Abstract
OBJECTIVE
Insulin resistance renders macrophages more prone to cholesterol-induced apoptosis by promoting nuclear localization of transcription factor FoxO1. But FoxO1 also decreases macrophage inflammation, raising the question of how is the balance between pro-apoptotic and anti-inflammatory effects determined. We sought to identify the mechanism whereby FoxO1 dampens inflammation without promoting apoptosis. We hypothesized that nutrient-dependent FoxO1 acetylation plays a role in this process.
METHODS & RESULTS
We generated knock-in mice bearing alleles that encode constitutively deacetylated FoxO1, and studied the ex vivo response of primary peritoneal macrophages. We show that macrophages derived from mice homozygous for constitutively deacetylated FoxO1 alleles retain anti-inflammatory properties in response to free cholesterol loading, without increasing apoptosis. Deacetylated FoxO1 inhibits free cholesterol-induced Akt phosphorylation and increases levels of the Nf-κB precursor p105, decreasing nuclear translocation of Nf-κB p65 subunit, and dampening Mek/Erk activation to prevent inflammation.
CONCLUSIONS
Deacetylated FoxO1 regulates p105 to prevent macrophage inflammation without causing apoptosis, suggesting a potential novel therapeutic approach to atherosclerosis through FoxO1 deacetylation.
Keywords: Genetically altered mice, Mechanism of atherosclerosis/growth factors, Type 2 diabetes, Apoptosis, Cell signaling/signal transduction
Complications from atherosclerotic cardiovascular (CV) disease are a leading cause of death 1. Macrophage inflammation in the vessel wall is a key event in the formation and rupture of atherosclerotic plaques. Various cytokines produced from lesional macrophages contribute to lesion progression 2.
In early atherosclerosis, macrophages take up modified cholesterol-rich lipoproteins, and store their cholesterol largely in esterified form, resulting in “foam cell” formation. As lesions advance, cholesteryl-ester content drops, while unesterified, or “free” cholesterol (FC) rises 3, 4, likely inducing endoplasmic reticulum (ER) stress and expression of transcription factor Chop 5. The in vivo relevance of ER stress-mediated Chop induction to atherosclerosis progression is demonstrated by studies in which Chop ablation halved the number of apoptotic lesional macrophages and reduced plaque size in ApoE−/− or Ldlr−/− mice 6. In addition to ER stress, FC activates the mitogen-activated kinase (Mapk, including Erk1/2, Jnk1/2, and p38) and Ikk/Nf-κB inflammatory pathways, possibly by inducing Tnf-α and Il-6 7.
Macrophages are insulin-sensitive cells 8. Defective macrophage insulin signaling predisposes to foam cell formation in insulin-resistant states 9, and impairs the ability of macrophages to relieve ER stress, resulting in greater macrophage apoptosis and plaque necrosis within advanced lesions 10. In macrophages, FoxO1, FoxO3 and FoxO4 appear to have dual functions, promoting apoptosis in the context of ER stress, but decreasing inflammation in response to FC 11 or LPS 12. FoxO1 activity is regulated by post-translational modifications to meet the cell’s metabolic demand or stress response 13. Falling nutrient levels activate FoxO1 through dephosphorylation and deacetylation, whereas rising nutrient levels inactivate FoxO1 through Akt-dependent phosphorylation, and Cbp/p300-dependent acetylation 14.
Purpose of this study was to understand the mechanism whereby FoxO1 activation can have effects that are seemingly at odds: more apoptosis and less inflammation. Our goal is to define approaches that leverage the anti-inflammatory actions of FoxO1 for therapeutic ends, without promoting apoptosis. It has been shown that hyperglycemia in type 2 diabetes leads to FoxO1 acetylation 15–17. Acetylation targets FoxO1 to nuclear PML bodies, where it undergoes deacetylation to become transcriptionally active 15. Less clear is the effect of FC signaling on macrophage FoxO1, and the role of FoxO1 acetylation in macrophage activation. To answer these questions, we generated knock-in mice bearing alleles that encode constitutively deacetylated FoxO1, and studied the ex vivo response of primary peritoneal macrophages to FC challenge. Here we show that deacetylated FoxO1 prevents FC-induced Akt phosphorylation and increases levels of p105, the precursor protein of Nf-κB subunits. p105, in turn, promotes Nf-κB p65 and p50 cytoplasmic retention and dampens Erk activation, decreasing inflammation. The data are consistent with a model in which FoxO1 deacetylation can uncouple inflammation from apoptosis in FC-loaded macrophages.
METHODS
Experimental animals
Acetylation-defective FoxO1 knock-in mice (FoxO1KR/KR) have been described 18.
Materials
A detailed list of manufacturers is provided in the online supplement.
Macrophage culture, FC loading, immunoprecipitation and immunoblotting
We harvested peritoneal macrophages from FoxO1+/+ or FoxO1KR/KR mice by peritoneal lavage 3 days after intraperitoneal injection of 4% thioglycolate, and cultured them in DMEM supplemented with 10% fetal bovine serum and 20% L929 cell-conditioned medium 19. We loaded cells with FC by incubation in medium supplemented with compound 58035 (10 mg/l) and acLDL (100 mg/l) for the indicated time periods 19. Protein analysis was carried out as described 11, 16.
Immunocytochemsitry
We performed FoxO1 immunocytochemstry as described 15. We measured apoptosis by Alexa 488-labeled annexin V and propidium iodide staining (Vybrant Apoptosis Assay kit, Invitrogen).
Quantitative RT-PCR and ELISA
We performed quantitative RT-PCR using DyNAmo HS SYBR Green qPCR Kit (Finnzymes) as described 15.
Adenoviruses
We have described adenoviruses encoding LacZ, GFP-tagged FoxO1-WT and FoxO1-KR, HA-tagged FoxO1-ADA, and nuclear factor-κB (Nf-κB)-luciferase 11, 15, 20, 21.
Luciferase assays
We measured luciferase activity in peritoneal macrophages transfected with Nf-κB reporter construct using Dual Luciferase reporter Assay System (Promega).
Statistical analysis
We show data as mean ± SE. We determined statistically significant differences (P < 0.05) using unpaired t-test or ANOVA with Dunn’s post hoc test.
RESULTS
FC loading induces FoxO1 acetylation in peritoneal macrophages
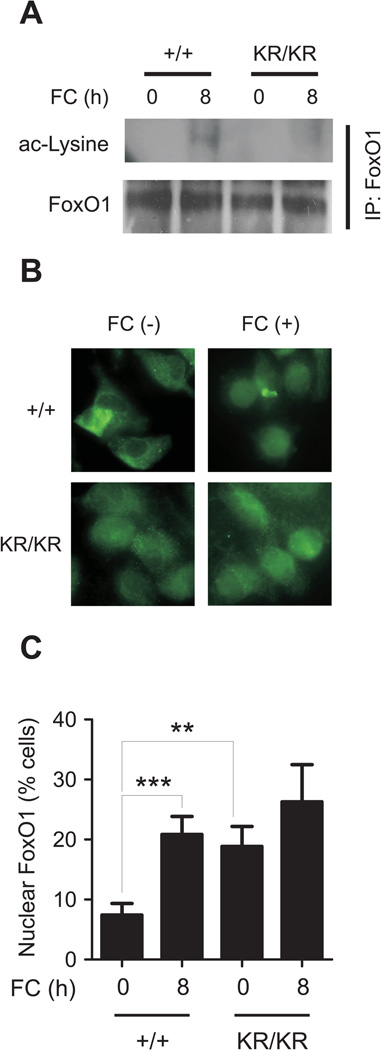
We examined the effect of FC loading on FoxO1 acetylation and subcellular localization. FC induced FoxO1 acetylation in FoxO1+/+ but not FoxO1KR/KR macrophages (Fig. 1A). The latter also showed increased nuclear FoxO1 in the basal state that was unaffected by FC loading, whereas the number of WT macrophages with predominantly nuclear FoxO1 doubled in response to FC loading (Fig. 1B, C). FoxO1 regulation by FC loading in macrophages is reminiscent of its regulation by high glucose/oxidative stress in pancreatic β-cells 15. The findings indicate that FC challenge phenocopies nutrient excess (high glucose) to promote FoxO1 acetylation, whereas deacetylation is required to keep FoxO1 in the nucleus.
Figure 1. FC-induced inflammatory cytokine expression and protein secretion in macrophages.
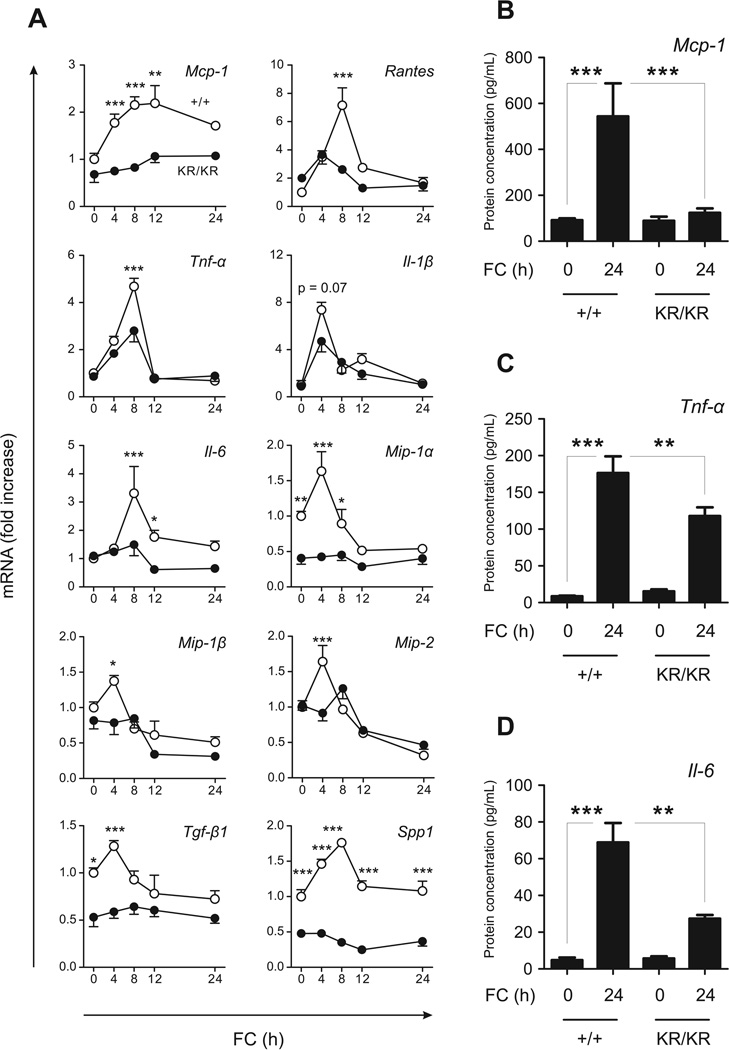
(A) Western blots of FC-loaded FoxO1+/+ (+/+) and FoxO1KR/KR macrophages (KR/KR) macrophages, immunoprecipitated (IP) with anti-FoxO1 antibody followed by immunoblotting with anti-acetyl-lysine (ac-Lysine) or total FoxO1 antibodies. (B) Representative immunohistochemical images of FoxO1 sub-cellular localization. (C) Percentage of cells with nuclear FoxO1. All images are representative of at least 3 independent experiments. Scale bar = 10 µm. ** P < 0.01 and *** P < 0.001 between the indicated groups. (D) Time course analyses of mRNAs expression in FoxO1+/+ (+/+, open circles) and FoxO1KR/KR macrophages (KR/KR, closed circles) loaded with FC (n=5). * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. +/+. (E–G) Peptide levels of Mcp-1 (E), Tnf-〈 (F), and Il-6 (G) in conditioned medium from macrophages loaded with FC for 24h (n=4). * P < 0.05, ** P < 0.01, and *** P < 0.001 between the indicated groups.
Blunted induction of inflammatory cytokines inFC-loaded FoxO1KR/KR macrophages
FC loading increased levels of mRNAs encoding Mcp-1, Tnf-α, Il-6, Il-1β, Rantes, Mip-1α, Mip-1β, Mip-2, Tgf-β1, and osteopontin (encoded by Spp1) between 2- and 8-fold in WT macrophages, but these effects were absent (Mcp-1, Tnf-α, Il-6, Mip-1β, Mip-2) or blunted (Rantes, Tnf-α, Il-1β, Mip-2) in FoxO1KR/KR macrophages (Fig. 1D). Levels of Mip-1α, Tgf-β1, and Spp1 were decreased also in the basal state in FoxO1KR/KR macrophages (Fig. 1D). In contrast, levels of the anti-inflammatory cytokine Il-10 were comparable between the two genotypes. The changes in mRNA were paralleled by a fivefold increase in Mcp-1 release in the culture medium in WT macrophages that was totally absent in FoxO1KR/KR macrophages (Fig. 1E). Release of Tnf-α and Il-6 increased >100-fold in FoxO1+/+ cells, and was blunted by 40% and 60%, respectively, in FoxO1KR/KR cells (Fig. 1F–G).
In contrast, the response of FoxO1KR/KR macrophages to the TLR2 agonist zymosan, and to the TLR4 agonist LPS did not show a clear-cut anti-inflammatory pattern and, in some instances (e.g., LPS-stimulated Il-6; zymosan- and LPS-stimulated Tnfα-induction) were more pronounced in FoxO1KR/KR than in FoxO1+/+ macrophages (Suppl. Fig. IA–B).
FoxO1-KR doesn’t increase FC-induced apoptosis
FC increases macrophage apoptosis by activating the Chop branch of the unfolded protein response 5, 6. The phosphorylation-defective mutant FoxO1, FoxO1-ADA (in which the three main sites of insulin-induced phosphorylation have been mutated) 22, is constitutively nuclear and exacerbates this effect 11. As the FoxO1-KR mutant was also predominantly nuclear, we expected it to induce apoptosis. But we failed to detect differences in Bip and Chop mRNA (Suppl. Fig. IIA), or in the number of apoptotic macrophages in response to FC between FoxO1+/+ and FoxO1KR/KR cells (Suppl. Fig. IIB, C). Apoptosis induced by thapsigargin, an inhibitor of the endoplasmic reticulum Ca2+ pump sarcoplasmic/endoplasmic reticulum calcium ATPase (Serca) that promotes ER stress 23, was also comparable between the two cell types, as was expression of type A scavenger receptor (SR-A)–a mediator of acLDL uptake 24–toll-like receptor-4 (TLR-4), and Tnf receptor 1 (TnfR1), all of which are required for ER stress-induced Mapk activation 25, 26 (Suppl. Fig. IID). These data indicate that FoxO1-KR dampens inflammation without predisposing to apoptosis, a critical difference between it and the phosphorylation-defective mutant.
FoxO1-KR inhibits FC-induced Akt phosphorylation
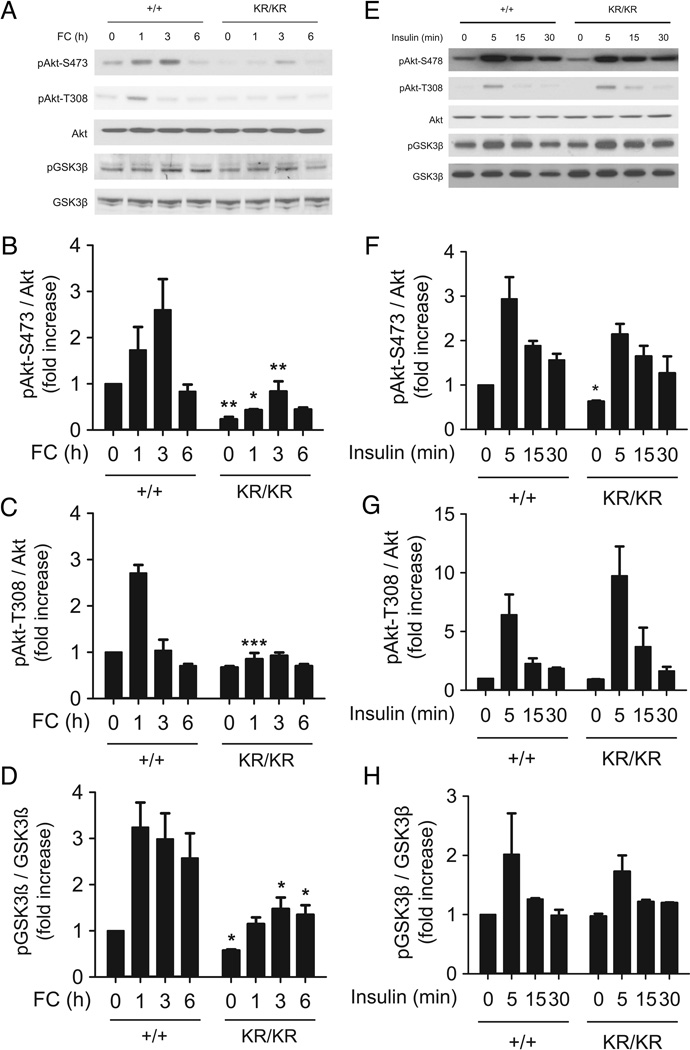
In view of the role of Akt in modulating the macrophage anti-apoptotic and anti-inflammatory responses 27, we examined the effects of FC loading on Akt activity in macrophages. In the early stages of FC loading, Akt phosphorylation on Ser-473 and Thr-308 was increased in FoxO1+/+ macrophages, but this effect was decreased by ~80% in FoxO1KR/KR macrophages (Fig. 2A–C). Phosphorylation of the Akt substrate glycogen synthase kinase 3β (GSK3β) was also decreased in FoxO1KR/KR macrophages (Fig. 2A, D). In contrast, there was no difference in insulin-induced Akt phosphorylation between the two cell types (Fig. 2E–H). Thus, Akt activation by FC is selectively impaired in FoxO1KR/KR macrophages.
Figure 2. FC– and insulin–induced Akt and GSK3β phosphorylation in macrophages.
(A) Representative immunoblots and (B–D) quantification of phospho-Akt Ser-473 (B), phospho-Akt Thr-308 (C), and phospho-GSK-3β (D) at the indicated times. (E) Representative immunoblots and (F–H) quantification of phospho-Akt Ser-473 (F), phospho-Akt Thr-308 (G), and phospho-GSK-3β (H) in FoxO1+/+ (+/+) and FoxO1KR/KR (KR/KR) macrophages stimulated with insulin (10µM) for the indicated times. Band intensity was quantified from three independent experiments. * P < 0.05, ** P < 0.01, and *** P < 0.001 vs. +/+.
Impaired Mek/Erk activation in FoxO1KR/KR macrophages
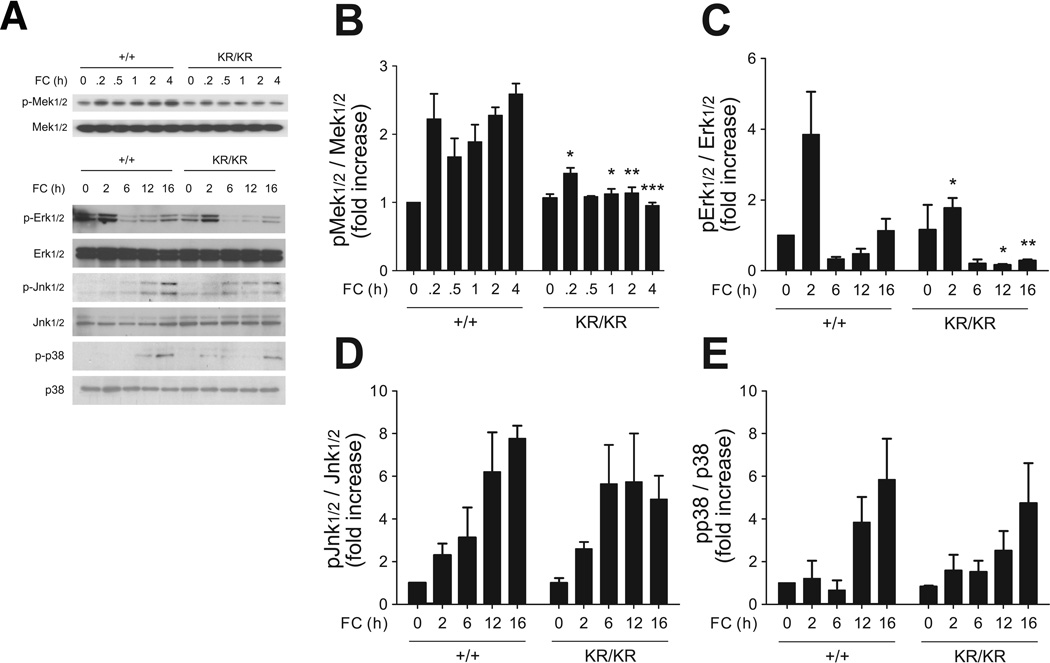
Macrophage FC loading increases Mcp-1, Tnf-α and Il-6 via Nf-κB and/or Mapk pathways 7 (Suppl. Fig. III). We interrogated the contribution of these two mechanisms to the blunted inflammatory response of FoxO1KR/KR macrophages. FC rapidly induced Mek phosphorylation by ~twofold, and promoted biphasic Erk phosphorylation in FoxO1+/+ macrophages; these responses were significantly attenuated in FoxO1KR/KR macrophages (Fig. 3A–C). In contrast, Jnk and p38 phosphorylation increased up to 8-fold and were comparable in both cell types (Fig. 3A, D–E).
Figure 3. FC activates Mapk in macrophages.
(A) Representative immunoblots and (B–E) quantification of phospho-Mek1/2 (B), phospho-Erk1/2 (C), phospho-Jnk1/2 (D), and phospho-p38 (E) in FoxO1+/+ (+/+) and FoxO1KR/KR (KR/KR) macrophages loaded with FC for the indicated times. Band intensity was quantified from three independent experiments. * P < 0.05, ** P < 0.01, and *** P < 0.001 vs. +/+.
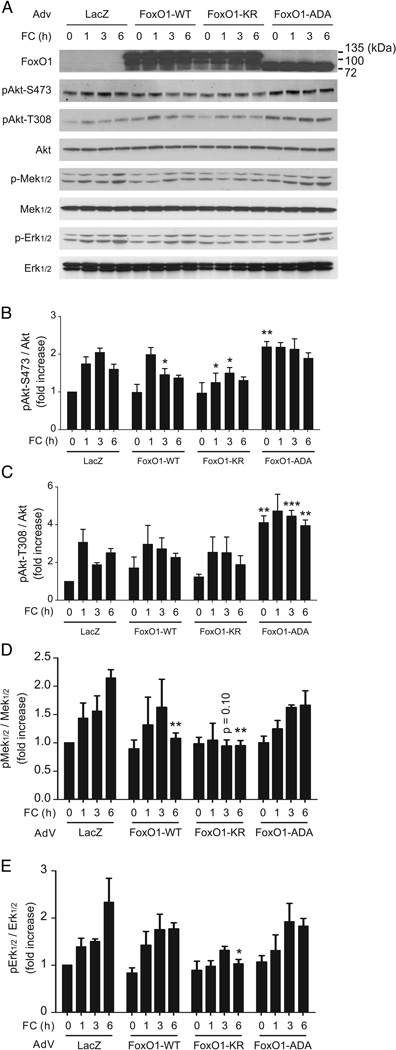
Distinct signaling pathways are elicited by phosphorylation-defective vs. acetylation-defective FoxO1 mutants
We have previously shown that the phosphorylation-defective FoxO1-ADA has anti-inflammatory effects in macrophages 11. As the FoxO1-KR mutant is also predominantly nuclear (although not to the same extent as the phosphorylation-defective mutant), we considered the possibility that the observed effects might simply reflect a gain of FoxO1 function, and be unrelated to its acetylation state. To investigate this point, we performed ex vivo experiments by transducing primary peritoneal macrophages with FoxO1-WT, FoxO1-ADA (phosphorylation-defective) and FoxO1-KR (acetylation-defective), and measured their response to FC loading. FoxO1-KR and WT-FoxO1 inhibited FC induced Akt phosphorylation on Ser-473 and Thr-308, whereas FoxO1-ADA increased it (Supp. Fig. IVA–C). Likewise, FC induced Mek and Erk phosphorylation (Suppl. Fig. IVA, D–E). FoxO1-WT or FoxO1-ADA had no effect on this response, whereas FoxO1-KR blunted it (Suppl. Fig. IVA, D–E). These data indicate that deacetylated FoxO1 has a unique effect that modulates inflammatory pathways in FC-loaded macrophages.
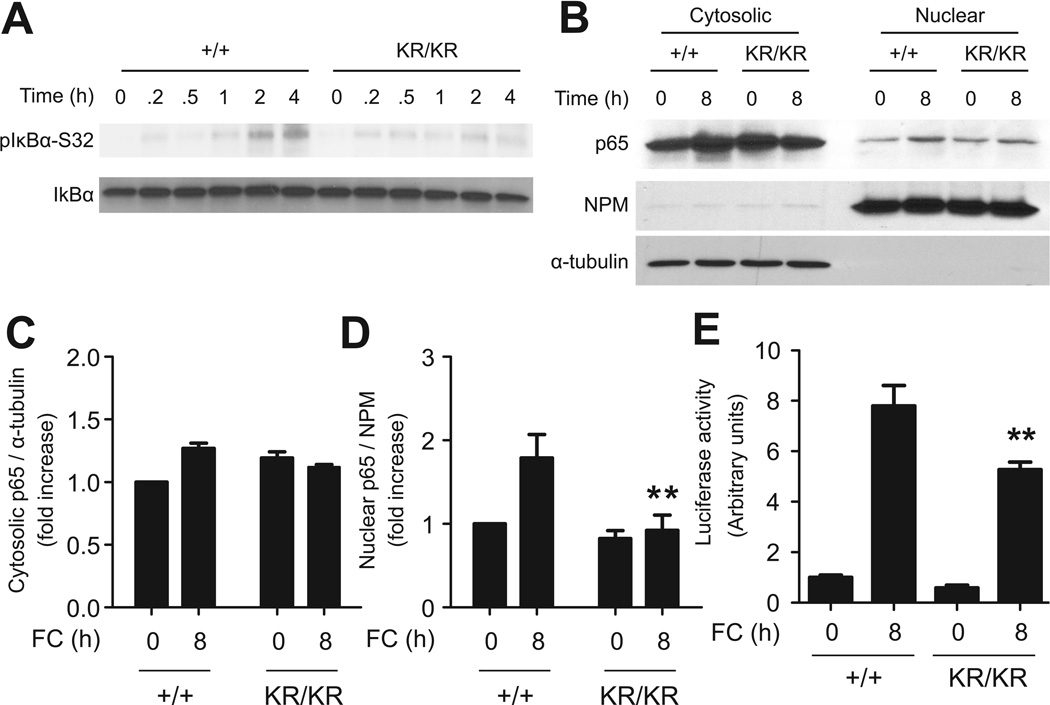
FoxO1-KR impairs activation of Nf-κB by FC
FC loading activated Nf-κB signaling in macrophages 7,25, stimulating IκBα phosphorylation on Ser-32 in a time-dependent manner. This response was nearly absent in FoxO1KR/KR macrophages (Fig. 4A).
Figure 4. FC activates Nf-κB in macrophages.
(A) Representative immunoblots of phospho-IκBα (Ser-32) and IκBα in FoxO1+/+ (+/+) and FoxO1KR/KR (KR/KR) macrophages loaded with FC for the indicated times. (B) Representative immunoblots and quantification of (C) cytosolic and (D) nuclear p65. Band intensity was quantified from three independent experiments. (E) Luciferase activity in FoxO1+/+ (+/+) and FoxO1KR/KR (KR/KR) macrophages transduced with recombinant adenovirus carrying an Nf-κB–responsive luciferase reporter construct for 24h followed by FC loading for 8h. NPM: nucleophosmin. * P < 0.05, ** P < 0.01, and *** P < 0.001 vs. +/+.
IκBα phosphorylation triggers its proteasomal degradation, resulting in Nf-κB nuclear translocation 28. Accordingly, we observed a twofold increase in nuclear content of the Nf-κB p65 subunit following FC loading of FoxO1+/+ macrophages (Fig. 4B–D). In contrast, levels of nuclear p65 were unchanged in FC-treated FoxO1KR/KR macrophages (Fig. 4B–D). To correlate these changes with Nf-κB function, we assessed the activity of an Nf-κB reporter gene transduced into FoxO1+/+ or FoxO1KR/KR cells. In FoxO1+/+ cells, FC induced an ~ 8-fold increase of Nf-κB-luciferase activity, whereas the effect of FC was decreased by 40% in FoxO1KR/KR cells (Fig. 4E).
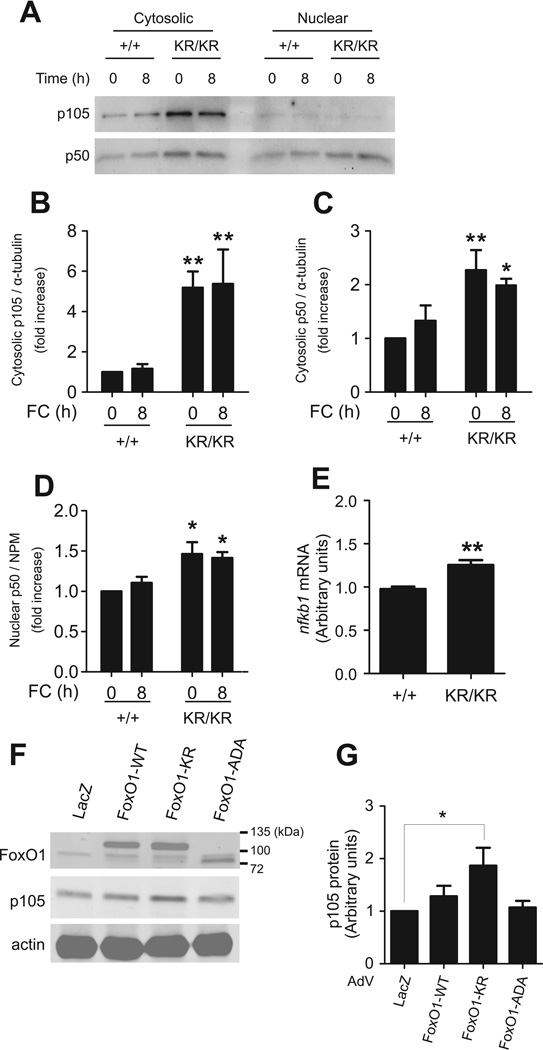
Identification of p105 (Nfkb1) as mediator of the anti-inflammatory effects of FoxO1-KR
The combined impairment of Nf-κB and Mek/Erk activities in FoxO1KR/KR macrophages led us to formulate the testable hypothesis that a shared regulator of both pathways was responsible for this reduction. The product of the Nfkb1 gene, p105, is the precursor of Nf-κB p50 29, and functions as an IκB-like molecule by sequestering p65, p50, and c-Rel in the cytoplasm 30–32 and preventing their binding to DNA 31. p105 also inhibits the Mek/Erk pathways by forming stoichiometric complexes with the Mek inhibitor TPL-2/Cot 32. We detected a fourfold increase in p105 protein levels (Fig. 5A–B), associated with a twofold rise of cytosolic p50 levels in FoxO1KR/KR macrophages (Fig. 5C), consistent with increased extra-nuclear retention of this subunit. In contrast, nuclear levels of p50 increased only by ~30% in FoxO1KR/KR cells (Fig. 5D). The increase of p105 protein was associated with a 30% rise of Nfkb1 mRNA in FoxO1KR/KR macrophages (Fig. 5E), suggesting that the main mechanism of increased p105 levels is not increased Nfkb1 transcription. Transduction of FoxO1-KR adenovirus in primary peritoneal macrophages also increased p105 protein levels (Fig. 5F), albeit to a lower extent, probably reflecting the limited efficiency of adenoviral transduction compared to studying a homogeneous population of FoxO1KR/KR macrophages. The increase was independent of changes in Nfkb1 mRNA (data not shown), consistent with a transcription-independent mechanism. In contrast, FoxO1-WT and FoxO1-ADA had no effect on p105 (Fig. 5F–G). These data indicate that p105 is a specific, albeit indirect (non-transcriptional) target of deacetylated FoxO1.
Figure 5. Nfkb1 and p105 levels in macrophages.
(A) Representative immunoblots and quantification of cytosolic (B) p105 and (C) p50, and (D) nuclear p105 in FoxO1+/+ (+/+) and FoxO1KR/KR (KR/KR) macrophages loaded with FC for the indicated times. Loading control (α-tubulin and NPM) is shown in Fig. 6B. (E) Nfkb1 levels in FoxO1+/+ (+/+) and FoxO1KR/KR (KR/KR) macrophages (n=6). (F) Representative immunoblots and (G) quantification of p105 and actin levels in cytosolic fractions from FoxO1+/+ macrophages transduced with adenovirus (AdV) encoding LacZ, FoxO1-WT, FoxO1-KR, or FoxO1-ADA. Band intensity was quantified from three independent experiments. * P < 0.05, and ** P <0.01 vs. +/+.
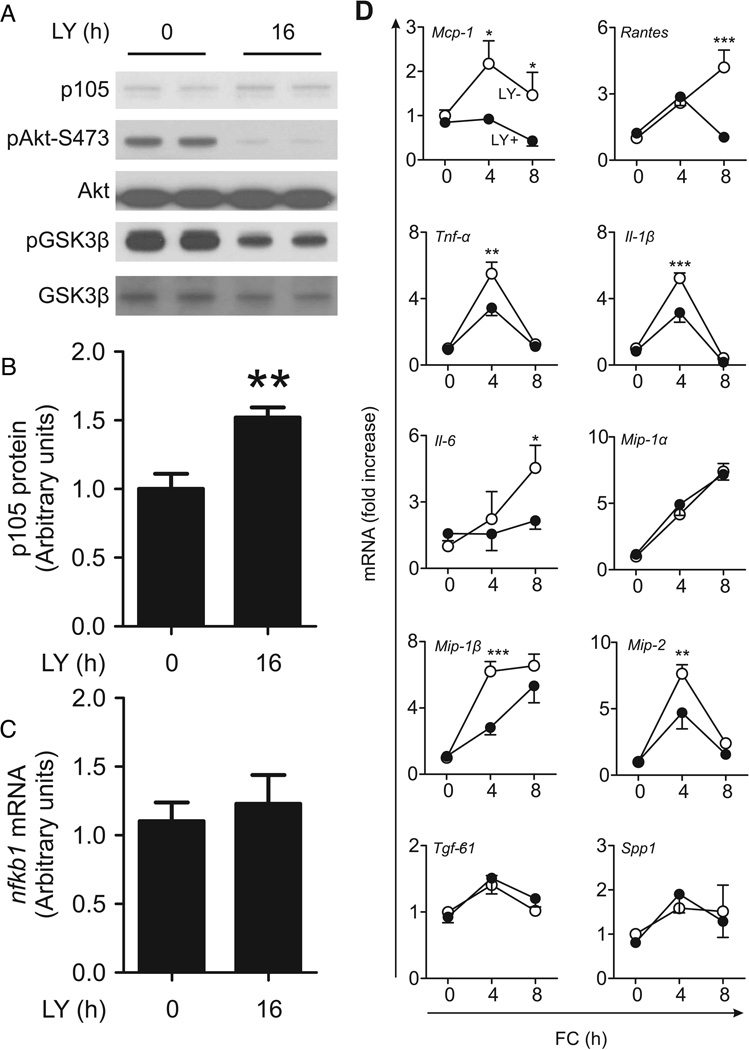
Pi3k inhibition mimics the effect of FoxO1-KR on p105
Akt phosphorylates Ikk 33, 34, triggering p105 proteolysis and Nf-κB/Mek/Erk activation. In addition, the Akt substrate GSK3β stabilizes p105 through phosphorylation, preventing its degradation 35. We reasoned that, if FoxO1-KR increased p105 by inhibiting Akt, its effects should be phenocopied by pharmacological inhibition of Pi 3-kinase (Pi3k). Indeed, the Pi3k inhibitor LY294002 inhibited Akt phosphorylation and GSK-3β phosphorylation, and increased p105 protein levels (Fig. 6A–B) independent of mRNA (Fig. 6C). LY294002 also inhibited FC-induced Mcp-1, Tnf-α, IL-6, IL-1β, Mip-1β, and Mip-2 expression in macrophages (Fig. 6D). In contrast, Mip-1α, Tgf-β1, and Spp1, whose induction by FC was blunted in FoxO1KR/KR macrophages, were unaffected by LY294002 (Fig. 6D). These data provide indirect support for the hypothesis that the anti-inflammatory effects of FoxO1-KR are mediated by its ability to decrease Akt phosphorylation.
Figure 6. Levels of p105, Nfkb1, and inflammatory cytokines in LY294002-treated macrophages.
(A) Representative immunoblots of p105, phospho-Akt Ser-473, Akt, phospho-GSK-3β and GSK-3β, and (B) quantification of p105 and (C) Nfkb1 levels in FoxO1+/+ macrophages treated with LY294002 (LY, 10μM) for 16h. (D) mRNAs encoding inflammatory cytokines in FoxO1+/+ macrophages pretreated with (LY+, closed circles) or without (LY-, open circles) LY294002 (10μM) for 16h followed by FC loading for the indicated times (n=5). * P < 0.05, ** P < 0.01, and *** P < 0.001 vs. LY- group.
FoxO1-KR does not act through IκBε, the main target gene of FoxO1-ADA
We have previously shown that phosphorylation-deficient FoxO1-ADA blunts Nf-κB activation by FC and increases IκBε expression 11, leading to p65 retention in the cytoplasm 28, 36–38. In the light of this precedent, we considered the possibility that FoxO1-KR regulated expression of IκBα, IκBβ, and IκBε (Suppl. Fig. VA). Consistent with prior observations, FC loading increased IκBε levels ~fourfold, whereas IκBα and IκBβ levels were unaffected (Suppl. Fig. VA). However, IκBε induction was similar in FoxO1+/+ and FoxO1KR/KR macrophages (Suppl. Fig. VA). We also examined the ability of FoxO1-WT, FoxO1-ADA, and FoxO1-KR to regulate the 3 IκBs in primary macrophages. Only FoxO1-ADA significantly increased IκBε 11, whereas neither FoxO1-WT nor FoxO1-KR affected any of the 3 IκBs (Suppl. Fig. VB). These data support the conclusion that FoxO1-KR inhibits FC-induced Nf-κB activity independent of IκBε, and provide more evidence for a deacetylation-specific mechanism of reduced inflammation.
DISCUSSION
The pathogenesis of inflammation in cardiovascular atherosclerotic disease is multi-factorial, and involves generation of cytokines, free radical formation, hemodynamic stress, hypertension, infections, and accumulation of oxidized phospholipids and 7-oxysterols 39, 40. FC accumulation in macrophages promotes inflammation through cytokine production 7, 11, 25, leading to increased levels of adhesion molecules and tissue factor 41, and migration of vascular smooth muscle cells 42. We now show that FC increases FoxO1 acetylation, and that constitutively deacetylated FoxO1 15 prevents FC-induced inflammation without promoting apoptosis. The latter finding sets this FoxO1 mutant apart from a phosphorylation-defective mutant that we have previously shown to inhibit inflammation AND promote FC-dependent apoptosis 11. Interestingly, constitutively active Akt prevents FC-induced macrophage apoptosis in insulin-resistant macrophages 11, while of PI3K/Akt inhibition by LY294002 increases it. These findings suggest that FoxO1-KR activates additional anti-apoptotic pathways to offset its pro-apoptotic properties macrophages. As FoxO1 phosphorylation and acetylation are regulated in response to different physiologic cues and disease states 13, the present findings have implications that go beyond the FC loading model.
We propose a novel mechanism to explain the findings (Suppl. Fig. VI). FoxO1 deacetylation blocks FC-dependent Akt phosphorylation, leading to decreased Ikk/Nf-κB and Mek/Erk activation, reduced cytokine expression, and reduced inflammation. We identify the product of the Nfkb1 gene p105 as a non-transcriptional target of deacetylated FoxO1 that potentially mediates its anti-inflammatory effects. The data provide further evidence that deacetylation fine-tunes FoxO1 function through target gene selection; thus, the phosphorylation-defective and acetylation-defective mutants have opposite effects on Akt phosphorylation and selective effects on Nfkb1 and IκBε expression 11, 21. Identifying the mechanism of the differential effects on Akt phosphorylation poses a daunting challenge for future work, as does the identification of co-regulatory mechanisms whereby FoxO proteins can regulate gene expression without direct DNA binding 20.
Akt regulates inflammation 33, 43–45 by phosphorylating Ikkα on Thr-23 and activating Nf-kB 45. Pharmacological inhibition of Ikk increases p105 levels, and inhibits LPS-stimulated Nf-κB and Mek/Erk activation in macrophages 30. On the other hand, p105 gain-of-function fails to affect LPS-induced Nf-κB and Mek/Erk signaling 30, suggesting that this mechanism is specific for certain, but not all inflammatory stimuli. Consistent with this observation, we have observed that FoxO1KR/KR macrophages are resistant to FC-induced, but not to Zymosan- or LPS-induced activation of inflammatory cytokines. It is also of interest to note that pharmacological inhibition of Pi3k-Akt mimics the FoxO1-KR effect to raise p105 levels, but has narrower effects on cytokine induction by FC, suggesting that not all effects of FoxO1-KR can be explained by its inhibition of Akt. We propose that decreased Akt activity by FoxO1-KR attenuates p105 proteolysis by two separate mechanisms: Ikk inhibition, leading to Nf-κB and Mapk inhibition; and decreased GSK3β phosphorylation, which has been shown to prevent constitutive processing/degradation of p105 by increasing its stability 35.
The present study confirms the biphasic activation pattern of Mek and Erk 46, 47. This could be due to delayed activation of certain subsets of Map kinases, or to autocrine signaling by cytokines produced from FC-stimulated macrophages, such as CXC-ligand 4 and IL-10 48, 49.
The role of FoxO1 in the development of atherosclerosis varies in different cells and tissue types. Insulin receptor-deficient macrophages, in which FoxO1 is constitutively active, display anti-inflammatory responses to FC 11 and LPS 12, but are prone to apoptosis 10. Conversely, in vascular endothelial cells FoxO1 gain-of-function promotes peroxynitrite and ROS generation 16, predisposing to inflammation. This finding is consistent with the observation that endothelial-cell specific ablation of insulin receptors (a condition associated with increased nuclear FoxO1) in ApoE−/− mice worsens atherosclerosis 50, as does Akt1 ablation 51, pinpointing vascular endothelial cells as a key site of atherosclerotic lesion development in insulin resistance.
In conclusion, our study demonstrates that FoxO1 deacetylation is an important regulatory mechanism in cholesterol-laden macrophages, with the potential to uncouple inflammation from apoptosis. As these mechanisms underlie the progression of atherosclerotic plaques from benign to unstable lesions 2, it can be envisioned that treatments promoting FoxO1 deacetylation will favorably affect cardiovascular outcomes in type 2 diabetes.
Supplementary Material
Figure 7.
Figure 8.
ACKNOWLEDGMENTS
We thank members of the Accili, Tabas and Tall laboratories for discussion of the data and critical reading of the manuscript.
SOURCES OF FUNDING
K.T. is the recipient of a research fellowship from the Uehara Memorial Foundation of Tokyo. This work was supported by National Institutes of Health grants P01HL087123, R01HL054591 (I.A.T.), F32DK79496 (A.S.B.), and DK63608 (Columbia Diabetes & Endocrinology Research Center).
Non-standard abbreviations and acronyms
- LPS
lipopolysaccharide
- FC
free cholesterol
- Mcp-1
monocyte chemoattractant protein-1
- ILs
interleukins
- TNF
tumor necrosis factor
- Mips
macrophage inflammatory proteins
- Mapk
Mitogen-activated protein kinase
- Nf-κB
nuclear factor kappa B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The Authors declare that they have no competing financial interest in the work described.
REFERENCES
- 1.National Institute of Diabetes and Digestive and Kidney Diseases. National diabetes statistics fact sheet: General information and national estimates on diabetes in the united states. 2005 [Google Scholar]
- 2.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruth HS. Histochemical detection of esterified cholesterol within human atherosclerotic lesions using the fluorescent probe filipin. Atherosclerosis. 1984;51:281–292. doi: 10.1016/0021-9150(84)90175-8. [DOI] [PubMed] [Google Scholar]
- 4.Small DM, Bond MG, Waugh D, Prack M, Sawyer JK. Physicochemical and histological changes in the arterial wall of nonhuman primates during progression and regression of atherosclerosis. J Clin Invest. 1984;73:1590–1605. doi: 10.1172/JCI111366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature cell biology. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 6.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of apoe−/− and ldlr−/− mice lacking chop. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: Model of nf-kappab- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 8.Tabas I, Tall A, Accili D. The impact of macrophage insulin resistance on advanced atherosclerotic plaque progression. Circ Res. 2010;106:58–67. doi: 10.1161/CIRCRESAHA.109.208488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR. Increased cd36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases er stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Senokuchi T, Liang CP, Seimon TA, Han S, Matsumoto M, Banks AS, Paik JH, DePinho RA, Accili D, Tabas I, Tall AR. Forkhead transcription factors (foxos) promote apoptosis of insulin-resistant macrophages during cholesterol-induced endoplasmic reticulum stress. Diabetes. 2008;57:2967–2976. doi: 10.2337/db08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Tobe K, Kadowaki T, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC. Myeloid lineage cell-restricted insulin resistance protects apolipoproteine-deficient mice against atherosclerosis. Cell Metab. 2006;3:247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Accili D, Arden KC. Foxos at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 14.van der Heide LP, Smidt MP. Regulation of foxo activity by cbp/p300-mediated acetylation. Trends Biochem Sci. 2005;30:81–86. doi: 10.1016/j.tibs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. Foxo1 protects against pancreatic beta cell failure through neurod and mafa induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka J, Qiang L, Banks AS, Welch CL, Matsumoto M, Kitamura T, Ido-Kitamura Y, DePinho RA, Accili D. Foxo1 links hyperglycemia to ldl oxidation and endothelial nitric oxide synthase dysfunction in vascular endothelial cells. Diabetes. 2009;58:2344–2354. doi: 10.2337/db09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks AS, Kim-Muller J-Y, Mastracci TL, Kofler NM, Qiang L, Haeusler RA, Jurczak MJ, Laznik D, Heinrich G, Samuel VT, Shulman GI, V.E. P, Accili D. Dissociation of the glucose and lipid regulatory functions of foxo1 by targeted knock-in of acetylation-defective alleles in mice. Cell Metab. 2011 doi: 10.1016/j.cmet.2011.09.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires er stress pathways and engagement of the type a scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura T, Kitamura YI, Funahashi Y, Shawber CJ, Castrillon DH, Kollipara R, DePinho RA, Kitajewski J, Accili D. A foxo/notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest. 2007;117:2477–2485. doi: 10.1172/JCI32054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor foxo1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- 23.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum ca-atpase family of calcium pumps. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- 24.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class a-i/ii and cd36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 25.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Q, Kim YS, Lin Y, Lewis J, Neckers L, Liu ZG. Tumour necrosis factor receptor 1 mediates endoplasmic reticulum stress-induced activation of the map kinase jnk. EMBO Rep. 2006;7:622–627. doi: 10.1038/sj.embor.7400687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui D, Thorp E, Li Y, Wang N, Yvan-Charvet L, Tall AR, Tabas I. Pivotal advance: Macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J Leukoc Biol. 2007;82:1040–1050. doi: 10.1189/jlb.0307192. [DOI] [PubMed] [Google Scholar]
- 28.Baeuerle PA, Baltimore D. I kappa b: A specific inhibitor of the nf-kappa b transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 29.Fan CM, Maniatis T. Generation of p50 subunit of nf-kappa b by processing of p105 through an atp-dependent pathway. Nature. 1991;354:395–398. doi: 10.1038/354395a0. [DOI] [PubMed] [Google Scholar]
- 30.Beinke S, Robinson MJ, Hugunin M, Ley SC. Lipopolysaccharide activation of the tpl-2/mek/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by ikappab kinase-induced proteolysis of nf-kappab1 p105. Mol Cell Biol. 2004;24:9658–9667. doi: 10.1128/MCB.24.21.9658-9667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice NR, MacKichan ML, Israel A. The precursor of nf-kappa b p50 has i kappa b-like functions. Cell. 1992;71:243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- 32.Waterfield MR, Zhang M, Norman LP, Sun SC. Nf-kappab1/p105 regulates lipopolysaccharide-stimulated map kinase signaling by governing the stability and function of the tpl2 kinase. Mol Cell. 2003;11:685–694. doi: 10.1016/s1097-2765(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 33.Romashkova JA, Makarov SS. Nf-kappab is a target of akt in anti-apoptotic pdgf signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 34.Salmeron A, Ahmad TB, Carlile GW, Pappin D, Narsimhan RP, Ley SC. Activation of mek-1 and sek-1 by tpl-2 proto-oncoprotein, a novel map kinase kinase kinase. EMBO J. 1996;15:817–826. [PMC free article] [PubMed] [Google Scholar]
- 35.Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3 beta regulates nf-kappa b1/p105 stability. J Biol Chem. 2003;278:39583–39590. doi: 10.1074/jbc.M305676200. [DOI] [PubMed] [Google Scholar]
- 36.Beg AA, Ruben SM, Scheinman RI, Haskill S, Rosen CA, Baldwin AS., Jr. I kappa b interacts with the nuclear localization sequences of the subunits of nf-kappa b: A mechanism for cytoplasmic retention. Genes Dev. 1992;6:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Nabel GJ. A new member of the i kappab protein family, i kappab epsilon, inhibits rela (p65)-mediated nf-kappab transcription. Mol Cell Biol. 1997;17:6184–6190. doi: 10.1128/mcb.17.10.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whiteside ST, Epinat JC, Rice NR, Israel A. I kappa b epsilon, a novel member of the i kappa b family, controls rela and crel nf-kappa b activity. EMBO J. 1997;16:1413–1426. doi: 10.1093/emboj/16.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dulak J, Jozkowicz A, Dichtl W, Alber H, Schwarzacher SP, Pachinger O, Weidinger F. Vascular endothelial growth factor synthesis in vascular smooth muscle cells is enhanced by 7-ketocholesterol and lysophosphatidylcholine independently of their effect on nitric oxide generation. Atherosclerosis. 2001;159:325–332. doi: 10.1016/s0021-9150(01)00520-2. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Oh P, Horner T, Rogers RA, Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J Biol Chem. 1997;272:7211–7222. doi: 10.1074/jbc.272.11.7211. [DOI] [PubMed] [Google Scholar]
- 41.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 42.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kane LP, Mollenauer MN, Xu Z, Turck CW, Weiss A. Akt-dependent phosphorylation specifically regulates cot induction of nf-kappa b-dependent transcription. Mol Cell Biol. 2002;22:5962–5974. doi: 10.1128/MCB.22.16.5962-5974.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr. Akt stimulates the transactivation potential of the rela/p65 subunit of nf-kappa b through utilization of the ikappa b kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 45.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. Nf-kappab activation by tumour necrosis factor requires the akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 46.Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT. Fibroblast growth factor 2 induction of the osteocalcin gene requires mapk activity and phosphorylation of the osteoblast transcription factor, cbfa1/runx2. J Biol Chem. 2002;277:36181–36187. doi: 10.1074/jbc.M206057200. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Zhong S, Dong Z, Chen N, Bode AM, Ma W. Uva induces ser381 phosphorylation of p90rsk/mapkap-k1 via erk and jnk pathways. J Biol Chem. 2001;276:14572–14580. doi: 10.1074/jbc.M004615200. [DOI] [PubMed] [Google Scholar]
- 48.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by mapk phosphatase 1 (mkp-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kasper B, Brandt E, Brandau S, Petersen F. Platelet factor 4 (cxc chemokine ligand 4) differentially regulates respiratory burst, survival, and cytokine expression of human monocytes by using distinct signaling pathways. J Immunol. 2007;179:2584–2591. doi: 10.4049/jimmunol.179.4.2584. [DOI] [PubMed] [Google Scholar]
- 50.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein e null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.