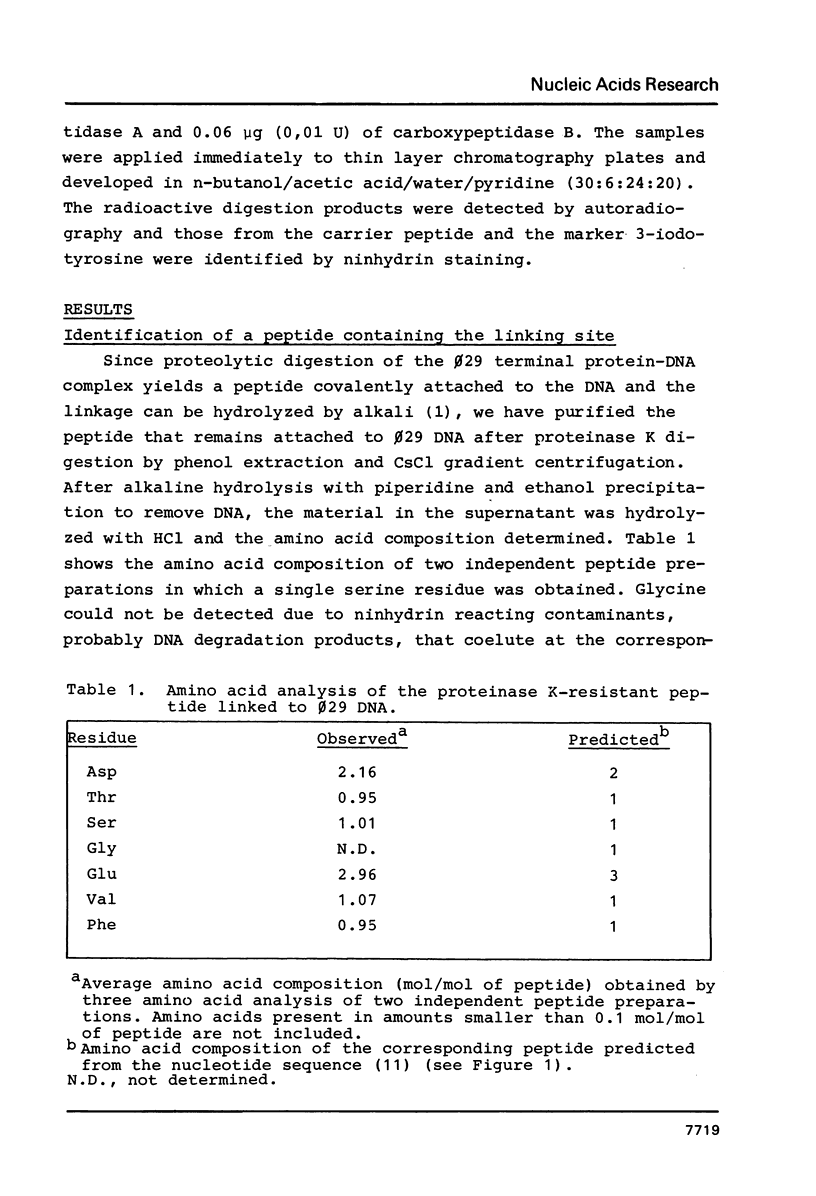
Abstract
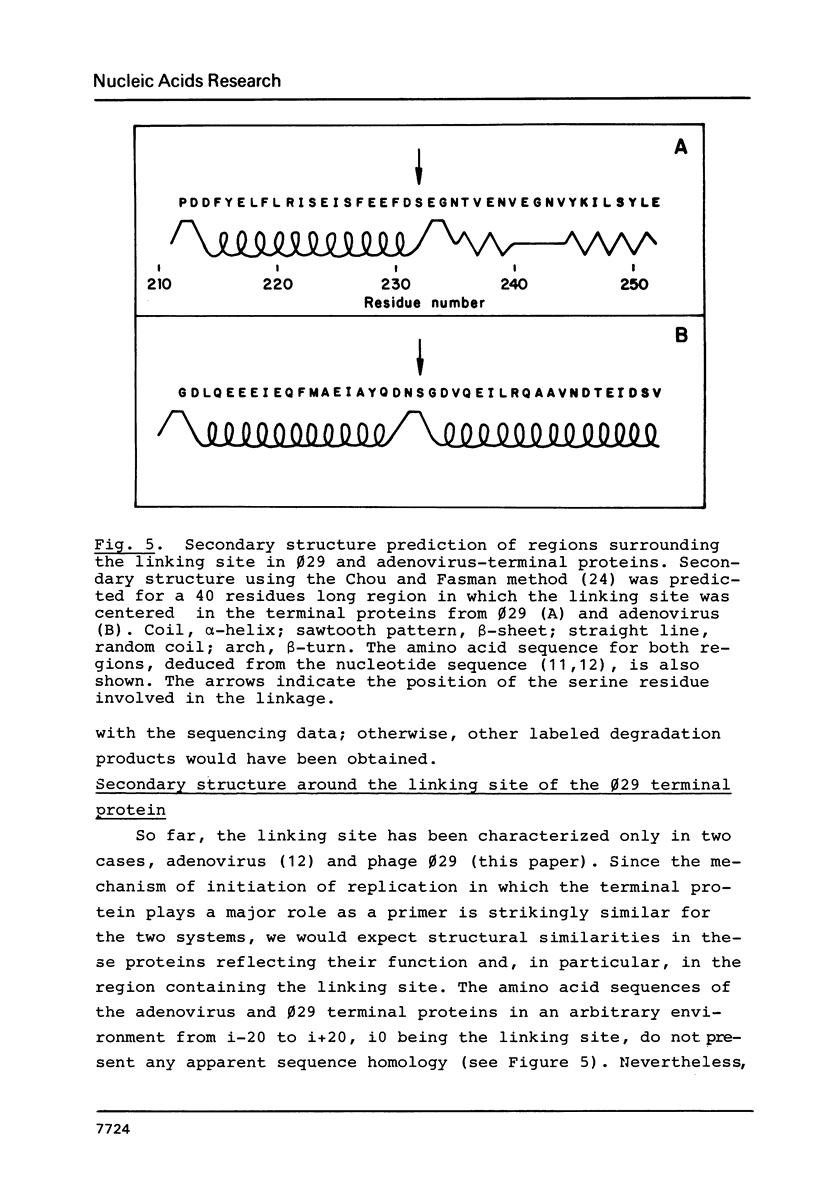
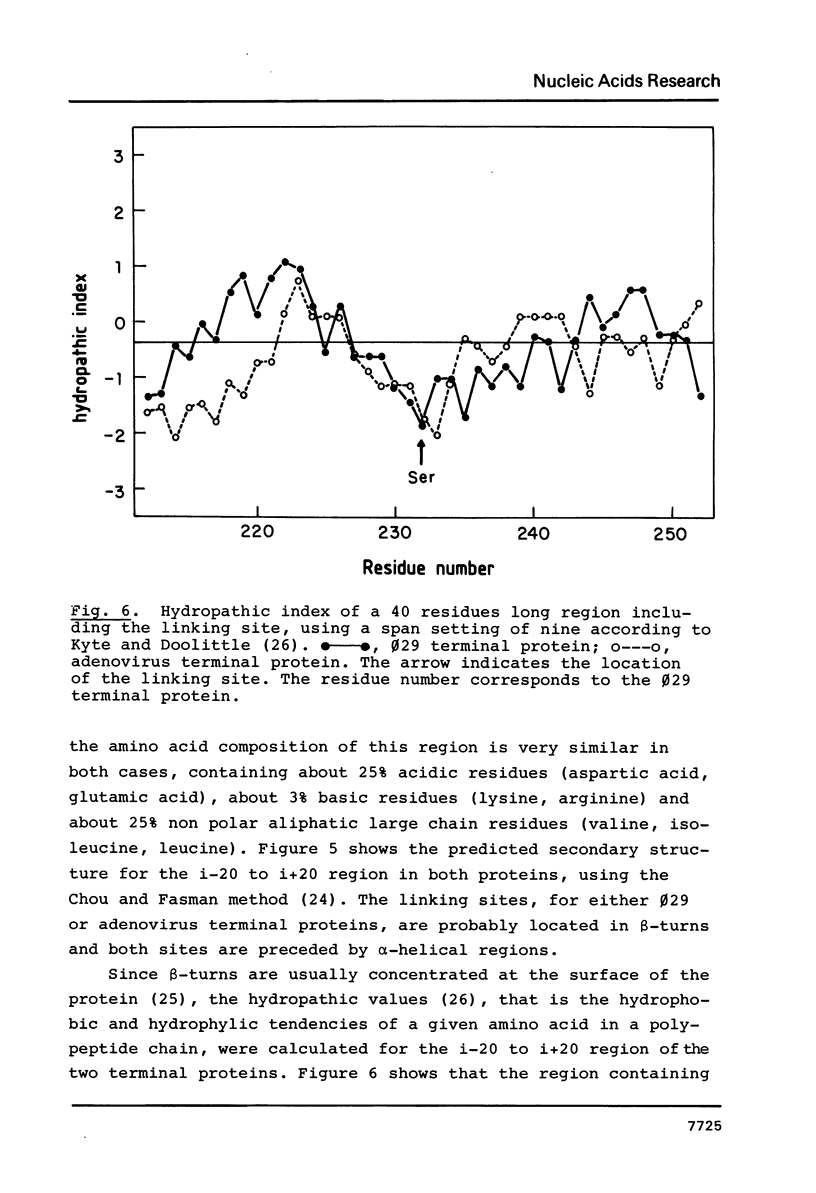
B. subtilis phage phi 29 has a terminal protein, p3, covalently linked to the 5' ends of the DNA through a phosphodiester bond between a serine residue and 5'-dAMP. This protein acts as a primer in DNA replication by forming an initiation complex with the 5'-terminal nucleotide dAMP. The amino acid sequence of the terminal protein, deduced from the nucleotide sequence of gene 3, showed the presence of 18 serine residues in a total of 266 amino acids. In this paper we have identified the serine involved in the linkage with the DNA as the residue 232, located close to the C-terminus of the molecule. This result was obtained by amino acid analysis of the peptide that remains linked to the DNA after proteinase K digestion of the terminal protein-phi 29 DNA complex and automated Edman degradation of the corresponding [125I]-labeled tryptic peptide. Prediction of the secondary structure of the terminal protein suggested that the serine residue involved in the linkage with the DNA is placed in a beta-turn, probably located on the external part of the molecule, as indicated by hydropathic values.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamford D. H., Mindich L. Characterization of the DNA-protein complex at the termini of the bacteriophage PRD1 genome. J Virol. 1984 May;50(2):309–315. doi: 10.1128/jvi.50.2.309-315.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford D., McGraw T., MacKenzie G., Mindich L. Identification of a protein bound to the termini of bacteriophage PRD1 DNA. J Virol. 1983 Aug;47(2):311–316. doi: 10.1128/jvi.47.2.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Kelly T. J., Jr Structure of the linkage between adenovirus DNA and the 55,000 molecular weight terminal protein. J Mol Biol. 1981 Jan 15;145(2):319–337. doi: 10.1016/0022-2836(81)90208-4. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Escarmís C., Salas M. Nucleotide sequence of the early genes 3 and 4 of bacteriophage phi 29. Nucleic Acids Res. 1982 Oct 11;10(19):5785–5798. doi: 10.1093/nar/10.19.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García E., Gómez A., Ronda C., Escarmis C., López R. Pneumococcal bacteriophage Cp-1 contains a protein bound to the 5' termini of its DNA. Virology. 1983 Jul 15;128(1):92–104. doi: 10.1016/0042-6822(83)90321-5. [DOI] [PubMed] [Google Scholar]
- Guyer R. L., Todd C. W. Protein sequencing: thermal conversion of thiazolinone derivatives of amino acids to thiohydantoins. Anal Biochem. 1975 Jun;66(2):400–404. doi: 10.1016/0003-2697(75)90607-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto F., Horigome T., Kanbayashi M., Yoshida K., Sugano H. An improved method for separation of low-molecular-weight polypeptides by electrophoresis in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1983 Feb 15;129(1):192–199. doi: 10.1016/0003-2697(83)90068-4. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Salas M. Protein p3 is linked to the DNA of phage phi 29 through a phosphoester bond between serine and 5'-dAMP. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6425–6428. doi: 10.1073/pnas.77.11.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirochika H., Nakamura K., Sakaguchi K. A linear DNA plasmid from Streptomyces rochei with an inverted terminal repetition of 614 base pairs. EMBO J. 1984 Apr;3(4):761–766. doi: 10.1002/j.1460-2075.1984.tb01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciarte M. R., Lázaro J. M., Salas M., Vińuela E. Physical map of bacteriophage phi29 DNA. Virology. 1976 Oct 15;74(2):314–323. [PubMed] [Google Scholar]
- Kemble R. J., Thompson R. D. S1 and S2, the linear mitochondrial DNAs present in a male sterile line of maize, possess terminally attached proteins. Nucleic Acids Res. 1982 Dec 20;10(24):8181–8190. doi: 10.1093/nar/10.24.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz I. D. Protein folding. J Am Chem Soc. 1972 May 31;94(11):4009–4012. doi: 10.1021/ja00766a060. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- López R., Ronda C., García P., Escarmís C., García E. Restriction cleavage maps of the DNAs of Streptococcus pneumoniae bacteriophages containing protein covalently bound to their 5' ends. Mol Gen Genet. 1984;197(1):67–74. doi: 10.1007/BF00327924. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Salas M. Initiation of phage phi 29 DNA replication by the terminal protein modified at the carboxyl end. Nucleic Acids Res. 1983 Nov 11;11(21):7397–7407. doi: 10.1093/nar/11.21.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez E. A manual subtractive end-group determination of oligopeptides using fluorescamine or o-phthalaldehyde. Anal Biochem. 1982 Nov 15;127(1):55–60. doi: 10.1016/0003-2697(82)90143-9. [DOI] [PubMed] [Google Scholar]
- Peñalva M. A., Salas M. Initiation of phage phi 29 DNA replication in vitro: formation of a covalent complex between the terminal protein, p3, and 5'-dAMP. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5522–5526. doi: 10.1073/pnas.79.18.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart J. E., Stillman B. W. Adenovirus terminal protein precursor. Partial amino acid sequence and the site of covalent linkage to virus DNA. J Biol Chem. 1982 Nov 25;257(22):13499–13506. [PubMed] [Google Scholar]
- Stillman B. W. The replication of adenovirus DNA with purified proteins. Cell. 1983 Nov;35(1):7–9. doi: 10.1016/0092-8674(83)90201-5. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]