Abstract
Schizophrenia patients (SZ) show early visual processing deficits in many, but not all, tasks. These deficits may be associated with dysregulation of intrinsic oscillatory activity that compromises signal-to-noise in the SZ brain. This question was studied using visual steady-state stimulation and post-steady-state presentation of transient visual stimuli. SZ had higher intrinsic oscillatory activity at the steady-state stimulation frequency (12.5 Hz) and at the 6.25 Hz subharmonic, showed a significant decrease in visual steady-state magnitude over 2 sec of stimulation, and were unable to promptly terminate the steady-state response following stimulation offset. If adjustment for levels of intrinsic brain activity were made, however, it would have appeared that SZ had activity of similar magnitude as healthy subjects following steady-state stimulus termination, indicating that such adjustments could substantially alter theoretical interpretations. Visual evoked potential abnormalities (N1/P2 amplitudes) present among SZ at the initiation of steady-state stimulation were less apparent in the 750 ms immediately following steady-state stimulation offset. Higher intrinsic oscillatory brain activity may be a fundamental characteristic of SZ that merits further evaluation for understanding this disorder’s neuropathological correlates and associated symptomatology.
Keywords: visual, VEP, steady-state, wavelets, baseline correction, intrinsic neural activity
1. Introduction
Schizophrenia patients (SZ) have early visual processing deficits under some, but not all, stimulus conditions (Butler et al., 2008; Clementz et al., 2008; Kieffaber et al., 2007; Luck and Gold, 2008; Wang et al., 2010). For instance, lower-tier (primary and extrastriate) visual electrocortical facilitation and attentional selectivity are relatively unimpaired in SZ when visual attention and target detection processes are differentiated (Clementz et al., 2008; Kieffaber et al., 2007). SZ also may have difficulty modulating gain (response amplitude relative to intrinsic neural activity) in the visual system (Clementz et al., 2008; Butler & Javitt, 2005). These diverse findings suggest that SZ visual processing abnormalities are a function of complex interactions between brain regions (Kemner et al., 2009) that are supported by oscillatory activities within cortical neural networks (Uhlhaas & Singer, 2006). To develop a better understanding of the neural basis for early visual processing deficits in SZ, it may be useful to study the oscillatory capability of their visual cortical neurons.
Oscillatory activity in visual cortex can be effectively evaluated by measuring the steady-state visual evoked potential (ssVEP) with electroencephalography (EEG). The ssVEP is brain activity that evolves to equal the stimulus flicker rate (Regan, 1989). In contrast to visual evoked potentials (VEPs) where changes occur at many frequencies, ssVEPs occur predominantly at known frequencies of interest, and are characterized by high signal-to-noise ratios (Mast and Victor, 1991), even among SZ (Clementz et al., 2008). At steady-state stimulus onset there is still a VEP (Clementz et al., 2004; Moratti et al., 2007), but after a few hundred ms, the neural response is primarily at the flicker frequency with neural sources in, or close to, lower-tier visual cortex (Di Russo et al., 2006; Clementz et al., 2008).
Few studies have evaluated the ssVEP in SZ (e.g., Clementz et al., 2004; Jin et al., 1990; Krishnan et al., 2005; Riečanský et al., 2010; see Brenner et al., 2009, for recent review). Patients have shown reduced amplitude ssVEPs in response to flickering stimuli in the theta, high alpha, beta, and gamma frequency ranges (Brenner et al., 2009). In response to extended steady-state stimulation (2 sec), however, Riečanský et al. (2010) found enhanced initial ssVEP in the gamma range among SZ that appeared to settle to more normal levels over time; SZ also had accentuated high alpha-to-low beta range oscillations following steady-state offset (a lingering oscillation effect reminiscent of Clementz et al., 2004). Interestingly, intrinsic pre-stimulus neural activity (see, e.g., Fox et al., 2007) among SZ also tends to be enhanced in lower (theta to alpha) frequencies. Most of the above studies (save Riečanský et al., 2010) considered the ssVEP to be a stable event (one that is stationary from beginning to end), even though this response evolves over the stimulation period (Moratti et al., 2007).
The present report addressed three issues. First, SZ often differ from healthy subjects on intrinsic prestimulus neural activity across many frequency bands (Clementz et al., 2008; Wang et al., 2010; Winterer et al., 2004). SZ also differ from other groups on instrinsic low frequency neural activity at rest (Clementz et al., 1994), but this difference is rarely considered and adjustments for baseline activity are routinely employed in studies of neural oscillations (see Brenner et al., 2009, for a discussion). It is important to consider, therefore, whether baseline adjustments contribute to group effects on visual oscillatory activity. Second, given that the ssVEP develops over stimulation time, differences in its evolution should be investigated to evaluate the stability of this visual sensory response among SZ. Third, Clementz et al. (2004) showed that the SZ visual system recovers sluggishly from stimulus processing. If so, subsequent stimulus registration may be affected. Presenting transient visual stimuli in the period immediately after steady-state offset provides a novel means for addressing this issue (Moratti et al., 2007).
2. Materials and Methods
Twelve right-handed chronic outpatient SZ (4 females; mean age = 36.4 years; Global Assessment of Functioning score M=34, SD=4) and twelve right-handed healthy (6 females; mean age = 35.2 years) participants were recruited from the community. SZ were clinically stable on atypical antipsychotic medications for >8 weeks prior to participation. Subjects were interviewed with the SCID (First et al., 1995) by two psychologists to either verify their clinical diagnosis (SZ) or rule out Axis I disorders (healthy subjects). Participants were absent of neurological hard signs, possibly confounding treatments for electrophysiological measurements (such as ECT or benzodiazepines), history of head trauma and current psychoactive substance use disorders. The study was approved by the Institutional Review Board at the University of Georgia; participants provided written informed consent prior to study involvement and were paid $10 US.
Subjects viewed steady-state stimuli on a 19-inch computer monitor (refresh rate 100 Hz) positioned 80 cm from their eyes. Stimuli were two red 8 × 8 checkerboards (four 1.2 cm square red and black boxes in alternating sequence), one each in left and right visual fields (same as Moratti et al., 2007). Checkerboards were positioned so their inner borders were 6.8 degrees from central fixation, and were luminance-modulated (40 ms on, 40 ms off) at a fixed rate of 12.5 Hz for 2000 ms. This oscillation frequency allowed for evaluation of both ssVEP development and lingering oscillatory effects following steady-state offset (Clementz et al., 2004; Moratti et al., 2007; Riečanský et al., 2010). Transient stimuli were presented at three times relative to steady-state offset: +240 (T240 condition), +480 (T480 condition), and +720 ms (T720 condition), and consisted of 9.6 cm square solid red blocks presented at central fixation for 240 ms. All trials were presented in one block of randomly interleaved trials, with 66 trials of each condition. Six trials of each condition were targets (the transient stimulus was pink instead of red). Subjects were to simultaneously press buttons on a response pad with their left and right index fingers upon target appearance; these trials were not included in analyses (their purpose was encourage attention to the task). Trials were followed by a random 6 to 10 sec interval. Participants briefly practiced the task to ensure their ability to discriminate pink and red boxes. Both groups performed at 100% target detection accuracy, and there was no between-groups difference on latency (based on F-tests).
EEG data were continuously recorded using a 257 sensor Electrical Geodesics net, digitized at 250 Hz, analog filtered from .01 - 100 Hz, and referenced to Cz. Sensor impedances were kept below 50 kΩ. Sensors at the outer canthi and above and below the eyes recorded eye movements and blinks. Sensors on the neck and cheeks were excluded, leaving 216 for further analyses. Blink and heart-rate artifacts were corrected using BESA (Berg and Scherg, 1994). Data were average-referenced, and filtered (zero phase) from .5 - 75 Hz. Epochs of 4000 ms including a 500 ms baseline were scored using BESA and Matlab 7.7 (Mathworks, Natick, MA).
2.1 ssVEP scoring
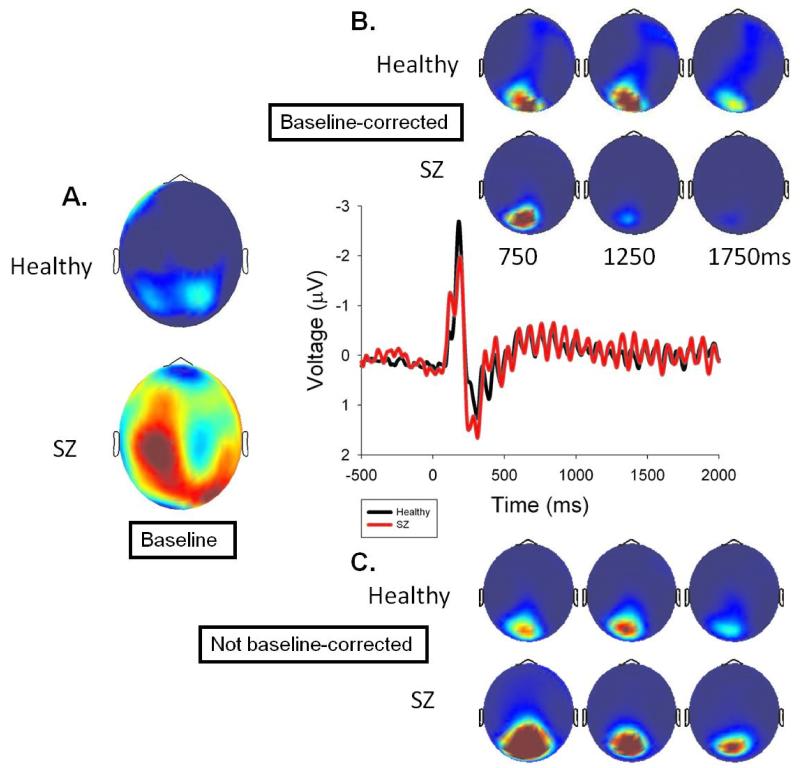
Grand averages during steady-state stimulation at the driving frequency (across all conditions because subjects did not know the transient condition at this point, so ssVEP was unaffected by condition) were analyzed using a Morlet wavelet with 3 cycles at 6.25 Hz (1/2f), 4 cycles at 12.5 Hz (1f), and 5 cycles at 25 Hz (2f), with wavelets centered on every time point, in order to balance time resolution at the lower frequencies with stability at the higher frequencies (Busch & VanRullen, 2010). The resulting power values from 29 sensors that captured the peak ssVEP activity (from the grand average topography across groups over the entire steady state stimulation period; see Figure 1) were analyzed both before and after baseline correction starting 500 ms after steady-state onset (to ensure establishment of ssVEP) in 500 ms bins (midpoints of 750, 1250, and 1750 ms; see Figure 1).
Figure 1.
Steady-state VEP from a sensor in the middle of the activated region over dorsal visual cortex. A.) Topography of baseline power at the driving frequency for healthy and SZ participants. B.) Baseline corrected grand average power topographies for healthy and SZ participants in 500 ms bins centered as indicated. B.) Same topographies without baseline correction.
2.2 VEP scoring
Data were filtered (zero phase) from 2 - 30 Hz with a 12.5 Hz notch (1 Hz width) to reduce ssVEP contribution to VEPs (although results were the same without the notch filter). EEG data were averaged across trials using 750 ms windows (including a 250 ms baseline). To integrate data recorded from every sensor, spatial principal components analysis (PCA) was implemented. For each group average and condition, a 216×216 covariance matrix was calculated (using time points as observations) and PCA was calculated with promax vector rotation and Kaiser normalization (Dien, 2006). Scree plots for each group and condition always identified 2 components, with the spatial distributions of these components and amount of variance accounted for (always close to 90%) being nearly identical between groups and within condition.
There was remarkable between-groups similarity in spatial distribution and factors loadings of (i) VEPs to onset of peripheral steady-state stimuli and (ii) across VEPs to all three central transient (post steady-state) conditions. Therefore, a grand average was created (i) across groups for VEPs to steady-state onset and (ii) across groups and conditions for VEPs to central post steady-state transients. This allowed for creation of spatial PCA components that were directly comparable between-groups and across conditions (post steady-state transients). For steady-state onset, the first two components accounted for 72.9% and 18.8% of the VEP variance; for post steady-state transients, the first two components accounted for 68.4% and 20.4% of the VEP variance.
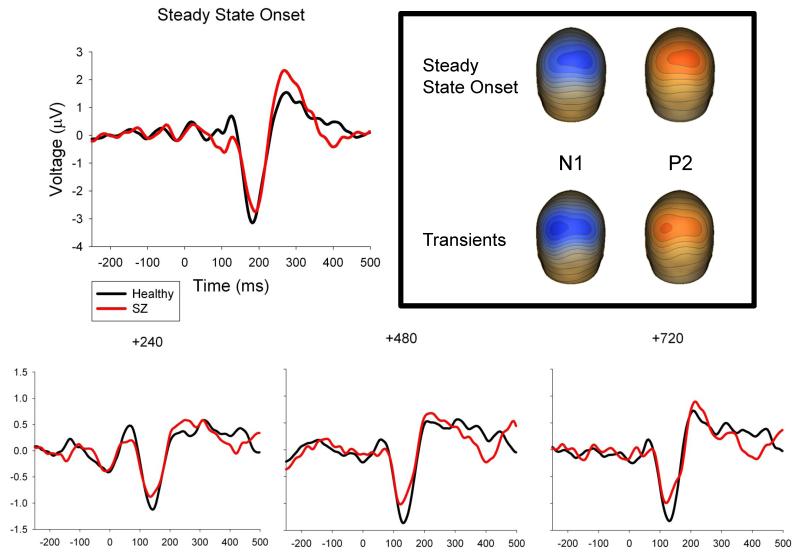
For both PCAs, factor weights were multiplied by each subject’s VEP data, summed across the sensors, and divided by the sum of the factor weights. For steady-state onset, eigenvalue-weighted averages of the two PCA factor waveforms were computed for each subject yielding a single waveform (Figure 2). For post steady-state transients, eigenvalue-weighted averages of the two PCA factor waveforms were computed for each subject and transient condition (T240, T480, and T720) yielding three different waveforms (Figure 2). N1 and P2 VEPs could be identified in these waveforms for all subjects and conditions; P1 was not similarly identifiable for all subjects and conditions so it was not scored. For every subject and condition, VEP peak amplitudes and latencies were quantified at the peaks closest in time to the grand average responses.
Figure 2.
ERPs to steady-state stimulus onset and transient stimuli. ERPs represent the weighted average of the sensor weights for the first two PCA components for that condition applied to each individual’s data and then averaged over the entire head. Topographies illustrate the weighted average of the first two PCA components for the steady-state onset and the averaged transient stimuli at the N1 and P2 peaks. Transient ERPs are differentially scaled from steady-state onset ERP in order to best show the peaks of interest, therefore absolute differences in peak amplitude between groups should be noted according to the scale.
3. Results
3.1 ssVEP analyses
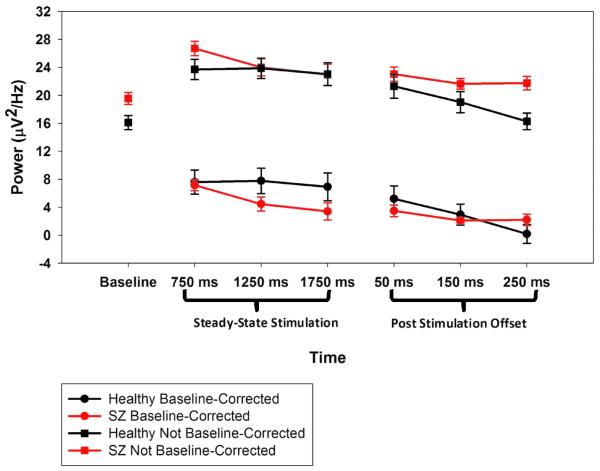
At 6.25 Hz (1/2f), SZ had higher baseline activity (M=20.0 μV2/Hz, SD=3.6) than healthy subjects (M=16.7 μV2/Hz, SD=3.6), t(22)=2.3, p=.03; the same was true at 12.5 Hz (1f), t(22)=2.6, p=.018 (Figures 1 and 3). Only the 12.5 Hz driving frequency, however, showed a significant ssVEP, or increase from baseline, following steady-state onset, F(1,22)= 60.5, p<.001, so subsequent statistical analyses were restricted to this frequency. There were significant Group by Time Bin interactions both with and without baseline adjustment, F(2,44)=6.9, p=.008, ε=.24 (see Figures 1 and 3). Simple main effects analyses showed an effect of bin for SZ, F(2,22)= 14.2, p=.001, ε=.56, but not for healthy subjects, F(2,22)=1.2, p=.315, ε=.09. For SZ; the 750 ms time bin had significantly stronger magnitude than the 1750 ms time bin, t(1,11)=3.89, p=.003.
Figure 3.
Dynamics of non-baseline-corrected power at the driving frequency across time for healthy and SZ participants. Time labels on the X axis represent the baseline period (−324 to −50 ms pre-stimulus), centers of 500 ms windows during steady-state stimulation, and 100 ms windows for the period following steady-state stimulation offset.
Clementz et al. (2004) showed that SZ had prolonged ssVEP activity, so linear contrasts were performed on three 100 ms bins (prior to VEP activity associated with the first transient) after steady-state stimulation offset. Healthy subjects, F(1,11)=12.5, p=.005, but not SZ, F(1,11)=1.4, p=.262, had a significant linear decrease in 12.5 Hz power during this interval (Figure 3). This effect was evident in a Time Bin by Group interaction, F(1,22)=3.6, p=.04, for both baseline corrected and non-baseline corrected data. For non-baseline corrected data, however, there was also a main effect of Group, F(1,22)= 4.5, p=.045, indicating that SZ power at the driving frequency remained higher than that of healthy subjects.
3.2 VEP analyses
At steady-state onset, there was a main effect of group on VEP amplitudes, F(1,22)= 5.1, p=.034, with healthy subjects having larger N1s (M=−1.6 μV, SD=0.7) than SZ (M=−1.3 μV, SD=0.6), and smaller P2s (M=0.9 μV, SD=0.6) than SZ (M=1.2 μV, SD=0.5). There were no significant differences between-groups on VEP amplitudes to the transients (Figure 2). There were also no group differences on VEP latencies at either steady-state onset or to the post-steady-state transients (Figure 2).
4. Discussion
This investigation yielded four noteworthy outcomes. First, SZ had higher intrinsic neural activity at both the 12.5 Hz steady-state frequency and its 6.25 Hz subharmonic than healthy persons; adjusting for this difference had implications for SZ ssVEP characteristics (see below). Second, SZ showed a significant decrease in ssVEP magnitude over the course of steady-state stimulation. Third, after termination of the steady-state stimulus, healthy persons returned to near baseline 12.5 Hz magnitude within 300 ms, but SZ had continued oscillatory activity that did not decrement over this same interval (Clementz et al., 2004). Fourth, group differences in stimulus registration (N1/P2 amplitudes) at steady state onset were attenuated to transients occurring after steady-state offset. The implications of these findings for understanding SZ neuropathology are briefly discussed below.
High levels of intrinsic neural activity characterize SZ (Clementz et al., 2004; Clementz et al, 2008; Krishnan et al. 2005; Rolls et al., 2008; Winterer et al., 2004), and might reveal a constitutionally mediated core deficit in SZ cortical functioning (Rolls et al., 2008). Intrinsic (not stimulus evoked, although perhaps stimulus-related) neural activity can be measured in multiple ways; nevertheless, studies in which such data are reported agree that SZ are elevated on this parameter compared to healthy subjects. This effect may be particularly evident in the theta to high-alpha range, although a systematic evaluation of this phenomenon across multiple frequencies has not appeared in the archival literature. Like in this report, which quantified activity in lower frequency ranges, high intrinsic neural oscillations can masquerade as accentuated responding in sensory cortices (Clementz et al., 2008; Spencer et al., 2004; Wang et al., 2010), but ultimately deleteriously affects signal-to-noise ratios. For instance, although SZ had modestly enhanced early ssVEP magnitudes (similar to that observed by Riečanský et al., 2010, in the gamma range), their percentage increase over baseline was sub-normal (38% for SZ versus 49% for healthy subjects); by the end of the steady-state stimulation period, the apparent difference in these values was more remarkable (19% for SZ versus 45% for healthy subjects). Diminished signal-to-noise could affect stimulus processing fidelity and perceptual experience, an issue that could be fruitfully addressed in subsequent investigations.
SZ did not maintain ssVEP at their initial magnitudes over the stimulation period. Without adjusting for baseline differences at 12.5 Hz, Figure 1 illustrates that SZ had modestly accentuated initial oscillatory responses that significantly diminished over time to equal those observed among healthy subjects (albeit with lower signal-to-noise). After baseline adjustment, however, SZ initial ssVEP appeared to be more similar to healthy persons but then significantly diminished over time to appear lower than healthy subjects. Use of baseline adjustment may depend on questions of interest; however, for understanding the nature of SZ ssVEP, baseline adjustments may be of limited value. Appreciating group differences on signal-to-noise may require separate quantification of baseline and ssVEP activity. For instance, it seems inaccurate to infer SZ had healthy-level initial ssVEP magnitudes (given signal-to-noise differences) even though this would be the conclusion when only analyzing baseline-adjusted data.
Consistent with other research, at steady-state onset SZ had smaller N1 (e.g., Clementz et al., 2004) and larger P2 (e.g., Krieger et al., 2001; Nagasawa et al., 1999) VEPs than healthy persons. In response to the post-steady-state transients, however, these effects were attenuated. The visual N1 is more closely related to sensory registration than the P2, which may be associated with stimulus classification processes (Garcia-Larrea et al., 1992). With increased attention, the N1 is typically enhanced but the P2 may be diminished in amplitude (Crowley and Colrain, 2004). The pattern observed here may be a consequence of a relative between-groups equalization of intrinsic brain activity in neurons tuned for the (central) transient stimuli location. Although SZ VEPs to the transient stimuli were still modestly different from those observed among healthy subjects, the groups did not differ significantly on transient VEP magnitudes, in contrast to what was observed for VEPs at steady-state onset. This phenomenon could be more completely investigated by manipulating the locations of transient stimuli in relation to steady-state stimulus location and timing (e.g., at the same or different location and during or following steady-state stimulation; see, e.g., Rockstroh et al., 1996). Future work will be needed to address whether there are differences in transient response amplitudes secondary to such manipulations.
One issue to consider is that after steady-state offset oscillatory activity among neurons tuned to the flickering stimuli locations was still not equal between-groups. With the present paradigm, we were unable to quantify intrinsic activity for neurons tuned specifically to the transient location. Perhaps enhancing neuronal investment at peripheral locations by steady-state stimulation modifies between-groups differences in intrinsic activity in neuronal pools tuned for other spatial locations. For instance, intrinsic activity is enhanced for neurons tuned to a spatial location requiring enhanced neural investment like the steady-state location in the present study (Ress et al., 2000), and the level of this enhancement correlates with task difficulty (Howard et al., 2003), with the same task perhaps requiring greater cognitive control for successful performance among SZ. Increasing cognitive control requirements may produce abnormally elevated intrinsic activity in specific neuronal pools among SZ, a maladaptive effort which may leave other neuronal pools unaffected. (see, e.g., Kapur, 2003). If so, transient stimuli may have been presented to SZ and healthy neuronal ensembles in more similar states of sensory processing readiness, which could reduce between-groups differences in neural responses to stimulus events.
Knowledge of SZ neuropathology may yield a partial explanation for the present findings. Low NMDA receptor function, perhaps a primary pathology in SZ (Kantrowitz & Javitt, 2010), prompts a secondary decrease of GABAergic interneuron activity, causing both disinhibition of pyramidal cells (Belforte et al., 2010; Gordon, 2010) and an inability to generate high signal-to-noise excitatory drive in cortical networks supporting specific stimulus processing requirements (Rolls et al., 2008; Tanaka, 2008; Yoon et al., 2010). Evidence for this ultimately maladaptive effort to restore homeostatic balance in local cortical circuits has been reported in multiple SZ brain regions, including visual cortex (Hashimoto et al., 2007). These cellular deviations are most evident in more superficial cortical layers where neurons support feature integration, communication with other cortical locations (Lewis, 2009), and are the most likely generators of N1/P2 VEPs. This neuropathology could account for both accentuated early visual processing in SZ (Clementz et al., 2008; Spencer et al., 2004; Wang et al., 2010) and lingering of the ssVEP after stimulus termination because such a system would be unable to efficiently recover from a bout of prolonged sensory processing (Clementz et al., 2004; Rolls et al., 2008).
Supplementary Material
Acknowledgement
The authors would like to acknowledge the National Institutes of Health for providing funding for this project.
Role of funding source Funding for this study was provided by NIH Grants MH057886 and MH051129. The NIH had no further role in development of the paradigm or interpretation of experimental findings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest The authors report no conflicts of interest.
References
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin Neurophysiol. 1994;90(3):229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, et al. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35(6):1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci U S A. 2010;107(37):16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18(2):151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008;64(1):40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Keil A, Kissler J. Aberrant brain dynamics in schizophrenia: delayed buildup and prolonged decay of the visual steady-state response. Brain Res Cogn Brain Res. 2004;18(2):121–129. doi: 10.1016/j.cogbrainres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sponheim SR, Iacono WG, Beiser M. Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology. 1994;31(5):486–494. doi: 10.1111/j.1469-8986.1994.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Wang J, Keil A. Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. J Neurosci. 2008;28(50):13411–13418. doi: 10.1523/JNEUROSCI.4095-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol. 2004;115(4):732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Taddei F, Apnile T, Spinelli D. Neural correlates of fast stimulus discrimination and response selection in top-level fencers. Neurosci Lett. 2006;408(2):113–118. doi: 10.1016/j.neulet.2006.08.085. [DOI] [PubMed] [Google Scholar]
- Dien J. Progressing towards a consensus on PCA of ERPs. Clin Neurophysiol. 2006;117(3):699–702. doi: 10.1016/j.clinph.2005.09.029. author reply 703-697. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for axis I DSM-IV disorders - patient edition (SCID-I/P) Biometrics Research Department, NY State Psychiatric Institute; New York: 1995. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56(1):171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Lukaszewicz AC, Mauguiere F. Revisiting the oddball paradigm. Non-target vs neutral stimuli and the evaluation of ERP attentional effects. Neuropsychologia. 1992;30(8):723–741. doi: 10.1016/0028-3932(92)90042-k. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Testing the glutamate hypothesis of schizophrenia. Nat Neurosci. 2010;13(1):2–4. doi: 10.1038/nn0110-2. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13(12):1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Jin Y, Potkin SG, Rice D, Sramek J, Costa J, Isenhart R, et al. Abnormal EEG responses to photic stimulation in schizophrenic patients. Schizophr Bull. 1990;16(4):627–634. doi: 10.1093/schbul/16.4.627. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kemner C, Foxe JJ, Tankink JE, Kahn RS, Lamme VA. Abnormal timing of visual feedback processing in young adults with schizophrenia. Neuropsychologia. 2009;47(14):3105–3110. doi: 10.1016/j.neuropsychologia.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP. Event related brain potential evidence for preserved attentional set switching in schizophrenia. Schizophr Res. 2007;93(1-3):355–365. doi: 10.1016/j.schres.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger S, Lis S, Gallhofer B. Reaction-times and bioelectrical brain signals of drug-naive schizophrenic first-onset patients in identification and classification tasks. Acta Psychiatr Scand Suppl. 2001;(408):42–59. doi: 10.1034/j.1600-0447.2001.104s408042.x. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, et al. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2005;116(3):614–624. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Neuroplasticity of excitatory and inhibitory cortical circuits in schizophrenia. Dialogues Clin Neurosci. 2009;11(3):269–280. doi: 10.31887/DCNS.2009.11.3/dalewis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64(1):34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast J, Victor JD. Fluctuations of steady-state VEPs: interaction of driven evoked potentials and the EEG. Electroencephalogr Clin Neurophysiol. 1991;78(5):389–401. doi: 10.1016/0013-4694(91)90100-i. [DOI] [PubMed] [Google Scholar]
- Moratti S, Clementz BA, Gao Y, Ortiz T, Keil A. Neural mechanisms of evoked oscillations: stability and interaction with transient events. Hum Brain Mapp. 2007;28(12):1318–1333. doi: 10.1002/hbm.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Kamiya T, Kawasaki Y, Higashima M, Urata K, Sakai N, et al. The relationship between auditory ERP and neuropsychological assessments in schizophrenia. Int J Psychophysiol. 1999;34(3):267–274. doi: 10.1016/s0167-8760(99)00083-5. [DOI] [PubMed] [Google Scholar]
- Regan D. Human brain electrophysiology: evoked potentials and evoked magnetic fields in science and medicine. Elsevier; New York: 1989. [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3(9):940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Riecansky I, Kasparek T, Rehulova J, Katina S, Prikryl R. Aberrant EEG responses to gamma-frequency visual stimulation in schizophrenia. Schizophr Res. 2010;124(1-3):101–109. doi: 10.1016/j.schres.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Rockstroh B, Muller M, Heinz A, Wagner M, Berg P, Elbert T. Modulation of auditory responses during oddball tasks. Biol Psychol. 1996;43(1):41–55. doi: 10.1016/0301-0511(95)05175-9. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9(9):696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101(49):17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S. Dysfunctional GABAergic inhibition in the prefrontal cortex leading to “psychotic” hyperactivation. BMC Neurosci. 2008;9:41. doi: 10.1186/1471-2202-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52(1):155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Wang J, Brown R, Dobkins KR, McDowell JE, Clementz BA. Diminished parietal cortex activity associated with poor motion direction discrimination performance in schizophrenia. Cereb Cortex. 2010;20(7):1749–1755. doi: 10.1093/cercor/bhp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161(3):490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30(10):3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.