR.
Livers were harvested at 2 and 6 h after reperfusion. Sphingosine-1-phosphate (S1P) in liver tissues was quantified by ELISA (A). SK2 mRNA was measured by real time-PCR (B). IR, ischemia/reperfusion; ABC, ABC294640; A.U., arbitrary units. **, p<0.01 vs the ABC-treated group at the corresponding time point (n = 4 per group).
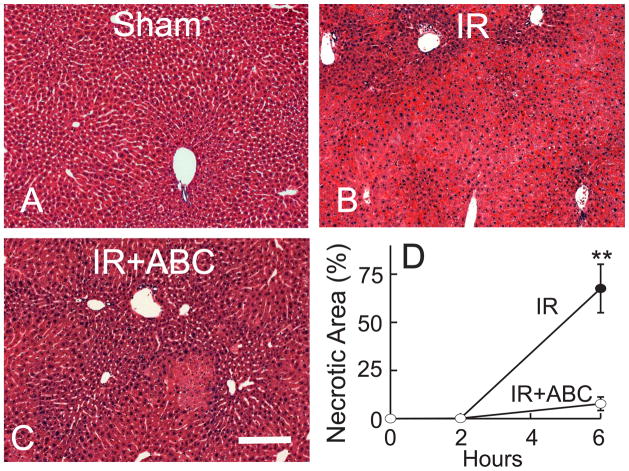
ABC294640 Prevents Hepatic Warm IR-Induced Cell Death
Because SK2 and S1P increased after hepatic IR, we assessed the effects of ABC294640 on several indicators of liver IR injury. After sham operation, no hepatic histopathological changes were observed (Fig. 3A). At 2 h after IR, necrosis was barely observable (Fig. 3D), but at 6 h after IR, extensive panlobular necrosis (68% of the liver tissue) was present (Fig. 3B and D). Pretreatment of the mice with ABC294640 attenuated IR-induced liver necrosis to only ~7% (Fig. 3C and D).
Fig. 3. ABC294640 attenuates necrosis after hepatic IR.
Livers were harvested at 2 and 6 h after reperfusion (IR), and liver slices were stained with H+E. Representative images at 6 h are shown (A to C). The bar is 100 μm. Necrotic areas were quantified by image analysis of 10 randomly selected fields (D). **, p<0.01 vs the ABC-treated group at the corresponding time point (n = 4 per group).
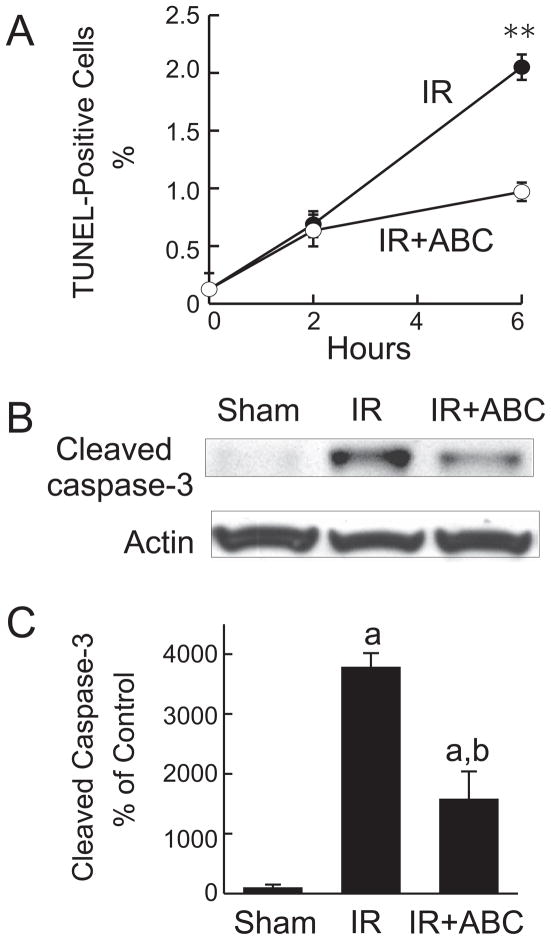
As another marker of liver injury, apoptosis was evaluated by TUNEL (Fig. 4A). TUNEL-positive hepatocytes increased from a basal level of 0.13% to 0.6% and 2% at 2 and 6 h after IR, respectively (Fig. 4A), and this increase in apoptosis was attenuated by ABC294640 (Fig. 4A). Cleaved caspase-3 was barely detectable after sham-operation but increased ~38-fold at 6 h after reperfusion, confirming the occurrence of apoptosis (Fig. 4B and C). ABC294640-treatment blunted the activation of caspase-3 by ~60% (Fig. 4B and C). Together, these data demonstrate that warm IR causes massive cell death in the liver, with necrosis being the predominant form of cell death over apoptosis. Pretreatment with ABC294640 effectively prevented hepatic cell death, suggesting that SK2 and S1P play important roles in IR-induced liver injury.
Fig. 4. ABC294640 decreases apoptosis after hepatic IR.
Livers were harvested at 2 and 6 h after reperfusion (IR). TUNEL-positive cells were counted in 10 randomly selected fields (A). **, p<0.01 vs the ABC-treated group at the corresponding time point. Representative Western blot images of cleaved caspase-3 are shown in B, and quantified by densitometry (C). a, p<0.05 vs sham-operation; b, p<0.05 vs IR (n = 4 per group).
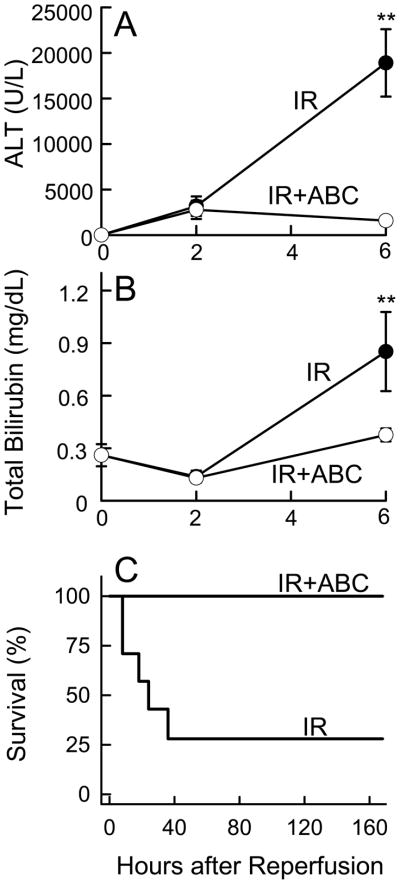
ABC294640 Improves Liver Function and Survival after Hepatic Warm IR
Prior to ischemia, serum ALT levels were 22 U/L, and increased to ~3200 U/L at 2 h and ~19,000 U/L at 6 h after reperfusion, indicating severe liver injury (Fig. 5A). Treatment of the mice with ABC294640 suppressed the peak ALT levels to ~1,600 U/L, (Fig. 5A).
Fig. 5. ABC294640 protects liver function and improves survival after hepatic IR.
Blood samples were collected at 2 and 6 h after reperfusion for ALT (A) and bilirubin measurement (B). **, p<0.01 vs the ABC-treated group at the corresponding time point (n = 4 per group). Survival probabilities (C) after IR were significantly different between vehicle- (n = 7) and ABC294640-treated mice (n = 9) by the Kaplan-Meier test.
Serum bilirubin was not significantly altered at 2 h after reperfusion, but increased 3-fold at 6 h after reperfusion, confirming poor liver function (Fig. 5B). Consistent with the ALT data, ABC294640-treatment almost completely prevented hyperbilirubinemia after IR (Fig. 5B).
Most importantly, the protection of liver function by ABC294640-treatment resulted in complete protection against death from IR injury. Specifically, all mice survived after sham operation (data not shown); however, only 28% of the vehicle-treated mice survived after IR, with death occurring mainly in the first 36 h (Fig. 5C). In marked contrast, the survival of ABC294640-pretreated mice was 100% after IR (Fig. 5C), indicating that ABC294640 prevented IR-induced acute liver failure.
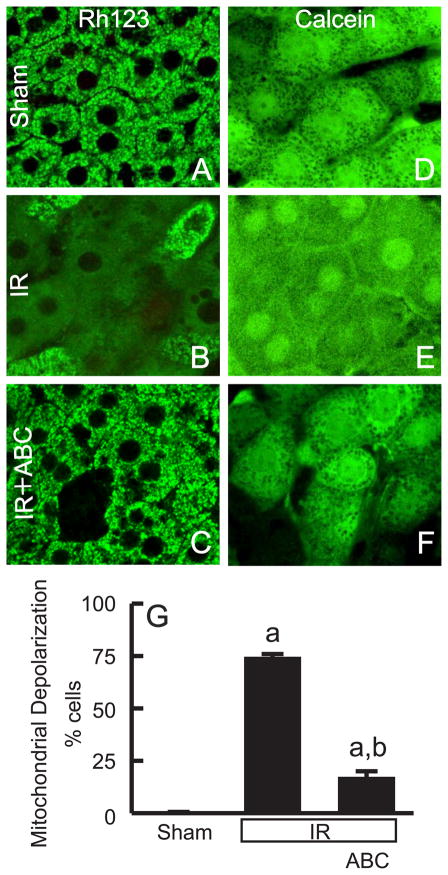
ABC294640 Prevents Mitochondrial Depolarization after Hepatic IR in vivo
The MPT plays an important role in hepatic IR injury (19). To determine if ABC294640-treatment prevents loss of mitochondrial function after IR, we used intravital multiphoton microscopy to visualize polarized hepatic mitochondria in living mice. In sham-operated mice, green Rh123 fluorescence was punctate in virtually all hepatocytes, indicating proper mitochondrial polarization (Fig. 6A). Red PI staining in nuclei, indicative of cell death, was negligible. In contrast, at 2 h after IR, mitochondria in many hepatocytes (74%) did not take up Rh123, indicating the occurrence of mitochondrial depolarization (Fig. 6B and G). Despite the absence of mitochondrial polarization, the majority of hepatocytes maintained plasma membrane integrity at 2 h after IR, as shown by low (only ~1% of cells) nuclear PI staining, indicating that mitochondrial depolarization had occurred in most hepatocytes, which preceded cell death. Importantly, mitochondrial depolarization occurred in only 17% of hepatocytes in ABC294640-treated mice following IR (Fig. 6C and G). This is likely to be the basis for the protection of hepatic function by ABC294640.
Fig. 6. ABC294640 prevents mitochondrial depolarization and onset of the MPT after hepatic IR.
At 2 h after sham-operation or reperfusion, Rh123 plus PI or calcein-AM alone was infused, and intravital multiphoton microscopy was performed. Representative images of Rh123 are shown in A to C, and images of calcein in D to F. Rows are: upper, sham operation (sham); middle, ischemia-reperfusion (IR); lower, IR plus ABC294640-treatment. Bars are 5 μm. Viable cells with depolarized mitochondria were counted in 10 random fields for each liver (G). a, p<0.05 vs sham-operation; b, p<0.05 vs IR (n = 5 in the IR group and n = 4 in the sham and IR+ABC group, respectively). The majority of the cells in E show the MPT except the cell in the left upper corner (identified by the arrow), where incomplete MPT with some dark voids remaining is seen.
To investigate if mitochondrial depolarization is caused by MPT onset, intravital multiphoton imaging of calcein was performed in livers following IR. Calcein, a fluorophore that loads into the cytosol, gains entrance to the mitochondrial matrix space only when PT pores open. Calcein outlined mitochondria as dark voids in hepatocytes of livers of sham-operated mice (Fig. 6D), and these dark voids disappeared after 2 h of reperfusion (Fig. 6E). Therefore, disappearance of these voids directly showed that MPT onset had occurred in vivo. Treatment of the mice with ABC294640 prevented the disappearance of these voids (Fig. 6F), indicating protection against the MPT.
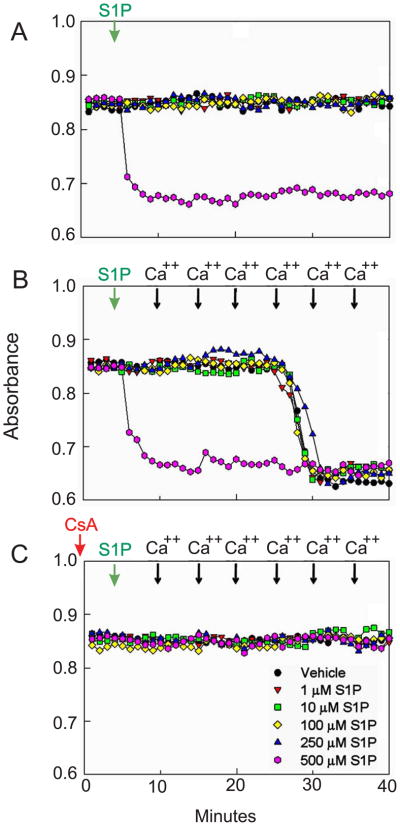
Effects of Sphingosine-1-Phosphate and Sphingosine on the Mitochondrial Permeability Transition in Isolated Liver Mitochondria
The potential for S1P and sphingosine to induce the MPT directly was investigated in isolated rat liver mitochondria. Addition of 10–100 μM S1P (Fig. 7A) or sphingosine (data not shown) to mitochondria did not induce MPT-dependent swelling. High concentration of S1P (500 μM) caused mitochondrial swelling as shown by a large decrease in absorbance at 540 nm (Fig. 7A) that was blocked by CsA, an MPT inhibitor (Fig. 7C). Sphingosine exceeding 100 μM was not soluble in the reaction buffer. S1P and sphingosine had no effect on calcium-induced mitochondrial swelling (Fig. 7B).
Fig. 7. Effects of Sphingosine-1-Phosphate on the Mitochondrial Permeability Transition in Isolated Rat Liver Mitochondria.
Mitochondrial swelling was assessed as a decrease in absorbance at 540 nm. A: Mitochondrial suspensions were treated with 0 to 500 μM S1P in the absence of cyclosporine A (CsA). B: After mitochondrial suspensions were treated with 0 to 500 μM S1P, aliquots of 50 μM CaCl2 were added every 5 minutes to induce the MPT. C: Mitochondrial suspensions were treated with 0 to 500 μM S1P in the presence of 2 μM CsA. Green arrows, addition of the vehicle or S1P; black arrows: addition of CaCl2; red arrow, addition of CsA. For all experiments, the response to 500 μM S1P is indicated by the pink circles. Shown are representative OD values of 4 independent experiments.
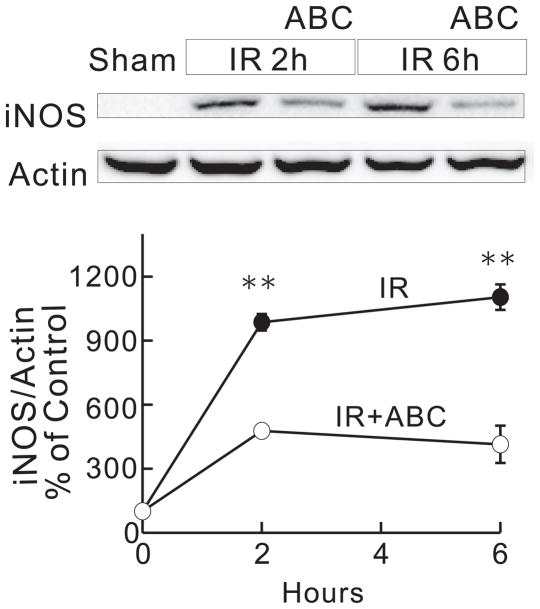
ABC294640 Prevents Hepatic IR-Induced Upregulation of iNOS
Since S1P causes swelling of isolated mitochondrial only at high, unphysiological concentrations, we investigated whether inhibition of SK2 blocked the MPT in vivo by indirect effects. Because IR upregulates iNOS and reactive nitrogen species promote the onset of the MPT, we assessed the effects of ABC294640 on iNOS expression following IR. As indicated in Figure 8, iNOS was barely detectable before IR, but increased markedly at 2 h and 6 h after reperfusion. Treatment of the mice with ABC294640 blunted this elevation of iNOS expression by ~50 to 60%.
Fig. 8. ABC294640 blunts upregulation of iNOS after hepatic IR.
Livers were harvested at 2 and 6 h after ischemia/reperfusion (IR). Levels of iNOS and actin were determined by Western blotting. Blot images were shown in A. Images of iNOS were quantified by densitometry (B). **, p<0.01 vs the ABC-treated group at the corresponding time point (n = 4 per group).
ABC294640 Prevents Hepatic Warm IR-Induced Inflammatory Processes
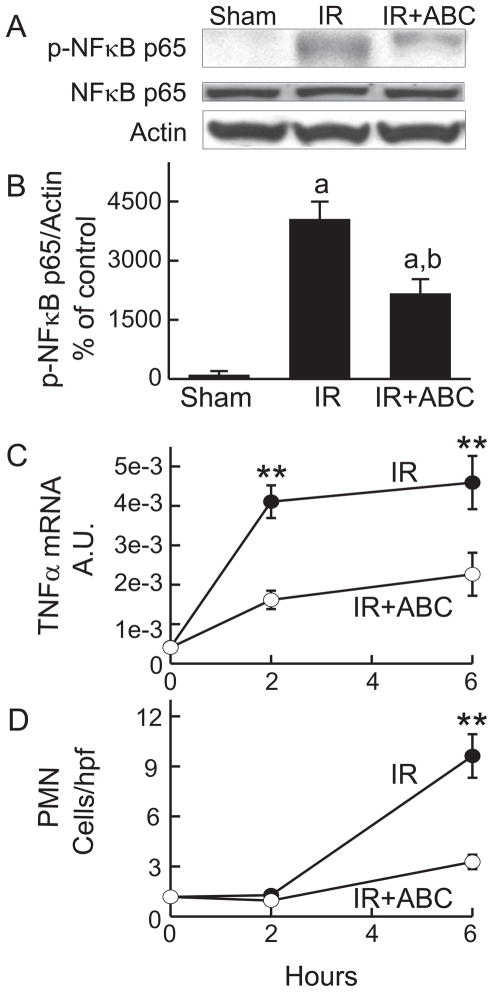
Because inflammation plays important roles in IR injury (8), we examined the effects of ABC294640 on inflammatory processes after hepatic IR. NF-κB activation prevents or promotes cell death in various settings (25), and is involved in inflammatory cytokine formation and the upregulation of intracellular adhesion molecules (25). At 2 h after IR, the phosphorylated p65 subunit of NF-κB increased ~40-fold, whereas total p65 subunit remained unchanged, indicating NF-κB activation (Fig. 9A and B). ABC294640-treatement blunted this response by half.
Fig. 9. ABC294640 blunts NF-κ B activation, TNFα mRNA increase and polymorphonuclear neutrophil infiltration after hepatic IR.
Livers were harvested at 2 and 6 h after ischemia/reperfusion (IR). Western blot images of NF-κB p65 and phosphorylated NF-κB p65 at 2 h are shown in A and quantified by densitometry (B). TNFα mRNA in liver tissue was measured by quantitative real-time PCR (C). A.U., arbitrary units. MPO-positive cells detected by immunohistochemistry were counted in a blinded manner in 10 randomly selected fields using a 40x objective lens (D). **, p<0.01 vs the ABC-treated group at the corresponding time point (n = 4 per group).
Toxic cytokine, e.g. TNFα, formation stimulates inflammatory processes and promotes cell death. TNFα mRNA increased ~10-fold at 2 h after reperfusion and remained elevated at 6 h (Fig. 9C). ABC294640-treatment inhibited this increase of TNFα mRNA by ~50 to 60%.
At 6 h after IR, MPO-positive cells in the liver increased by 9-fold, indicating infiltration of polymorphonuclear neutrophils (PMNs) (Fig. 9D). Infiltration of PMNs was not apparent after 2 h. ABC294640 blunted PMN infiltration after 6 h by ~70% (Fig. 9D). Thus, inhibition of SK2 reduced a number of hepatic inflammatory processes caused by IR.
DISCUSSION
SK2 Plays an Important Role in Hepatic IR Injury
Products of sphingolipid metabolism are important second messengers that regulate a variety of cell processes including cell death, proliferation, motility, differentiation, oncogenesis and inflammation (3, 4, 26). S1P is produced by 2 mammalian subtypes of SK (SK1 and SK2) in vivo. A variety of proliferative factors and cytokines rapidly elevate cellular SK activity (2–4), and the resulting S1P acts as a second messenger (3, 4, 26). Extracellular S1P causes diverse biological and pathophysiological effects by acting largely through the five members of the S1P receptor family (S1P1–5) which are coupled to distinct G-protein-mediated signaling pathways in different tissues (26, 27). The actions of extracellular S1P likely depend upon the receptor subtype-specific repertoire of G protein coupling, as well as the tissue- and cell type-specific expression patterns of the S1P receptors themselves (26, 27).
The role of SKs in IR injury is controversial. SK expression has been shown to increase in cultured cells exposed to hypoxia (24, 28, 29). Conflictingly, plasma S1P levels increase during myocardial infarction (9) and N,N,N-trimethylsphingosine, a SK inhibitor, protects against myocardial IR injury (30), while S1P and adenoviral gene transfer of SK1 protect myocardium against IR injury (12, 13). S1P3 deficiency attenuates renal and pulmonary IR injury (10, 11), and FTY720, which rapidly internalizes S1P receptors thereby attenuating S1P signaling, decreases liver injury after liver transplantation (31). Therefore, the roles of SKs may be organ specific, perhaps relating to different subtypes of SKs or S1P receptors; however, the preponderance of the data suggests that reduction of S1P production and/or signaling is protective against IR injury.
In this study, we investigated the roles of SK2 in hepatic IR injury using a novel, highly specific SK2 inhibitor, ABC294640 (18). In spite of the high interest in sphingolipid-related signaling, few inhibitors of SK have emerged. Dimethylsphingosine, D,L-threo-dihydrosphingosine and N,N,N-trimethylsphingosine are often used to inhibit SK in cultured cells; however, they also affect a variety of other enzymes (32, 33). The selectivity of several natural inhibitors of SK that have been isolated remains unknown (34). In recent years, a series of structurally novel inhibitors of SK have been identified (35, 36). These inhibitors are selective for SK and do not interfere with the ATP binding site on the enzyme. Consequently, their biological effects are probably not mediated by off-target inhibition of other lipid and protein kinases (35, 36). These novel SK inhibitors have activity in cells and inhibit ulcerative colitis, arthritis, and tumor growth in animal models in the absence of systemic toxicity (5, 8, 36).
In this study, we tested the affects of ABC294640 on hepatic warm IR injury. Our studies showed that SK2 is upregulated and the production of S1P is increased markedly after IR. ABC294640 inhibited this overproduction of S1P in hepatocytes in culture and liver in vivo (Fig. 1 and 2). Interestingly, ABC294640 also partially suppressed the upregulation of SK2 mRNA following IR (Fig. 1 and 2). Elevation of SK2 mRNA in hepatic tissue was suppressed by ABC294640 within 2 hours of reperfusion, which was before onset of either necrotic or apoptotic cell death. SK2 mRNA and S1P levels did not increase more after 6 h in either the presence or absence of ABC294640, when both apoptotic and necrotic cell death became strongly evident. These results suggest that the changes in SK2 mRNA were unlikely secondary to cell death/injury. Indeed, it would be paradoxical for SK2 mRNA to actually increase as cells died, since cell death would inhibit mRNA synthesis and activate RNases both in vivo and in vitro. Inhibition of SK2 in vivo and in cultured hepatocytes substantially attenuated IR injury as indicated by decreased cell death (Figs. 1, 3 and 4), improved liver function (Fig. 5A and B) and prevention of acute liver failure after hepatic warm IR (Fig. 5C). Knockdown of SK2 by siRNA also effectively prevented hepatocyte death (Fig. 1). These results support the hypothesis that activation and upregulation of SK2 play important roles in hepatic IR pathology. It remains unclear whether intracellular or extracellular S1P is more important in injury. Moreover, inhibition of SK2 likely alters other sphingolipids, such as ceramide, sphingosine, ceramide-1-phosphate in addition to S1P, and many of these molecules have physiological/pathological activities. Ceramide, which increases after SK inhibition, is reported to increase apoptotic tumor cell death, but we observed instead a decrease of hepatocyte death both in vitro and in vivo by ABC294640-treatment. Thus, it seems unlikely that ceramide directly induces the killing of normal hepatocytes. An examination of each of the several SK-linked sphingolipid metabolites during IR-induced cell death is beyond the scope of the present study and will be the subject of future experiments.
In apparent contrast to the present findings, a recent study shows that repeated injection of S1P decreases hepatic and renal injury after hepatic IR, possibly through activation of S1P1 receptors (37). Theoretically, supplementation with S1P should have the opposite effect of SK inhibition. However, after injection of S1P or dihydro-S1P (i.e. sphinganine 1-phosphate), serum phosphatases will degrade the lipid to sphingosine and dihydrosphingosine, which is a known SK inhibitor (38). Additionally, FTY720, an S1P receptor superagonist that internalizes and down-regulates S1P receptors, protects the liver from IR injury (31). Therefore, injection of S1P after IR could alter the expression and intracellular localization of S1P receptors. Furthermore, SK inhibition in addition to decreasing S1P may alter other bioactive sphingolipid species that modulate cytotoxicity. Future studies will be needed to investigate these possibilities.
Inhibition of SK2 Prevents MPT Onset after IR in vivo
Our studies demonstrating a role in regulating the MPT shed considerable light on the mechanism by which SK2 activation leads to IR injury. The MPT leads to the collapse of the mitochondrial membrane potential, failure of oxidative phosphorylation, and onset of necrotic cell death (39). In addition, the MPT causes release of cytochrome c from mitochondria, triggering apoptosis (40, 41). ROS and RNS formation, calcium mobilization, TNFα formation, Bax translocation, and JNK activation also promote MPT onset (40, 42–45). S1P causes mitochondrial swelling directly only at very high concentrations which unlikely occur in vivo (Fig. 7). Therefore, activation of SK most likely leads to the MPT by indirect mechanisms. For example, S1P causes upregulation of TNFα formation, Ca2+ influx into cells and stimulates JNK activation in cultured cells (7, 46, 47), and these S1P-dependent changes are capable of inducing MPT onset. Using intravital multiphoton microscopy, we observed that mitochondrial depolarization occurred in many viable hepatocytes after IR in vivo (Fig. 6). The onset of MPT in response to IR was blocked by ABC294640, in association with suppression of the increased TNFα synthesis and upregulation of iNOS that follow this insult (Figs. 8 and 9). Therefore, ABC294640 may be inhibiting the MPT by preventing increases in TNFα and iNOS expression and/or action. Importantly, prevention of the MPT onset decreased cell death and prevented acute liver failure in mice following IR (Fig. 3–5), consistent with cytoprotection by specific MPT inhibitors against storage/reperfusion injury reported previously (22). Therefore, inhibition of the SK2 signaling cascade minimizes hepatic IR injury, at least in part, by preventing mitochondrial dysfunction.
Inhibition of SK2 Prevents Inflammation after IR in vivo
Activation of SK results in pro-inflammatory responses (3, 5–8, 48). For example, S1P induces NF-κB, which in turn increases toxic cytokines, proinflammatory enzymes such as cyclooxygenase-2 (COX-2), and adhesion molecules (3, 6, 48). S1P causes granulocyte activation and decreases their apoptosis, thus enhancing chronic inflammation (7). The non-specific SK inhibitor dimethylsphingosine blocks inflammatory cytokine-induced adhesion molecule expression in vitro (3). Inhibition of SK attenuates ulcerative colitis and arthritis, diseases with a large inflammatory component (5, 8).
In vivo, inflammatory responses due to the initial IR insult act to extend and magnify the extent of IR injury. Formation of proinflammatory cytokines is a crucial step in inflammatory responses, which stimulates subsequent expression of adhesion molecules and infiltration and activation of white blood cells. In this study, activation of NF-κB and production of TNFα increased dramatically after hepatic IR well before onset of overt necrotic cell death (Fig. 9). Infiltration of PMNs into the liver followed the increase of TNFα, and treatment with ABC294640 blunted these inflammatory reactions. Therefore, inhibition of inflammation by ABC294640 is unlikely due to an antinecrotic effect, but more likely due to suppression of proinflammatory cytokine formation. Moreover, previous studies show that SK is activated in inflammatory cells such as monocytes and RAW macrophages (49, 50). It is also well established that Kupffer cells (hepatic macrophages) become activated very soon after IR to produce toxic cytokines and promote inflammation (51). Therefore, inhibition of inflammatory cells may also help to protect against IR injury. In vivo, multiple mechanisms/pathways often act in parallel or in sequence.
In conclusion, inhibition of SK2 by ABC294640 protected against IR-induced MPT onset and inflammation, major events precipitating and extending hepatic cell death after IR. Thus, it is likely that SK2 positively modulates MPT onset and inflammation to promote hepatic IR injury, which selective SK2 inhibition prevents. Accordingly, selective SK2 inhibition by ABC294640 represents a promising new strategy to attenuate hepatic IR injury in clinical settings.
Supplementary Material
Acknowledgments
This study was supported, in part, by Grants DK84632, DK70844, and DK37034 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007 May;12(5):923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 2.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006 Dec;1758(12):2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, et al. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993 Oct 7;365(6446):557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 5.Lai WQ, Irwan AW, Goh HH, Howe HS, Yu DT, Valle-Onate R, et al. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol. 2008 Dec 1;181(11):8010–8017. doi: 10.4049/jimmunol.181.11.8010. [DOI] [PubMed] [Google Scholar]
- 6.Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003 Aug;17(11):1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 7.MacKinnon AC, Buckley A, Chilvers ER, Rossi AG, Haslett C, Sethi T. Sphingosine kinase: a point of convergence in the action of diverse neutrophil priming agents. J Immunol. 2002 Dec 1;169(11):6394–6400. doi: 10.4049/jimmunol.169.11.6394. [DOI] [PubMed] [Google Scholar]
- 8.Maines LW, Fitzpatrick LR, French KJ, Zhuang Y, Xia Z, Keller SN, et al. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci. 2008 Apr;53(4):997–1012. doi: 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, et al. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003 Jul;146(1):62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 10.Jo SK, Bajwa A, Ye H, Vergis AL, Awad AS, Kharel Y, et al. Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int. 2008 Oct 29; doi: 10.1038/ki.2008.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, et al. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci U S A. 2005 Jun 28;102(26):9270–9275. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Duan HF, Wang H, Yi J, Liu HJ, Zhang QW, Li LB, et al. Adenoviral gene transfer of sphingosine kinase 1 protects heart against ischemia/reperfusion-induced injury and attenuates its postischemic failure. Hum Gene Ther. 2007 Nov;18(11):1119–1128. doi: 10.1089/hum.2007.036. [DOI] [PubMed] [Google Scholar]
- 13.Vessey DA, Kelley M, Li L, Huang Y, Zhou HZ, Zhu BQ, et al. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit. 2006 Oct;12(10):BR318–BR324. [PubMed] [Google Scholar]
- 14.Husted TL, Lentsch AB. The role of cytokines in pharmacological modulation of hepatic ischemia/reperfusion injury. Curr Pharm Des. 2006;12(23):2867–2873. doi: 10.2174/138161206777947597. [DOI] [PubMed] [Google Scholar]
- 15.Jaeschke H. Role of reactive oxygen species in hepatic ischemia-reperfusion injury and preconditioning. J Invest Surg. 2003;16:127–140. [PubMed] [Google Scholar]
- 16.Zhong Z, Froh M, Connor HD, Li X, Conzelmann LO, Mason RP, et al. Prevention of hepatic ischemia-reperfusion injury by green tea extract. Am J Physiol Gastrointest Liver Physiol. 2002 Oct;283(4):G957–G964. doi: 10.1152/ajpgi.00216.2001. [DOI] [PubMed] [Google Scholar]
- 17.Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, et al. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008 Jan;47(1):236–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010 Apr;333(1):129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong Z, Ramshesh VK, Rehman H, Currin RT, Sridharan V, Theruvath TP, et al. Activation of the oxygen-sensing signal cascade prevents mitochondrial injury after mouse liver ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2008 Oct;295(4):G823–G832. doi: 10.1152/ajpgi.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehman H, Ramshesh VK, Theruvath TP, Kim I, Currin RT, Giri S, et al. NIM811, a Mitochondrial Permeability Transition Inhibitor, Attenuates Cholestatic Liver Injury But Not Fibrosis in Mice. J Pharmacol Exp Ther. 2008 Sep 18;327:699–706. doi: 10.1124/jpet.108.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemasters JJ. The ATP-to-oxygen stoichiometries of oxidative phosphorylation by rat liver mitochondria. An analysis of ADP-induced oxygen jumps by linear nonequilibrium thermodynamics. J Biol Chem. 1984 Nov 10;259(21):13123–13130. [PubMed] [Google Scholar]
- 22.Theruvath TP, Zhong Z, Currin RT, Pediaditakis P, Lemasters JJ. Minocycline mitigates storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47:235–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol. 1997 Dec;273(6 Pt 1):C1783–C1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad M, Long JS, Pyne NJ, Pyne S. The effect of hypoxia on lipid phosphate receptor and sphingosine kinase expression and mitogen-activated protein kinase signaling in human pulmonary smooth muscle cells. Prostaglandins Other Lipid Mediat. 2006 May;79(3–4):278–286. doi: 10.1016/j.prostaglandins.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Schwabe RF, Brenner DA. Nuclear factor-kappaB in the liver: friend or foe? Gastroenterology. 2007 Jun;132(7):2601–2604. doi: 10.1053/j.gastro.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 26.Means CK, Brown JH. Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc Res. 2009 May 1;82(2):193–200. doi: 10.1093/cvr/cvp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biochim Biophys Acta. 2008 Sep;1781(9):483–488. doi: 10.1016/j.bbalip.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Anelli V, Gault CR, Cheng AB, Obeid LM. Sphingosine kinase 1 is up-regulated during hypoxia in U87MG glioma cells. Role of hypoxia-inducible factors 1 and 2. J Biol Chem. 2008 Feb 8;283(6):3365–3375. doi: 10.1074/jbc.M708241200. [DOI] [PubMed] [Google Scholar]
- 29.Ader I, Brizuela L, Bouquerel P, Malavaud B, Cuvillier O. Sphingosine kinase 1: a new modulator of hypoxia inducible factor 1alpha during hypoxia in human cancer cells. Cancer Res. 2008 Oct 15;68(20):8635–8642. doi: 10.1158/0008-5472.CAN-08-0917. [DOI] [PubMed] [Google Scholar]
- 30.Murohara T, Buerke M, Margiotta J, Ruan F, Igarashi Y, Hakomori S, et al. Myocardial and endothelial protection by TMS in ischemia-reperfusion injury. Am J Physiol. 1995 Aug;269(2 Pt 2):H504–H514. doi: 10.1152/ajpheart.1995.269.2.H504. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y, Man K, Lo CM, Ng KT, Li XL, Sun CK, et al. Attenuation of small-for-size liver graft Injury by FTY720: significance of cell survival Akt signaling pathway. Am J Transplant. 2004;4:1399–1407. doi: 10.1111/j.1600-6143.2004.00527.x. [DOI] [PubMed] [Google Scholar]
- 32.Igarashi Y, Hakomori S, Toyokuni T, Dean B, Fujita S, Sugimoto M, et al. Effect of chemically well-defined sphingosine and its N-methyl derivatives on protein kinase C and src kinase activities. Biochemistry. 1989 Aug 22;28(17):6796–6800. doi: 10.1021/bi00443a002. [DOI] [PubMed] [Google Scholar]
- 33.Megidish T, White T, Takio K, Titani K, Igarashi Y, Hakomori S. The signal modulator protein 14-3-3 is a target of sphingosine- or N,N-dimethylsphingosine-dependent kinase in 3T3(A31) cells. Biochem Biophys Res Commun. 1995 Nov 22;216(3):739–747. doi: 10.1006/bbrc.1995.2684. [DOI] [PubMed] [Google Scholar]
- 34.Kono K, Sugiura M, Kohama T. Inhibition of recombinant sphingosine kinases by novel inhibitors of microbial origin, F-12509A and B-5354c. J Antibiot (Tokyo) 2002 Jan;55(1):99–103. doi: 10.7164/antibiotics.55.99. [DOI] [PubMed] [Google Scholar]
- 35.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003 Sep 15;63(18):5962–5969. [PubMed] [Google Scholar]
- 36.French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006 Aug;318(2):596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 37.Park SW, Kim M, Chen SW, D’Agati VD, Lee HT. Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock. 2010 Jan;33(1):31–42. doi: 10.1097/SHK.0b013e3181c02c1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buehrer BM, Bell RM. Inhibition of sphingosine kinase in vitro and in platelets. Implications for signal transduction pathways. J Biol Chem. 1992 Feb 15;267(5):3154–3159. [PubMed] [Google Scholar]
- 39.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003 May 9;304(3):463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 40.Kantrow SP, Tatro LG, Piantadosi CA. Oxidative stress and adenine nucleotide control of mitochondrial permeability transition. Free Radic Biol Med. 2000 Jan 15;28(2):251–260. doi: 10.1016/s0891-5849(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 41.Zamzami N, Susin SA, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, et al. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996 Apr 1;183(4):1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theruvath TP, Snoddy MC, Zhong Z, Lemasters JJ. Mitochondrial Permeability Transition in Liver Ischemia and Reperfusion: Role of c-Jun N-Terminal Kinase 2. Transplantation. 2008 May 27;85(10):1500–1504. doi: 10.1097/TP.0b013e31816fefb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sestili P, Tommasini I, Cantoni O. Peroxynitrite promotes mitochondrial permeability transition-dependent rapid U937 cell necrosis: survivors proliferate with kinetics superimposable on those of untreated cells. Free Radic Res. 2001 May;34(5):513–527. doi: 10.1080/10715760100300451. [DOI] [PubMed] [Google Scholar]
- 44.Takeyama N, Matsuo N, Tanaka T. Oxidative damage to mitochondria is mediated by the Ca(2+)-dependent inner-membrane permeability transition. Biochem J. 1993 Sep 15;294( Pt 3):719–725. doi: 10.1042/bj2940719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem. 1998 Mar 27;273(13):7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- 46.Itagaki K, Hauser CJ. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J Biol Chem. 2003 Jul 25;278(30):27540–27547. doi: 10.1074/jbc.M301763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ki SH, Choi MJ, Lee CH, Kim SG. Galpha12 specifically regulates COX-2 induction by sphingosine 1-phosphate. Role for JNK-dependent ubiquitination and degradation of IkappaBalpha. J Biol Chem. 2007 Jan 19;282(3):1938–1947. doi: 10.1074/jbc.M606080200. [DOI] [PubMed] [Google Scholar]
- 48.Limaye V, Xia P, Hahn C, Smith M, Vadas MA, Pitson SM, et al. Chronic increases in sphingosine kinase-1 activity induce a pro-inflammatory, pro-angiogenic phenotype in endothelial cells. Cell Mol Biol Lett. 2009 Feb 23; doi: 10.2478/s11658-009-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhi L, Leung BP, Melendez AJ. Sphingosine kinase 1 regulates pro-inflammatory responses triggered by TNFalpha in primary human monocytes. J Cell Physiol. 2006 Jul;208(1):109–115. doi: 10.1002/jcp.20646. [DOI] [PubMed] [Google Scholar]
- 50.Hammad SM, Crellin HG, Wu BX, Melton J, Anelli V, Obeid LM. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat. 2008 Mar;85(3–4):107–114. doi: 10.1016/j.prostaglandins.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wanner GA, Ertel W, Muller P, Hofer Y, Leiderer R, Menger MD, et al. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock. 1996 Jan;5(1):34–40. doi: 10.1097/00024382-199601000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.