Abstract
Host hematopoietic derived antigen presenting cells are important for induction of graft-versus-host disease. The relative importance of various subsets of hematopoietic derived APCs is not well-understood. Recent data suggest that basophils can function as antigen presenting cells and induce T helper 2 (Th2) lymphocyte responses. We investigated the role of host basophils in the induction of donor T cell responses and graft-versus-host disease after allogeneic bone marrow transplantation. Elimination of host basophils did not alter the severity of graft-versus-host disease induced mortality across multiple clinically relevant models of allogeneic BMT. Furthermore induction of donor T cell proliferation and Th2 polarization was not significantly altered following depletion of host basophils. In contrast to their role in the induction of Th2 responses under certain contexts, our results demonstrate that basophils are dispensable for induction of donor Th2 responses and for the severity of GVHD.
Keywords: Antigen presenting cells, basophils, BMT, GVHD, Th2 polarization
INTRODUCTION
Basophils are basophilic granulocytes circulating in the peripheral blood. They account for less than 1% of blood leukocytes and share certain features with mast cells1. However in light of their smaller numbers and short life-span, basophils have been considered to be of minor relevance when compared with mast cells2,3. Exciting new data have demonstrated that basophils play a significant role on their own3,4. Basophils have been shown to provide IL-4 and present antigens to naïve T cells5–8. Several studies have suggested that basophils can act as potent APCs and are necessary and sufficient for induction of Th2 responses8–13. Although other emerging data have challenged this notion on the ability of basophils to prime Th2 responses, their role in allogeneic T responses is not known14–16. Specifically, the role of basophils in modulating allo-antigen driven Th differentiation and in vivo allo-responses such as GVHD is not known.
Host hematopoietic derived antigen presenting cells play an important role in the induction of host allo-antigen driven donor T cell response leading to GVHD17. Alloreactive cytopathic donor T cells, upon being presented by the allo-antigens in the right context, proliferate, differentiate into either Th1 or/and Th2 or/and Th17 effector cells and cause target organ damage18–21. While the exact and specific role different Th subsets in causing GVHD is complex and being increasingly studied22, the role of host hematopoietic derived APCs in causing Th differentiation after allogeneic BMT is not well-understood. Furthermore, the importance of different host hematopoietic derived APC subsets in causing GVHD is not completely understood22. We investigated the role of the contribution of host basophils in the presence of other APCs in inducing donor T cell polarization and GVHD severity. Utilizing multiple clinically relevant murine models of GVHD, we show that depletion of host basophils did not alter the Th1/Th2 balance of donor T cells or regulate the severity of GVHD after allogeneic BMT.
MATERIALS AND METHODS
Mice
Female C57BL/6 (B6, H-2b, CD45.2+) mice were purchased from the Charles River Laboratories (Wilmington, MA). B6-Ly5.2/Cr (B6-CD45.1, H-2b, CD45.1+) mice were purchased from the National Cancer Institute-Frederick (Frederick, MD). BALB/c (H-2d, CD45.2+) and B6D2F1(H-2b/d, CD45.2+) mice were purchased from Taconic (Hudson, NY). B6.129S7-Ifngtm1TS/J (B6-background IFNγ-KO, H-2b, CD45.2+) mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
Cell culture
For the generation of bone marrow dendritic cells (DCs), bone marrow cells were harvested from tibias and femurs and cultured for 7 days in RPMI1640 media (Invitrogen, Carlsbad, CA) supplemented with 10% FCS (Invitrogen) and 20 ng/ml recombinant murine GM-CSF (Peprotech, Rocky Hill, NJ)23. Samples were enriched for DCs by positive selection of CD11c+ cells using MACS CD11c-microbeads and LS columns (Miltenyi, Auburn, CA). For the generation of bone marrow basophils, bone marrow cells were cultured for 9–10 days in RPMI1640 media supplemented with 10% FCS and 10 ng/ml recombinant murine IL-3 (Peprotech)1,6,8,9. Samples were enriched for basophils by positive selection of DX5+ cells using MACS DX5-microbeads and LS columns (Miltenyi).
Antibodies and flow cytometric analysis
FITC-, PE, PerCP-Cy5.5 or APC-conjugated monoclonal antibodies (mAbs) to mouse CD4 (clone; RM4-4), CD8a (53-6.7), CD45.1 (A20) and CD45.2 (104), CD49b(DX5), CD80(16-10A1), CD86(GL1), FcεR1(MAR-1), H-2Kb(AF6-88.5.5.3), H-2Kd(SF1-1.1) I-A/I-E(M5/114.15.2) from eBioscience (San Diego, CA). Anti-mouse CD40(3/23), I-Ab(AF6-120.1) and I-Ad(AMS-32.1) were purchased from BD Pharmingen (San Diego, CA). Cultured bone marrow cells, spleen cells from bone marrow transplant mice, peripheral blood cells from mAb-treated mice were incubated with anti-CD16/CD32 (2.4G2; Fc block™, BD Pharmingen) mAb for 15 min at 4°C in staining buffer (2% FCS-containing PBS) then the cells were stained with fluorochrome-conjugated mAbs for 15 min at 4°C in the staining buffer. After washing, the cells were analyzed using C6 flowcytometer (Accuri Cytometers, Ann Arbor, MI)24. Basophils were gated for on SSC low cells as described previously25.
Bone marrow transplantation
Host mice were irradiated (137Cs source) with 9–11 Gy total body irradiation (TBI) 1 day before bone marrow transplant (BMT). Donor bone marrow cells were harvested from femurs and tibias and T cells in the bone marrow were magnetically removed using CD90.2-microbeads (Miltenyi, Auburn, CA) and MACS LS columns (Miltenyi)26. Spleen T cells were magnetically isolated using CD90.2 microbeads and MACS LS columns (Miltenyi). T cell numbers were determined based on cell count and purity. Syngeneic or allogeneic T cell-depleted bone marrow (TCDBM) and T cells were infused through tail vein26. For basophil-depletion experiments, host mice were treated with 10 μg of anti-FcεRI mAb (MAR-1 functional grade purified, eBioscience) on day −1, 0 and 1. Control group host mice were treated with 10 μg of Armenian Hamster IgG (functional grade purified, eBioscience). Host mice were housed in sterilized microisolator cages and maintained on acidified water (pH < 3) for 3 weeks as described previously26. Survival was monitored daily, clinical GVHD was assessed weekly as described previously26. All animal studies were performed per the institutional IACUC guidelines of the University of Michigan.
Cytokine analysis
Levels of IFN-γ, IL-4, IL-5 and IL-17A in sera were determined by by ELISA (BD Pharmingen) per manufacturer’s instructions. Spleen cells harvested from bone marrow transplant hosts were stimulated with soluble anti-mouse CD3e mAb (2 μg/ml, eBio500A2 functional grade purified, eBioscience) and anti-mouse CD28 (2 μg/ml, 37.51 functional grade purified, eBioscience) in the presence of brefeldin A (1/1000 dilution; eBioscience) for 5 hours24. After stimulation, cells were fixed for 20 min using FACS Lysing solution (BD Bioscineces, San Jose, CA) and processed for intracellular cytokine staining (ICC). Fixed cells were washed with permeabilization buffer (eBioscience) and stained with PE-conjugated anti-mouse IFN-γ (XMG1.2, BD Pharmingen), APC-conjugated anti-mouse IL-4 (11B11, eBioscience) or PE-conjugated anti-mouse IL-17A (eBio17B7, eBioscience) for 30 min at 4°C24. The cells were washed with permeabilization buffer and staining buffer (2 times), re-suspended with staining buffer and analyzed on a C6 flowcytometer.
Histology
Formalin-preserved gut and liver were embedded in paraffin, cut into 5-μm thick sections, and stained with haematoxylin and eosin for histologic examination. Slides were coded without reference to prior treatment and examined in a blinded fashion by a pathologist (C. Liu). A semiquantitative scoring system was used to assess the following abnormalities known to be associated with GVHD27, 28.
Statistical analysis
Student t test was used for the statistical analysis of in vitro data while the Wilcoxon rank test was used to analyze survival data. P< 0.05 was considered statistically significant.
RESULTS
Basophils are poor stimulators of allogeneic T cells
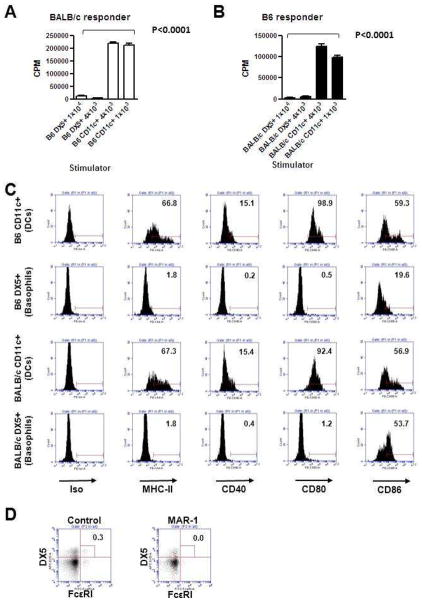
We determined the ability of basophils to stimulate allogeneic T cells in vitro. We cultured BALB/c T cells with basophils cultured from allogeneic B6 donors in a standard mixed leukocyte reaction (MLR) assay. The stimulatory capacity was compared with BM derived B6 DCs, the most potent APCs. The B6 DCs, as expected caused robust proliferation of allogeneic BALB/c T cells (Figure 1A). By contrast, as shown in Figure 1A, B6 basophils caused significantly less proliferation of allogeneic T cells (P< 0.0001). In addition, changing the stimulator to responder ratio did not alter the inability of basophils to stimulate allogeneic T cells. To rule out ant strain dependent effects, we also tested the ability of BALB/c DCs and basophils to stimulate B6 T cells in an MLR. BALB/c derived basophils also demonstrated significantly poor ability to stimulate proliferation of allogeneic B6 T cells when compared with DCs (Figure 1B).
Figure 1. Basophils are poor stimulators of allogeneic T cells.
(A) Indicated numbers of in vitro IL-3-induced bone marrow B6 basophils (DX5+) or GM-CSF-induced bone marrow dendritic cells (DCs, CD11c+) and 4×105 BALB/c CD90+ T cells were co-cultured in 96-well round bottom plate for 5 days. Incorporation of 3H-thymidine (1 μCi/well) by proliferating cells during the last 6 h of culture was measured. (B) Indicated numbers of in vitro IL-3-induced BALB/c bone marrow basophils (DX5+) or GM-CSF-induced bone marrow dendritic cells (CD11c+) and 4×105 B6 CD90+ T cells were co-cultured in 96-well round bottom plate for 5 days. Incorporation of 3H-thymidine (1 μCi/well) by proliferating cells during the last 6 h of culture was measured. (C) Flow cytometric analysis of B6 and BALB/c bone marrow DCs and basophils. MHC class II (I-Ab for B6, I-Ad/I-Ed for BALB/c), CD40, CD80 and CD86 expression levels on CD11c+ cells (DCs, top row) and DX5+ cells (basophils, bottom row) are shown. Numbers shown in each histogram indicate % positivity of the surface marker. (D) Flow cytometric analysis of control hamster mAb (left) or MAR-1 (right) treated BALB/c mouse peripheral blood. Mice were treated with 10 μg of control mAb or MAR-1 three times every 24 hours and peripheral blood samples were corrected from the mice and analyzed. Numbers shown in dot plots indicate % of DX5+FcεRI+ cells.
We next analyzed the phenotype of the bone marrow derived basophils and compared it with the BM DCs from B6 animals. As shown in Figure 1C, bone marrow derived basophils demonstrated significantly lower expression of MHC class II, CD80, CD86 and CD40 than BM DCs. Bone marrow derived basophils from BALB/c similarly demonstrated lower expression of class II and CD80, 86 and 40 when compared to BALB/c BM DCs, thus ruling out potential strain dependent artifacts (Figure 1C). These data suggest that basophils are poor in vitro APCs for stimulation of allogeneic T cells due to lack of appropriate expression levels of co-stimulatory molecules.
Host basophils do not alter in vivo generation of allogeneic Th2 polarization
In order to determine whether basophils are critical for induction of in vivo allogeneic donor T cell responses we utilized the BALB/c (H-2d) → B6 (H-2b) mouse model of allogeneic BMT. The B6 recipients were injected with MAR-1 (anti-Fcε1) or control IgG on day −1, 0 and 1 to eliminate host basophils as in Methods. This schedule was chosen based on previous reports and as shown in Figure 1D, successfully depleted basophils in the BM and spleen regardless of irradiation 7,8,10,11. It is nonetheless possible that this schedule can also deplete the early engrafting basophils from the infused TCD donor BM. The B6 animals were lethally irradiated and transplanted with TCD BM and naïve splenic T cells from allogeneic (BALB/c) or syngeneic (B6) donors on day 0. Donor T cell expansion and differentiation was analyzed in the spleen on day +7 after allogeneic BMT. As shown in Figure 2A, allogeneic BALB/c donor T cells demonstrated equivalent expansion and also similar numbers of IFNγ+ (Th1) or IL-4+ (Th2) or IL-17A+ (Th17) regardless of depletion of the basophils in the B6 hosts. Furthermore, consistent with expansion of the Th1, Th2 and Th17 cells, depletion of basophils had no statistically significant impact on the serum levels IFN-γ, IL-4/Il-5 and IL-17A.
Figure 2. Effect of basophil depletion on donor T cell expansion and cytokine production.
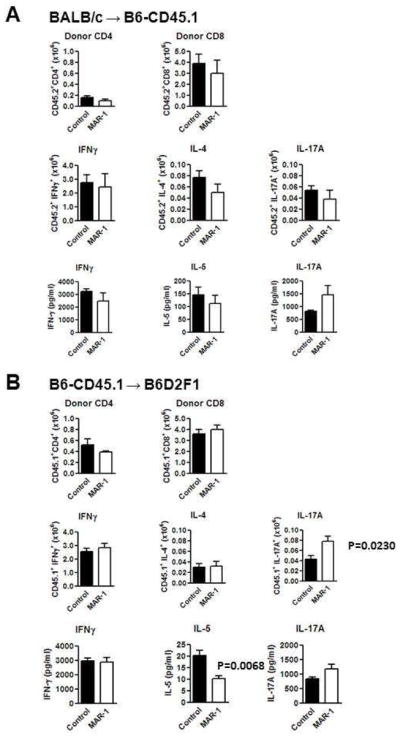
(A) B6-CD45.1 (CD45.1+, CD45.2−) recipients were irradiated (10 Gy) on day -1 and injected with 5×106 BALB/c (CD45.2+) TCDBM and 4×106 CD90+ T cells on day 0. Recipients were treated with 10 μg of Hamster IgG (control Ab)(■, n =4) or anti-mouse FcεRI (MAR-1)(□, n =4) on day −1, 0 and 1. Spleen cells and sera were collected from recipients on day 7. Spleen cells were counted, stained with anti-CD45.2, CD4 and CD8 mAbs and analyzed by flowcytometry. Donor CD4 and CD8 T cell expansion was determined based on donor marker (CD45.2), CD4 and CD8 positivity. Intracellular IFNγ, IL-4 and IL-17A staining was performed after stimulation of spleen cells with anti-CD3ε and anti-CD28 mAbs. Numbers of cytokine-producing cells were determined based on donor marker and cytokine positivity. Serum cytokine level was determined by ELISA. (B) B6D2F1 (CD45.1−, CD45.2+) recipients were irradiated (10 Gy) on day −1 and injected with 5×106 B6 (CD45.1−, CD45.2+) TCDBM and 2×106 B6-CD45.1 (CD45.1+, CD45.2−) CD90+ T cells on day 0. Recipients were treated with 10 μg of control Ab (■, n =4) or MAR-1 (□, n =4) on day −1, 0 and 1. Spleen cells and sera were collected from recipients on day 7. Spleen cells were counted, stained with anti-CD45.1, CD4 and CD8 mAbs and analyzed by flowcytometry. Donor CD4 and CD8 T cell expansion was determined based on donor marker (CD45.1), CD4 and CD8 positivity. Intracellular IFNγ, IL-4 and IL-17A staining was performed after stimulation of spleen cells with anti-CD3ε and anti-CD28 mAbs. Numbers of cytokine-producing cells were determined based on donor marker and cytokine positivity. Serum cytokine level was determined by ELISA.
Because of the potential for strain dependent differential effects on T cell polarization, we next determined the effect of basophil depletion on allogeneic T cell expansion and Th differentiation in a second, clinically relevant, B6 (H-2b) → B6D2F1 (H-2b/d) model of allogeneic BMT29. The B6D2F1animals were injected with MAR-1 (anti-Fcε1) or control IgG and were then used as recipients in an allogeneic BMT. As shown in Figure 2B, B6 donor T cells demonstrated similar expansion and Th1 or Th2 (P=NS) but a higher expansion of Th17 cells (P=0.023) in the basophil depleted when compared with control animals. Serum levels of the respective cytokines, IFN-γ, IL-4 and IL-17A (P=NS) were similar between the groups, although the serum levels of IL-5 was decreased (P=0.006). By contrast donor T cells from the naïve, non-transplanted BALB/c and B6 did not constitutively demonstrate any significant numbers of either Th1 (IFN-γ), or Th2 (IL-4) or Th17 (IL-17A) cells. These data collectively demonstrate that host basophils are not required for in vivo expansion and Th2 polarization of allogeneic T cells.
Host basophils are dispensable for induction of mortality from GVHD
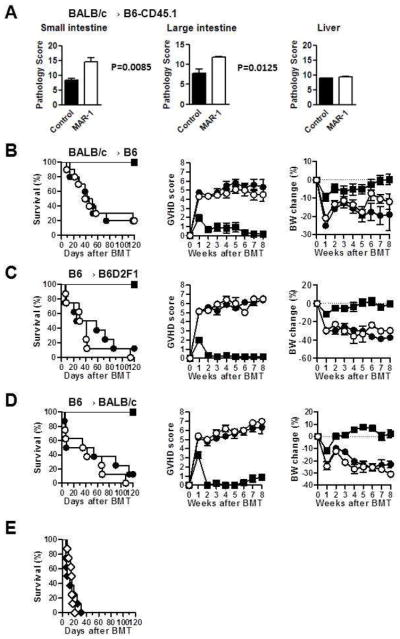
Host APCs are critical for induction of GVHD30–32. The relative roles of various host hematopoietic derived host APC subsets in causing GVHD are not well understood. We therefore next examined whether host basophils modulated the severity of GVHD. Even though depletion of host basophils did not alter the expansion or Th polarization of donor T cells, it is formally possible that they might modulate the severity of distinct target organs21. Utilizing the BALB/c→B6 model, we therefore analyzed whether depletion of host basophils differentially affected the pathology of GVHD specific target organs, the gastrointestinal tract, liver and skin. The target organs from the allogeneic animals were harvested on day +7 after BMT and analyzed for histopathological severity. Elimination of host basophils enhanced the histo-pathological severity of in the large and small bowels (P<0.05) but not the liver (Figure 3A) while no significant GVHD specific skin changes were observed (data not shown). We next evaluated the effects of basophil depletion administration on the clinical severity and mortality of acute GVHD. Basophil depletion did not have a significant impact on survival or the severity of clinical GVHD scores and weight after allogeneic BMT (Fig. 3B; P =NS). All surviving mice showed greater complete donor chimerism by fluorescence-activated cell sorter analysis (data not shown), ruling out graft failure or mixed chimerism as a cause for the GVHD.
Figure 3. Effect of basophil depletion on GVHD severities.
(A) B6 recipients were irradiated (10 Gy) on day −1 and injected with 5×106 BALB/c (CD45.2+) TCDBM and 4×106 CD90+ T cells on day 0. Recipients were treated with 10 μg of control Ab (■, n =4) or MAR-1 (□, n =4) on day −1, 0 and 1. GVHD target organs (small, large intestines and liver) were removed on day 7 for pathological analysis. GVHD pathology scores are shown. (B) B6 recipients were irradiated (10 Gy) on day −1 and injected with 5×106 syngeneic B6 TCDBM (●, n=6). Allogeneic BMT recipients were treated with 10 μg of control Ab (●, n =10) or MAR-1(○, n =10) on day −1, 0 and 1, and injected with 5×106 BALB/c TCDBM and 4×106 CD90+ T cells. Survival was monitored daily, and GVHD clinical score and body weight (BW) change were monitored weekly. (C) B6D2F1 recipients were irradiated (10 Gy) on day −1 and received 5×106 syngeneic B6 TCDBM (●, n=6), Allogeneic BMT recipients were treated with 10 μg of control Ab (●, n =8) or MAR-1 (○, n =8) on day −1, 0 and 1, and injected with 5×106 B6 TCDBM and 2×106 CD90+ T cells. Survival was monitored daily, and GVHD clinical score and body weight change were monitored weekly. (D) BALB/c recipients were irradiated (8 Gy) on day −1 and received 5×106 syngeneic BALB/c TCDBM (●, n=7). Allogeneic BMT recipients were treated with 10 μg of control Ab (●, n =8) or MAR-1 (○, n =8) on day −1, 0 and 1, and injected with 5×106 B6 TCDBM and 0.5×106 CD90+ T cells. Survival was monitored daily, and GVHD clinical score and body weight change were monitored weekly. (E) B6D2F1 recipients treated with 10 μg of control Ab (◆, n =8, day −1, 0, 1) or MAR-1 (◇, n =8, day −1, 0, 1) were irradiated (10 Gy) on day −1 and injected with 5×106 wild type B6 TCDBM and 2×106 IFNγ-KO CD90+ T cells on day 0. Survival was monitored daily. P=NS.
To rule out strain dependent artifacts, we also examined the impact of host basophil depletion on GVHD severity in the B6→B6D2F1 model of allo-BMT29. The B6D2F1 recipients showed similar target organ histopathological, clinical severity of GVHD and weight loss after allogeneic BMT (Figure 3C; P =NS). Similar lack of impact was also observed in the third (B6→BALB/c) model of allogeneic BMT (Figure 3D; P =NS). Because absence of Th1 polarization has been suggested to enhance Th2 polarization, we also examined the impact of depletion of host basophils in the INF-γ deficient T cell polarization and GVHD severity after allogeneic BMT. Depletion of host basophils did not modulate T cell polarization of GVHD severity despite the absence of Th1 (INF-γ) cytokine secretion by the mature donor T cells (Figure 3E, P= NS). Collectively these data demonstrate that host basophils do not play a significant in the induction and severity of GVHD.
DISCUSSION
Recent data have brought into focus the role for basophils as antigen presenting cells and in priming Th2 responses3,4,8–13. The role of basophils in causing Th2 polarization and regulating in vivo allogeneic responses is not known. Because host hematopoietic derived APC subsets are important for priming donor T cells after allogeneic BMT we tested the hypothesis that depletion of host basophils will reduce Th2 polarization (and enhance Th1/Th17) polarization and modulate GVHD30,31. In contrast to our hypothesis, we found that host basophils were dispensable, both for priming of Th2 responses and for modulation of the severity of GVHD after allogeneic BMT. The lack of impact of basophil depletion was observed in multiple models of allogeneic BMT, thus ruling out strain dependent artifact. This is in contrast to the recent reports that demonstrated in distinct experimental settings that basophils, rather than DCs, are the critical APCs for driving Th2 cell differentiation8–11,13. In immunization models with protease allergens IgE mediated or with parasite T. muris, depletion of basophils as was performed in our experiments (by pretreatment with the MAR-1 antibody to Fc RI) lead to the loss of Th2 differentiation8–13. The APC function and induction of Th2 responses was critically dependent on the interaction of MCH class II on basophils with the CD4+ T cells8,10. Our data demonstrate that although basophils constitutively express MHC class II and other conventional co-stimulatory molecules such as CD80, CD86 and CD40, the level of expression of these molecules are much lower than conventional DCs. Furthermore, in vitro studies also demonstrated that basophils are less effective than DCs in priming allogeneic T cell responses. Thus, in contrast to earlier observations, we found that basophils are poor inducers of T cell proliferation and were dispensable for Th2 priming in the context of in vivo allogeneic responses.
Our results are however consistent with the observations that demonstrated only a minor role for basophils as APCs and Th2 inducers13–16. Hammad et al reported that the anti-FcεRIα mAb (MAR-1) not only efficiently depletes basophils but also a subset of DCs that express FcεRI14. Our data would suggest that these DC subsets, in addition to the basophils, too may be dispensable to Th2 differentiation after allogeneic BMT. Thus basophils are the not potent or the primary inducers of Th2 polarization in the context of allogeneic stimulation. The relative contribution of basophils and DCs on in vivo Th2 differentiation therefore may be dependent on the type of model and stimulus.
In the context of allogeneic BMT, host basophils thus do not have significant impact on the severity GVHD mortality. Following allogeneic BMT other host APCs might be sufficient for driving Th2 differentiation, perhaps because of the presence of the right cytokine milieu from the conditioning and the allo-T cell responses. We did not directly address the role of donor BM derived basophils, which may play a role in the maintenance of the Th2 phenotype of donor T cells. Basophils have an estimated in vivo lifespan of only 60 hours and they are very fragile cells with poor survival after standard sorting procedures1–3,5. Thus, it is technically difficult to perform transfer experiments with basophils. Nonetheless, it is important to point out that our observations do not negate a critical role for basophils in the maintenance of allo-antigen driven Th2 and memory responses, either alone or in concert with other accessory or antigen presenting cells33. Alternatively, in the context of allo-responses, at least in vivo, DCs and other APCs likely dominate the allo-antigen presentation and donor T responses. Thus, it is possible that basophils may target different types of antigens and lead to subsequent generation of Th2 response, while they are poor presenters of allo-antigens.
Both host and donor hematopoietic derived APCs play a role in GVHD (34,32,35,36). Host hematopoietic derived APCs have been shown to be crucial for induction of GVH responses30,31. Nonetheless, the role of specific host hematopoietic derived APC subsets remains is not well-understood. Emerging data suggest a role for DCs in the absence of all other APCs, but whether DCs are necessary in the presence of other APC subsets is not known37–39. Host B cells have been shown to be either dispensable for induction of GVHD or be regulatory on the severity of GVHD 39,40. Korngold and colleagues demonstrated that host mast cells may play a role in enhancing skin GVHD41. While basophils share many similarities with mast cells, they are also different in other ways. For example mast cells, in contrast to basophils, have a longer half-life, primarily reside in peripheral tissues and can proliferate even after maturation1,3. Thus, while host mast cells may be critical for modulating GVHD target organ damage, host basophils do not play a significant role in either enhancing or mitigating GVHD specific target organ damage.
In summary, we demonstrate that host basophils are dispensable for induction of Th2 differentiation in the context of allogeneic stimulation. While the role of Th1/Th2/Th17 effector pathways in the type, severity and specificity of GVHD is complex and being increasingly understood, our data suggest that targeting host basophils will not mitigate mortality from GVHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Min B. Basophils: what they ‘can do’ versus what they ‘actually do’. Nat Immunol. 2008;9:1333–9. doi: 10.1038/ni.f.217. [DOI] [PubMed] [Google Scholar]
- 2.Arinobu Y, Iwasaki H, Gurish MF, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A. 2005;102:18105–10. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Nonredundant roles of basophils in immunity. Annu Rev Immunol. 2011;29:45–69. doi: 10.1146/annurev-immunol-031210-101257. [DOI] [PubMed] [Google Scholar]
- 4.Sokol CL, Medzhitov R. Role of basophils in the initiation of Th2 responses. Curr Opin Immunol. 2010;22:73–7. doi: 10.1016/j.coi.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh K, Shen T, Le Gros G, Min B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood. 2007;109:2921–7. doi: 10.1182/blood-2006-07-037739. [DOI] [PubMed] [Google Scholar]
- 6.Min B, Prout M, Hu-Li J, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–17. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mack M, Schneider MA, Moll C, et al. Identification of antigen-capturing cells as basophils. J Immunol. 2005;174:735–41. doi: 10.4049/jimmunol.174.2.735. [DOI] [PubMed] [Google Scholar]
- 10.Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimoto T, Yasuda K, Tanaka H, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–12. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan BM, Liang HE, Bando JK, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–35. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–74. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Hammad H, Plantinga M, Deswarte K, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang H, Cao W, Kasturi SP, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–17. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phythian-Adams AT, Cook PC, Lundie RJ, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–96. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–70. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 18.Paczesny S, Hanauer D, Sun Y, Reddy P. New perspectives on the biology of acute GVHD. Bone Marrow Transplant. 45:1–11. doi: 10.1038/bmt.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tawara I, Maeda Y, Sun Y, et al. Combined Th2 cytokine deficiency in donor T cells aggravates experimental acute graft-vs-host disease. Exp Hematol. 2008;36:988–96. doi: 10.1016/j.exphem.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy WJ, Welniak LA, Taub DD, et al. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102:1742–8. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105:1289–98. doi: 10.1172/JCI7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–52. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 23.Toubai T, Malter C, Tawara I, et al. Immunization with host-type CD8{alpha}+ dendritic cells reduces experimental acute GVHD in an IL-10-dependent manner. Blood. 115:724–35. doi: 10.1182/blood-2009-06-229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tawara I, Koyama M, Liu C, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JJ, McGarry MP. When is a mouse basophil not a basophil? Blood. 2007;109:859–61. doi: 10.1182/blood-2006-06-027490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy P, Sun Y, Toubai T, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–73. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy P, Maeda Y, Hotary K, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci U S A. 2004;101:3921–6. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: The role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–13. [PubMed] [Google Scholar]
- 29.Reddy P, Negrin R, Hill GR. Mouse models of bone marrow transplantation. Biol Blood Marrow Transplant. 2008;14:129–35. doi: 10.1016/j.bbmt.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–5. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 31.Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–81. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 32.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11:1244–9. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 33.Denzel A, Maus UA, Rodriguez Gomez M, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–42. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald KP, Palmer JS, Cronau S, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–63. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 35.Matte CC, Liu J, Cormier J, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–92. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173:5467–75. doi: 10.4049/jimmunol.173.9.5467. [DOI] [PubMed] [Google Scholar]
- 37.Duffner UA, Maeda Y, Cooke KR, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393–8. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 38.Markey KA, Banovic T, Kuns RD, et al. Conventional dendritic cells are the critical donor APC presenting alloantigen after experimental bone marrow transplantation. Blood. 2009;113:5644–9. doi: 10.1182/blood-2008-12-191833. [DOI] [PubMed] [Google Scholar]
- 39.Rowe V, Banovic T, MacDonald KP, et al. Host B cells produce IL-10 following TBI and attenuate acute GVHD after allogeneic bone marrow transplantation. Blood. 2006;108:2485–92. doi: 10.1182/blood-2006-04-016063. [DOI] [PubMed] [Google Scholar]
- 40.Matte-Martone C, Wang X, Anderson B, et al. Recipient B cells are not required for graft-versus-host disease induction. Biol Blood Marrow Transplant. 2010;16:1222–30. doi: 10.1016/j.bbmt.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy GF, Sueki H, Teuscher C, Whitaker D, Korngold R. Role of mast cells in early epithelial target cell injury in experimental acute graft-vs-host disease. J InvestDermatol. 1994;102:451–61. doi: 10.1111/1523-1747.ep12373016. [DOI] [PubMed] [Google Scholar]