Abstract
Purpose
Aims of this cross-sectional study were to assess health status and care dependency in patients with advanced chronic obstructive pulmonary disease (COPD) or chronic heart failure (CHF) and to identify correlates of an impaired health status.
Methods
The following outcomes were assessed in outpatients with advanced COPD (n = 105) or CHF (n = 80): clinical characteristics; general health status (EuroQol-5 Dimensions (EQ-5D); Assessment of Quality of Life instrument (AQoL); Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36)); disease-specific health status (St. Georges Respiratory Questionnaire (SGRQ), Minnesota Living with Heart Failure Questionnaire (MLHFQ)); physical mobility (timed ‘Up and Go’ test); and care dependency (Care Dependency Scale).
Results
Patients with advanced COPD or CHF have an impaired health status and may be confronted with care dependency. Multiple regression analyses have shown that physical and psychological symptoms, care dependency and number of drugs were correlated with impaired health status in advanced COPD or CHF, while demographic and clinical characteristics like age, gender, disease severity and co-morbidities were not correlated.
Conclusions
Clinical care should regularly assess symptom burden and care dependency to identify patients with advanced COPD or CHF at risk for an impaired health status.
Keywords: Chronic obstructive pulmonary disease, Chronic heart failure, Health status, Health-related quality of life, Care dependency, Functional status
Introduction
Patient’s self-reported health status, defined as the impact of health on a person’s ability to perform and derive fulfilment from the activities of daily life, is an important outcome [1]. Patients with advanced chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF) often have an impaired health status [2, 3]. Previous studies suggest that health status of patients with advanced COPD or CHF is equally or even more affected than health status of patients with incurable cancer [4, 5]. Furthermore, a decreased disease-specific health status is associated with reduced rates of survival in COPD and CHF [6–8].
Management plans of patients with advanced COPD or CHF should strive to optimize daily functioning and stabilize disease-specific health status [9–11]. Identifying clinical correlates of an impaired health status may allow clinicians to better monitor health status and intervene more effectively in patients with advanced COPD or CHF. Several correlates of diminished general or disease-specific health status in COPD or CHF have been suggested before, like gender, age, educational level, symptoms, psychological symptoms, disease severity, body mass index (BMI), co-morbidities, smoking status and number of physician-prescribed drugs [2, 3, 12–19]. However, currently available literature does not provide definitive evidence; hence, it is still unknown whether and to what extent these clinical correlates are interrelated in advanced COPD or CHF.
Health status includes patient’s self-reported quality of life and functional status [1]. Functional impairment may have significant consequences for patients and their families, such as social isolation of the patient and their loved ones in the case of impaired mobility [20, 21]. One observational study showed that patients with COPD or CHF admitted to the hospital may experience disability in basic and instrumental activities of daily living [22]. Impairment in the ability to perform normal daily tasks can lead to patients becoming dependent on caregivers [20]. A qualitative study of patients with end-stage COPD, CHF or renal disease and their family caregivers showed that increased dependency may lead to frustration, depression and social isolation and increases the burden on family caregivers [20]. Finally, there is some suggestion that care dependency is associated with increased mortality in patients hospitalized for an acute exacerbation of COPD [8]. However, quantitative studies comparing care dependency in clinically stable outpatients with advanced COPD or CHF are lacking. Moreover, it remains unknown whether and to what extent care dependency and health status are interrelated in patients with advanced COPD or CHF.
Aims of this cross-sectional study were to assess health status and care dependency in patients with advanced COPD or CHF and to identify correlates of impaired health status. The present authors hypothesized a priori that care dependency is an important correlate of general and disease-specific health status in patients with advanced COPD or CHF, irrespective of the underlying disease.
Methods
Design
This cross-sectional study is part of a longitudinal study concerning self-perceived symptoms and care needs in patients with severe to very severe COPD or CHF and the consequences for their closest relatives [23]. Details of the methodology of this study and data on advance care planning and symptom burden have been published before [23–25].
The study was registered at the Dutch Trial Register (NTR 1552).
Study population
Patients with advanced COPD or CHF were recruited by their physician specialist during an outpatient consultation of one academic and five general hospitals in the Netherlands in 2008 and 2009. Patients were eligible if they had a diagnosis of advanced COPD (Global initiative for chronic Obstructive Lung Disease (GOLD) stage III or IV) or CHF (New York Heart Association (NYHA) class III or IV). For patients who were referred for the study but refused participation, data like severity of their disease, age and gender were collected to compare characteristics of participating and non-participating patients. All participating patients gave written informed consent. The Medical Ethical Commission of the Maastricht University Medical Centre + (MUMC +), Maastricht, the Netherlands, approved this study (MEC 07-3-054).
Instruments
Patients were visited by a member of the research team in their home environment. The following outcomes were assessed: demographics; weight and height; self-reported co-morbidities (Charlson comorbidity index [26]); current medication; and forced expiratory volume in the first second (FEV1). FEV1 was calculated from the flow–volume curve measured by a handheld pulmonary spirometer [27]. Symptoms in the previous 2 weeks were assessed using visual analogue scales (VAS) [23, 24]. Severity of dyspnea was measured using the modified Borg scale (range 0 (nothing at all) to 10 (maximal)) [28]. Symptoms of anxiety and depression were studied using the Hospital Anxiety and Depression Scale (HADS) [29]. The HADS is divided into an anxiety subscale (HADS-A) and a depression subscale (HADS-D). Total scores for each subscale range from 0 (optimal) to 21 (worst) points.
General health status
General health status was assessed using the self-administered questionnaires EuroQol-5 Dimensions (EQ-5D) [30], Assessment of Quality of Life instrument (AQoL) [31] and the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) [32].
EQ-5D is a five-item questionnaire consisting of mobility, self-care, usual activity, pain/discomfort and anxiety/depression [30]. Each item has three levels: no problems, some problems and extreme problems. An index score is provided which ranges from −0.59 (worst) to 1.0 (best) [30]. In addition, patients rated their current health using VAS. VAS scores range from 0 (death or worst possible health) to 100 (best possible health).
AQoL consists of 15 items divided into five domains: illness; independent living; social relationships; physical senses; and psychological well-being [31]. Total score ranges from −0.04 (worst) to +1.00 (best) [33].
SF-36 consists of 36 items divided into eight domains: physical functioning; role-physical; bodily pain; general health; vitality; social functioning; role-emotional; and mental health. For each domain, scores range from 0 (worst) to 100 points (best) [32]. A physical component summary measure and mental component summary measure are provided using norm-based methods with scores from a Dutch general population [34]. These summary measure scores are transformed to make a minimum and maximum possible score of 0 and 100 points. All scores below 50 points can be interpreted as below the general population norm [32].
Disease-specific health status
Disease-specific health status was assessed in COPD patients using the St. Georges Respiratory Questionnaire (SGRQ) [35]. SGRQ provides three domain scores (symptoms; activities; and impact) and a total score, ranging from 0 (optimal) to 100 points (worst) [35].
In CHF patients, disease-specific health status was assessed using the Minnesota Living with Heart Failure Questionnaire (MLHFQ) [36]. MLHFQ consists of two domains: physical (ranges from 0 (best) to 40 points (worst)) and emotional (ranges from 0 (best) to 25 points (worst)). Total score ranges from 0 (best) to 105 points (worst) [36]. A total score >45 points is defined as poor health status, 24–45 points as moderate health status, while <24 points represent good health status [37].
Physical mobility
Physical mobility was assessed using the timed ‘Up and Go’ (TUG) test [38]. TUG test measures in seconds the time needed to stand up from a chair, walk a distance of three metres, turn and walk back to the chair and sit down again [38].
Care dependency
Care dependency was assessed using the Care Dependency Scale (CDS). CDS consists of 15 items regarding basic and instrumental activities of daily living, like personal care, household activities, social and recreational activities [39]. The score ranges from 15 (worst) to 75 points (best). Patients with a CDS score ≤68 points are considered as care dependent [40].
Statistics
Categorical variables are described as frequencies, while continuous variables have been tested for normality and are presented as mean and standard deviation (SD) or median and inter-quartile range (IQR). Categorical variables were compared between patients with COPD and CHF using Chi-square test. Continuous variables were compared between patients with COPD and CHF using independent sample T-test or Mann–Whitney U test, as appropriate. CDS score was compared between COPD and CHF after adjusting for age, gender, BMI and Charlson comorbidity index score using linear regression analysis with robust standard errors. To study correlates of general and disease-specific health status, multiple regression analysis models were developed for EQ-5D index score; AQoL total score; SGRQ total score; and MLHFQ total score. The following possible variables were tested for correlation with the dependent variables: age, gender, disease, Charlson comorbidity index score, FEV1, BMI, smoking status, marital status, level of education, modified Borg scale, number of self-perceived symptoms with VAS score greater than 30 mm, long-term oxygen therapy (LTOT), TUG score, HADS-A score, HADS-D score, CDS score, presence of a family caregiver, need for assistance with personal care by a professional caregiver and the number of physician-prescribed drugs. The variables that showed bivariate correlation with the dependent variables (Pearson correlation coefficient >0.30) were entered as independent variables in the standard multiple regression analysis models. Statistical analyses were performed using SPSS 17.0. STATA 11.1 was used for linear regression with robust standard errors. A priori, a two-sided level of significance was set at p ≤ 0.05 [41].
See online supplement for details.
Results
General patient characteristics
In total, 105 COPD patients and 80 CHF patients were included. The proportion of eligible patients who participated in the study was 62.9% for COPD and 46.0% for CHF patients, P < 0.05. Most COPD patients had very severe COPD (GOLD stage IV: n = 77, 73.3%). CHF patients were mainly classified as NYHA III (n = 74, 92.5%). On average, CHF patients were older and more often living alone than COPD patients (Table 1).
Table 1.
Patient characteristics
| COPD (n = 105) | CHF (n = 80) | |
|---|---|---|
| Gender (male), n (%) | 65 (61.9%) | 54 (67.5%) |
| Age (years), mean (SD) | 66.3 (9.2)* | 76.2 (8.3) |
| BMI (kg/m2), mean (SD)# | 26.3 (6.7)* | 28.6 (5.6) |
| Marital status (married/living with partner), n (%) | 78 (74.3%)* | 45 (56.3%) |
| Current smokers, n (%) | 26 (24.8%)* | 11 (13.8%) |
| FEV1 (% predicted), mean (SD)# | 34.1 (13.5)* | 75.5 (24.5) |
| Charlson index (pts), mean (SD) | 2.5 (1.7)* | 4.4 (2.0) |
| Long-term oxygen therapy, n (%) | 62 (59.0%)* | 10 (12.5%) |
| Need for professional caregiver, n (%) | 23 (21.9%)* | 32 (40.0%) |
| Family caregiver, n (%) | 90 (85.7%) | 61 (76.3%) |
| Number of symptoms, mean (SD) | 8.1 (3.7) | 9.1 (3.5) |
| Borg scale (points), mean (SD) | 4.8 (2.0)* | 4.0 (2.5) |
| HADS-A score (points), mean (SD) | 5.9 (4.5) | 5.6 (4.3) |
| HADS-D score (points), mean (SD) | 6.3 (4.0) | 6.9 (4.0) |
| Number of physician-prescribed drugs, mean (SD)# | 8.5 (3.9)* | 10.8 (3.7) |
Abbreviations: Body Mass Index (BMI); Chronic Obstructive Pulmonary Disease (COPD); Chronic Heart Failure (CHF); Forced Expiratory Volume in the first second (FEV 1); Hospital Anxiety and Depression Scale, Anxiety subscale (HADS-A); Hospital Anxiety and Depression Scale, Depression subscale (HADS-D)
* P < 0.05; # non-parametric statistical tests were used because of skewed data
Participating patients differed from patients who refused participation in some respects. COPD and CHF participants were younger than non-participants. Mean age of non-participants was 69.7 (9.7) years for COPD and 78.5 (9.0) years for CHF, P < 0.05. In addition, COPD participants had more advanced disease than patients who refused participation. Only 38.7% of non-participants was classified as GOLD-stage IV, P < 0.05. Finally, CHF participants were more often men compared to non-participants (40.4%), P < 0.05.
General health status
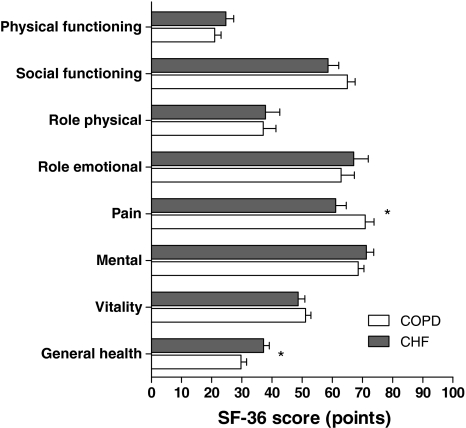
General health status was considerably impaired in patients with advanced COPD or CHF. Indeed, mean (SD) EQ-5D index score was 0.51 (0.33) for COPD patients and 0.47 (0.32) for CHF patients, P > 0.05. A higher proportion of CHF compared to COPD patients reported problems regarding pain/discomfort (Table 2). In addition, AQoL scores showed impairment in general health status for both diseases. However, mean (SD) total AQoL score was worse for CHF than COPD patients (0.35 (0.26) vs. 0.46 (0.28), respectively, P < 0.05) (Table 3). AQoL physical senses score was higher (better) for COPD than CHF patients. Other AQoL domain scores were comparable for COPD and CHF. The SF-36 showed impaired physical health status compared with a Dutch standard population [34]. Mean (SD) SF-36 physical component summary measure scores were comparable for COPD and CHF: 22.4 (9.6) and 22.2 (10.1) points, respectively, P > 0.05. Mean (SD) SF-36 mental component summary measure scores were 47.3 (14.5) points for COPD and 47.5 (14.6) points for CHF, respectively, P > 0.05. For most SF-36 domains, impairment was comparable for COPD and CHF patients. However, some differences were present. COPD patients reported more impairment in the domain ‘general health’, while CHF patients reported more impairment in the domain ‘pain’ (Fig. 1).
Table 2.
General health status assessed with EuroQol-5 Dimensions (EQ-5D)
| COPD (n = 105) | CHF (n = 80) | ||
|---|---|---|---|
| EQ-5D index score, mean (SD) | 0.51 (0.33) | 0.47 (0.32) | |
| EQ-5D VAS, mean (SD)# | 62.6 (14.0) | 62.1 (13.5) | |
| EQ-5D Mobility, n (%) | No problems | 16 (15.2%) | 8 (10.0%) |
| Some problems | 87 (82.9%) | 69 (86.2%) | |
| Extreme problems | 2 (1.9%) | 3 (3.8%) | |
| EQ-5D Self-care, n (%) | No problems | 43 (41.0%) | 27 (33.8%) |
| Some problems | 50 (47.6%) | 38 (47.4%) | |
| Extreme problems | 12 (11.4%) | 15 (18.8%) | |
| EQ-5D Usual activities, n (%) | No problems | 18 (17.1%) | 12 (15.0%) |
| Some problems | 61 (58.1%) | 50 (62.5%) | |
| Extreme problems | 26 (24.8%) | 18 (22.5%) | |
| EQ-5D Pain/discomfort*, n (%) | No problems | 57 (54.3%) | 29 (36.2%) |
| Some problems | 38 (36.2%) | 43 (53.8%) | |
| Extreme problems | 10 (9.5%) | 8 (10.0%) | |
| EQ-5D Anxiety/depression, n (%) | No problems | 62 (59.1%) | 44 (55.0%) |
| Some problems | 33 (31.4%) | 29 (36.2%) | |
| Extreme problems | 10 (9.5%) | 7 (8.8%) |
Abbreviations: Chronic Obstructive Pulmonary Disease (COPD); Congestive Heart Failure (CHF); EuroQol-5 Dimensions (EQ-5D); Visual Analogue Scale (VAS)
* P < 0.05; # non-parametric statistical tests have been used because of skewed data
Table 3.
General health status assessed with Assessment of Quality of Life (AQoL)
| COPD (n = 105) | CHF (n = 80) | |
|---|---|---|
| AQoL illness | 0.17 (0.17) | 0.18 (0.14) |
| AQoL independent living | 0.58 (0.30) | 0.50 (0.30) |
| AQoL social relationships# | 0.83 (0.20) | 0.78 (0.27) |
| AQoL physical senses# | 0.92 (0.11)* | 0.84 (0.15) |
| AQoL psychological well-being# | 0.87 (0.13) | 0.85 (0.15) |
| AQoL total | 0.46 (0.28)* | 0.35 (0.26) |
Values expressed as mean (SD)
Abbreviations: Assessment of Quality of Life instrument (AQoL); Chronic Obstructive Pulmonary Disease (COPD); Congestive Heart Failure (CHF)
* P < 0.05; # non-parametric statistical tests have been used because of skewed data
Fig. 1.
General health status assessed with Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36). Mean (SEM) domain scores of the SF-36 in patients with advanced chronic obstructive pulmonary disease (COPD, n = 105) or chronic heart failure (CHF, n = 80). * P < 0.05
Disease-specific health status
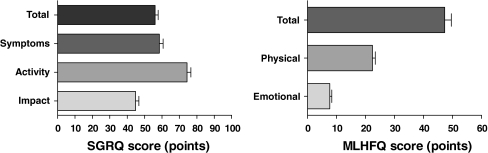
SGRQ scores and MLHFQ scores are shown in Fig. 2. Poor health status (>45 points) was reported by 43 CHF patients (53.7%), moderate health status (24–45 points) by 25 CHF patients (31.3%) and good health status (<24 points) by 12 patients (15.0%) with advanced CHF.
Fig. 2.
Disease-specific health status assessed with St. Georges Respiratory Questionnaire (SGRQ) and Minnesota Living with Heart Failure Questionnaire (MLHFQ). Mean (SEM) total and domain scores of the SGRQ in patients with advanced chronic obstructive pulmonary disease (COPD, n = 104) (left panel) and mean (SEM) total and domain scores of the MLHFQ in patients with advanced chronic heart failure (CHF, n = 80) (right panel)
Care dependency
CHF patients had a lower (worse) median (IQR) CDS score than COPD patients: 65.0 (58.3–71.0) points versus 70.0 (63.0–73.0) points, respectively, P < 0.05. However, CDS score was comparable for COPD and CHF, after adjusting for age, gender, BMI and Charlson comorbidity index score (adjusted P = 0.57). The linear regression model showed an association between age and CDS score (adjusted P = 0.03) and Charlson comorbidity index score and CDS score (adjusted P = 0.02). Older patients and patients with a higher Charlson comorbidity index score had lower CDS scores.
Correlates of general health status
The number of self-perceived symptoms, TUG test, HADS-A score, HADS-D score, Borg scale, CDS score, need for assistance by a professional caregiver and the number of physician-prescribed drugs showed bivariate correlation with EQ-5D index score (Pearson correlation coefficient >0.30). Age, gender, disease, Charlson comorbidity index score, FEV1, BMI, smoking status, marital status, level of education, LTOT and presence of a family caregiver did not show a bivariate correlation (Pearson correlation coefficient ≤ 0.30). The multiple regression analysis model, with EQ-5D index score as dependent variable and the aforementioned variables that showed bivariate correlation as independent variables, was able to explain 56.4% of the variance in EQ-5D index score. Patients who experienced more symptoms, impairment in physical mobility, more symptoms of anxiety, a higher level of care dependency, the need for a professional caregiver to assist with personal care or patients who were using a higher number of physician-prescribed drugs reported worse general health status, as assessed with EQ-5D (Table 4).
Table 4.
Correlates of general health status: results of standard multiple regression models
| Model | Correlates | Standardized Beta | P value |
|---|---|---|---|
| EQ-5D index score (R 2 = 0.564, P < 0.0005) | Number of symptoms | −0.149 | 0.022 |
| TUG test | −0.228 | 0.000 | |
| HADS-A | −0.182 | 0.007 | |
| HADS-D | −0.033 | 0.640 | |
| Borg scale | −0.096 | 0.083 | |
| CDS | 0.253 | 0.000 | |
| Need professional caregiver | −0.157 | 0.006 | |
| Number of drugs | −0.124 | 0.024 | |
| AQol total score (R 2 = 0.661, P < 0.0005) | Number of symptoms | −0.192 | 0.001 |
| TUG test | −0.006 | 0.914 | |
| HADS-A | −0.072 | 0.228 | |
| HADS-D | −0.170 | 0.006 | |
| Borg scale | −0.086 | 0.078 | |
| CDS | 0.347 | 0.000 | |
| Need professional caregiver | −0.225 | 0.000 | |
| Number of drugs | −0.137 | 0.005 |
n = 185
Abbreviations: Assessment of Quality of Life instrument (AQoL); Care Dependency Scale (CDS); EuroQol-5 Dimensions (EQ-5D); Hospital Anxiety and Depression Scale, Anxiety subscale (HADS-A); Hospital Anxiety and Depression Scale, Depression subscale (HADS-D); Timed ‘Up and Go’ test (TUG test)
The number of self-perceived symptoms, TUG test, HADS-A score, HADS-D score, Borg scale, CDS score, need for a professional caregiver and the number of physician-prescribed drugs also showed a bivariate correlation with AQoL total score, while the other variables did not show correlation. The standard multiple regression model showed that the number of symptoms, HADS-D score, CDS score, the need for a professional caregiver and the number of physician-prescribed drugs explained 66.1% of the variance in AQoL total score. Patients who experienced more symptoms, more symptoms of depression, a higher level of care dependency, the need for a professional caregiver or patients who were using more physician-prescribed drugs reported worse general health status, as assessed with AQoL (Table 4).
See online supplement for multiple regression analysis of general health status for COPD and CHF separately.
Correlates of disease-specific health status
The number of self-perceived symptoms, HADS-A score, HADS-D score, Borg scale and CDS score showed bivariate correlation with SGRQ total score (Pearson correlation coefficient >0.30). A bivariate correlation was not found for age, gender, Charlson comorbidity index score, FEV1, BMI, smoking status, marital status, level of education, TUG test, LTOT, presence of a family caregiver, the need for a professional caregiver or the number of medications the subject was using. The multiple regression analysis model showed that the number of symptoms, HADS-D score, Borg scale and CDS score were able to explain 57.5% of the variance in SGRQ total score in advanced COPD (Table 5). COPD patients who experienced increased symptoms, increased symptoms of depression, increased severe dyspnea or a higher level of care dependency reported more severe impairment in disease-specific health status.
Table 5.
Correlates of disease-specific health status: results of standard multiple regression models
| Model | Correlates | Standardized beta | P value | |
|---|---|---|---|---|
| COPD n = 104* | SGRQ total score (R 2 = 0.575, P < 0.0005) | Number of symptoms | 0.259 | 0.003 |
| HADS-A | −0.027 | 0.768 | ||
| HADS-D | 0.213 | 0.018 | ||
| Borg scale | 0.358 | 0.000 | ||
| CDS | −0.225 | 0.004 | ||
| CHF n = 80 | MLHFQ total score (R 2 = 0.529, P < 0.0005) | Number of symptoms | 0.251 | 0.020 |
| HADS-A | 0.311 | 0.005 | ||
| HADS-D | 0.074 | 0.511 | ||
| Borg scale | 0.165 | 0.076 | ||
| CDS | −0.212 | 0.020 |
Abbreviations: Care Dependency Scale (CDS); Chronic Obstructive Pulmonary Disease (COPD); Congestive Heart Failure (CHF); Hospital Anxiety and Depression Scale, Anxiety subscale (HADS-A); Hospital Anxiety and Depression Scale, Depression subscale (HADS-D); Minnesota Living with Heart Failure Questionnaire (MLHFQ); St. Georges Respiratory Questionnaire (SGRQ); Timed ‘Up and Go’ test (TUG test)
* 1 patient excluded because of missing values
The number of self-perceived symptoms, HADS-A score, HADS-D score, Borg scale and CDS score were shown to exhibit bivariate correlation with MLHFQ total score in patients with advanced CHF (Pearson correlation coefficient >0.30). The multiple regression analysis model showed that the number of symptoms, HADS-A score and CDS score were statistically significantly correlated with disease-specific health status. These variables predicted 52.9% of the variance in MLHFQ score. Patients with advanced CHF, who experienced a higher number of symptoms, more anxiety and a higher level of care dependency reported more impairment in disease-specific health status (Table 5).
Discussion
Key findings
The present study shows that patients with advanced COPD or CHF have an impaired health status, irrespective of the underlying disease. A substantial number of these patients are confronted with care dependency. Symptom burden, symptoms of anxiety and/or depression and care dependency are correlates of general and disease-specific health status in advanced COPD and/or CHF. In addition, the number of physician-prescribed drugs is correlated with general health status in advanced COPD and/or CHF. Demographic and clinical characteristics like age, gender, disease severity and co-morbidities are not correlated with general or disease-specific health status.
Health status, care dependency and correlates of health status
The present study has shown that age, gender, co-morbidities, lung function, smoking status, marital status, level of education, LTOT and presence of a family caregiver were not correlated with general and/or disease-specific health status in patients with advanced COPD or CHF. These findings conflict with some of the currently available literature. Previously, it was suggested that impaired general health status is associated with female gender and higher age in patients with CHF [13, 16]. However, most patients in these studies had mild to moderate CHF [13, 16], while the present study included only patients with severe to very severe CHF. Spencer and colleagues showed an association between smoking status and SGRQ total score in patients with COPD [17]. We did not confirm these findings in our sample of patients with advanced COPD. Other studies suggested an association between FEV1 and total SGRQ score in patients with severe COPD [14, 15], another finding that was not confirmed in the present study. Indeed, the present study showed lack of correlation between FEV1 and SGRQ total score (Spearman’s rho −0.093 (P = 0.354)). Then again, the present study showed also lack of correlation between FEV1 and EQ-5D index score (Spearman’s rho 0.087 (P = 0.387)) or AQoL total score (Spearman’s rho 0.161 (P = 0.107)) in patients with advanced COPD. Oga and colleagues have shown that change in health status and change in pulmonary function were not related [42]. In a recent study in clinically stable patients with COPD, severity of airflow limitation was poorly related to health status [43]. Thus, there is substantial evidence that FEV1 is not appropriate for identifying patients with advanced COPD at increased risk for an impaired health status.
Clinical correlates, like symptom burden, symptoms of anxiety and/or depression, care dependency and number of physician-prescribed drugs were able to explain 56% and 66% of EQ-5D index score and AQoL total score, respectively. Higher symptom burden is associated with worse general and disease-specific health status in patients with advanced COPD or CHF. The correlation between severity of dyspnea and disease-specific health status in COPD confirms previous findings [14, 15, 18, 44]. Furthermore, the correlation between symptom distress and health status has been shown before in patients with COPD or CHF and emphasizes again the importance of addressing symptom burden in patients with advanced COPD or CHF [2, 3, 19, 45].
The present study shows that care dependency is a major correlate of general and disease-specific health status in patients with advanced COPD or CHF. Regular assessment of care dependency should be part of routine clinical care for patients with advanced COPD or CHF. Management programmes aimed at optimizing health status of patients with advanced COPD or CHF should assess impairments in basic and instrumental activities of daily living and should try to minimize care dependency.
Limitations of the present study
The study population consisted of a convenience sample of patients. While the majority of eligible COPD patients were willing to participate, the response rate for CHF patients was below 50%. The current response rate confirms the previously reported difficulty of recruitment of older patients with CHF [46] and may limit the generalizability of the results.
Some differences were present between COPD and CHF patients. Although differences between COPD and CHF patients in age, marital status and smoking status are statistically significant, the clinical relevance for the present study may be limited, as is shown by the lack of correlation between health status and age, marital status or smoking status. Furthermore, patients with co-morbidities were not excluded from the analyses. Indeed, co-morbidities in patients with COPD and CHF are common [47–49] and might have influenced the results of the present study. Finally, the present study is a cross-sectional study, and health status and care dependency are likely to change during the course of the disease [7, 50]. A longitudinal follow-up study is warranted to further reveal how health status and care dependency change over time in patients with advanced COPD or CHF.
Conclusions and implications
This study demonstrates that health status is considerably impaired in patients with advanced COPD or CHF and confirms our hypothesis that care dependency is an important correlate of general and disease-specific health status. Patients confronted with care dependency, patients who experience more physical and psychological symptoms or patients who are using a higher number of physician-prescribed drugs are at risk for having an impaired health status. Therefore, clinicians should routinely assess symptom burden as well as care dependency in patients with advanced COPD or CHF.
Acknowledgments
This project was supported by: Proteion Thuis, Horn, The Netherlands; CIRO + , Horn, The Netherlands; Grant 3.4.06.082 of the Netherlands Asthma Foundation, Leusden, The Netherlands; Stichting Wetenschapsbevordering Verpleeghuiszorg (SWBV), Utrecht, The Netherlands. The present authors are grateful to research nurses Mrs. Els Verstraeten en Mrs. Jamila Dekker-Heuts for collection of the data and to Mrs. Linda Koolen for input of the data. The present authors are grateful to the doctors of the following collaborating hospitals and departments for their participation in this study: Maastricht University Medical Centre + (MUMC +), Maastricht, the Netherlands: Department of Respiratory Medicine, Department of Cardiology; Laurentius Hospital, Roermond, the Netherlands: Department of Respiratory Medicine; St Jans Gasthuis, Weert, the Netherlands: Department of Cardiology; Màxima Medical Centre, Veldhoven/Eindhoven, the Netherlands: Department of Cardiology; Catharina hospital, Eindhoven, the Netherlands: Department of Respiratory Medicine; Atrium Medical Centre, Heerlen, the Netherlands: Department of Cardiology. BioSci Consulting, Maasmechelen, Belgium, Scott Wagers for reviewing the text for clarity.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- AQoL
Assessment of Quality of Life instrument
- BMI
Body mass index
- CDS
Care Dependency Scale
- COPD
Chronic obstructive pulmonary disease
- CHF
Chronic heart failure
- EQ-5D
EuroQol-5 Dimensions
- FEV1
Forced expiratory volume in the first second
- GOLD
Global initiative for chronic Obstructive Lung Disease
- HADS
Hospital Anxiety and Depression Scale
- LTOT
Long-term oxygen therapy
- SF-36
Medical Outcomes Study 36-Item Short-Form Health Survey
- MLHFQ
Minnesota Living with Heart Failure Questionnaire
- NYHA
New York Heart Association
- SGRQ
St. Georges Respiratory Questionnaire
- TUG
Timed ‘Up and Go’
- VAS
Visual analogue scale
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s11136-012-0165-z.
References
- 1.Curtis JR, Patrick DL. The assessment of health status among patients with COPD. European Respiratory Journal. 2003;41:36s–45s. doi: 10.1183/09031936.03.00078102. [DOI] [PubMed] [Google Scholar]
- 2.Blinderman CD, Homel P, Andrew Billings J, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. Journal of Pain and Symptom Management. 2009;38(1):115–123. doi: 10.1016/j.jpainsymman.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. Journal of Pain and Symptom Management. 2008;35(6):594–603. doi: 10.1016/j.jpainsymman.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55(12):1000–1006. doi: 10.1136/thorax.55.12.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Leary N, Murphy NF, O’Loughlin C, Tiernan E, McDonald K. A comparative study of the palliative care needs of heart failure and cancer patients. European Journal of Heart Failure. 2009;11(4):406–412. doi: 10.1093/eurjhf/hfp007. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal J, Francis L, Reid J, Murray S, Denvir M. Quality of life in patients with chronic heart failure and their carers: a 3-year follow-up study assessing hospitalization and mortality. European Journal of Heart Failure. 2010;12(9):1002–1008. doi: 10.1093/eurjhf/hfq114. [DOI] [PubMed] [Google Scholar]
- 7.Habraken JM, van der Wal WM, Ter Riet G, Weersink EJ, Toben F, Bindels PJ. Health-related quality of life and functional status in end-stage COPD: longitudinal study. European Respiratory Journal. 2011;37(2):280–288. doi: 10.1183/09031936.00149309. [DOI] [PubMed] [Google Scholar]
- 8.Almagro P, Calbo E, Ochoa de Echaguen A, Barreiro B, Quintana S, Heredia JL, Garau J. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448. doi: 10.1378/chest.121.5.1441. [DOI] [PubMed] [Google Scholar]
- 9.Curtis JR. Palliative and end-of-life care for patients with severe COPD. European Respiratory Journal. 2008;32(3):796–803. doi: 10.1183/09031936.00126107. [DOI] [PubMed] [Google Scholar]
- 10.Jaarsma T, Beattie JM, Ryder M, Rutten FH, McDonagh T, Mohacsi P, Murray SA, Grodzicki T, Bergh I, Metra M, Ekman I, Angermann C, Leventhal M, Pitsis A, Anker SD, Gavazzi A, Ponikowski P, Dickstein K, Delacretaz E, Blue L, Strasser F, McMurray J. Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure. 2009;11(5):433–443. doi: 10.1093/eurjhf/hfp041. [DOI] [PubMed] [Google Scholar]
- 11.Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, Levy M, Mularski RA, Osborne ML, Prendergast TJ, Rocker G, Sibbald WJ, Wilfond B, Yankaskas JR. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. American Journal of Respiratory and Critical Care Medicine. 2008;177(8):912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 12.Janssen DJ, Spruit MA, Leue C, Gijsen C, Hameleers H, Schols JM, Wouters EF. Symptoms of anxiety and depression in COPD patients entering pulmonary rehabilitation. Chronic Respiratory Disease. 2010;7(3):147–157. doi: 10.1177/1479972310369285. [DOI] [PubMed] [Google Scholar]
- 13.Azevedo A, Bettencourt P, Alvelos M, Martins E, Abreu-Lima C, Hense HW, Barros H. Health-related quality of life and stages of heart failure. International Journal of Cardiology. 2008;129(2):238–244. doi: 10.1016/j.ijcard.2007.07.091. [DOI] [PubMed] [Google Scholar]
- 14.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T. Stages of disease severity and factors that affect the health status of patients with chronic obstructive pulmonary disease. Respiratory Medicine. 2000;94(9):841–846. doi: 10.1053/rmed.2000.0804. [DOI] [PubMed] [Google Scholar]
- 15.Miravitlles M, Alvarez-Sala JL, Lamarca R, Ferrer M, Masa F, Verea H, Zalacain R, Murio C, Ros F. Treatment and quality of life in patients with chronic obstructive pulmonary disease. Quality of Life Research. 2002;11(4):329–338. doi: 10.1023/A:1015520110663. [DOI] [PubMed] [Google Scholar]
- 16.de Rivas B, Permanyer-Miralda G, Brotons C, Aznar J, Sobreviela E. Health-related quality of life in unselected outpatients with heart failure across Spain in two different health care levels. Magnitude and determinants of impairment: the INCA study. Quality of Life and Research. 2008;17(10):1229–1238. doi: 10.1007/s11136-008-9397-3. [DOI] [PubMed] [Google Scholar]
- 17.Spencer S, Calverley PM, Sherwood Burge P, Jones PW. Health status deterioration in patients with chronic obstructive pulmonary disease. American Journal of Respiratory Critical Care Medicine. 2001;163(1):122–128. doi: 10.1164/ajrccm.163.1.2005009. [DOI] [PubMed] [Google Scholar]
- 18.de Torres JP, Casanova C, Hernandez C, Abreu J, Montejo de Garcini A, Aguirre-Jaime A, Celli BR. Gender associated differences in determinants of quality of life in patients with COPD: a case series study. Health Quality of Life Outcomes. 2006;4:72. doi: 10.1186/1477-7525-4-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heo S, Moser DK, Lennie TA, Zambroski CH, Chung ML. A comparison of health-related quality of life between older adults with heart failure and healthy older adults. Heart and Lung. 2007;36(1):16–24. doi: 10.1016/j.hrtlng.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Fitzsimons D, Mullan D, Wilson JS, Conway B, Corcoran B, Dempster M, Gamble J, Stewart C, Rafferty S, McMahon M, MacMahon J, Mulholland P, Stockdale P, Chew E, Hanna L, Brown J, Ferguson G, Fogarty D. The challenge of patients’ unmet palliative care needs in the final stages of chronic illness. Palliative Medicine. 2007;21(4):313–322. doi: 10.1177/0269216307077711. [DOI] [PubMed] [Google Scholar]
- 21.Habraken JM, Pols J, Bindels PJ, Willems DL. The silence of patients with end-stage COPD: a qualitative study. British Journal of General Practice. 2008;58(557):844–849. doi: 10.3399/bjgp08X376186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Incalzi RA, Corsonello A, Pedone C, Corica F, Carbonin P, Bernabei R. Construct validity of activities of daily living scale: a clue to distinguish the disabling effects of COPD and congestive heart failure. Chest. 2005;127(3):830–838. doi: 10.1378/chest.127.3.830. [DOI] [PubMed] [Google Scholar]
- 23.Janssen DJ, Wouters EF, Schols JM, Spruit MA. Self-perceived symptoms and care needs of patients with severe to very severe chronic obstructive pulmonary disease, congestive heart failure or chronic renal failure and its consequences for their closest relatives: The research protocol. BMC Palliative Care. 2008;7:5. doi: 10.1186/1472-684X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen, D. J., Spruit, M. A., Uszko-Lencer, N. H., Schols, J. M., & Wouters, E. F. Symptoms, co-morbidities and healthcare in advanced COPD or chronic heart failure.Journal Palliative Medicine (In press). [DOI] [PubMed]
- 25.Janssen, D. J., Spruit, M. A., Schols, J. M., & Wouters, E. F. (2010) A call for high-quality advance care planning in outpatients with severe COPD or chronic heart failure. Chest (published online ahead of print). [DOI] [PubMed]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Disease. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Standardization of Spirometry, 1994 Update. American Thoracic Society. American Journal of Respiratory Critical Care Medicie, 152(3), 1107–1136 (1995). [DOI] [PubMed]
- 28.Wilson RC, Jones PW. A comparison of the visual analogue scale and modified Borg scale for the measurement of dyspnoea during exercise. Clinical Science (London) 1989;76(3):277–282. doi: 10.1042/cs0760277. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 30.Dolan P. Modeling valuations for EuroQol health states. Medical Care. 1997;35(11):1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Quality of Life Research. 1999;8(3):209–224. doi: 10.1023/A:1008815005736. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Snow KK, Kosinski M. SF-36 health survey manual and interpretation guide. Boston, MA: The Health Institute, New England Medical Center Hospitals; 1993. [Google Scholar]
- 33.Hawthorne G, Osborne R. Population norms and meaningful differences for the Assessment of Quality of Life (AQoL) measure. Australian and New Zealand Journal of Public Health. 2005;29(2):136–142. doi: 10.1111/j.1467-842X.2005.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 34.Kruijshaar ME, Hoeymans N, Bijl RV, Spijker J, Essink-Bot ML. Levels of disability in major depression: findings from the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Journal of Affective Disorders. 2003;77(1):53–64. doi: 10.1016/S0165-0327(02)00099-X. [DOI] [PubMed] [Google Scholar]
- 35.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. American Review Respiratory Disease. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 36.Riegel B, Moser DK, Glaser D, Carlson B, Deaton C, Armola R, Sethares K, Shively M, Evangelista L, Albert N. The Minnesota Living With Heart Failure Questionnaire: sensitivity to differences and responsiveness to intervention intensity in a clinical population. Nursing Research. 2002;51(4):209–218. doi: 10.1097/00006199-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Behlouli, H., Feldman, D. E., Ducharme, A., Frenette, M., Giannetti, N., Grondin, F., Michel, C., Sheppard, R., & Pilote, L. (2009). Identifying relative cut-off scores with neural networks for interpretation of the minnesota living with heart failure questionnaire. In Conference on Proceedings of IEEE on Engineering Medical and Biological Society, 1 (pp. 6242–6246). [DOI] [PubMed]
- 38.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 39.Dijkstra A, Tiesinga LJ, Goossen WT, Dassen TW. Further psychometric testing of the Dutch Care Dependency Scale on two different patient groups. International Journal of Nursing Practice. 2002;8(6):305–314. doi: 10.1046/j.1440-172X.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 40.Dijkstra A, Tiesinga LJ, Plantinga L, Veltman G, Dassen TW. Diagnostic accuracy of the care dependency scale. Journal of Advanced Nursing. 2005;50(4):410–416. doi: 10.1111/j.1365-2648.2005.03406.x. [DOI] [PubMed] [Google Scholar]
- 41.Altman DG, Gore SM, Gardner MJ, Pocock SJ. Statistical guidelines for contributors to medical journals. British Medical Journal (Clinical Research Ed) 1983;286(6376):1489–1493. doi: 10.1136/bmj.286.6376.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oga T, Nishimura K, Tsukino M, Hajiro T, Sato S, Ikeda A, Hamadas C, Mishima M. Longitudinal changes in health status using the chronic respiratory disease questionnaire and pulmonary function in patients with stable chronic obstructive pulmonary disease. Quality of Life Research. 2004;13(6):1109–1116. doi: 10.1023/B:QURE.0000031345.56580.6a. [DOI] [PubMed] [Google Scholar]
- 43.Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, MacNee W, Miller BE, Rennard S, Silverman EK, Tal-Singer R, Wouters E, Yates JC, Vestbo J. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respiratory Research. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spruit MA, Pennings HJ, Janssen PP, Does JD, Scroyen S, Akkermans MA, Mostert R, Wouters EF. Extra-pulmonary features in COPD patients entering rehabilitation after stratification for MRC dyspnea grade. Respiratory Medicine. 2007;101(12):2454–2463. doi: 10.1016/j.rmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Bekelman DB, Rumsfeld JS, Havranek EP, Yamashita TE, Hutt E, Gottlieb SH, Dy SM, Kutner JS. Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. Journal of General Internal Medicine. 2009;24(5):592–598. doi: 10.1007/s11606-009-0931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnes S, Gott M, Payne S, Parker C, Seamark D, Gariballa S, Small N. Recruiting older people into a large, community-based study of heart failure. Chronic Illness. 2005;1(4):321–329. doi: 10.1177/17423953050010040201. [DOI] [PubMed] [Google Scholar]
- 47.Rutten FH, Cramer MJ, Grobbee DE, Sachs AP, Kirkels JH, Lammers JW, Hoes AW. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. European Heart Journal. 2005;26(18):1887–1894. doi: 10.1093/eurheartj/ehi291. [DOI] [PubMed] [Google Scholar]
- 48.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: Role of comorbidities. European Respiratory Journal. 2006;28(6):1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 49.Bausewein C, Booth S, Gysels M, Kuhnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. Journal of Palliative Medicine. 2010;13(9):1109–1118. doi: 10.1089/jpm.2010.0068. [DOI] [PubMed] [Google Scholar]
- 50.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Mishima M. Longitudinal deteriorations in patient reported outcomes in patients with COPD. Respiratory Medicine. 2007;101(1):146–153. doi: 10.1016/j.rmed.2006.04.001. [DOI] [PubMed] [Google Scholar]