Abstract
Environmental chemicals have significant impacts on biological systems. Chemical exposures during early stages of development can disrupt normal patterns of development and thus dramatically alter disease susceptibility later in life. Endocrine disrupting chemicals (EDCs) interfere with the body's endocrine system and produce adverse developmental, reproductive, neurological, cardiovascular, metabolic and immune effects in humans. A wide range of substances, both natural and man-made, are thought to cause endocrine disruption, including pharmaceuticals, dioxin and dioxin-like compounds, polychlorinated biphenyls, DDT and other pesticides, and components of plastics such as bisphenol A (BPA) and phthalates. EDCs are found in many everyday products– including plastic bottles, metal food cans, detergents, flame retardants, food additives, toys, cosmetics, and pesticides. EDCs interfere with the synthesis, secretion, transport, activity, or elimination of natural hormones. This interference can block or mimic hormone action, causing a wide range of effects. This review focuses on the mechanisms and modes of action by which EDCs alter hormone signaling. It also includes brief overviews of select disease endpoints associated with endocrine disruption.
Introduction
EDs are synthetic chemicals that were originally designed for a specific action such as a pesticide, plastisizer, or solvent, but now have been found to have a side effect that when absorbed into the body causes them to either mimic or block hormones and disrupt the body's normal functions. This disruption can occur by altering normal hormone levels, inhibiting or stimulating the production of hormones, or changing the way hormones travel through the body, thus affecting the functions that these hormones control. EDCs were originally thought to exert their actions solely through nuclear hormone receptors, including estrogen receptors (ERs), androgen receptors (ARs), progesterone receptors, thyroid receptors (TRs), and retinoid receptors, among others (Table 1) [1]. However, recent evidence shows that the mechanisms by which EDCs act are much broader than originally recognized. Indeed, studies have shown that in addition to altering nuclear receptor signaling, EDs are capable of acting through nonsteroid receptors, transcriptional coactivators, enzymatic pathways involved in steroid biosynthesis and/or metabolism, and numerous other mechanisms that converge upon endocrine and reproductive systems [1, 2]. Other less well known mechanisms of action of EDCs include direct effects on genes [3] and their epigenetic impact [4]. These effects are particularly troubling since alterations in genetic programming during early stages of development may have profound effects years later and may also lead to transgenerational inheritance of disease (Figure 1.) [5].
Table 1.
Select human nuclear receptors and related functions
| Receptor | Abbreviation | Physiological Function | Endogenous Ligand | Examples of Endocrine Disrupting Chemicals |
|---|---|---|---|---|
| Androgen | AR | Male sexual development | Testosterone | Pesticides Phthalates Plasticisers Polyhalogenated compounds |
| Estrogen | ER α, β GPR30 (non-nuclear) | Female sexual development | Estradiol | Alklyphenols BPA Dioxins Furans Halogenated hydrocarbons Heavy metals |
| Thyroid Hormone | TR α, β | Metabolism Heart rate | Thyroid Hormone | BPA Dioxins Furans PBDEs PCBs Perchlorates Pesticides Phalates Phytoestrogens |
| Progesterone | PR | Female sexual development | Progesterone | BPA Fungicides Herbicides Insecticides |
| Arylhydrocarbon | AhR | Circadian rhythm Metabolism Neurogenesis Organ development Stress response |
Unknown | Dioxins Flavonoids Herbicides Indoles PCBs Pesticides |
| Peroxisome Proliferator-Activated | PPAR α, β, λ | Lipid homeostasis | Lipids/Fatty Acids | BPA Organotins |
| Glucocordicoid | GR α, β | Development Metabolism Stress response |
Cortisol | Arsenic BPA Phthalates |
Figure 1.
Model of the endocrine systems targeted by EDCs. This figure illustrates that all major endocrine organs are vulnerable to endocrine disruption, including the HPA axis, reproductive organs, the pancreas, and the thyroid gland. EDCs are also known to impact hormone-dependent metabolic systems and brain function.
There are several characteristics of the endocrine system that must be accounted for in order to develop a full understanding of the mechanisms of actions and the consequences of exposure to EDCs. For instance, similar to hormones, EDCs can function at very low doses in a tissue specific manner. EDCs may also exert non-traditional dose-responses due to the complicated dynamics of hormone receptor occupancy and saturation. Thus low doses may have more impact on a target tissue than higher doses, and the effects may be entirely different. The age at which an individual is exposed to an EDC also has important implications on resulting health consequences. Indeed, it is now clear that exposure to EDCs during development results in different effects than exposures during adulthood. Adults require higher concentrations for EDCs to cause toxicity and their effects only last as long as the EDC is present. Low dose exposure during development can result in that lasts long after the EDC is gone from the body. For this reason, the field of endocrine disruption coined the term “the fetal basis of adult disease”, or FeBAD, to describe the interactions between the developing organism and the environment that determine the propensity of that individual to develop disease later in life [6]. This concept has been extended beyond the fetal period to include the early postnatal developmental period when organs continue to undergo substantial development. DOHaD (developmental origins of health and disease) describe the interactions between the developing organism and the environment that determine the propensity of that individual to develop disease later in life [1].
Growing evidence also suggests that EDCs may affect not only the exposed individual but also the children and subsequent generations. The mechanism of transmission involves non-genomic modifications of the germ line such as changes in DNA methylation and histone acetylation. Altogether, EDCs pose a significant challenge to our industrialized society and to the health of humans and the environment. Indeed, due to their wide commercial use and direct link to adverse human health outcomes the Endocrine Society published a scientific statement in 2009 indicating that endocrine disruptors pose a “significant concern for public health” [1].
Modes of Action
1. Nuclear receptor signaling
EDCs are structurally similar to many hormones, function at extremely low concentrations, and many have lipophilic properties. EDCs are capable of mimicking natural hormones and maintain similar modes of action, transport, and storage within tissues. The properties of these chemicals, while unintended, make them particularly well suited for activating or antagonizing nuclear hormone receptors. Thus, there is virtually no endocrine system immune to these substances, because of the shared properties and similarities of receptors and enzymes involved in the synthesis, release, and degradation of hormones (Table 1) [1].
The nuclear hormone receptors are a superfamily of transcription factors that play important roles in both physiology and disease. In humans, there are some 48 nuclear receptors and many remain “orphans” as their endogenous ligands are yet to be determined. Research on the roles of nuclear receptors has been limited largely to the use of synthetic agonists, as well as genetic approaches to alter expression. This contrasts with the estrogen receptors (ERα and ERβ), which have been extensively studied [7]. These receptors remain at the center of endocrine disruption research, as outlined below, and results from these studies may provide a model for how other nuclear receptors interact with hormone mimics.
The estrogens are a group of steroid hormones produced by enzymatic modification of cholesterol. The primary estrogen of the reproductive years is 17β-estradiol (estradiol), which is derived from testosterone by aromatase activity. There are a wide range of natural and synthetic molecules that can activate ERα and ERβ. Natural estrogens include those produced by plants (phytoestrogens) and fungi (mycoestrogens). Synthetic ER activators include those intentionally produced for use in humans (e.g., diethylstilbestrol) as well as chemicals targeted for other uses that have unintended ER-modulating activities (e.g., DDT, methoxychlor). Identifying chemicals that display estrogenic activity is nowa major focus of the of research done on endocrine disruption.
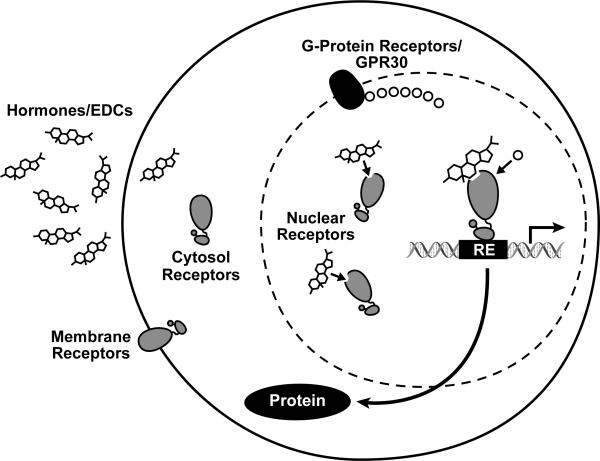
The primary means by which estrogenic compounds disrupt normal development is via interaction with one of the estrogen receptors. There are three types of receptors for estrogens: the nuclear estrogen receptors (ERs), the membrane bound estrogen receptors (which are variants of the nuclear ERs), and the estrogen G protein-coupled receptor (GPR30), which is a membrane-bound protein with a high affinity toward estrogen. The main function of the ER is as a DNA-binding transcription factor that regulates gene expression and subsequent downstream responses (Figure 2).
Figure 2.
Illustration of steroid hormone receptor signaling pathway. Hormones, or hormone mimics, bind to membrane or cytosol receptors, which in turn shuttle to the nucleus and attach themselves to response elements (REs), where they work to regulate gene transcription and ultimately protein production. Some receptors reside solely in the nucleus atop REs in inactive forms and become activated upon hormone binding. EDCs can alter this signaling process by binding to steroid receptors and either activating or inhibiting transcriptional response.
While some EDCs act as estrogen mimics, others have estrogenic activity but they are not true estrogens. For example, BPA was designed as a synthetic estrogen and has been shown to bind to the estrogen receptors ( ERα, ERβ, and to the membrane ER, resulting in a cellular signal transduction cascade that is indicative of an estroenic response. [8, 9] However, detailed examination of its effects on gene expression in a variety of tissues indicates that, while there is significant overlap, it does not stimulate the same suite of genes as estradiol. In addition there is mounting evidence that EDCs such as BPA interact with other nuclear receptors, albeit at higher concentrations. For example, one study found that BPA binds to the thyroid hormone receptor (TR) with a lower affinity than the estrogen receptor [10]. However, others believe BPA acts as an indirect antagonist of thyroid hormone (TH) and that its effects on TH action in vivo are likely dependent on the composition and relative abundance of cofactors available in the cell [11]. Studies have also shown that BPA binds to the ubiquitous aryl hydrocarbon receptor (AhR) [12]. This is not surprising because AhR is thought to be activated by many chemicals and likely mediates toxicity through several signaling pathways [12].
The focus of EDC research has been on estrogens, androgens and thyroid agonists and antagonists, but it is now clear that there are EDCs that affect other receptors and metabolic systems. Another nuclear hormone receptor targeted by EDCs is the peroxisome proliferator-activated receptor gamma (PPARγ) [reviewed in 13]. PPARγ was shown to be a key regulator of adipogenesis in vitro and in vivo and is important clinically as a target for drugs that ameliorate insulin resistance in type II diabetes. PPARγ functions as a heterodimer with the retinoid ‘X’ receptor, RXR; the RXR-PPARγ heterodimer is a ligand modulated transcription factor that directly regulates the expression of its target genes [14]. PPARγ is thought to be the master regulator of adipogenesis because it plays an important role in nearly all aspects of adipocyte biology [reviewed in 15, 16]. Activation of PPARγ2 in pre-adipocytes induces them to differentiate into adipocytes and PPARγ is required for this process in vitro and in vivo [17, 18]. Moreover, expression of PPARγ is sufficient to transform susceptible stem cells into preadipocytes [19]. Activating the PPARγ pathway drives mutipotent stromal stem cells to enter the adipogenic pathway whereas inhibition of PPARγ expression promotes an osteogenic fate [reviewed in 20, 21]. It is known that humans whose diabetes is being treated with rosiglitazone (a drug that activates PPARγ) develop more adipocytes and gain weight [22]. Therefore it is reasonable to hypothesize that chemicals capable of activating PPARγ might have the same effect [reviewed in 23].
Low Dose Effects
For many years, toxicologists have relied on the presumption that “the dose makes the poison”, first proposed by the Swiss physician and alchemist, Paracelsus in the 1500's. This view predicts that higher doses of a chemical will cause greater harm than low doses. This model is traditionally used by regulators to establish risk assessment profiles of chemicals. It relies on a monotonic dose response curve generated from high and moderate dose measurements that are linearly extrapolated downward to predict toxicity at very low doses. However, multiple studies on EDCs contradict this concept and question the adequacy of traditional toxicology testing paradigms for detecting low dose effects of EDCs. Indeed, these reports suggest that, similar to hormones, EDCs are capable of eliciting bi-phasic dose responses for many different endpoints at many levels of organization. These U-shaped and inverted U-shaped non-monotonic dose response (NMDR) curves are used as evidence that very low concentrations of EDCs can effect endpoints such as cell proliferation and organ development.
NMDR curves have been described for numerous EDCs [24]. However, much controversy surrounds determining internal concentrations, the active metabolites, and the actual daily exposure levels of EDCs, particularly in the case studies of BPA. The duration and route of exposure may also have a big influence on how the chemical is metabolized and whether or not the chemical remains biologically active. Additionally, the “low dose” levels at which these chemicals function are lower than those typically used in standard toxicology testing. This makes it difficult for toxicologists to use traditional rodent models to predict relevant endpoints for human exposures, when testing proceeds from a high dose and stops when a “no observed adverse effects level” (NOAEL) is reached.
Despite the controversy surrounding the “low dose” concept, there are several reasons why dose response curves to toxicants may be non-monotonic. For example, the induction of metabolizing enzymes or conjugation of substrates my result in a U-shaped dose response for some endpoints. A recent study by Gualtieri et al., using Sertoli cells exposed to various doses of BPA (0.5nM- 100μM), demonstrated that only intermediate doses (10 μM -50 μM), not high or low doses, induced an incremental increase in cell protecting glutathione levels [25]. Their findings show that detoxification through direct conjugation was enhanced at intermediate levels and cell viability was negatively affected at high and low doses where the cells were incapable of eliciting a response mechanism.
Several studies have suggested that non-monotonic responses can be explained by the down-regulation of receptors at higher hormone levels [26, 27]. There is also evidence that NMDR curves are generated by the integration of two or more monotonic dose response curves that occur through different pathways affecting a common endpoint with opposing effects [28, 29]. Furthermore, adaptive responses through complex cell signaling pathways and feed-back mechanisms could cause non-monotonic effects that are inconsistent with traditional dose-response curves. For example, Bouskine et al. reported that BPA stimulates JKT-1 cell proliferation in vitro in an inverse U-shape dose-response curve [30]. The authors propose that BPA activates two different signaling pathways that are distinct in both signaling mechanism and the time frame of response. In summary, making predictions about the safety of chemicals by testing at moderate or high doses is not appropriate when very low doses of endocrine disruptors can alter biochemical and morphological endpoints in a manner that is not necessarily predicted by exposures at much higher doses [31]. Lastly, it was proposed in a theoretical treatment that non-monotonic systems result from a loss of negative feedback and that such systems can be converted back into monotonic systems by adding back negative feedback [32]. This has important implications for EDCs since it well known that most hormonal signaling pathways are regulated by negative feedback and it has been demonstrated that EDCs differentially affect the stability of nuclear receptor proteins and ligands [reviewed in 33].
2. Developmental windows of susceptibility
Adult exposure to endocrine disrupting chemicals is certainly an important factor in adverse health outcomes, however focus on the fetus and/or neonate is of primary concern since developing organisms are extremely sensitive to perturbation by chemicals with hormone-like activity [34]. Adverse effects may be most pronounced in the developing organism and occur at concentrations of the chemical that are far below levels that would be considered harmful in the adult [35]. For example, fetal chemical exposure may result in permanent alterations in reproductive or neurological function. While fetal development is commonly known to be a period of increased sensitivity to chemical insult, childhood and adolescence are also marked by continued maturation of key endocrine systems, and are therefore susceptible to chemical exposure. Indeed, the developmental basis of health and disease DOHaD hypothesis first proposed by David Barker in 1997, showed that poor in-utero nutrition resulted in high rates of disease manifested later in life [36]. This concept has now includes non-nutritional early life exposures that have been shown to alter the body's physiology. Thus the DOHaD paradigm, provides a framework to assess the effect of not only early nutrition but also EDCs on long-term health (Figure 3) [37].
Figure 3.
Model illustrating early life exposures may cause functional changes at cellular levels that lead to changes in physiological status, and ultimately adult disease.
During early fetal and childhood development, a wide variety of genes are activated and inactivated in a sequential manner, providing numerous targets for environmental exposures. The rapid nature of the processes that occur during critical developmental periods give rise to concerns about vulnerability during early stages of life. Exposures during the early stages of fetal development can impact development of the developing central nervous system, heat, limbs, skeleton, and reproductive system. For instance, mercury exposure has been shown to affect cell migration during fetal development leading to central nervous system anomalies and mental retardation in children. Fetal and embryonic exposures to EDCs have been linked to childhood cancers [38]. Many of the adverse effects on development in late pregnancy and early childhood show themselves as functional deficits in organs or systems, instead of overt malformations or growth retardation [39, 40]. There is growing concern that exposure to EDCs may affect sexual maturation in children. This concern has focused primarily on the decreases in average age of puberty in some ethnic groups in the U.S. and other countries. Changes in age at puberty are indicative of other impairments in the endocrine and reproductive systems and are also associated with increased risk of multiples diseases later in life.
Normal development is an intricate process that involves establishment of a proper hormone milieu necessary for providing the biological signaling processes for the growth of organ systems. Basic biological processes involved in development and in the body's ability to respond to environmental exposures progress at different rates [41]. Since the protective processes involved in metabolizing harmful chemicals may not be fully functional until later in development, environmental exposures pose unique challenges to the fetus and young child. For example, studies have shown that PON1, the enzyme which detoxifies activated organophosphorus pesticides (OPs) and reduces oxidative stress, does not become fully functional until the age of 9 [42]. Moreover, children with PON1 polymorphisms display differences in paraoxonase enzymatic activity which is necessary to clear harmful chemicals from the body. These differences in metabolic phenotypes during periods of development demonstrate the challenges environmental exposures pose to the developing fetus.
Of special concern are man-made hormone mimicking chemicals capable of evading defense mechanisms and misdirecting developmental decisions. Recent studies document detectable amounts of a variety of EDCs such as phthalates, polybrominated diphenyl ethers, polycyclic aromatic hydrocarbons, and BPA in pregnant women, fetuses, newborns, young children and adolescents [43-48]. Since each organ system has a different developmental trajectory, the effects of exposures are dependent not only on the type and dose of the chemical, but also when the exposure occurs [49]. Exposures that occur early in pregnancy can have influence on in short-term health effects of birth outcome, such as the length of gestation period, birth weight, and head circumference [45], while exposures later in pregnancy or during early childhood can lead to cognitive and developmental deficiencies [47]. These studies illustrate that the in utero developmental period is a critically sensitive window of vulnerability. Disruptions during this time-frame can lead to subtle functional changes that may not emerge until later in life [50]. Evidence now suggests that early life exposures to toxic chemicals can be directly associated with subsequent increases in the rates of breast and prostate cancer, Parkinson's disease, obesity and other diseases (Table 2) [50-52].
Table 2.
Developmentally-Induced Diseases (Human*)
| System | Disease | Chemicals |
|---|---|---|
| Reproductive/Endocrine | Breast/Prostate Cancer Endometriosis Infertility Diabetes/Metabolic Syndrome Early puberty* Obesity* |
BPA Dioxin, PCBs Estrogens, Pesticides, Phthalates BPA Estrogens, BPA BPA, Organochlorine pesticides, Organotins |
| Immune/Autoimmune | Susceptibility to infections Autoimmune disease |
Dioxin Dioxin |
| Pulmono-cardiovascular |
Asthma* Heart disease/Hypertension Stroke |
Air pollution BPA PCBs |
| Brain/Nervous | Alzheimer's Disease Parkinson's Disease ADHD/Learning disabilities* |
Lead Pesticides PCBs, Lead, Ethanol, Organochlorine pesticides |
Heindel and Newbold [53] described several important principles that demonstrate how early life environmental exposures contribute to increased risks of adult disease. First, chemical exposures can have both tissue-specific and time-specific consequences on growth and development. As long as tissue is developing, it is susceptible to disruptions from environmental exposures. These disruptions can result from changes in gene expression, protein activity, cell communication or other mechanisms. Secondly, the initiating in utero exposure may act alone or in concert with other environmental stressors. That is, the risk of developing disease in adulthood can be due to the combined insults over a lifetime. Thirdly, the pathophysiology may be manifested in a disease that otherwise might not have occurred and disease progression may have variable latent periods. Finally, the effects of environmental chemical exposures can be transgenerational, thus affecting future generations.
3. Transgenerational actions of endocrine disruptors
The majority of environmental factors and toxins do not have the ability to alter DNA sequence or promote genetic mutations at normal physiological doses [54-56]. This is due, in part, to the stability of the genome, which has developed multiple mechanisms to ensure that DNA stability and replication proceed with a high degree of fidelity [57]. Therefore, many environmental factors promote abnormal phenotypes or disease, independent of any change in the DNA sequence [5]. However, early life chemical exposures lead to later life adult onset diseases [58]. One general mechanism by which prenatal and postnatal exposures could be linked to phenotypic changes later in life is through the alteration of epigenetic marks, which have a central role in determining the functional output of the information that is stored in the genome [54].
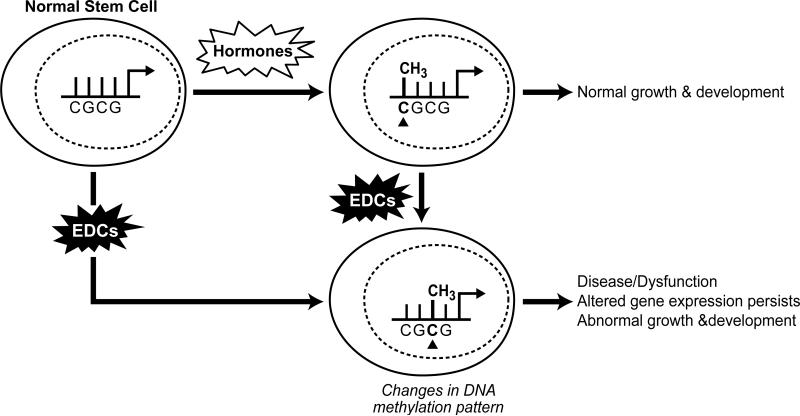
The term epigenetics refers to the factors around DNA that regulate its activity but are independent of the DNA sequence. While there are several factors that can modify DNA to alter gene expression, such as histone remodeling and regulation by small non-coding RNAs, we focus here on the ability of environmental chemicals to reprogram DNA through changes in methylation patterns. DNA methylation takes place at the carbon-5 position of cytosine in CpG dinucleotides due to DNA methyltransferases [59]. Methyl-binding proteins then attach to these sites and subsequently attract other chromatin modifying proteins, with the end result being a silencing of the methylated gene. On the other hand, hypomethylated genes tend to be more accessible to transcriptional machinery and can generate increased and inappropriate gene expression (Figure 4) [60]. The majority of environmental factors act on somatic tissues and influence the physiology of the individual exposed [5]. However, in some cases these environmental factors promote a heritable transmission of the disease phenotype through successive generations [5, 56, 61]. The heritable transmission of this environmentally induced phenotype is referred to as epigenetic transgenerational inheritance.
Figure 4.
Model depicting how EDCs can alter methylation patterns and normal epigenetic programming in cells. Alterations in the epigenetic status of somatic cells can lead to disease in developing tissues, whereas changes in the epigenetic programming in stem cells can lead to multi- and transgeneration effects in the offspring.
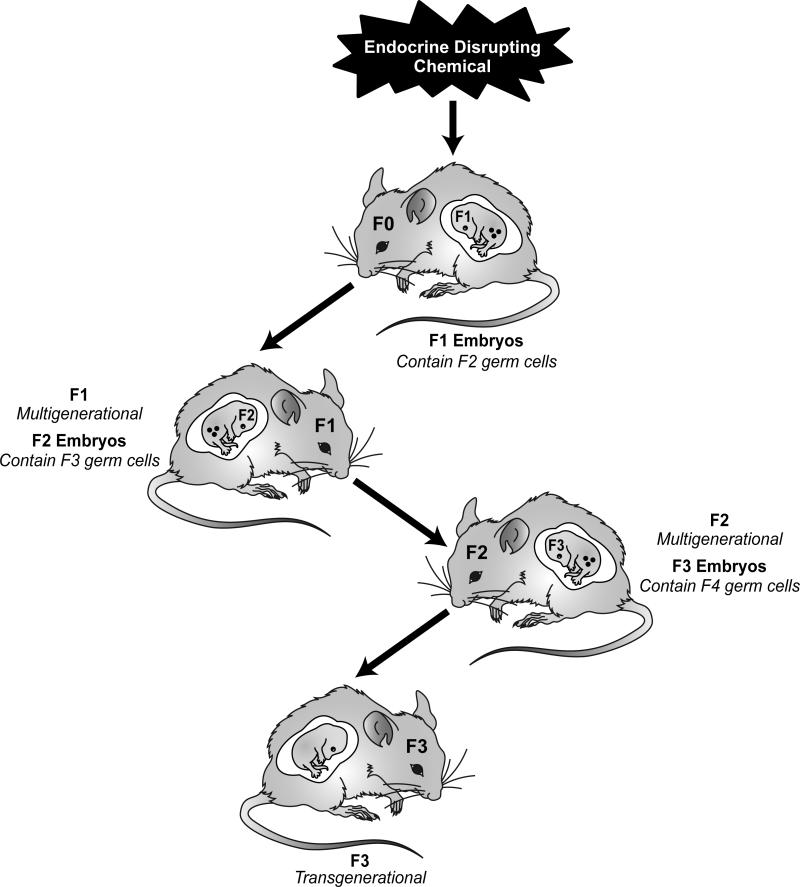
A classic example of a multigenerational phenotype resulting from an environmental chemical involves prenatal exposure to the potent synthetic estrogen diethylstilbestrol (DES), which was prescribed to reduce the risk of pregnancy complications and losses during the 1950s and 1960s [62, 63]. Exposure of a gestating female to DES was found to promote an abnormal reproductive tract and gonadal dysfunction in the F1 generation males and females, as well as abnormal female reproductive tract function in the F2 generation [35]. It is interesting that the F1 and F2 generations display different disease phenotypes. Studies are currently underway to determine whether early life exposure to DES promotes multigenerational phenotypes [64, 65]. Another example of multigenerational exposure was demonstrated using the anti-androgenic drug, flutamide. Many other chemicals have also been implicated in promoting toxicity for multiple generations, including BPA [66, 67], polycyclic hydrocarbons [68, 69], cocaine [69], pesticides [70], and phytoestrogens [71-73]. It is important to note that the multigenerational effects mentioned above involving direct exposures and phenotypes are not considered transgenerational because they are not transmitted solely through the germline. Only effects appearing in the F3 generation are considered to be truly transgenerational (Figure 5) [5].
Figure 5.
EDCs many promote epigenetic alterations that influence somatic cells and so the disease status of the individual exposed (F0 generation). In pregnant females, EDC exposure could also cause epigenetic modifications in the next two generations (F1 and F2) through the fetus and its germ line. The effect of such multigenerational exposure in subsequent generations (F3 and beyond) would be considered a transgenerational phenotype.
One of the first studies to demonstrate epigenetic, transgenerational effects of an endocrine disruptor involved the analysis of vinclozolin actions on the male germ line in rats [74]. Vinclozolin is a fungicide commonly used in agriculture that is known for its anti-androgenic endocrine action [75]. In this study Skinner et al. showed that exposing a pregnant rat to vinclozolin or methoxychlor (an estrogeneic pesticide) during embryonic days 8-14 caused defects in spermatogenic capacity, which were transmitted through at least four subsequent generations. Interestingly, the transgenerational phenotypes observed in the animals also included adult onset diseases, such as kidney disease, immune abnormalities, prostate lesions and cancer [74, 76]. Subsequently, others have observed changes in behavior and learning capacity following vinclozolin exposure [77-81], including transgenerational changes in mate preferences and anxiety behavior [81]. These transgenerational effects were only seen when the exposure window overlapped with critical developmental processes such as germ cell methylation in the differentiating testis.
Epigenetic marks such as methylation patterns are laid down during development and are responsible for the programming necessary to transform stem cells into differentiated cells and tissues. The loss and subsequent reestablishment of the epigenetic profile in the developing embryo comprise a critically sensitive period during which the system is particularly vulnerable to environmental influences. While exposure to environmental agents may not result in obvious phenotypes, these exposures can alter the epigenetic programming of both somatic and germ cells inducing subtle functional changes leading to disease later in life and in future generations. The exact mechanism whereby environmental chemicals alter the epigenome has not been definitively established. It is known that following fertilization, the DNA methylation pattern in the sperm-derived pronucleus is actively removed (excluding imprinted genes). However, the enzymatic machinery responsible for demethylation is largely unknown. Recently, Activation-Induced cytidine Deaminase (AID) has been found to be highly expressed in primordial germ cells. AID was previously thought to act as a single-stranded DNA deaminase involved in recombination of the immunoglobulin genes during class switching [82]. However, AID activity may also play a role in DNA methylation in primordial germ cells and in the early embryo and in disrupting the action of DNA methytransferase activity during periods of programming. Emerging developments in technology necessary for accurate mapping of the epigenome during developmental periods will undoubtedly lead to a better understanding of transgenerational epigenetic inheritance.
Selected Disease endpoints
1. Male reproduction and development
Given the fact that both that hormone production and action are regulated in large part by the reproductive tissue, it is not surprising that EDCs contribute to many adverse reproductive health outcomes in developing and adult humans. Epidemiological data has revealed an increase in male reproductive function disorders over the past 50 years, suggesting a correlative relationship with the increasing amounts of EDCs in the environment [83]. In the context of male reproductive health, EDCs have been linked to 1) disrupted reproductive function, displayed as reduced semen quality and infertility; 2) altered fetal development, displayed as urogenital tract abnormalities, including hypospadias and cryptorchidism, and 3) testicular germ cell cancer (TGCC) [1, 84]. As previously mentioned, the potential lag between exposure to EDCs and the manifestation of a clinical reproductive disorder is of critical concern. In humans, this period may be years or decades post exposure because sexual maturity and fertility cannot be assessed until the exposed individual has attained a certain age [1].
Skakkebaek et al. [85] have suggested that the incidences of cryptorchidism, hypospadias and poor semen quality are risk factors for one another and that they are all predictive of developing testicular germ cell cancers. This quartet is defined as the testicular dysgenesis syndrome (TDS). They propose that the etiology of TDS lies in the diminished androgen action in fetal developmental periods and has a negative impact on the proper functioning of Sertoli cells (the cells supporting germ cells) and Lydig cells (where androgen synthesis occurs). This hypothesis proposes a strong association between environmental exposures and development of TDS.
Identifying environmental causes of TDS in humans is difficult because developing fetal tissues are inaccessible for examination. Thus the majority of mechanistic evidence linking EDCs to TDS comes from animal experiments. It is possible to experimentally induce all the elements of TDS, except for germ cell cancer, by exposing pregnant rats to phthalates and other chemicals that block androgen action [86]. This model is referred to as the “phthalate syndrome” model, and it is comprises non-descent of testis, malformations of the external genitalia, poor semen quality, and malformations of other sex organs [87]. The causes of phthalate syndrome center on suppression of fetal androgen action, which is the key driver of male reproductive organ development. Phthalates lower levels of testosterone and its derivatives by interfering with the uptake of steroid hormone precursors into fetal Leydig cells where steroid synthesis takes place. The net results are malformations of internal reproductive organs, hypospadias, retained nipples, and feminized anal-genital distance (AGD) [87]. Certain pesticides are able to block the androgen receptor, or interfere with the conversion of testosterone into dihydrotestosterone, thus producing effects similar to phthalate syndrome. Androgen action also is essential for proliferation and development of Sertoli cells, which are necessary for sperm production. Altogether, EDC-mediated disruption of androgen action during fetal development results in reduced fertility later in life [88].
Epidemiological studies have identified an association between chemical exposure (e.g., to phthalates, polychlorinated biphenyls (PCBs), dioxins, and nonpersistent pesticides) and reduced semen quality. In a U.S.-based study, Duty et al. [89] found links between monobutyl phthalate exposure and poor sperm motility and concentrations. A study of dioxin exposure conducted by Mocarelli et al. [90], suggests that timing of exposure has a significant impact on semen quality. This study was based on men whom were exposed to high levels of TCCD as a result of a chemical plant explosion in 1976 in Seveso, Italy. Men who were exposed prepubertally (1-9 years of age) demonstrated poor semen quality as adults. Interestingly, men who were exposed between 10-17 and 18-27 years of age showed slightly positive or no differences in semen quality, respectively. Several occupational studies have found associations between pesticide exposure and reduced semen quality [91-97]. In a study on male partners of pregnant women, Swan et al [98] found elevated odds ratios for poorer semen quality in relation to urinary concentrations of several common pesticides. Meeker et al. [99] also found an inverse relationship between urinary pesticide levels in men and sperm concentration and motility. While there are clear association between EDCs and diminished male reproductive health, there is a clear need for further epidemiological studies to identify the classes of chemicals, exposure levels, and the most critical windows of susceptibility important to male reproductive health.
2. Female reproduction and development
The ability of EDCs to alter reproductive function and health in females has been clearly demonstrated by the consequences of DES use in pregnant women. The daughters of women given DES while pregnant were shown to have rare cervicovaginal cancers [100, 101], decreased fertility and increases in rates of ectopic pregnancy [102], and early menopause [103]. Many of these disorders have been replicated in laboratory animals treated with DES during gestation [62, 104-108]. As Newbold points out [106], the lessons learned from 40 years of DES research in humans and animals are that the female fetus is susceptible to environmentally induced reproductive abnormalities, that gonadal organogenesis is sensitive to synthetic hormones and hormone mimics during critical exposure windows, and that reproductive disease may not appear until decades after exposures.
Proper development of ovarian follicles in the fetus is dependent on estrogen exposure during critical periods of development. For instance, mice treated with DES on postnatal day 1 to 5 develop multioocytic follicles as adults [109]. Therefore, maintaining a homeostatic balance of local and systemic hormones during follicle development is necessary for normal follicle development and germ cell quality [110]. Perturbations in hormone signaling resulting from chemical exposures during developmental periods could contribute to ovarian disorders and declining conception rates in human populations [111]. And while the mechanisms by which EDCs alter follicle development are not fully understood, there is evidence that these chemicals are contributing to increased rates of aneuploidy [112], polycyctic ovary syndrome (PCOS) [113, 114], premature ovarian failure (POF) [113, 114], and altered cyclicity and fecundity [115-119]. For example, studies have shown that prenatal exposure to BPA causes irregular cycles in mice, which is likely due to hypothalamic alterations in the circuitry that controls luteinizing hormone (LH) secretion and ovulation [116, 120]. In humans, altered cyclicity has been reported in individuals exposed to organochlorine pesticides. Indeed, cycle irregularities have been noted in women whose mothers were exposed in utero to DES [64].
Uterine fibroids (leiomyomas) are the most common tumor of the female reproductive system [121], occurring in 25% to 50% of all women. The risk of the development of uterine fibroids increases with age during premenopausal years, but tumors typically regress with the onset of menopause [122]. Obesity, age at menarche and unopposed estrogen signaling have been shown to increase the risks for tumors [123]. The best characterized animal model for study of uterine fibroids is the Eker rat. A mutation of the tuberous sclerosis complex 2 (Tsc2) tumor suppressor gene causes females to develop spontaneous uterine fibroids at a high frequency [124]. Studies using this model have shown that exposure to EDCs increases the incidence of fibroids in these animals [125]. Developmental exposure to DES causes rats that are genetically predisposed to uterine tumors to develop even more tumors of a larger size, but fails to induce tumors in wild-type rats. Importantly, DES exposure imparts a hormonal imprint on the developing uterus that causes an increase in estrogen-responsive gene expression [110]. The potential for DES to cause uterine fibroids in humans is less clear. Two studies on DES daughters came to different conclusions. In a study of 2,570 women born during the period DES was prescribed, no association was found between prenatal exposure and uterine fibroids [126]. Another study of 1.188 women found a significant relationship between DES exposure and uterine fibroids [127]. On analysis of these studies, Baird and Newbold concluded that there was a definitive increase in uterine fibroids in DES daughter and the discrepancies between the studies was due to the differences and sensitivities of the methods used to detect the tumors.
In summary, both animal and human studies suggest a role of EDCs in altering female reproductive development. Data from animal experiments show that EDC exposure during critical periods of development, both prenatal and neonatal, can induces functional changes that appear later in life. There are data gaps in understanding the mechanisms by which EDCs carry out their action, but it is clear that to reduce the risk of reproductive disorders we must take action to reduce exposure to these chemicals.
3. Obesity and metabolic disorders
There is now compelling evidence linking prenatal exposures to a variety of chemicals with altered developmental programming that may lead to weight gain [23] and metabolic disturbances such as diabetes later in life[128, 129]. One well-studied example concerns the effects of maternal tobacco smoking. Babies born to mothers who smoke are typically born with a low birth weight and increased risk of obesity, cardiovascular disease and metabolic syndrome later in life, thus some component(s) of tobacco smoke that are transported to the fetus are “obesogens” [130].
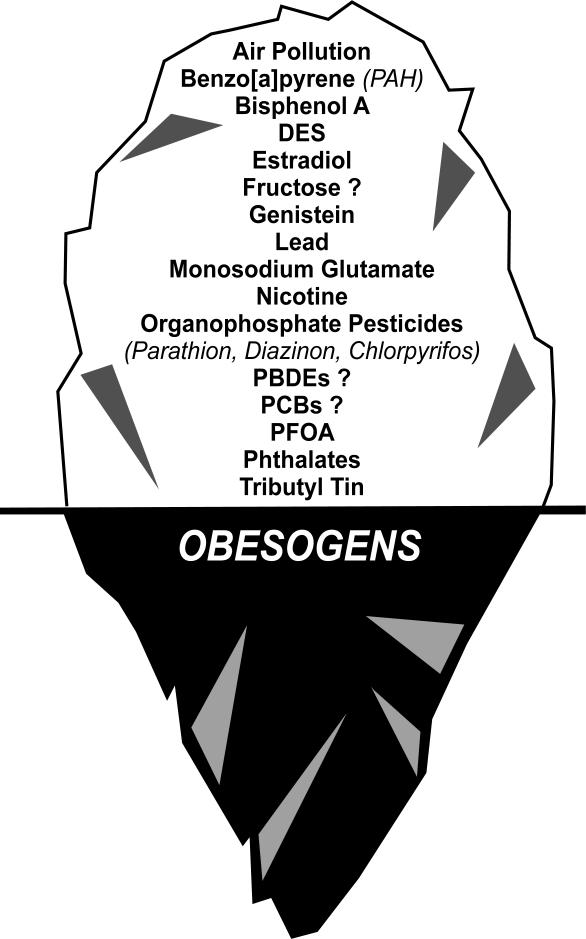
Obesogens are functionally defined as chemicals that promote weight gain by acting directly on fat cells (to increase their number or the storage of fat) or indirectly by altering mechanism through which the body regulates appetite and satiety, by altering basal metabolic rate, or by altering energy balance to favor the storage of calories [reviewed in 23, 131]. Many known obesogens are EDCs that can act as direct ligands for nuclear hormone receptors, or affect components in metabolic signaling pathways under hormonal control [23]. Indeed, environmental chemicals such as tributyltin(TBT) and triphenyltin (TPT) are known to stimulate adipogenesis in vitro and in vivo. TBT and TPT are nanomolar affinity ligands for the RXR-PPARγ heterodimer [132, 133] and stimulate 3T3-L1 preadipocytes to differentiate into adipocytes [132-134] in a PPARγ-dependent manner [135, 136]. The ligand-binding pocket of PPARγ is large and considered to be promiscuous [137]; therefore, it is not surprising that an increasing number of other chemicals with dissimilar structures have been shown to be PPARγ ligands [reviewed in 13]. It is currently unknown how many environmental chemicals activate PPARγ and whether some or all of these will ultimately turn out to be obesogens but there is little doubt that activating PPARγ is an important pathway for adipogenesis and obesity (figure 6) [13, 23].
Figure 6.
The iceberg illustration indicates that there is evidence that exposure to certain EDCs during results in obesity in animal models. Only a few chemicals have been thoroughly studied in humans, thus possible that many more chemicals will be found below “the tip of the iceberg” that impact obesity.
Mature adipocytes are generated from multipotent stromal cells (MSCs) found in almost all fetal and adult tissues [138]. MSCs can differentiate into bone, adipose tissue, cartilage, muscle, in vitro and are thought to help maintain these tissues in the adult. Exposure of pregnant mice to TBT or the pharmaceutical obesogen rosiglitazone produced an MSC population that was predisposed to differentiate into adipocytes at the expense of bone [135]. Although the effects of TBT exposure on adults remains unexplored, it is known that rosiglitazone treatment increases weight and fat cell number in humans [139]; therefore, it is likely that TBT has the same effect. Intriguingly, MSCs derived from mice exposed to TBT in utero showed epigenetic alterations in the methylation status of the CpG islands of adipogenic genes such as AP2 and PPARγ which presumably led to the observed increase in the number of preadipocytes in the MSC compartment and in the frequency with which MSCs differentiated into adipocytes upon adipogenic stimulation [135]. Ultimately, it will be quite important to understand how the setpoint for adipocyte number is programmed in humans and how this can be altered by EDC exposure.
There is growing concern in the scientific community that EDCs may be contributing to the rapid increased rates of diabetes and metabolic syndrome. It is of particular concern that the incidence of both obesity and diabetes are rising rapidly in the young. While there can be no argument that eating calorie-dense, nutrient-poor food in large portions plays an important role, the rapid rise in obesity and diabetes in the young suggests the influence of early life exposures to chemicals may be playing an important role. Indeed, there is a growing body of literature linking exposure to EDCs such as BPA, dioxins, organochlorine and organophosphate pesticides with the incidence of metabolic syndrome and diabetes [128, 129, 140]. It is know that obesity and diabetes are linked and many of these same chemicals are associated with weight gain/obesity [23, 131, 141] and diabetes. While the precise metabolic pathways targeted by most of these chemicals are uncertain at present, the data linking EDCs with obesity, metabolic syndrome and diabetes are strong and the number of studies finding positive association is growing. Understanding the molecular mechanisms involved in the role of epigenetics and early life exposures will provide important insights into the etiology of these chronic disorders and should play an important role in designing effective prevention strategies.
Conclusion
Humans are exposed to thousands of chemicals during their lifetime, through the air they breathe, the food they eat, and the water they drink. A significant number of these chemicals are toxic due to the fact that they can disrupt the endocrine system. Over the past decade, the list of chemicals with endocrine disrupting activity has dramatically increased [142]. Evidence has shown that EDCs compromise the reproductive system, thyroid signaling mechanisms, as well as tissues and organs associated with energy metabolism, glucose control, fat cell development and satiety. Indeed, it is plausible that all endocrine systems are to some degree affected by environmental chemical exposures. Since EDCs activate the same receptors and signaling pathways as hormones and act at low concentrations, they are subject to the same biological regulatory systems as hormones. And since hormones control all aspects of physiology across the lifespan, the same can be expected from chemicals with endocrine disrupting activity.
Hormones play a critical role in tissue development and the programming of stem cells and tissues during the developmental process. The same can be said for EDCs. The DOHaD paradigm illustrates that many, if not all, diseases have their origin during development. EDCs pose the most risk during the development period as they alter programming, which leads to increased susceptibility to disease later in life. Testing for chemicals with endocrine disrupting activity can be challenging as the effects are often subtle (functional changes such as alterations in epigenetic marks, and changes in gene expression), and they can manifest effects later in life, long after the EDC is eliminated from the body. Over the past 40 years, there has been a significant increase in a variety of endocrine-associated diseases including, infertility, premature puberty, ADHD, obesity and diabetes, and endocrine cancers such as prostate, ovarian and breast. It is biologically plausible that EDCs are playing a significant role in these and other diseases.
The notion that EDCs are significantly impacting human health is of great concern. More data is needed to expand the list of tissues affected by EDCs, and more effort is needed to identify and classify the diseases and dysfunctions they are causing in humans and animal models. Nevertheless, current data is sufficient to identify a public health problem that must be addressed. There must be concerted efforts to reduce exposures to EDCs across the lifespan, with particular emphasis in pregnant women and infants. In addition, it is important for scientists to develop biomarkers to measure exposure to EDCs during development periods. These biomarkers could be used to identify windows of susceptibility to EDCs and to develop early therapeutic interventions.
Highlights: Endocrine Disrupting Chemicals and Disease Susceptibility.
Chemical exposures during development can alter disease susceptibility later in life.
Endocrine disrupting chemicals (EDCs) can produce adverse developmental, reproductive, neurological, cardiovascular, metabolic and immune effects in humans.
EDCs interfere with the synthesis, secretion, transport, activity, or elimination of natural hormones.
Acknowledgments
Statement
This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamanti-Kandarakis E, Palioura E, Kandarakis SA, Koutsilieris M. The impact of endocrine disruptors on endocrine targets. Horm Metab Res. 2010;42(8):543–552. doi: 10.1055/s-0030-1252034. Epub 2010 Apr 2023. [DOI] [PubMed] [Google Scholar]
- 3.Moral R, Wang R, Russo IH, Lamartiniere CA, Pereira J, Russo J. Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J Endocrinol. 2008;196(1):101–112. doi: 10.1677/JOE-07-0056. [DOI] [PubMed] [Google Scholar]
- 4.Anway MD, Skinner MK. Epigenetic programming of the germ line: effects of endocrine disruptors on the development of transgenerational disease. Reprod Biomed Online. 2008;16(1):23–25. doi: 10.1016/s1472-6483(10)60553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner MK. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res C Embryo Today. 2011;93(1):51–55. doi: 10.1002/bdrc.20199. doi: 10.1002/bdrc.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 8.Watson CS, Bulayeva NN, Wozniak AL, Alyea RA. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids. 2007;72:124–134. doi: 10.1016/j.steroids.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. Journal of Steroid Biochemistry and Molecular Biology. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- 11.Gauger KJ, Kato Y, Haraguchi K, Lehmler HJ, Robertson LW, Bansal R, Zoeller RT. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ Health Perspect. 2004;112(5):516–523. doi: 10.1289/ehp.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pocar P, Fischer B, Klonisch T, Hombach-Klonisch S. Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction. 2005;129(4):379–389. doi: 10.1530/rep.1.00294. [DOI] [PubMed] [Google Scholar]
- 13.Janesick A, Blumberg B. Minireview: PPARgamma as the target of obesogens. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 15.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 16.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 17.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4(4):611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 18.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 19.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5(8):442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 21.Takada I, Kouzmenko AP, Kato S. PPAR-gamma Signaling Crosstalk in Mesenchymal Stem Cells. PPAR Res 2010. 2010 doi: 10.1155/2010/341671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim WS, Do MY, Kim SK, Kim HJ, Hur KY, Kang ES, Ahn CW, Lim SK, Lee HC, Cha BS. The long-term effects of rosiglitazone on serum lipid concentrations and body weight. Clin Endocrinol (Oxf) 2006;65(4):453–459. doi: 10.1111/j.1365-2265.2006.02614.x. [DOI] [PubMed] [Google Scholar]
- 23.Janesick AS, Blumberg B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Research (Part C) 2011;91 doi: 10.1002/bdrc.20197. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conolly RB, Lutz WK. Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Tox. Sci. 2004;77:151–157. doi: 10.1093/toxsci/kfh007. [DOI] [PubMed] [Google Scholar]
- 25.Gualtieri AF, Iwachow MA, Venara M, Rey RA, Schteingart HF. Bisphenol A effect on glutathione synthesis and recycling in testicular Sertoli cells. J Endocrinol Invest. 2010 doi: 10.1007/BF03347468. [DOI] [PubMed] [Google Scholar]
- 26.Medlock KL, Lyttle CR, Kelepouris N, Newman ED, Sheehan DM. Estradiol down-regulation of the rat uterine estrogen receptor. Proc. Soc. Exp. Biol. Med. 1991;196(3):293–300. doi: 10.3181/00379727-196-43191. [DOI] [PubMed] [Google Scholar]
- 27.Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol. Reprod. 1998;59:1143–1152. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- 28.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30(1):75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soto AM, Lin T-M, Sakabe K, Olea N, Damassa DA, Sonnenschein C. Variants of the human prostate LNCaP cell line as a tool to study discrete components of the androgen-mediated proliferative response. Oncology Research. 1995;7:545–558. [PubMed] [Google Scholar]
- 30.Bouskine A, Nebout M, Brucker-Davis F, Benahmed M, Fenichel P. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect. 2009;117(7):1053–1058. doi: 10.1289/ehp.0800367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111(8):994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enciso GA, Smith HL, Sontag ED. Non-monotone systems decomposable into monotone systems with negative feedback. Journal of Differential Equations. 2005;224:205–227. [Google Scholar]
- 33.Tabb MM, Blumberg B. New modes of action for endocrine-disrupting chemicals. Mol Endocrinol. 2006;20(3):475–482. doi: 10.1210/me.2004-0513. [DOI] [PubMed] [Google Scholar]
- 34.Newbold RR, Padilla-Banks E, Snyder RJ, Phillips TM, Jefferson WN. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol. 2007;23(3):290–296. doi: 10.1016/j.reprotox.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147(6 Suppl):S11–17. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 36.Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. 1997;2(2):105–112. doi: 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- 37.Silveira PP, Portella AK, Goldani MZ, Barbieri MA. Developmental origins of health and disease (DOHaD) J Pediatr (Rio J) 2007;83(6):494–504. doi: 10.2223/JPED.1728. [DOI] [PubMed] [Google Scholar]
- 38.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 39.Naruse I, Keino H. Apoptosis in the developing CNS. Prog Neurobiol. 1995;47(2):135–155. doi: 10.1016/0301-0082(95)00024-p. [DOI] [PubMed] [Google Scholar]
- 40.Rice D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suk WA, Collman GW. Genes and the environment: their impact on children's health. Environ Health Perspect. 1998;106(Suppl 3):817–820. doi: 10.1289/ehp.98106817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huen K, Harley K, Brooks J, Hubbard A, Bradman A, Eskenazi B, Holland N. Developmental changes in PON1 enzyme activity in young children and effects of PON1 polymorphisms. Environ Health Perspect. 2009;117(10):1632–1638. doi: 10.1289/ehp.0900870. Epub 2009 Jun 1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assist environmental medicine physicians. Altern Med Rev. 2010;15(2):101–109. [PubMed] [Google Scholar]
- 44.Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, Godbold J, Biro F, Kushi LH, Pfeiffer CM, Calafat AM. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ. Health Perspect. 2007;115(1):116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Apelberg BJ, Goldman LR, Calafat AM, Herbstman JB, Kuklenyik Z, Heidler J, Needham LL, Halden RU, Witter FR. Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. Environ Sci Technol. 2007;41(11):3891–3897. doi: 10.1021/es0700911. [DOI] [PubMed] [Google Scholar]
- 47.Lovasi GS, Quinn JW, Rauh VA, Perera FP, Andrews HF, Garfinkel R, Hoepner L, Whyatt R, Rundle A. Chlorpyrifos exposure and urban residential environment characteristics as determinants of early childhood neurodevelopment. Am J Public Health. 2011;101(1):63–70. doi: 10.2105/AJPH.2009.168419. Epub 2010 Mar 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118(5):712–719. doi: 10.1289/ehp.0901340. Epub 2010 Jan 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of disease and dysfunction. Reprod Toxicol. 2007;23(3):257–259. doi: 10.1016/j.reprotox.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Grandjean P, Heindel JJ. In utero and early-life conditions and adult health and disease. N Engl J Med. 2008;359(14):1523. doi: 10.1056/NEJMc081629. author reply 1524. [DOI] [PubMed] [Google Scholar]
- 51.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115(10):1406–1414. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, Rubin BS, Soto AM. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol. 2008;26:210–219. doi: 10.1016/j.reprotox.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heindel JJ, Newbold R. Early Life Origins of Human Health and Disease Karger. Basel: 2009. Developmental origins of health and disease: the importance of environmental exposures; pp. 42–51. [Google Scholar]
- 54.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szyf M. The dynamic epigenome and its implications in toxicology. Toxicol Sci. 2007;100(1):7–23. doi: 10.1093/toxsci/kfm177. Epub 2007 Aug 2003. [DOI] [PubMed] [Google Scholar]
- 56.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21(4):214–222. doi: 10.1016/j.tem.2009.12.007. Epub 2010 Jan 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schar P, Fritsch O. DNA repair and the control of DNA methylation. Prog Drug Res. 2011;67:51–68. doi: 10.1007/978-3-7643-8989-5_3. [DOI] [PubMed] [Google Scholar]
- 58.Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102(2):90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 59.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31(2):89–97. doi: 10.1016/j.tibs.2005.12.008. Epub 2006 Jan 2005. [DOI] [PubMed] [Google Scholar]
- 60.Tang WY, Ho SM. Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metab Disord. 2007;8(2):173–182. doi: 10.1007/s11154-007-9042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitelaw NC, Whitelaw E. Transgenerational epigenetic inheritance in health and disease. Curr Opin Genet Dev. 2008;18(3):273–279. doi: 10.1016/j.gde.2008.07.001. Epub 2008 Aug 2012. [DOI] [PubMed] [Google Scholar]
- 62.Newbold RR. Prenatal exposure to diethylstilbestrol (DES) Fertil Steril. 2008;89(2 Suppl):e55–56. doi: 10.1016/j.fertnstert.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 63.Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 2000;21(7):1355–1363. [PubMed] [Google Scholar]
- 64.Titus-Ernstoff L, Troisi R, Hatch EE, Hyer M, Wise LA, Palmer JR, Kaufman R, Adam E, Noller K, Herbst AL, Strohsnitter W, Cole BF, Hartge P, Hoover RN. Offspring of women exposed in utero to diethylstilbestrol (DES): a preliminary report of benign and malignant pathology in the third generation. Epidemiology. 2008;19(2):251–257. doi: 10.1097/EDE.0b013e318163152a. [DOI] [PubMed] [Google Scholar]
- 65.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reprod Toxicol. 2011;31(3):337–343. doi: 10.1016/j.reprotox.2010.10.012. Epub 2010 Nov 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yaoi T, Itoh K, Nakamura K, Ogi H, Fujiwara Y, Fushiki S. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun. 2008;376(3):563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 68.Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, Ho SM. Relation of DNA methylation of 5'-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009;4(2):e4488. doi: 10.1371/journal.pone.0004488. Epub 2009 Feb 4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Novikova SI, He F, Bai J, Cutrufello NJ, Lidow MS, Undieh AS. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One. 2008;3(4):e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andersen HR, Schmidt IM, Grandjean P, Jensen TK, Budtz-Jorgensen E, Kjaerstad MB, Baelum J, Nielsen JB, Skakkebaek NE, Main KM. Impaired reproductive development in sons of women occupationally exposed to pesticides during pregnancy. Environ Health Perspect. 2008;116(4):566–572. doi: 10.1289/ehp.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 72.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114(4):567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guerrero-Bosagna CM, Sabat P, Valdovinos FS, Valladares LE, Clark SJ. Epigenetic and phenotypic changes result from a continuous pre and post natal dietary exposure to phytoestrogens in an experimental population of mice. BMC Physiol. 2008;8:17. doi: 10.1186/1472-6793-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE., Jr. Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol. 1994;126(2):276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- 76.Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J Androl. 2006;27(6):868–879. doi: 10.2164/jandrol.106.000349. Epub 2006 Jul 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andre SM, Markowski VP. Learning deficits expressed as delayed extinction of a conditioned running response following perinatal exposure to vinclozolin. Neurotoxicol Teratol. 2006;28(4):482–488. doi: 10.1016/j.ntt.2006.04.002. Epub 2006 Jun 2009. [DOI] [PubMed] [Google Scholar]
- 78.Ottinger MA, Lavoie E, Thompson N, Barton A, Whitehouse K, Barton M, Abdelnabi M, Quinn M, Jr., Panzica G, Viglietti-Panzica C. Neuroendocrine and behavioral effects of embryonic exposure to endocrine disrupting chemicals in birds. Brain Res Rev. 2008;57(2):376–385. doi: 10.1016/j.brainresrev.2007.08.011. Epub 2007 Sep 2019. [DOI] [PubMed] [Google Scholar]
- 79.Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104(14):5942–5946. doi: 10.1073/pnas.0610410104. Epub 2007 Mar 5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ottinger MA, Quinn MJ, Jr., Lavoie E, Abdelnabi MA, Thompson N, Hazelton JL, Wu JM, Beavers J, Jaber M. Consequences of endocrine disrupting chemicals on reproductive endocrine function in birds: establishing reliable end points of exposure. Domest Anim Endocrinol. 2005;29(2):411–419. doi: 10.1016/j.domaniend.2005.02.038. Epub 2005 Apr 2007. [DOI] [PubMed] [Google Scholar]
- 81.Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3(11):e3745. doi: 10.1371/journal.pone.0003745. Epub 2008 Nov 3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maul RW, Gearhart PJ. AID and somatic hypermutation. Adv Immunol. 2010;105:159–191. doi: 10.1016/S0065-2776(10)05006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delbes G, Levacher C, Habert R. Estrogen effects on fetal and neonatal testicular development. Reproduction. 2006;132(4):527–538. doi: 10.1530/rep.1.01231. [DOI] [PubMed] [Google Scholar]
- 84.Vos JG, Dybing E, Greim HA, Ladefoged O, Lambre C, Tarazona JV, Brandt I, Vethaak AD. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation. Crit Rev Toxicol. 2000;30(1):71–133. doi: 10.1080/10408440091159176. [DOI] [PubMed] [Google Scholar]
- 85.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16(5):972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 86.Foster PM. Mode of action: impaired fetal leydig cell function--effects on male reproductive development produced by certain phthalate esters. Crit Rev Toxicol. 2005;35(8-9):713–719. doi: 10.1080/10408440591007395. [DOI] [PubMed] [Google Scholar]
- 87.Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29(1):140–147. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181-145. Epub 2005 Aug 2011. [DOI] [PubMed] [Google Scholar]
- 88.Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365(1546):1697–1712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani DC, Hauser R. Phthalate exposure and human semen parameters. Epidemiology. 2003;14(3):269–277. [PubMed] [Google Scholar]
- 90.Mocarelli P, Gerthoux PM, Patterson DG, Jr., Milani S, Limonta G, Bertona M, Signorini S, Tramacere P, Colombo L, Crespi C, Brambilla P, Sarto C, Carreri V, Sampson EJ, Turner WE, Needham LL. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116(1):70–77. doi: 10.1289/ehp.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abell A, Ernst E, Bonde JP. Semen quality and sexual hormones in greenhouse workers. Scand J Work Environ Health. 2000;26(6):492–500. doi: 10.5271/sjweh.573. [DOI] [PubMed] [Google Scholar]
- 92.Juhler RK, Larsen SB, Meyer O, Jensen ND, Spano M, Giwercman A, Bonde JP. Human semen quality in relation to dietary pesticide exposure and organic diet. Arch Environ Contam Toxicol. 1999;37(3):415–423. doi: 10.1007/s002449900533. [DOI] [PubMed] [Google Scholar]
- 93.Oliva A, Spira A, Multigner L. Contribution of environmental factors to the risk of male infertility. Hum Reprod. 2001;16(8):1768–1776. doi: 10.1093/humrep/16.8.1768. [DOI] [PubMed] [Google Scholar]
- 94.Larsen SB, Giwercman A, Spano M, Bonde JP. A longitudinal study of semen quality in pesticide spraying Danish farmers. The ASCLEPIOS Study Group. Reprod Toxicol. 1998;12(6):581–589. doi: 10.1016/s0890-6238(98)00047-1. [DOI] [PubMed] [Google Scholar]
- 95.Padungtod C, Savitz DA, Overstreet JW, Christiani DC, Ryan LM, Xu X. Occupational pesticide exposure and semen quality among Chinese workers. J Occup Environ Med. 2000;42(10):982–992. doi: 10.1097/00043764-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 96.Lifeng T, Shoulin W, Junmin J, Xuezhao S, Yannan L, Qianli W, Longsheng C. Effects of fenvalerate exposure on semen quality among occupational workers. Contraception. 2006;73(1):92–96. doi: 10.1016/j.contraception.2005.06.067. Epub 2005 Nov 2016. [DOI] [PubMed] [Google Scholar]
- 97.Whorton MD, Milby TH, Stubbs HA, Avashia BH, Hull EQ. Testicular function among carbaryl-exposed exployees. J Toxicol Environ Health. 1979;5(5):929–941. doi: 10.1080/15287397909529802. [DOI] [PubMed] [Google Scholar]
- 98.Swan SH, Kruse RL, Liu F, Barr DB, Drobnis EZ, Redmon JB, Wang C, Brazil C, Overstreet JW. Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect. 2003;111(12):1478–1484. doi: 10.1289/ehp.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meeker JD, Ryan L, Barr DB, Herrick RF, Bennett DH, Bravo R, Hauser R. The relationship of urinary metabolites of carbaryl/naphthalene and chlorpyrifos with human semen quality. Environ Health Perspect. 2004;112(17):1665–1670. doi: 10.1289/ehp.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine. 1971;284(15):878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 101.Barclay DL. Congenital diethylstilbestrol-associated vaginal/cervical adenosis (DES babies) J Ark Med Soc. 1979;75(12):451–452. [PubMed] [Google Scholar]
- 102.Goldberg JM, Falcone T. Effect of diethylstilbestrol on reproductive function. Fertil Steril. 1999;72(1):1–7. doi: 10.1016/s0015-0282(99)00153-3. [DOI] [PubMed] [Google Scholar]
- 103.Hatch EE, Troisi R, Wise LA, Hyer M, Palmer JR, Titus-Ernstoff L, Strohsnitter W, Kaufman R, Adam E, Noller KL, Herbst AL, Robboy S, Hartge P, Hoover RN. Age at natural menopause in women exposed to diethylstilbestrol in utero. Am J Epidemiol. 2006;164(7):682–688. doi: 10.1093/aje/kwj257. Epub 2006 Aug 2003. [DOI] [PubMed] [Google Scholar]
- 104.Newbold RR, Jefferson WN, Banks EP. Endocrine Society. San Diego, CA: 1999. Developmental exposure to low doses of diethylstilbestrol (DES) results in permanent alterations in the reproductive tract; p. 261. [Google Scholar]
- 105.Newbold RR, Jefferson WN, Grissom SF, Padilla-Banks E, Snyder RJ, Lobenhofer EK. Developmental exposure to diethylstilbestrol alters uterine gene expression that may be associated with uterine neoplasia later in life. Mol Carcinog. 2007;46(9):783–796. doi: 10.1002/mc.20308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199(2):142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 107.Miyagawa S, Sato M, Iguchi T. Molecular mechanisms of induction of persistent changes by estrogenic chemicals on female reproductive tracts and external genitalia. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 108.Kim H, Hayashi S, Chambon P, Watanabe H, Iguchi T, Sato T. Effects of diethylstilbestrol on ovarian follicle development in neonatal mice. Reprod Toxicol. 2009;27(1):55–62. doi: 10.1016/j.reprotox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 109.Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE. Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology. 2007;148(5):1968–1976. doi: 10.1210/en.2006-1083. Epub 2007 Jan 1925. [DOI] [PubMed] [Google Scholar]
- 110.Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ., Jr. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90(4):911–940. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Braw-Tal R. Endocrine disruptors and timing of human exposure. Pediatr Endocrinol Rev. 2010;8(1):41–46. [PubMed] [Google Scholar]
- 112.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Hagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol A causes meiotic aneuploidy in the female mouse, Current Biology. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 113.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 114.Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(1):162–168. doi: 10.1210/jc.2007-1834. Epub 2007 Oct 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jefferson WN, Padilla-Banks E, Newbold RR. Adverse Effects on Female Development and Reproduction in CD-1 Mice Following Neonatal Exposure to the Phytoestrogen Genistein at Environmentally Relevant Doses. Biol Reprod. 2005 June 1; doi: 10.1095/biolreprod.105.041277. Online. [DOI] [PubMed] [Google Scholar]
- 116.Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol. Dev. 2003;5(1):67–75. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 117.Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, Soto AM. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146(9):4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 119.Bonassi S, Znaor A, Norppa H, Hagmar L. Chromosomal aberrations and risk of cancer in humans: an epidemiologic perspective. Cytogenet Genome Res. 2004;104(1-4):376–382. doi: 10.1159/000077519. [DOI] [PubMed] [Google Scholar]
- 120.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109(7):675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 122.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94(4):435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 123.Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41(7):483–490. [PubMed] [Google Scholar]
- 124.Everitt JI, Wolf DC, Howe SR, Goldsworthy TL, Walker C. Rodent model of reproductive tract leiomyomata. Clinical and pathological features. Am J Pathol. 1995;146(6):1556–1567. [PMC free article] [PubMed] [Google Scholar]
- 125.Newbold RR, Moore AB, Dixon D. Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES) Toxicol Pathol. 2002;30(5):611–616. doi: 10.1080/01926230290105839. [DOI] [PubMed] [Google Scholar]
- 126.Wise LA, Palmer JR, Stewart EA, Rosenberg L. Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women's Health Study. Obstet Gynecol. 2005;105(3):563–568. doi: 10.1097/01.AOG.0000154161.03418.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baird DD, Newbold R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod Toxicol. 2005;20(1):81–84. doi: 10.1016/j.reprotox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 128.Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7(6):346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- 129.Alonso-Magdalena P, Ropero AB, Soriano S, Quesada I, Nadal A. Bisphenol-A: a new diabetogenic factor? Hormones (Athens) 2010;9(2):118–126. doi: 10.1007/BF03401277. [DOI] [PubMed] [Google Scholar]
- 130.Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. Int J Epidemiol. 2002;31(2):413–419. [PubMed] [Google Scholar]
- 131.Grun F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009;304(1-2):19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006;20(9):2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 133.Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol. 2005;67(3):766–774. doi: 10.1124/mol.104.008409. [DOI] [PubMed] [Google Scholar]
- 134.Inadera H, Shimomura A. Environmental chemical tributyltin augments adipocyte differentiation. Toxicol Lett. 2005;159(3):226–234. doi: 10.1016/j.toxlet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 135.Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol. 2010;24(3):526–539. doi: 10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, Rosenfeld MG, Willson TM, Glass CK, Milburn MV. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395(6698):137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 138.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 139.Shadid S, Jensen MD. Effects of pioglitazone versus diet and exercise on metabolic health and fat distribution in upper body obesity. Diabetes Care. 2003;26(11):3148–3152. doi: 10.2337/diacare.26.11.3148. [DOI] [PubMed] [Google Scholar]
- 140.Hectors TL, Vanparys C, van der Ven K, Martens GA, Jorens PG, Van Gaal LF, Covaci A, De Coen W, Blust R. Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. Diabetologia. 2011;54(6):1273–1290. doi: 10.1007/s00125-011-2109-5. [DOI] [PubMed] [Google Scholar]
- 141.Grun F, Blumberg B. Minireview: the case for obesogens. Mol Endocrinol. 2009;23(8):1127–1134. doi: 10.1210/me.2008-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Colborn T. TEDX List of Potential Endocrine Disruptors. [August 3, 2011];The Endocrine Disruptor Exchange. ( http://www.endocrinedisruption.com/endocrine.TEDXList.overview.php)