Abstract
Derivatization of peptides as quaternary ammonium salts (QAS) is a promising method for sensitive detection by electrospray ionization tandem mass spectrometry (Cydzik et al. J. Pept. Sci. 2011, 17, 445–453). The peptides derivatized by QAS at their N-termini undergo fragmentation according to the two competing mechanisms – charge remote (ChR) and charge directed (ChD). The absence of mobile proton in the quaternary salt ion results in ChR dissociation of a peptide bond. However, Hofmann elimination of quaternary salt creates an ion with one mobile proton leading to the ChD fragmentation. The experiments on the quaternary ammonium salts with deuterated N-alkyl groups or amide NH bonds revealed that QAS derivatized peptides dissociate according to the mixed ChR-ChD mechanism. The isotopic labeling allows differentiation of fragments formed according to ChR and ChD mechanisms.
Electronic supplementary material
The online version of this article (doi:10.1007/s13361-011-0245-2) contains supplementary material, which is available to authorized users.
Key words: Quaternary ammonium salts, Derivatization of peptides, Peptide sequencing
Introduction
Structural characterization of molecules by mass spectrometry relies on the fragmentation of precursor ions during the analysis, yielding a variety of structurally significant fragment ions [1]. There are two distinct fragmentation pathways of protonated peptides, based on charge-directed (ChD) and charge-remote (ChR) mechanisms. ChD reactions occur for peptides containing a proton attached to amino group. The proton is mobile and can migrate from one protonation site to another [2–4]. The protonation of amide nitrogen weakens the amide bond, resulting in fragmentation of the peptide backbone after gas-phase collisional activation [5].
The first examples of ChR fragmentation were observed during a study of fatty acids [6]. For peptides, the ChR processes occur when the charge is fixed on one particular atom, e.g., at arginine residue, which has high proton affinity [7–9]. The effect of arginine residue can be mimicked by derivatizing a peptide with a fixed positive charge-carrying molecule, including quaternary ammonium salts (QAS) [10]. The ChR fragmentation does not depend on the mobile proton but it is rather caused by the intramolecular hydrogen shifting within the precursor ion. To pinpoint which of the hydrogens (α, β, and amide) is involved in the mechanism, peptide derivatives with selective deuterium labeling were analyzed [11, 12]. The results suggest that it is the amide hydrogen of the residue at which the cleavage occurs that shifts during the fragmentation. Previously, Reid et al. described the competition of both fragmentation pathways during ESI-MS/MS experiment using protonated serine and its derivatives [13]. To the best of our knowledge, the coexistence of both mechanisms during the fragmentation of the nonprotonated [M]+ molecular ion of QAS-peptide derivatives has not been reported.
Recently, we presented a new method of derivatization of peptides as QAS on solid support [14]. The proposed methodology offers a novel approach to increase sensitivity in analysis of peptides by electrospray ionization tandem mass spectrometry. In this study, we present the fragmentation pathways of model peptides conjugated with various linear QAS. We performed ESI-MS/MS experiments on [M]+ ions of all the synthesized QAS-peptide derivatives. With the aid of deuterium-labeled analogs the contribution of two known distinct fragmentation pathways, charge-directed and charge-remote mechanisms, was examined using collision-induced dissociation (CID).
Experimental
Materials
The details of performed syntheses are given as Supplementary Data.
Hydrogen/Deuterium Exchange
H/D exchange was initiated by dissolving 0.1 mg of QAS-peptide derivatives in 200 μL of D2O at room temperature. After 20 min, all protons from the amide bonds and amino acid side chains were exchanged for deuterons, as judged from the ESI-MS analysis.
Mass Spectrometry
All experiments were performed on a Fourier transform ion cyclotron resonance (FT-ICR) Apex-Qe Ultra 7T instrument (Bruker Daltonics, Bremen, Germany). The instrumental settings and measurement details are described in Supplementary Data.
Results and Discussion
To investigate the influence of QAS on the mechanism of peptide fragmentation we synthesized on solid phase a series of model tetrapeptides containing four different QAS: trimethyl-, triethyl-, tripropyl- and tributyl-ammonium acetyl (TMAA, TEAA, TPAA, TBAA, respectively) derivatized peptides (Table 1S in Supplementary Data). We performed ESI-MS/MS experiments on [M]+ ions of all the synthesized QAS-peptide derivatives. As we indicated in our previous article [14], a series of fragment ions [a-C2H4] and [b-C2H4] for TEAA, [a-C3H6] and [b-C3H6] for TPAA, and [a-C4H8] and [b-C4H8] for TBAA peptides derivatives were observed as the consequence of partial QAS group fragmentation by Hofmann elimination (Supplementary Data, Figure 2S). The neutral loss of the alkene molecule (CnH2n) results in appearance of a mobile proton in the QAS-derivatized peptide ion, which was further used for studying the hydrogen scrambling in deuterated peptide ions.
The aim of our study was to prove whether the hydrogen atom located at the 1' or the 2' carbon atom of the QAS alkyl group participates in the Hofmann elimination. We synthesized two TEAA peptide derivatives with deuterons located at the 1' carbon atoms of N-alkyl groups of QAS (d6-Et3N+-CH2CO-Asp-Val-Tyr-Thr-NH2 ((d 6 -Et 3 N)-1b), d6-Et3N+-CH2CO-Ala-Ala-Ala-Ala-NH2 ((d 6 -Et 3 N)-5b)). For this purpose we developed a new method of the synthesis of QAS-peptides on solid support by direct N-alkylation of the amino group by deuterated ethyl iodide CH3CD2I.
The representative MS/MS spectrum of the parent ion [M]+ for peptide (d 6 -Et 3 N)-1b is presented in Figure 1. The analysis reveals a series of b-type ions, ranging from b2 to b4, accompanied by the corresponding a-type ions. A series of a- and b-fragment ions with neutral losses of 30.0439 were observed as the consequence of C2H2D2 elimination from the QAS group. A similar fragmentation pathway was also observed for the second synthesized deuterated peptide, (d 6 -Et 3 N)-5b (Supplementary Data, Figure 3S). The neutral loss of C2H2D2 rather than C2H3D indicates that it is the proton from methyl, not methylene group which is left on nitrogen after Hofmann elimination of the ethylene. This mobile proton can then migrate to amide groups of peptide backbone thus allowing the ChD fragmentation mechanism (Figure 1).
Figure 1.
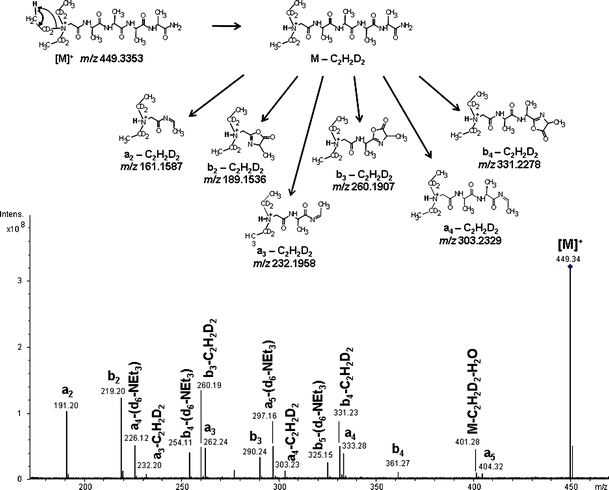
ESI MS/MS spectrum of the [M]+ molecular ion of peptide (d 6 -Et 3 N)-1b. Neutral losses of the alkene molecule from (d 6 -Et 3 N)-1b and its a- and b-fragment ions resulting from Hofmann elimination are presented (theoretical values of m/z are given)
ESI- MS/MS Analysis of Deuterated QAS-Peptide Derivatives
To check the contribution of two fragmentation pathways, ChD and ChR, during fragmentation of the QAS-peptides, we obtained a series of derivatives (Table 1S in Supplementary Data) and replaced all exchangeable protons by deuterons. Therefore, in ChR mechanism, the amide deuteron should contribute to the fragment formation. On the other hand, the ChD fragmentation pathway may occur when a proton is transferred from the 2' carbon atom of the N-alkyl group of a QAS-residue during Hofmann elimination in the initial alkene loss during CID.
We performed the ESI-MS/MS analysis on the monoisotopic ion of the deuterated precursor to determine the participation of both mechanisms. Figure 2c presents a high resolution ESI-MS/MS spectrum of peptide d 9 -1d, with all exchangeable protons substituted by deuterons. The parent ion [M]+ selected for fragmentation contains specific species of d 9 -1d, with negligible amount (less than 2%) of molecules containing 13C atoms. Therefore, the intensity ratio of isotopic peaks of the resulting fragments that differ from each other by one Da depends on the contribution of protons and deuterons (assigned as L and H peaks) but not contribution of 13C atoms. The obtained results indicate that the formation of a- and b-ions (without neutral losses) occurs with amide deuteron shift, since the formed fragments were represented by single peaks [Figure 2c (insets)]. This strongly supports the ChR fragmentation mechanism during formation of a- and b-ions. In contrast, all fragment ions [a-C4H8] and [b-C4H8] are represented by pairs of peaks, differing by one mass unit. The peak, located at higher m/z (H) corresponds to the fragment with all protons exchanged by deuterons, whereas the second one, located at lower m/z (L), represents the fragment with one remaining proton.
Figure 2.
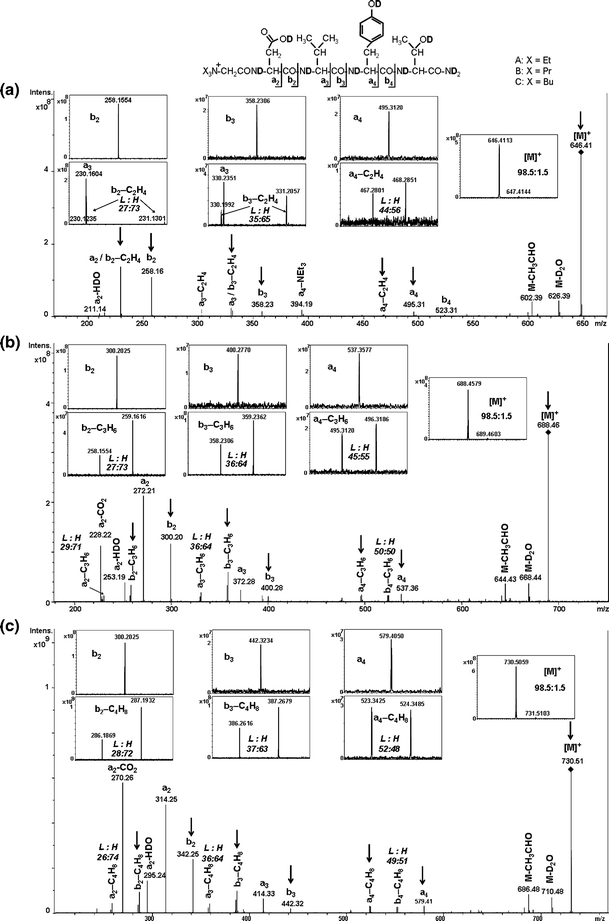
ESI-MS/MS spectra of deuterated QAS-peptides. (a) d 9 -1b, X = Et, (b) d 9-1c, X = Pr, and (c) d 9 -1d, X = Bu. The peaks of representative fragments (L and H peaks) are shown in insets. ESI-MS/MS spectra for other deuterated QAS-peptides and ratios of L and H peak intensities for all QAS-peptides are presented in Supplementary Data (Figures 4–6S and Table 2S)
The presence of both isotopic L and H peaks may be explained assuming that Hofmann elimination (Figure 1) results in formation of a protonated peptide, which therefore undergoes the ChD fragmentation according to the mobile proton model [15], with fast intramolecular proton exchange (hydrogen scrambling) and peptide bond dissociation initiated by protonation. The ion of analyzed peptide d 9 -1d possesses nine exchangeable deuterons (Figure 2c). Hofmann elimination results in protonation of an alkylated N-terminal nitrogen. As a result, the [M – C4H8] ion contains nine deuterons and one proton. Assuming that the hydrogen scrambling is not impaired, each protonation site contains 90% D and 10% H. Therefore, the intensities of L and H peaks should depend on the number of available sites in consecutive fragment ions. For example, the [a4 – C4H8] fragment retains five protonation sites from [M – C4H8] (50%), therefore, the expected L:H ratio is 50:50. This value is in good agreement with the experimental L:H peak ratio (52:48). The experimental proton contents for [b3 – C4H8] and [b2 – C4H8] ions (37% and 27%) were slightly higher as compared to the calculated ones (30% and 20%, respectively). One of the possible explanations of this phenomenon is that b – C4H8 ions (L and H) may be formed from d 9 -1d precursor according to two parallel fragmentation pathways. The first one is based on the described above assumption that after the initial Hofmann elimination the proton located at the N-terminal nitrogen participates in hydrogen scrambling with deuterated amides of peptide backbone. The following ChD fragmentation leads to two b4 – C4H8 ions represented by L and H peaks, since the discussed proton may remain in the b4 – C4H8 fragment or in the neutral C-terminal fragment. The alternative pathway leading to the b – C4H8 ions may be initiated by ChR fragmentation of the d 9 -1d precursor, with the proceeding Hofmann elimination. The second pathway may only produce proton-containing b-fragments (represented by L peaks), as the cleavage of amide bond occurs before the proton from Hofmann elimination is available for scrambling.
Similar results were obtained for other tested QAS-peptide derivatives (supplementary data, Figures 4–6S), which indicates that neither the carboxyl group of aspartic acid side chain nor the hydroxyl groups of tyrosine and threonine residues seem to affect the fragmentation pathways. The scheme of proposed fragmentation pathways for a representative peptide d 6 -5d is presented in Figure 3.
Figure 3.
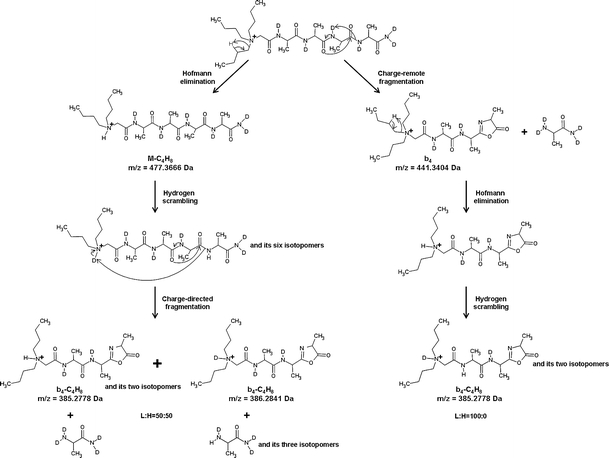
Two possible pathways of b4-C4H8 formation from d 6 -5d precursor ion. Theoretical m/z values and intensity ratios of L and H peaks are given
Conclusions
Peptides derivatized with quaternary ammonium salts are characterized by a high ionization efficiency, which allows a sensitive mass spectrometric detection of these compounds. The fragmentation spectra are dominated by a- and b-type ions, with abundant neutral losses corresponding to Hofmann elimination. The use of isotopic labeling allows the identification of contributions of the ChR and ChD mechanisms to the fragmentation of QAS-derivatized peptides. The mobile proton required for ChD fragmentation ion is generated from Hofmann elimination of ammonium salt, which creates a convenient model for studying hydrogen scrambling in peptide ions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 504 kb)
Acknowledgments
The authors acknowledge support for this work by grant no. N N204 180040 from the Ministry of Science and Higher Education of Poland.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Hunt DF, Yates JR, Shabanowitz J, Winston S, Hauer CR. Protein sequencing by tandem mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6233–6237. doi: 10.1073/pnas.83.17.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsaprailis G, Nair H, Somogyi A, Wysocki VH, Zhong W, Futrell JH, Summerfield SG, Gaskell SJ. Influence of secondary structure on the fragmentation of protonated peptides. J. Am. Chem. Soc. 1999;121:5142–5154. doi: 10.1021/ja982980h. [DOI] [Google Scholar]
- 3.Paizs B, Suhai S. Fragmentation pathways of protonated peptides. Mass Spectrom. Rev. 2005;24:508–548. doi: 10.1002/mas.20024. [DOI] [PubMed] [Google Scholar]
- 4.Dongre AR, Jones JL, Somogyi A, Wysocki VH. Influence of peptide composition, gas-phase basicity, and chemical modification on fragmentation efficiency: Evidence for the mobile proton model. J. Am. Chem. Soc. 1996;118:8365–8374. doi: 10.1021/ja9542193. [DOI] [Google Scholar]
- 5.McCormack AL, Somogyi A, Dongre AR, Wysocki VH. Fragmentation of protonated peptides: Surface-induced dissociation in conjunction with a quantum mechanical approach. Anal. Chem. 1993;65:2859–2872. doi: 10.1021/ac00068a024. [DOI] [PubMed] [Google Scholar]
- 6.Tomer KB, Crow FW, Gross ML. Location of double bond position in unsaturated fatty acids by negative ion MS/MS. J. Am. Chem. Soc. 1983;105:5487–5488. doi: 10.1021/ja00354a055. [DOI] [Google Scholar]
- 7.Cheng C, Gross ML. Applications and mechanisms of charge-remote fragmentation. Mass Spectrom. Rev. 2000;19:398–420. doi: 10.1002/1098-2787(2000)19:6<398::AID-MAS3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Adams J. Charge-remote fragmentations: Analytical applications and fundamental studies. Mass Spectrom. Rev. 1990;9:141–186. doi: 10.1002/mas.1280090202. [DOI] [Google Scholar]
- 9.Syrstad EA, Turecek F. Toward a general mechanism of electron capture dissociation. J. Am. Soc. Mass Spectrom. 2005;16:208–224. doi: 10.1016/j.jasms.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Zaia J, Biemann K. Comparison of charged derivatives for high energy collision-induced dissociation tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 1995;6:428–436. doi: 10.1016/1044-0305(95)00018-9. [DOI] [PubMed] [Google Scholar]
- 11.Liao PC, Huang ZH, Allison J. Charge remote fragmentation of peptides following attachment of a fixed positive charge: A matrix-assisted laser desorption/ionization post-source decay study. J. Am. Soc. Mass Spectrom. 1997;8:501–509. doi: 10.1016/S1044-0305(97)81513-9. [DOI] [Google Scholar]
- 12.Sadagopan N, Watson JT. Mass spectrometric evidence for mechanism of fragmentation of charge-derivatized peptides. J. Am. Soc. Mass. Spectrom. 2001;12:399–409. doi: 10.1016/S1044-0305(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 13.Reid GE, Simpson RJ. Leaving group and gas phase neighboring group effects in the side chain losses from protonated serine and its derivatives. J. Am. Soc. Mass Spectrom. 2000;11:1047–1060. doi: 10.1016/S1044-0305(00)00189-6. [DOI] [PubMed] [Google Scholar]
- 14.Cydzik M, Rudowska M, Stefanowicz P, Szewczuk Z. Derivatization of peptides as quaternary ammonium salts for sensitive detection by ESI-MS. J. Pept. Sci. 2011;17:445–453. doi: 10.1002/psc.1342. [DOI] [PubMed] [Google Scholar]
- 15.Laskin J, Lifshitz C. Principles of Mass Spectrometry Applied to Biomolecules. New Jersey: Wiley; 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 504 kb)


